Abstract
Objectives
The purpose was to investigate two types of feed-forward postural adjustments associated with preparation to predictable external perturbations.
Methods
Nine subjects stood on a wedge, toes-up or toes-down while a pendulum impacted their shoulders. EMGs of leg and trunk muscles were analyzed within the framework of the uncontrolled manifold hypothesis.
Results
Early postural adjustments (EPAs) were seen 400–500 ms and anticipatory postural adjustments (APAs), 100–150 ms prior to the impact. EPAs and APAs were also seen in the time profiles of muscle modes representing muscle groups with linear scaling of the activation levels. Center of pressure shifts were stabilized by co-varied adjustments in muscle mode magnitudes across trials. The index of these multi-muscle synergies showed two drops (anticipatory synergy adjustments, ASAs), prior to EPA and APA in each subject. The findings were consistent between the two conditions.
Conclusions
The results show that feed-forward postural adjustments represent a sequence of two phenomena, EPAs and APAs. Each of those is preceded by ASAs that reduce stability of a variable that is to be adjusted during the EPAs and APAs. The findings fit a hierarchical scheme with synergic few-to-many mappings at each level of the hierarchy based on the referent body configuration hypothesis.
Significance
The results show the complexity of the postural preparation to action. Potentially, they have implications for the current strategies of rehabilitation of patients with neuro-motor disorders characterized by impaired postural control.
Keywords: Posture, anticipatory postural adjustment, synergy, anticipatory synergy adjustment, EMG
Introduction
Maintaining equilibrium during standing is a challenging task due to a number of factors including the small support area and the high center of mass location. Postural control has been viewed as an example of the famous problem of motor redundancy (Bernstein, 1967) in this case formulated as the problem of coordinated changes in the activation of numerous muscles of the lower extremities and the trunk. The notion of multi-muscle synergies has been used to address the problem of multi-muscle coordination. “Synergies”, however, have been defined differently by different authors. Several groups used matrix factorization techniques to identify groups of muscles with parallel scaling of the activation levels and addressed those as “synergies” (d’Avella et al., 2003, Ivanenko et al., 2006, Ting and Macpherson, 2005); for recent reviews see (Ting and Chvatal, 2010, Tresch and Jarc, 2009).
A different approach to synergies has been developed based on the principle of motor abundance (Gelfand and Latash, 1998). Within this approach synergies are associated with covaried adjustments in elemental variables that stabilize (decrease variability) of a potentially important performance variable to which all the elemental variables contribute (reviewed in (Latash et al., 2002, 2007)). Note that in this context, the word “stabilization” implies not mechanical stability (response to a small perturbation) but low variability as compared to what could be expected if co-variation were absent. In fact, this meaning of “stability” is intimately linked to dynamic stability of the system (Martin et al., 2009). Analysis within this approach also begins with identification of muscle groups with parallel scaling of activation levels. These groups, however, are viewed not as synergies but as elemental variables. Further, co-variation of magnitudes of those variables is analyzed to discover and quantify synergies stabilizing such potentially important variables for postural control as coordinate of the center of pressure and shear force (Danna-dos-Santos et al., 2007, Klous et al., 2010, Krishnamoorthy et al., 2003a, Krishnamoorthy et al., 2003b, Robert et al., 2008).
Recently, a phenomenon of anticipatory synergy adjustments (ASAs) has been demonstrated in studies of multi-digit action (Olafsdottir et al., 2005, Shim et al., 2005). During steady-state actions, individual digits show high indices of co-variation of their outputs stabilizing such variables as total force and total moment of force (Latash et al., 2001, Scholz et al., 2002, Shim et al., 2003, Zatsiorsky and Latash, 2008). If a subject is required to produce a quick action from a steady-state, the indices of co-variation drop about 150–200 ms prior to the action initiation (see also (Kim et al., 2006)). ASAs have been shown to shift towards the time of action initiation under the reaction-time instruction (Olafsdottir et al., 2005) and be reduced in magnitude in the elderly (Olafsdottir et al., 2007).
The mentioned features of ASAs make them similar to the well-known mechanism of feed-forward postural control addressed as anticipatory postural adjustments (APAs, (Aruin and Latash, 1995b, Belenkiy et al., 1967, Bouisset and Zattara, 1990); reviewed in (Massion, 1992)). However, the roles of ASAs and APAs have been assumed to be different. ASAs reflect attenuation of a synergy stabilizing a variable in preparation to a quick change in that variable, while APAs have been viewed as the means of generating forces and moments of force that minimize the effects of perturbation on posture. A recent study (Klous et al., 2011) documented ASAs and APAs in a quick bilateral arm movement task.
There is another group of phenomena that have traditionally been addressed as APAs. These are postural changes in preparation to a whole-body voluntary action, such as stepping, or preparing to deal with a predictable perturbation. COP shifts are seen several hundred ms prior to the time of swing foot-off (Crenna and Frigo, 1991) or an external perturbation (Santos et al., 2010).
In this study, we address three major hypotheses. First, we plan to show that early postural adjustments (EPAs), defined as those seen more than 200 ms prior to the action or perturbation initiation, and typically timed APAs can be seen in a sequence during preparation to a predictable perturbation. Until now, EPAs and APAs have been reported only separately, in different tasks (Belenkiy et al. 1967, Bouisset and Zattara, 1987, Lepers and Breniere, 1995, Wang et al., 2006). Demonstrating them as a sequence is important because this would support an idea of two components of postural preparation rather than a single component with modifiable timing. Our second hypothesis is that both EPAs and APAs (observed as changes in muscle activation levels) would be preceded by changes in indices of synergies stabilizing COP coordinate. In other words, we plan to show that ASAs and APAs (and also ASAs and EPAs) come as a sequence of, first, destabilizing a variable that has to be changed and then changing that variable. Our third hypothesis is that the observed sequence of EPAs and APAs will be preserved with changes in body position. If supported, the hypotheses will have important implications for rehabilitation of populations with demonstrated impairment of feed-forward postural control such as in healthy elderly, persons with atypical development, and neurological patients (Aruin and Almeida, 1997, Bouisset and Zattara, 1990, Inglin and Woollacott, 1988, Latash et al., 1995, Slijper et al., 2002).
Methods
Participants
Nine healthy participants, 5 males and 4 females, age 25 ± 4 years (mean ± SD), body mass 60 ±10 kg, height 1.68 ± 0.11 m, without any known neurological or motor disorders participated in the experiment. Seven subjects were right-handed and two subjects were left-handed based on the Edinburgh Inventory (Oldfield, 1971). The experimental procedure was approved by the Institutional Review Board of the University of Illinois at Chicago and the participants provided written informed consent.
Apparatus
A force platform (AMTI, OR-5, USA) was used to record the shear force in the anterior-posterior direction (FY), the vertical component of the reaction force (FZ) and the moment of force around the frontal axis (MX). Disposable self-adhesive electrodes (Red Dot 3M) were used to record the surface muscle activity (EMG) of the following muscles: tibialis anterior (TA), soleus (SOL), gastrocnemius medialis (GM), gastrocnemius lateralis (GL), biceps femoris (BF), semitendinosus (ST), vastus lateralis (VL), rectus femoris (RF), vastus medialis (VM), lumbar erector spinae (ES), latissimus dorsi (LD) and rectus abdominis (RA). The pairs of electrodes were placed over the right side of the subject’s body over the muscle bellies, spaced 3 cm apart. Prior to the placement of the electrodes, the skin area was cleaned with alcohol. A ground electrode was attached to the anterior aspect of the leg over the tibial bone. The EMG signals were collected, filtered and pre-amplified (10–500 Hz, gain 2000) with a commercially available EMG system (Myopac, RUN Technologies, USA). An accelerometer (Model 208CO3, PCB Piezotronics Inc., USA) was taped to the lateral aspect of the participant’s left clavicle to record the moment of the pendulum impact (see further text for details). All signals were sampled at 1000 Hz with a 16-bit resolution. A personal computer with customized LabView software (LabView 8.6 National Instruments, Austin, TX) was used to collect the data.
Procedures
The experiment consisted of: (1) control trials and (2) perturbation trials. First, two control trials were conducted; these data were later used for normalization of the EMG signals. In the control trials, the participants were standing barefoot in front of a metal frame either facing it or facing away from the frame. In both cases they were holding a bar with both hands with the shoulders flexed to 90° and elbows extended fully. The bar was connected by a rope to a 4.5 kg load via a pulley (Figure 1A). When the subjects were facing the pulley, they counteracted the load by activating the dorsal muscles of the trunk and legs in an isometric manner; when they were facing away from the pulley, the ventral muscles were activated in an isometric way. Each task was performed for 5 s and the subjects were required not to lean forward or backwards (controlled by the experimenter). The tasks were presented in a random order. The time interval between the two trials was 1 min. The subjects were standing with eyes open in all experimental conditions.
Figure 1.
