Abstract
In addition to cigarette smoking, alcohol exposure is also associated with increased lung infections and decreased mucociliary clearance. However, little research has been conducted on the combination effects of alcohol and cigarette smoke on lungs. Previously, we have demonstrated in a mouse model that the combination of cigarette smoke and alcohol exposure results in the formation of a very stable hybrid malondialdehyde-acetaldehyde (MAA)-adducted protein in the lung. In in vitro studies, MAA-adducted protein stimulates bronchial epithelial cell interleukin-8 via the activation of protein kinase C epsilon (PKCε). We hypothesized that direct MAA-adducted protein exposure in the lungs would mimic such a combination of smoke and alcohol exposure leading to airway inflammation. To test this hypothesis, C57BL/6J female mice were intranasally instilled with either saline, 30 µL of 50 µg/mL BSA-MAA, or unadducted BSA for up to 3 wk. Likewise, human lung surfactant proteins A and D (SPA, SPD) were purified from human pulmonary proteinosis lung lavage fluid and successfully MAA-adducted in vitro. Similar to BSA-MAA, SPD-MAA was instilled into mouse lungs. Lungs were necropsied and assayed for histopathology, PKCε activation, and lung lavage chemokines. In control mice instilled with saline, normal lungs had few inflammatory cells. No significant effects were observed in un-adducted BSA- or SPD-instilled mice. However, when mice were instilled with BSA-MAA or SPD-MAA for 3 wk, a significant peribronchiolar localization of inflammatory cells was observed. Both BSA-MAA and SPD-MAA stimulated increased lung lavage neutrophils and caused a significant elevation in the chemokine, KC, which is a functional homologue to human interleukin-8. Likewise, MAA-adducted protein stimulated the activation of airway and lung slice PKCε. These data support that MAA-adducted protein induces a pro-inflammatory response in the lungs and that lung surfactant protein is a biologically relevant target for malondialdehyde and acetaldehyde adduction. These data further implicate MAA-adduct formation as a potential mechanism for smoke and alcohol induced lung injury.
Keywords: innate immunity, aldehydes, cigarette smoke, alcohol, lung
Introduction
Heavy alcohol use is associated with the increased occurrence of lung diseases including pneumonia. In fact, meta-analyses have demonstrated that alcohol has a dose-dependent association as a risk factor for pneumonia (Samokhvalov et al., 2010). Part of the adverse health effect of alcohol emanates from direct exposure of the lungs to alcohol. Alcohol diffuses from the bronchial circulation into the airways where it is exposed to air, vaporizes, and then condenses back on to the airways. This ‘rain’ effect results in both acute and chronic alterations to innate lung defense and mucociliary clearance (Sisson, 2007). Unfortunately, the vast majority (between 80% and 95%) of alcoholics also smoke cigarettes (Patten et al., 1996).
There are approximately 46.6 million adult cigarette smokers in the United States representing 20.6% of the US population with the Midwest population reaching as high as 23.1% (Centers for Disease Control and Prevention [CDC], 2010). Unfortunately, there has been no decline in this number from 2005–2009. One-third of heavy smokers are alcoholics (Miller and Gold, 1998). Even though the association of cigarette smoking and alcohol consumption is universally recognized, relatively few co-exposure studies exist compared to mechanistic studies of the biologic effects of either alcohol alone or smoke alone exposure on lung injury.
One shared mechanism for the pathogenesis of lung injury from alcohol and cigarette smoke is via reactive aldehyde formation. Alcohol is rapidly metabolized into acetaldehyde (AA) by the action of alcohol dehydrogenase (ADH). In addition to the AA released during the first pass from the liver into the bloodstream, both ADH and CYP2E1 are present in lung and act to metabolize alcohol. In addition, oxidative stress-induced lipid peroxidation occurs in the lung resulting in the alcohol-induced formation of malondialdehyde (MDA). While lipid peroxidation-derived MDA formation can also be stimulated by cigarette smoke, the direct pyrrolysis of tobacco generates large concentrations of AA. These aldehydes are highly reactive and nonenzymatically bind to proteins covalently to form Schiff bases. Such aldehyde-protein binding in vivo generally results in a deleterious outcome due to the modification of nucleophilic residue side chains found on amino acids such as lysine and hydroxylysine (Harding, 1985). These reactions between aldehyde and proteins are commonly known as protein adducts.