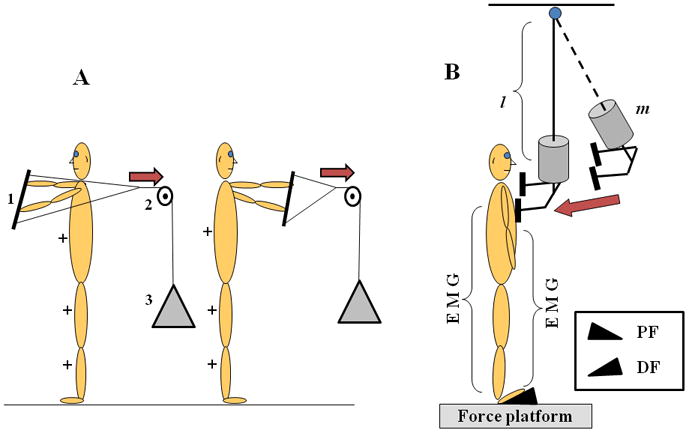
A. Schematic representation of the setup used to perform control trials. 1 is a bar, 2 is a pulley, and 3 is a 10 lbs load. The direction of the force applied to the bar is show by arrows. + indicate activation of the muscles associated with the direction of application of force. B. Schematic representation of the experimental setup. The subjects were exposed to the external perturbations while standing on a wedge that was oriented in such a way that either dorsiflexion (DF) or plantarflexion (PF) in the ankle joints was induced. l is the length and m is the 3% of subject’s body weight additional mass.
In the perturbation trials, two body positions were used, with a wedge (15 deg angle, 40 cm length and 40 cm width) placed directly on the force platform: (1) the participant was standing on the wedge with the feet plantar flexed (PF), such that the toes were down; and (2) the participant was standing with the feet dorsiflexed (DF), such that the toes were up. The PF and DF order was randomized. The participants stood barefoot with their feet shoulder width apart, parallel to each other. The foot position was marked on the wedge and reproduced across the trials. The subjects were instructed to keep upright body position using, as feedback, a pointer that was placed laterally to the subject at the shoulder level. Standing on the wedge resulted in substantial levels of activation of postural muscles, which were necessary to quantify multi-muscle synergies at steady-state (see later). We did not use standing without the wedge, because in this condition activation levels of most muscles were very low and did not allow quantitative analysis.
The participants were positioned in front of an aluminum pendulum attached to the ceiling. The pendulum consisted of a height adjustable central rod with the distal end designed as two padded pieces positioned shoulder width apart and projected towards the participant (Figure 1B). A load (3% of the body mass of the participant) was attached to the distal end of the central rod, immediately above the padded pieces. A rope fastened to the distal end of the central rod of the pendulum was passed through a pulley system and used to release the pendulum (for more details see (Krishnan and Aruin, 2011, Santos and Aruin, 2008)). The experimenter secured the pendulum to a trigger 0.5 m away from the participant and released the trigger by pulling the rope. A beep signaled the release of the pendulum providing an auditory cue. Although we tried to ensure that pendulum release occurred unexpectedly, some subjects started to adjust posture even before the pendulum release (see Figures 4 and 5 in Results). The participant watched the pendulum moving towards his/her body at all times; he/she received the impact of the pendulum on the shoulders and was required to maintain balance at all time. For safety purpose, a safety harness with two straps loosely attached to the ceiling was used. Before collecting data, participants were given three practice trials; then, a total of 25 trials were collected in each of the DF and PF conditions. The rest intervals between trials were 10 s. Additional rest periods were provided as needed.
Figure 4.
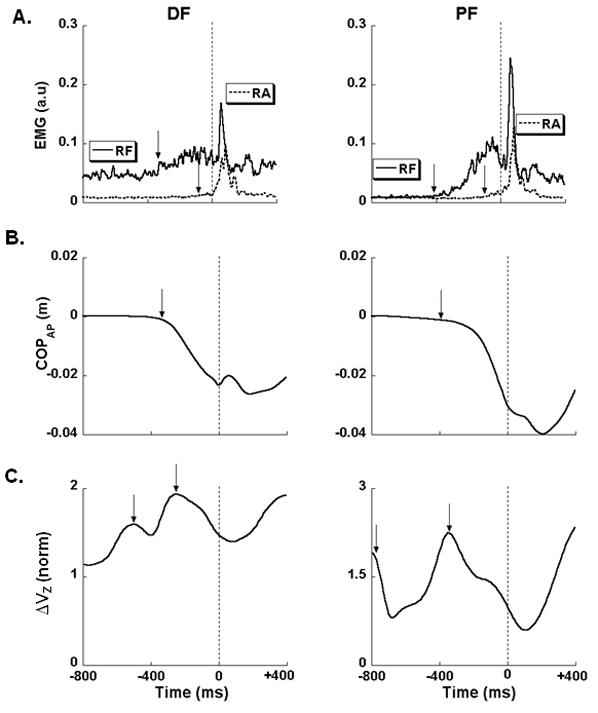
EMG traces for RF and RA muscles (A), COPAP displacements (B) and synergy index (C) are shown for a representative participant. Vertical dashed line at the center of each panel corresponds to time zero, t0 (the time of perturbation). Note that the pendulum was released −600 ms before t0. The arrows in EMG panels (RF = rectus femoris and RA = rectus abdominis) represent the instant in time where the magnitude of the EMG exceeded the baseline value ± 2 standard deviations. Note the two arrows that reflect two moments of time that belong to EPA and APA. The arrow in COPAP panel represents the instant in time where the magnitude of COPAP differs from the baseline, which is included under the EPA period. Negative values in COPAP panel correspond to backward displacements. The arrows in the synergy index panel show two drops of the peaks, each of them representing EPA and APA periods. Note that in PF condition, the drops in the synergy index occur earlier than the DF condition. ΔVZ is in arbitrary units.
Figure 5.
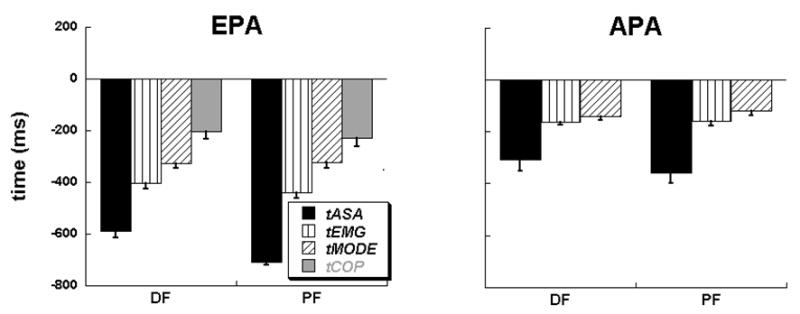
Timing indices for the early postural adjustments (EPA) and later postural adjustments (APA) averaged across the participants with standard error bars are shown for DF and PF conditions. Note that in the EPA period, PF condition produced early changes in the synergy index, EMGs, M-modes, and COPAP when compared to the DF condition. We present only one value for tCOP and it is included in the EPA time period because tCOP in both the conditions for majority of the subjects was in the time interval before −200ms with respect to the time of perturbation, t0. Note also that the order of their appearance is: tASA < tEMG < tMODE < tCOP.for the EPA period and tASA < tEMG < tMODE for the APA period.
Data processing
All signals were processed offline using customized Matlab 7.6 software (MathWorks, Natick, MA). For both the DF and the PF conditions, EMG signals were rectified and filtered with a 50 Hz low-pass, 2nd order, zero-lag Butterworth filter. The accelerometer signal was corrected for offset, and the time of impact (‘time zero’, t0=0) was calculated by a computer algorithm as a point in time at which the signal exceeded 5% of the maximum acceleration in that particular trial. This value was confirmed by visual inspection of the data. The period from −1000 to t0 was used to calculate baseline and components of feed-forward postural adjustments while period from t0 to + 400 ms was used to calculate components of feedback postural adjustments. The calculation of baseline activity was based on the period from −1000 ms to −850 ms before t0.
The vertical component of the reaction force (FZ), the horizontal component of the reaction force in the anterior-posterior direction (FY), and the moment of force around the frontal axes (MX) were filtered with a 20 Hz low-pass, 2nd order, zero-lag Butterworth filter. Time-varying COPAP was calculated using the following approximation (Winter et al., 1996):
| (1) |
where dz pertains to the distance from the surface to the platform origin (0.038 m). The data were shifted 50 ms forward with respect to t0 to account for the electro-mechanical delay (Cavanagh and Komi, 1979, Corcos et al., 1992). First, anterior-posterior COPAP displacement in each trial was computed by subtracting the average COPAP baseline activity. Second, the average COPAP displacement was calculated for the trial and compensatory peak COPAP was computed during the t0 to t0 + 400 ms interval. Lastly, COPAP shift initiation (tCOP) was defined as the instant in time when the change in COPAP (ΔCOPAP) from the baseline exceeded 5 mm. These parameters were calculated for each perturbation condition (DF or PF) and each subject.
The initiation of burst/inhibition time (tEMG) in the averaged across trials data for each condition was defined as the instant in time when the muscle activation differed by more than ± 2 standard deviations from the baseline activity for at least 25 ms continuously. An algorithm picked up the tEMG for each muscle and the defined tEMG was visually confirmed by an experienced researcher. Each muscle was assigned one tEMG value that corresponded to either a burst or an inhibition.
The rectified and filtered EMG signals for each experimental condition were integrated using a trapezoidal numerical integration with 10 ms time windows (IEMG) from −800 to +400 ms with respect to t0. IEMG data were normalized (IEMGNORM) using the method described in (Krishnamoorthy et al., 2003a, Krishnamoorthy and Latash, 2003b):
| (2) |
where IEMGSS is the average rectified EMG during the baseline activity (steady state standing, SS) which is integrated over a time window of the same duration as for IEMG. IEMGREF is the average rectified EMG in the middle of the trial obtained during the two control trials when holding the bar with 4.5 kg load attached in front or behind the body, integrated over a time window of the same duration as for IEMG.
Defining M-modes with principal component analysis (PCA)
The objective of this step of analysis was to identify groups of muscles (muscle modes or M-modes) that showed parallel scaling of changes in their levels of activation. For this purpose, IEMGNORM data for all the trials within the time window {−200 ms; t0} were analyzed. This time window was selected because both APAs and ASAs had been previously documented starting not earlier than 200 ms prior to a predictable perturbation or action initiation (Kim et al., 2006, Olafsdottir et al., 2005, Santos et al., 2010, Shim et al., 2005). A detailed explanation of this procedure can be found in the Appendix A. Briefly, principal component analysis (PCA) was applied to correlation matrices of IEMGNORM data within the above mentioned time frame. At least one muscle was significantly loaded (loading value over ±0.5; see (Hair et al., 1995)) on at least one of the first four PCs. Visual inspections of the scree plots confirmed the validity of this criterion. The 4 PCs were subjected to Varimax rotation with factor extraction. The factors (eigenvectors in the muscle activation space) will further be addressed as M-modes; M-modes were used as the elemental variables for further analysis of M-mode synergies. To calculate the magnitudes of the M-modes, the eigenvectors were multiplied by the IEMGNORM data (that ranges from −800 to +400 ms with respect to t0). The M-mode magnitudes formed time series.
The time of the initiation of change in the baseline value, tMODE was determined for the M-mode magnitude for each M-mode, each of the experimental condition (DF and PF) and for each of the subjects. Similarly to the computation based on EMGs, the time of the earliest change in M-mode magnitude was defined at the instant in time when the M-mode magnitude differed from the average baseline value by ±2 SDs. The earliest across the four M-modes value was accepted as tMODE for each subject and each condition.
Defining the Jacobian
Linear relations were assumed between small changes in the magnitude of the M-modes ( ) and the change in the performance variable ΔCOPAP within the time window {−200 ms; t0} over which M-modes were defined. Within each condition, multiple linear regressions over all trials were used to define the relationship between and ΔCOPAP for each subject separately. The resulting set of regression coefficients is the Jacobian matrix for each experimental condition. Further details about the procedure to define the Jacobian can be found in the Appendix A.
Uncontrolled manifold analysis: Computation of the synergy index
The UCM hypothesis assumes that the controller manipulates a set of elemental variables and tries to limit their variance to a sub-space corresponding to a desired value of a performance variable. Within this analysis, the trial-to-trial variance in the space of elemental variables is divided into two components. The first component lies within a subspace that keeps the performance variable unchanged (VUCM). The second component of the variance lies within the orthogonal complement to the UCM (VORT). Comparing the two components of the variance, normalized by the dimensionality of their respective sub-spaces, produces an index of variance that is compatible with stabilization of the selected performance variable.
In the current study, M-mode magnitudes were the elemental variables, while COPAP displacement was the performance variable. We would like to emphasize that the M-mode composition and the Jacobian were defined over the APA time interval but later applied to the whole time of data analysis. In two earlier studies (Klous et al., 2011, Krishnan et al., 2011), sets of M-modes (and corresponding Jacobians) were defined over different time intervals. Despite the documented differences in the M-mode composition, when different sets of M-modes were used to analyze the whole data set, very similar values and time patterns of the synergy indices were observed. Therefore, in this study, we used only one set of M-modes defined over the most relevant time interval.
First, for each condition and each subject, the mean magnitude of the M-modes over all the trials was subtracted from the magnitudes of the M-modes observed in individual trials resulting in a demeaned set of values (ΔM). The null-space of the Jacobian was calculated as an approximation of the UCM. The demeaned vector (ΔM) was projected onto the null-space and its orthogonal component. Finally, the trial-to-trial variance in each of the two sub-spaces (VUCM and VORT) and the total variance (VTOT) normalized by their respective degrees of freedom were calculated. VUCM reflects the amount of variance in the M-mode space that does not lead to changes in the average across trials COPAP coordinate; VORT reflects the amount of variance that does. We assume that a statistically significant difference between VUCM and VORT (per degree-of-freedom in each sub-space) reflects a purposeful control strategy by the central nervous system directed at reducing the across-trials COPAP variability.
To quantify the relative amount of variance that is compatible with stabilization of the COPAP, ΔV was calculated as an index of synergy (Danna-dos-Santos et al. 2007, Krishnamoorthy et al., 2003b, Robert et al., 2008) to facilitate comparison across subjects and conditions: . This index was calculated for each experimental condition (DF and PF) and for each time sample. Figure 2 schematically summarizes of the main steps in the data processing. A more detailed description of data processing can be found in the Appendix A. Note that the method of ΔV computation makes its positive values corresponding to a multi-M-mode synergy stabilizing COPAP coordinate. A larger value corresponds to a stronger synergy.
Figure 2.
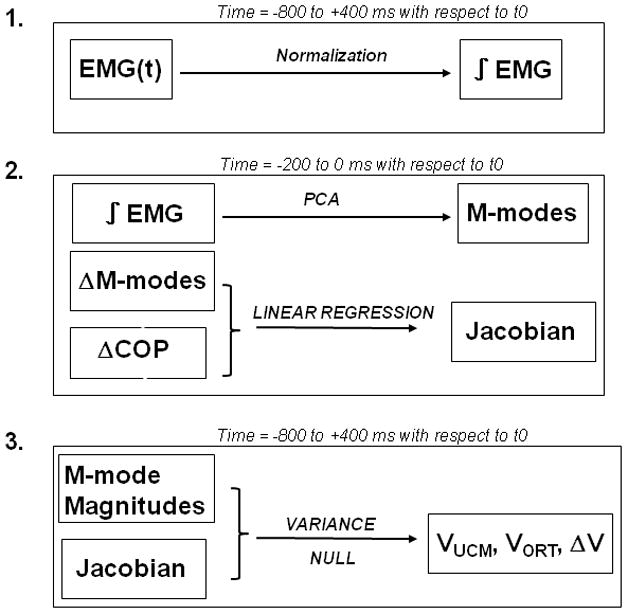
Steps involved in data processing.
Anticipatory synergy adjustments (ASA) were identified as a drop in the ΔV time profile. The time of ASA initiation, tASA, was calculated using the z-transformed time series of ΔV (ΔVZ). The rate of change of ΔVZ was computed from t0 to −800 ms backwards; the time tASA was defined by two criteria: (1) when the magnitude of this rate was equal to zero (dΔVZ/dt=0), which was considered as the start of a drop, and (2) the drop had to be larger than 20% of the ΔVZ magnitude at the time of dΔVZ/dt=0. Similarly, the next drop was identified during moving in the same direction until this rate approached zero and the magnitude of the drop was larger than the described threshold. Both tASA values were then checked visually by an experienced researcher at an optimal time resolution. All the subjects showed two such anticipatory drops in the ΔVZ time profiles before t0. The outcomes of calculation of the tASA, tEMG, and tMODE were grouped into two time intervals with respect to the moment of pendulum impact, t0 = 0: one from −600 to −200 ms (EPA, early postural adjustment) and second from −200 to 0 ms (APA, anticipatory postural adjustment). For example, for tEMG, the earliest one in the EPA group was termed the tEMG-EPA and the earliest in the APA group was termed tEMG-APA. Since there was a single threshold for tCOP, it was categorized under EPA period.
Statistics
Data are presented in the text and figures as means and standard errors. The data were subjected to Shapiro-Wilk test for normality. The peak magnitude of the COPAP displacement was compared between the two conditions (DF and PF) using paired Student’s t-test. Two-way repeated measures ANOVA was performed with factors Variance (2 levels: VUCM and VORT) and Condition (2 levels: DF and PF) to analyze possible differences in the values of these two variance indices across the two conditions. In the EPA period, to compare the initiation times of the synergy index (tASA), EMG (tEMG), M-mode (tMODE), and COPAP displacement (tCOP), two-way repeated measures ANOVA was applied with factors Time (4 levels: tASA, tEMG. tMODE and tCOP) and Condition (2 levels: DF and PF). In the APA period, to compare the initiation times of the synergy index (tASA), EMG (tEMG) and M-mode (tMODE), two-way repeated measures ANOVA was applied with factor Time (3 levels: tASA, tEMG, and tMODE) and Condition (2 levels: DF and PF). Pairwise comparisons with Bonferroni corrections were used for further analyses of significant effects. In all the ANOVAs, whenever the Mauchly’s test of sphericity was not met, Greenhouse-Geisser correction was made. The statistical significance was set at p < 0.05 in all the tests. Statistical analysis was performed in SPSS 17 for Windows XP (SPSS Inc., Chicago, USA).
RESULTS
EMG and COPAP Patterns
Figure 3 shows four EMG time profiles for a representative subject in DF condition, with the SOL and VM showing the tEMG-EPA, and with the TA and ST showing tEMG-APA. In addition, Figure 4A illustrates two EMG time profiles in both DF and PF conditions, with arrows showing the tEMG-EPA and tEMG-APA. In general, tEMG-EPA in SOL was seen in all 9 subjects in both DF and PF conditions and in RF it was seen in 7 subjects in DF and 8 subjects in PF condition. Similarly, tEMG-APA in RA was seen in 7 subjects in DF and 9 subjects in PF conditions. There were no cases when a single muscle showed bursts in both EPA and APA time intervals. The average across subjects’ time of the COPAP shift initiation (tCOP) was −204 ± 27 ms in the DF condition and −228 ± 32 ms in the PF condition. The COPAP shift continued till t0; so, it was not possible to identify two periods in COPAP shifts. Therefore tCOP was categorized under EPA time interval only. The peak COPAP in the DF condition was smaller when compared to the peak COPAP in the PF condition (paired t-test; Peak COPAP-DF= −0.028 ± 0.006 m < Peak COPAP-PF = −0.032 ± 0.008 m; t=2.3, p<0.05).
Figure 3.
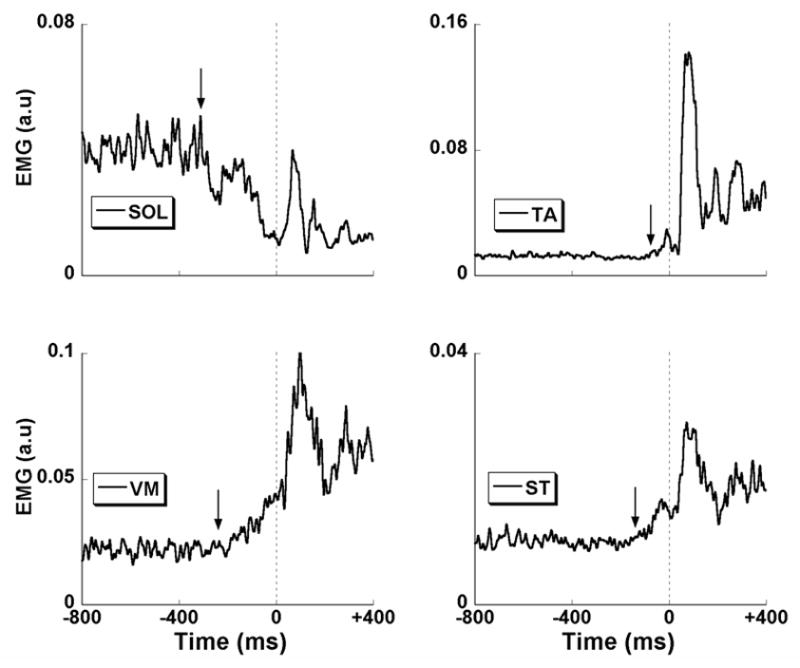
EMG traces for soleus (SOL), vastus medialis (VM), tibialis anterior (TA) and semi-tendinosis (ST) muscles are shown for a representative participant. Vertical dashed line at the center of each panel corresponds to time zero, t0 (the time of perturbation). The arrows show the instant in time where the magnitude of the EMG exceeded the baseline value ± 2 standard deviations. EPA time interval is seen in SOL and VM, while APA time interval is observed in TA and ST.
PCA and multiple regression
On average, the four M-modes accounted for 65.0 ± 5.5% of the total variance in the muscle activation space in the DF condition and 65.1 ± 6.1% of the total variance in the PF condition. A typical set of M-modes is presented in Table 1 with the significant loadings shown in bold. The first M-mode composition in the DF condition showed high loading values with the same sign for the IEMG indices of the dorsal and ventral muscles acting at the same joint, which we address as a ‘co-contraction’ pattern. A co-contraction pattern is defined as a pattern with significant loading coefficients on the same PC with the same sign (positive or negative) for two muscles with opposing actions at a particular joint (ankle, knee or hip). The second M-mode showed high loading values for the IEMG indices of the ventral muscles (‘push-forward’ M-mode; cf. (Krishnamoorthy et al., 2003b, Krishnamoorthy et al., 2004), while the third M-mode showed high loading values for the IEMG indices of the RA (ventral) and the ES and LD (dorsal) muscles, also with the same sign (co-contraction pattern). The fourth PC again showed a co-contraction pattern between the hamstrings and vastus lateralis. In the PF condition, the first and the second modes were ‘push-back’ and ‘push-forward’ M-modes, respectively, while the third and the fourth contained co-contraction patterns. Overall, in the PF condition, 21 M-modes with co-contraction patterns were seen, while in the DF condition, the number of M-modes with co-contraction patterns was 19 (from a total of 36 M-modes).
Table 1.
Results of the Principal Component Analysis
| DF | PF | |||||||
|---|---|---|---|---|---|---|---|---|
| Muscle | M1- mode | M2- mode | M3- mode | M4- mode | M1- mode | M2- mode | M3- mode | M4- mode |
| TA | 0.59 | −0.09 | −0.12 | −0.05 | 0.12 | 0.11 | 0.03 | −0.82 |
| VL | −0.02 | −0.58 | 0.04 | −0.50 | 0.07 | −0.60 | 0.25 | −0.45 |
| VM | 0.11 | −0.81 | −0.03 | −0.03 | −0.03 | −0.84 | −0.04 | −0.19 |
| RF | −0.06 | −0.56 | −0.03 | −0.14 | 0.09 | −0.80 | −0.04 | 0.06 |
| RA | 0.05 | −0.32 | 0.58 | 0.26 | 0.12 | 0.04 | 0.58 | −0.05 |
| SOL | 0.92 | 0.04 | 0.02 | −0.13 | 0.91 | 0.02 | 0.08 | 0.00 |
| GM | 0.93 | 0.00 | 0.03 | −0.14 | 0.92 | −0.07 | 0.11 | −0.06 |
| GL | 0.92 | 0.02 | 0.04 | −0.10 | 0.76 | −0.09 | 0.04 | −0.41 |
| BF | 0.14 | −0.26 | −0.03 | −0.82 | −0.03 | −0.30 | 0.26 | −0.63 |
| ST | 0.23 | −0.06 | −0.02 | −0.79 | 0.19 | −0.20 | −0.11 | −0.59 |
| ES | −0.14 | 0.17 | 0.79 | −0.02 | −0.06 | −0.05 | 0.70 | 0.15 |
| LD | 0.03 | 0.05 | 0.83 | −0.09 | 0.10 | −0.01 | 0.54 | −0.24 |
Data for a representative subject for the DF and PF conditions are shown. Loading factors are presented for the first four PCs (M-modes). Loadings over 0.5 are shown in bold.
TA = tibialis anterior; VL = vastus lateralis; VM = vastus medialis; RF = rectus femoris; RA = rectus abdominis; SOL = Soleus; GM = gastrocnemius medialis; GL = gastrocnemius lateralis; BF = biceps femoris; ST = semitendinosus; ES = erector spinae; LD = latissimus dorsi
Linear regression analysis was performed to define the Jacobian mapping small changes in the M-mode magnitudes onto COPAP shifts (ΔCOPAP). Results of the linear regression analysis were significant in all subjects (p < 0.001) for each of the two conditions. On average, the analysis accounted for 76.1 ± 13% and 67.7 ± 24% of variance in ΔCOPAP in the DF and PF conditions respectively.
Analysis of the COPAP stabilizing synergy
The results of analysis of the COPAP stabilizing synergy involved quantitative estimation of variance in the M-mode subspaces corresponding to no changes in COPAP (UCM) and the orthogonal to the UCM sub-space (see Methods). This analysis revealed higher VUCM values as compared to those of VORT in both DF and PF conditions. This finding was confirmed by a two-way repeated measures ANOVA Condition × Variance, which showed a main effect of Condition [F(1,159) = 59.1, p < 0.001], with the DF condition (VUCM = 0.68 ± 0.45; VORT = 0.24 ± 0.21) showing higher variance than the PF condition (VUCM = 0.59 ± 0.39; VORT = 0.14 ± 0.08). The main effect of Variance [F(1,159) = 418.5, p < 0.001], confirmed that VUCM had significantly higher values than VORT in both conditions; there was no interaction. This result confirms that COPAP was stabilized by co-variation of M-mode magnitudes (VUCM>VORT).
The difference between the two variance indices was reflected in consistently positive values of ΔV, a synergy index computed as the normalized difference between VUCM and VORT. For statistical analysis, ΔV data were log-transformed resulting in an index ΔVZ. Typically, there were two transient drops in ΔVZ (namely, tASA), the earlier one was observed during the EPA time interval and the second one was seen during the APA time interval (see Methods) The magnitude of the ΔV drop (arbitrary units) in the EPA interval was 0.31 ± 0.23 for the DF condition and 0.46 ± 0.22 for the PF condition. The magnitude of the second drop in APA time interval was 0.47 ± 0.36 for the DF condition and 0.61 ± 0.45 for the PF condition.
Figure 4C illustrates for the DF and PF conditions, in a typical subject, time profiles of ΔVZ. Visual inspection of the individual ΔVZ profiles showed a consistent pattern across subjects that included a transient drop in ΔVZ during the EPA time interval (seen prior to the initiation of changes in the RF activation level) followed by another drop (seen prior to the initiation of changes in the RA activation) during the APA time interval. The magnitude of the drop and its timing varied across subjects such that averaged across subjects data did not show consistent behavior. Therefore, we illustrate the mentioned finding with ΔVZ time profiles for one of the subjects (Figure 4). For statistical analysis, we focused on the two drops in ΔVZ (tASA) computed using the criterion mentioned in the Methods.
In the PF condition, on average, almost all the time variables tASA, tEMG, tMODE, tCOP occurred earlier than in the DF condition (see the right panels of Figure 4). Averaged across subjects timing indices with error bars for the EPA and APA periods are presented in Figure 5 for both conditions. Two-way ANOVA on the timing indices for the EPA period with the factors Condition (DF and PF) and Time (tASA, tEMG, tMODE, tCOP) confirmed the main effect of Condition [F(1,6) = 15.8, p < 0.01] reflecting earlier timing indices in the PF condition, the main effect of Time [F(3,18) = 77.5, p < 0.001], and a significant interaction [F(3,18) = 5.3, p < 0.01]. The interaction reflected the fact that tMODE in the DF condition occurred prior to tMODE in the PF condition, whereas all the other timing indices (tASA, tEMG, tCOP) occurred earlier in the PF condition. Pairwise comparisons confirmed significant differences within all pairs of the timing indices (p < 0.05).
Two-way ANOVA on the timing indices for the APA period with the factors Condition (DF and PF) and Time (tASA, tEMG, tMODE) showed a main effect of Time [F(2,12) = 9.2, p < 0.01], and no other effects. Pairwise comparisons, however, failed to show difference between the levels of Time. The timing indices appear in the order: tASA < tEMG < tMODE < tCOP for the EPA period and tASA < tEMG < tMODE for the APA period in both conditions as shown in Figure 5. Note the large absolute magnitudes of the negative time indices for the EPA. In some cases, these were longer than the pendulum flight time suggesting that some of the subjects in at least some of the trials anticipated pendulum release and started slow postural adjustment more than 600 ms prior to time zero.
Discussion
The results of the experiments support the three hypotheses formulated in the Introduction. Indeed, we observed two clearly different time intervals of postural preparation to the perturbation: One of them (EPA) started 400–500 ms prior to the impact followed by the second one (APA), 100–150 ms prior to the impact. It is of importance that both time intervals could be observed as a sequence in the same person; indeed, both time intervals were seen in each of the subjects. Moreover, the sequence was preserved with changes in body position (DF, PF). The analysis of multi-muscle synergies based on the uncontrolled manifold (UCM) hypothesis ((Scholz and Schoner, 1999); reviewed in (Latash et al., 2007)) has shown that each of the two time intervals consisted of two phenomena. First, there was a transient drop in the index of multi-M-mode synergies stabilizing the coordinate of the center of pressure in the anterior-posterior direction (COPAP), that is, anticipatory synergy adjustment (ASA, (Klous et al., 2011, Krishnan and Aruin, 2011, Olafsdottir et al, 2005)). During the first 100–200 ms of ASAs, no changes were seen in averaged across trials patterns of electromyographic (EMG) signals and COPAP. Such changes were observed later superimposed on the ongoing ASA. Further in the Discussion, we address implications of the results for such issues as the complex phenomena comprising feed-forward postural preparation to perturbation, feed-forward adjustments in postural multi-muscle synergies, and the place of these phenomena in a general scheme of the neural control of posture and movement.
Early and late feed-forward postural preparation
Both early and late postural adjustments, EPAs and APAs (Belenkiy et al., 1967, Elble et al., 1994), have been known for a long time Both, however, have frequently been addressed as APAs despite the evident differences in their characteristic timing. APAs were originally described by (Belenkiy et al., 1967) as changes in the postural muscle activation levels prior to a quick arm action. Later, many researchers explored APAs in preparation to fast arm movements (Aruin and Latash, 1995a, Bouisset and Zattara, 1987, Cordo and Nashner, 1982), load manipulations, (Aruin and Latash, 1995b, 1996), and expected external perturbations (Santos et al., 2010, Shiratori and Latash, 2000, Shiratori and Latash, 2006). All these studies reported changes in the activation levels of postural muscles about 100±50 ms prior to the action initiation or external object impact. The purpose of the APAs has been assumed to generate forces and moments of force directed against those expected from the planned action or upcoming perturbation (Bouisset and Zattara, 1987, Massion, 1992, Ramos and Stark, 1990).
Studies of postural adjustments prior to taking a step reported reproducible patterns of COP shifts (and muscle activations) starting several hundred ms prior to the take-off of the leading foot (Couillandre et al., 2002, Elble et al., 1994, Lepers and Breniere, 1995, Wang et al, 2006). Despite the large differences in the timing of these postural adjustments as compared to APAs in the mentioned studies, they were also addressed as APAs. Our current study strongly suggests that such early postural adjustments (EPAs) and APAs are two different phenomena. Indeed, we observed both EPAs and APAs as a sequence of events in the same person that occur about 400 ms and about 150 ms prior to the impact, respectively (see Figures 3 and 4A,). So, EPAs and APAs are not a single phenomenon with variable timing but two distinct aspects of postural preparation to perturbation.
The difference in the timing suggests that EPAs and APAs are likely to have different purposes. The generation of net forces and moments of force about 50–100 ms prior to an expected perturbation, that is, during APAs, may be mechanically justified (as supported by the modeling study of (Ramos and Stark, 1990). Note, however, that even with this timing, APAs may themselves turn into postural perturbations, for example if the induced COP shifts move close to the edge of a narrow support (Aruin et al., 1998, Slijper and Latash, 2000). Generating substantial forces and moments of force 400 ms prior to an expected perturbation may by itself be a balance perturbation. Based on these considerations, we suggest a hypothesis that the purpose of EPAs is different from that of APAs. EPAs adjust posture such that mechanical effects of an expected perturbation (or action, as in the mentioned step initiation studies) on balance are minimized. For example, during step initiation, lifting a foot without first shifting the COP towards the other foot would result in a loss of balance in the medio-lateral direction. APAs produce net forces and moments (reflected in COP shifts) adequate to counteract the expected effects of the perturbation. This hypothesis is indirectly supported by the rather long time delay between changes in the EMG signals during EPAs and detectable COP shifts. These time delays were on the order of 200 ms, which is substantially larger than typical electromechanical delays, about 50 ms (Cavanagh and Komi, 1979, Corcos et al., 1992).
Many earlier studies (cited above) reported APAs in the absence of EPAs. We believe that the presence of both components of postural adjustment in our study was partly due to the unusual initial body posture. In more comfortable postures, EPAs may be reduced or absent – a topic for a future study.
Role of body position in the EPA – APA sequence
The changes in the body position (standing with DF or PF) did not affect the sequence of the EPA and APA. Thus, our third hypothesis was supported. At the same time, we observed differences in the timing of different phenomena during EPAs (tASA, tEMG, tMODE, and tCOP) between the two conditions. In PF, all the time indices were earlier than in the DF condition. There are several possible explanations for this fact. First, there are different requirements to the stabilization of the ankle joint. Indeed, it is known that the close packed position (locked) for the ankle joint is dorsiflexion (Field, 2001, Gray et al., 2005, Hamill and Knutzen, 1995). Therefore, smaller compensatory COP displacements (shift of the COP as a reaction to the perturbation) and later timing indices during the EPA and APA intervals observed in the DF condition could be due to such a stabilization of the ankle joint. Alternatively, changes in the base of support could affect the magnitude of anticipatory postural adjustments as it was shown in the literature (Yiou et al., 2007). However, the base of support in the current experiment was the same in both the DF and the PF conditions and as such could not be a reason for the differences in timing indices. Another issue relates to differences in the body mobility associated with different body position. Indeed, standing on the differently oriented wedges could affect the available range of motion in the ankle joint which in turn could affect body mobility. We can speculate that in the PF condition the availability of the greater range of motion in the ankle joint could be associated with an earlier occurrence of changes in the computed indices.
Postural synergies and their feed-forward adjustments
The idea that muscles are organized into groups during whole-body actions dates back to classical studies by (Hughlings Jackson (1889) and Bernstein (1935). Recently, this idea has been explored experimentally using a variety of matrix factorization methods applied to integrated indices of muscle activation. These methods have been applied to studies of postural tasks (Krishnamoorthy et al., 2003b, 2004, Ting and Macpherson, 2005, Torres-Oviedo et al., 2007), locomotion (Ivanenko et al., 2005, Ivanenko et al., 2006), and arm actions (Krishnamoorthy et al., 2007). Although a few recent papers question the utility of the notion of multi-muscle synergy (Tresch and Jarc, 2009, Valero-Cuevas et al., 2009), most researchers agree that organization of muscles into groups with parallel scaling of muscle activation levels within a group is a method commonly used by the central nervous system (in addition to the mentioned references, see (d’Avella et al., 2003, Holdefer and Miller, 2002, Kutch and Buchanan, 2001, Sabatini, 2002, Ting, 2007), possibly to decrease the number of variables and alleviate the notorious problem of motor redundancy (Bernstein, 1967).
While there is substantial experimental support for existence of muscle groups with parallel scaling of activation levels, there is also substantial difference in views on the function of such groups. Some researchers views such groups as synergies (d’Avella, Saltiel, 2003, Ivanenko, Cappellini, 2005, Ting, 2007, Ting and Macpherson, 2005, Torres-Oviedo et al., 2006) while others view them as elemental variables (muscle modes or M-modes) forming a basis on which synergies are built (Danna-dos-Santos et al., 2007, Klous et al., 2010, Krishnamoorthy et al., 2004, Robert et al., 2008). According to the latter approach, gains at M-modes co-vary across repetitive trials to stabilize time profiles of potentially important variables, such as COPAP coordinate and shear force magnitude.
In our experiments, during steady-state standing, we observed that variance across trials in the M-mode space was primarily limited to a sub-space corresponding to an unchanged COPAP coordinate (the null-pace of the corresponding Jacobian matrix, approximating the UCM). As in earlier studies (Danna-dos-Santos et al., 2007, Klous et al., 2010, Klous et al., 2011, Krishnamoorthy et al., 2004), we interpret this result as a reflection of a synergy stabilizing COPAP coordinate. There were, however, reproducible transient changes in the index of this synergy (ΔV) in all subjects that were timed to the two mentioned phenomena of feed-forward postural adjustments, EPAs and APAs. These changes involved a transient drop in ΔV, a phenomenon addressed as anticipatory synergy adjustment (ASA).
Until recently, ASAs have only been reported in preparation to multi-digit fast actions (Kim et al., 2006, Latash, 2010a, Olafsdottir et al., 2005, Shim et al., 2005, Shim et al., 2006). Some of their features (see references in the Introduction) have suggested that ASAs and APAs could be tightly linked to each other. In particular, both ASAs and APAs showed a shift in their timing towards action initiation under the simple reaction time instruction (De Wolf et al., 1998), and both were delayed and reduced in magnitude in healthy elderly persons (Olafsdottir et al., 2007).
Only recently, have ASAs been documented during postural tasks involving a quick arm movement (Klous, et al., 2011). Our study confirms the findings of Klous and her colleagues and extends them to EPAs. Another important difference is that our study used predictable external perturbations, while in the mentioned study by Klous and her colleagues no external perturbation was applied. Overall, these studies confirm that ASAs can be observed during postural tasks in preparation to changes in muscle activation patterns. We would like to emphasize once again that the early portion of ASAs is not associated with any detectable changes in the muscle activation levels, only with changes in an index of co-variation among the M-mode magnitudes. This finding fits naturally a scheme of control with two types of control variables (Latash et al., 2007, Latash et al., 2005), those that define general patterns of performance (observed in averaged across trials patterns), and those that define stability of performance (observed in co-variation patterns).
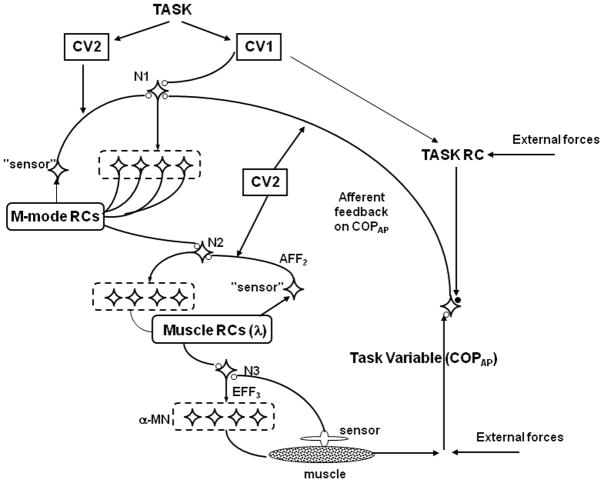
Hierarchical synergic control
We would like to put the main results of this study within the framework of a recent hypothesis that combines the ideas of synergic control within a hierarchical system and those of control with referent configurations (Latash, 2010a, b). According to the referent configuration (RC) hypothesis (which is a daughter of the equilibrium-point hypothesis, (Feldman, 1966, 1986)), control signals define a RC of the body, which is a configuration at which all muscles would be at their thresholds for activation via the tonic stretch reflex loop (Feldman, 2009, Feldman and Levin, 1995). Deviations between the RC and actual body configuration lead to muscle activations and body movement towards the RC. Commonly, external conditions and anatomical constraints do not allow the body to reach the RC; in such cases, non-zero muscle activations are observed in a new postural state.
Within a hierarchical control scheme, RC may be associated with setting referent values for important performance variables. This relatively low-dimensional input is mapped on higher-dimensional spaces of body variables (for example, joints, muscles, and motor units) via a hierarchy of few-to-many mappings organized in a synergic way with the help of feedback loops. The last statement means that the “many” variables co-vary across trials or along time to produce a stable value of the “few” variables. In our particular case, a high-level control variable (which may be associated with subthreshold depolarization of a neuronal pool, (Feldman and Levin, 1995, Latash, 2010a) is assumed to define RC for a one-dimensional variable (COPAP). This control variable is mapped on a higher-dimensional set of control variables defining RCs for M-modes, while further mapping produces an even higher-dimensional set of muscle-specific control variables, thresholds of the tonic stretch reflex, λ (not studied in this experiment).
Synergies stabilizing an input RC by co-varied adjustments of RCs at a lower hierarchical level are a natural feature of this scheme. APAs may be associated with a change in the highest level RC resulting in COP shifts and variable patterns of muscle activation co-varying across trials to produce the desired COP shift. The interpretation of EPAs is less trivial. Indeed, according to our hypothesis, EPAs are associated with postural adjustments to minimize the impact of the expected perturbation without producing major COP shifts. So, the highest-level RC is unchanged during EPAs. The control scheme, however, allows generating independent control signals to intermediate steps of the hierarchy. Such signals, for example, could produce subtle adjustments in the body configuration without generating net COP shifts. In particular, these may result in changes in muscle co-contraction levels and changes in body configuration compatible with the same center of mass projection. Obviously, this addition requires going outside the simplest hierarchy COP => M-modes => muscles.
As mentioned earlier, the phenomenon of ASAs suggests that two types of control variables are used by the controller for the control of multi-element, redundant systems. One of them defines desired profiles of performance, while the other one defines strength of co-variation stabilizing the performance. This scheme was suggested in an earlier study (Latash, Shim, 2005) and associated with setting a matrix of gains in local feedback loops that produce patterns of covariation of elemental variables stabilizing performance. It is illustrated schematically in Figure 6. In this figure, control variables of the first type (CV1) define desired RCs for an important task-specific performance variable. This input, after several steps within the hierarchy, results in changes in muscle activation levels shifting the performance variable to its referent value. Control variables of the second type (CV2), define gains in the feedback loops, which affect the computed indices of co-variation (ΔV in our study). We hypothesize that changes in CV2 precede changes in CV1 when the controller plans to change the performance variable quickly. They result in ASAs.
Figure 6.
A hypothetical hierarchical control scheme. At each step of the hierarchy, a few-to-many mapping takes place with feedback loops stabilizing the combined action of the “many” output (elemental) variables. Input variables specify depolarization of neuronal pools that translate into changes in referent values for corresponding variables (referent configurations). The feedback on the task variable (COPAP) ensures low variability of this variable produced by variable patterns within the hierarchy. Two types of control variables are assumed to be used: CV1 define desired magnitudes of performance variables while CV2 define gains in the feedback loops, which affect the computed indices of co-variation among elemental variables.
The main results of this study potentially have important implication for the current practice of rehabilitation of persons with postural disorders. Problems with feed-forward postural adjustments have been documented in a variety of conditions such as aging, atypical development, and neurological disorders (Aruin and Almeida, 1997, Inglin and Woollacott, 1988, Latash and Aruin, 1995, Slijper and Latash, 2002). Current rehabilitation strategies do not take into account possible changes in synergies stabilizing important mechanical variables during voluntary actions with postural components and in adjustments in those synergies in anticipation of a quick action. They also do not distinguish between the two components of postural preparation to action, EPAs and APAs. A more fine-grained approach to postural disorders is needed to move the field of postural rehabilitation ahead.
We have to admit a number of limitations of the study. In particular, we focused only on synergies stabilizing the anterior-posterior COP trajectory. Earlier studies have documented synergies in similar tasks stabilizing both COP trajectory and shear force magnitude in the anterior-posterior direction (Klous et al., 2010, Robert et al., 2008). We also did not consider COP shifts in the medio-lateral direction that have been shown in less symmetrical tasks such as stepping (Wang et al., 2006) and shifts of the moment of force about the vertical body axis that happens during asymmetrical arm movements (Aruin et al., 2001). These are topics for future studies.
HIGHLIGHTS.
Two components of feed-forward postural adjustments are demonstrated.
The sequence of the two components was preserved with changes in body position; each component was preceded by anticipatory synergy adjustments.
The results fit the referent body configuration hypothesis.
Acknowledgments
This study was supported in part by NIH grant NS-035032 and NIDRR grant H133P060003. We thank Maria Reyes Lopez Cerezo for her assistance in the data collection and Neeta Kanekar and Sambit Mohapatra for their helpful suggestions regarding the data collection.
APPENDIX A
Defining M-modes
IEMG data for all the trials within the time window {−200 ms; t0} were analyzed. The window was divided into twenty 10-ms windows. The IEMGNORM formed a matrix with twelve columns representing twelve muscles and the number of rows corresponding to the number of time windows times the number of trials analyzed (e.g., 20×25=500 rows and 12 columns) for each experimental condition. The correlation matrices for the IEMGNORM were subjected to PCA. The 4 PCs were subjected to Varimax rotation with factor extraction. The factors (eigenvectors) will further be addressed as M-modes, which were used as the elemental variables for further analysis of M-mode synergies.
Then, the M-mode magnitudes ( ) were computed by multiplying the loadings of the individual M-modes by the IEMGNORM data of the trial from −800 ms to +400 ms with respect to t0, using the following equation:
| (A1) |
Specifically, formed a matrix with 3000 rows and 4 columns [(120 time windows × 25 trials) × 4]. For each subject, the mean magnitude over the trials of each of the M-modes ( ) was calculated. This value was subtracted from vectors of the individual changes in the magnitudes of the M-modes ( ) for each trial, for each time window. The residual demeaned vectors were calculated for each of the subjects:
| (A2) |
where ( ) is the mean magnitude of the M-modes.
Defining the Jacobian
Linear relations were assumed between small changes in the magnitude of the M-modes ( ) and the change in the performance variable ΔCOPAP for the time window −200 ms to 0 ms with respect to t0. Both the magnitudes of the M-modes and the COPAP were filtered with a 20 Hz, low pass, 2nd order, zero-lag Butterworth filter before calculating the changes between time windows. Multiple linear regressions over all trials were performed. For each subject, and ΔCOPAP were computed over all trials for each of the data points:
| (A3) |
Hence, this analysis resulted in one Jacobian matrix for each experimental condition.
| (A4) |
where T is the sign of transpose.
Uncontrolled manifold analysis
The UCM was approximated with the null-space of the corresponding J. The null-space of J is a set of all vector solutions x of a system of equations Jx = 0. The null-space is spanned by the basis eigenvectors εi. The demeaned vector (ΔMdemeaned) was projected onto the null-space and its orthogonal component.
| (A5) |
Finally, the trial-to-trial variance in each of the two sub-spaces (VUCM and VORT) and the total variance (VTOT) normalized by their respective degrees of freedom was calculated as follows:
| (A6) |
To quantify the relative amount of variance that is compatible with stabilization of COPAP, an index of synergy ΔV was calculated.
| (A7) |
where all variance indices are computed per degree of freedom. Since VUCM, VORT, and VTOT are computed per DOF, the index of synergy ΔV ranges between 1.33 (all variance is within the UCM) and −4 (all variance is in the orthogonal sub-space). For further analyses, the ΔV values were transformed using a Fisher’s z-transformation (ΔVz) adapted to the boundaries of ΔV:
| (A8) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aruin A, Almeida G. A coactivation strategy in anticipatory postural adjustment in persons with Down syndrome. Motor control. 1997;2:178–97. [Google Scholar]
- Aruin AS, Forrest WR, Latash ML. Anticipatory postural adjustments in conditions of postural instability. Electroencephalogr Clin Neurophysiol. 1998;109:350–9. doi: 10.1016/s0924-980x(98)00029-0. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. Directional specificity of postural muscles in feed-forward postural reactions during fast voluntary arm movements. Experimental brain research Experimentelle Hirnforschung. 1995a;103:323–32. doi: 10.1007/BF00231718. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. The role of motor action in anticipatory postural adjustments studied with self-induced and externally triggered perturbations. Experimental brain research Experimentelle Hirnforschung. 1995b;106:291–300. doi: 10.1007/BF00241125. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. Anticipatory postural adjustments during self-initiated perturbations of different magnitude triggered by a standard motor action. Electroencephalogr Clin Neurophysiol. 1996;101:497–503. doi: 10.1016/s0013-4694(96)95219-4. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Ota T, Latash ML. Anticipatory postural adjustments associated with lateral and rotational perturbations during standing. J Electromyogr Kinesiol. 2001;11:39–51. doi: 10.1016/s1050-6411(00)00034-1. [DOI] [PubMed] [Google Scholar]
- Belenkiy V, Gurfinkel V, Pal’tsev Y. Elements of control of voluntary movements. Biofizika. 1967;10:135–41. [PubMed] [Google Scholar]
- Bernstein NA. The problem of interrelation between coordination and localization. Archives of Biological Science. 1935;38:1–35. (in Russian) [Google Scholar]
- Bernstein NA. The coordination and regulation of movements. Pergamon Press; London: 1967. [Google Scholar]
- Bouisset S, Zattara M. Biomechanical study of the programming of anticipatory postural adjustments associated with voluntary movement. J Biomechanics. 1987;20:735–42. doi: 10.1016/0021-9290(87)90052-2. [DOI] [PubMed] [Google Scholar]
- Bouisset S, Zattara M. Segmental movement as a perturbation to balance? Facts and concepts. In: Winters JM, SL-Y W, editors. Multiple Muscle Systems: Biomechanics and Movement Organization. New York: Springer-Verlag; 1990. pp. 498–506. [Google Scholar]
- Cavanagh PR, Komi PV. Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. European journal of applied physiology and occupational physiology. 1979;42:159–63. doi: 10.1007/BF00431022. [DOI] [PubMed] [Google Scholar]
- Corcos DM, Gottlieb GL, Latash ML, Almeida GL, Agarwal GC. Electromechanical delay: An experimental artifact. J Electromyogr Kinesiol. 1992;2:59–68. doi: 10.1016/1050-6411(92)90017-D. [DOI] [PubMed] [Google Scholar]
- Cordo PJ, Nashner LM. Properties of postural adjustments associated with rapid arm movements. Journal of neurophysiology. 1982;47:287–302. doi: 10.1152/jn.1982.47.2.287. [DOI] [PubMed] [Google Scholar]
- Couillandre A, Maton B, Breniere Y. Voluntary toe-walking gait initiation: electromyographical and biomechanical aspects. Experimental brain research Experimentelle Hirnforschung. 2002;147:313–21. doi: 10.1007/s00221-002-1254-y. [DOI] [PubMed] [Google Scholar]
- Crenna P, Frigo C. A motor programme for the initiation of forward-oriented movements in humans. J Physiol. 1991;437:635–53. doi: 10.1113/jphysiol.1991.sp018616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Avella A, Saltiel P, Bizzi E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci. 2003;6:300–8. doi: 10.1038/nn1010. [DOI] [PubMed] [Google Scholar]
- Danna-dos-Santos A, Slomka K, Zatsiorsky VM, Latash ML. Muscle modes and synergies during voluntary body sway. Experimental brain research Experimentelle Hirnforschung. 2007;179:533–50. doi: 10.1007/s00221-006-0812-0. [DOI] [PubMed] [Google Scholar]
- De Wolf S, Slijper H, Latash ML. Anticipatory postural adjustments during self-paced and reaction-time movements. Experimental brain research Experimentelle Hirnforschung. 1998;121:7–19. doi: 10.1007/s002210050431. [DOI] [PubMed] [Google Scholar]
- Elble RJ, Moody C, Leffler K, Sinha R. The initiation of normal walking. Mov Disord. 1994;9:139–46. doi: 10.1002/mds.870090203. [DOI] [PubMed] [Google Scholar]
- Feldman AG. On the functional tuning of the nervous system in movement control or preservation of stationary pose. II. Adjustable parameters in muscles. Biofizika. 1966;11:498–508. [PubMed] [Google Scholar]
- Feldman AG. Once more on the equilibrium-point hypothesis (lambda model) for motor control. J Mot Behav. 1986;18:17–54. doi: 10.1080/00222895.1986.10735369. [DOI] [PubMed] [Google Scholar]
- Feldman AG. Origin and advances of the equilibrium-point hypothesis. Advances in experimental medicine and biology. 2009;629:637–43. doi: 10.1007/978-0-387-77064-2_34. [DOI] [PubMed] [Google Scholar]
- Feldman AG, Levin MF. The origin and use of positional frames of reference in motor control. Behav and Brain Sci. 1995;18:723–44. [Google Scholar]
- Field D. Anatomy: palpation and surface markings. 3. Oxford; Boston: Butterworth Heinemann; 2001. [Google Scholar]
- Gelfand IM, Latash ML. On the problem of adequate language in motor control. Motor control. 1998;2:306–13. doi: 10.1123/mcj.2.4.306. [DOI] [PubMed] [Google Scholar]
- Gray H, Standring S, Ellis H, Berkovitz BKB. Gray’s anatomy: the anatomical basis of clinical practice. 39. Edinburgh; New York: Elsevier Churchill Livingstone; 2005. [Google Scholar]
- Hair JF, Anderson RE, Tatham RL, Black WC. Factor analysis. In: Borkowski D, editor. Multivariate data analysis. Englewood Cliffs: Prentice Hall; 1995. pp. 364–404. [Google Scholar]
- Hamill J, Knutzen K. Biomechanical basis of human movement. Baltimore: Williams & Wilkins; 1995. [Google Scholar]
- Hughlings Jackson J. On the comparative study of disease of the nervous system. British Medical Journal. 1889:55–362. [Google Scholar]
- Holdefer RN, Miller LE. Primary motor cortical neurons encode functional muscle synergies. Experimental brain research Experimentelle Hirnforschung. 2002;146:233–43. doi: 10.1007/s00221-002-1166-x. [DOI] [PubMed] [Google Scholar]
- Inglin B, Woollacott M. Age-related changes in anticipatory postural adjustments associated with arm movements. Journal of gerontology. 1988;43:M105–13. doi: 10.1093/geronj/43.4.m105. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Cappellini G, Dominici N, Poppele RE, Lacquaniti F. Coordination of locomotion with voluntary movements in humans. J Neurosci. 2005;25:7238–53. doi: 10.1523/JNEUROSCI.1327-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Wright WG, Gurfinkel VS, Horak F, Cordo P. Interaction of involuntary post-contraction activity with locomotor movements. Experimental brain research Experimentelle Hirnforschung. 2006;169:255–60. doi: 10.1007/s00221-005-0324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Shim JK, Zatsiorsky VM, Latash ML. Anticipatory adjustments of multi-finger synergies in preparation for self-triggered perturbations. Exp Brain Res. 2006;174:604–12. doi: 10.1007/s00221-006-0505-8. [DOI] [PubMed] [Google Scholar]
- Klous M, Danna-dos-Santos A, Latash ML. Multi-muscle synergies in a dual postural task: evidence for the principle of superposition. Experimental brain research Experimentelle Hirnforschung. 2010;202:457–71. doi: 10.1007/s00221-009-2153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klous M, Mikulic P, Latash ML. Two aspects of feedforward postural control: anticipatory postural adjustments and anticipatory synergy adjustments. Journal of neurophysiology. 2011;105:2275–88. doi: 10.1152/jn.00665.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy V, Goodman S, Zatsiorsky V, Latash ML. Muscle synergies during shifts of the center of pressure by standing persons: identification of muscle modes. Biological cybernetics. 2003a;89:152–61. doi: 10.1007/s00422-003-0419-5. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy V, Latash ML, Scholz JP, Zatsiorsky VM. Muscle synergies during shifts of the center of pressure by standing persons. Experimental brain research Experimentelle Hirnforschung. 2003b;152:281–92. doi: 10.1007/s00221-003-1574-6. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy V, Latash ML, Scholz JP, Zatsiorsky VM. Muscle modes during shifts of the center of pressure by standing persons: effect of instability and additional support. Experimental brain research Experimentelle Hirnforschung. 2004;157:18–31. doi: 10.1007/s00221-003-1812-y. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy V, Scholz JP, Latash ML. The use of flexible arm muscle synergies to perform an isometric stabilization task. Clin Neurophysiol. 2007;118:525–37. doi: 10.1016/j.clinph.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Aruin AS. Postural control in response to a perturbation: role of vision and additional support. Experimental brain research Experimentelle Hirnforschung. 2011;212:385–97. doi: 10.1007/s00221-011-2738-4. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Aruin AS, Latash ML. Two stages and three components of the postural preparation to action. Experimental brain research Experimentelle Hirnforschung. 2011;212:47–63. doi: 10.1007/s00221-011-2694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutch JJ, Buchanan TS. Human elbow joint torque is linearly encoded in electromyographic signals from multiple muscles. Neuroscience letters. 2001;311:97–100. doi: 10.1016/s0304-3940(01)02146-2. [DOI] [PubMed] [Google Scholar]
- Latash ML. Motor synergies and the equilibrium-point hypothesis. Motor control. 2010a;14:294–322. doi: 10.1123/mcj.14.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML. Stages in learning motor synergies: a view based on the equilibrium-point hypothesis. Hum Mov Sci. 2010b;29:642–54. doi: 10.1016/j.humov.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML, Aruin AS, Neyman I, Nicholas JJ. Anticipatory postural adjustments during self inflicted and predictable perturbations in Parkinson’s disease. Journal of neurology, neurosurgery, and psychiatry. 1995;58:326–34. doi: 10.1136/jnnp.58.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML, Scholz JF, Danion F, Schoner G. Structure of motor variability in marginally redundant multifinger force production tasks. Experimental brain research Experimentelle Hirnforschung. 2001;141:153–65. doi: 10.1007/s002210100861. [DOI] [PubMed] [Google Scholar]
- Latash ML, Scholz JP, Schoner G. Motor control strategies revealed in the structure of motor variability. Exerc Sport Sci Rev. 2002;30:26–31. doi: 10.1097/00003677-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Latash ML, Scholz JP, Schoner G. Toward a new theory of motor synergies. Motor control. 2007;11:276–308. doi: 10.1123/mcj.11.3.276. [DOI] [PubMed] [Google Scholar]
- Latash ML, Shim JK, Smilga AV, Zatsiorsky VM. A central back-coupling hypothesis on the organization of motor synergies: a physical metaphor and a neural model. Biological cybernetics. 2005;92:186–91. doi: 10.1007/s00422-005-0548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepers R, Breniere Y. The role of anticipatory postural adjustments and gravity in gait initiation. Experimental brain research Experimentelle Hirnforschung. 1995;107:118–24. doi: 10.1007/BF00228023. [DOI] [PubMed] [Google Scholar]
- Martin V, Scholz JP, Schoner G. Redundancy, self-motion, and motor control. Neural computation. 2009;21:1371–414. doi: 10.1162/neco.2008.01-08-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Olafsdottir H, Yoshida N, Zatsiorsky VM, Latash ML. Anticipatory covariation of finger forces during self-paced and reaction time force production. Neuroscience letters. 2005;381:92–6. doi: 10.1016/j.neulet.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafsdottir H, Zhang W, Zatsiorsky VM, Latash ML. Age-related changes in multifinger synergies in accurate moment of force production tasks. J Appl Physiol. 2007;102:1490–501. doi: 10.1152/japplphysiol.00966.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ramos CF, Stark LW. Postural maintenance during fast forward bending: a model simulation experiment determines the “reduced trajectory”. Experimental brain research Experimentelle Hirnforschung. 1990;82:651–7. doi: 10.1007/BF00228807. [DOI] [PubMed] [Google Scholar]
- Robert T, Zatsiorsky VM, Latash ML. Multi-muscle synergies in an unusual postural task: quick shear force production. Experimental brain research Experimentelle Hirnforschung. 2008;187:237–53. doi: 10.1007/s00221-008-1299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini AM. Identification of neuromuscular synergies in natural upper-arm movements. Biological cybernetics. 2002;86:253–62. doi: 10.1007/s00422-001-0297-7. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Aruin AS. Role of lateral muscles and body orientation in feedforward postural control. Experimental brain research Experimentelle Hirnforschung. 2008;184:547–59. doi: 10.1007/s00221-007-1123-9. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture: 1. Electromyographic analysis. J Electromyogr Kinesiol. 2010;20:388–97. doi: 10.1016/j.jelekin.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Danion F, Latash ML, Schoner G. Understanding finger coordination through analysis of the structure of force variability. Biological cybernetics. 2002;86:29–39. doi: 10.1007/s004220100279. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Schoner G. The uncontrolled manifold concept: identifying control variables for a functional task. Experimental brain research Experimentelle Hirnforschung. 1999;126:289–306. doi: 10.1007/s002210050738. [DOI] [PubMed] [Google Scholar]
- Shim JK, Latash ML, Zatsiorsky VM. Prehension synergies: trial-to-trial variability and hierarchical organization of stable performance. Experimental brain research Experimentelle Hirnforschung. 2003;152:173–84. doi: 10.1007/s00221-003-1527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JK, Olafsdottir H, Zatsiorsky VM, Latash ML. The emergence and disappearance of multi-digit synergies during force-production tasks. Exp Brain Res. 2005;164:260–70. doi: 10.1007/s00221-005-2248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JK, Park J, Zatsiorsky VM, Latash ML. Adjustments of prehension synergies in response to self-triggered and experimenter-triggered load and torque perturbations. Experimental brain research Experimentelle Hirnforschung. 2006;175:641–53. doi: 10.1007/s00221-006-0583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori T, Latash ML. The roles of proximal and distal muscles in anticipatory postural adjustments under asymmetrical perturbations and during standing on rollerskates. Clin Neurophysiol. 2000;111:613–23. doi: 10.1016/s1388-2457(99)00300-4. [DOI] [PubMed] [Google Scholar]
- Shiratori T, Latash ML. Postural preparation for sequential perturbations. JHK. 2006;15:3–24. [Google Scholar]
- Slijper H, Latash ML. The effects of instability and additional hand support on anticipatory postural adjustments in leg, trunk, and arm muscles during standing. Experimental brain research Experimentelle Hirnforschung. 2000;135:81–93. doi: 10.1007/s002210000492. [DOI] [PubMed] [Google Scholar]
- Slijper H, Latash ML, Rao N, Aruin AS. Task-specific modulation of anticipatory postural adjustments in individuals with hemiparesis. Clin Neurophysiol. 2002;113:642–55. doi: 10.1016/s1388-2457(02)00041-x. [DOI] [PubMed] [Google Scholar]
- Ting LH. Dimensional reduction in sensorimotor systems: a framework for understanding muscle coordination of posture. Prog Brain Res. 2007;165:299–321. doi: 10.1016/S0079-6123(06)65019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting LH, Chvatal SA. Decomposing muscle activity in motor tasks. In: Danion F, Latash ML, editors. Motor Control Theories, Experiments and Applications. Oxford University Press; New York: 2010. pp. 102–38. [Google Scholar]
- Ting LH, Macpherson JM. A limited set of muscle synergies for force control during a postural task. Journal of neurophysiology. 2005;93:609–13. doi: 10.1152/jn.00681.2004. [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Macpherson JM, Ting LH. Muscle synergy organization is robust across a variety of postural perturbations. Journal of neurophysiology. 2006;96:1530–46. doi: 10.1152/jn.00810.2005. [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Ting LH. Muscle synergies characterizing human postural responses. Journal of neurophysiology. 2007;98:2144–56. doi: 10.1152/jn.01360.2006. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Jarc A. The case for and against muscle synergies. Curr Opin Neurobiol. 2009;19:601–7. doi: 10.1016/j.conb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cuevas FJ, Venkadesan M, Todorov E. Structured variability of muscle activations supports the minimal intervention principle of motor control. Journal of neurophysiology. 2009;102:59–68. doi: 10.1152/jn.90324.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zatsiorsky VM, Latash ML. Muscle synergies involved in preparation to a step made under the self-paced and reaction time instructions. Clin Neurophysiol. 2006;117:41–56. doi: 10.1016/j.clinph.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. Journal of neurophysiology. 1996;75:2334–43. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]
- Yiou E, Hamaoui A, Le Bozec S. Influence of base of support size on arm pointing performance and associated anticipatory postural adjustments. Neuroscience letters. 2007;423:29–34. doi: 10.1016/j.neulet.2007.06.034. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM, Latash ML. Multifinger prehension: an overview. J Mot Behav. 2008;40:446–76. doi: 10.3200/JMBR.40.5.446-476. [DOI] [PMC free article] [PubMed] [Google Scholar]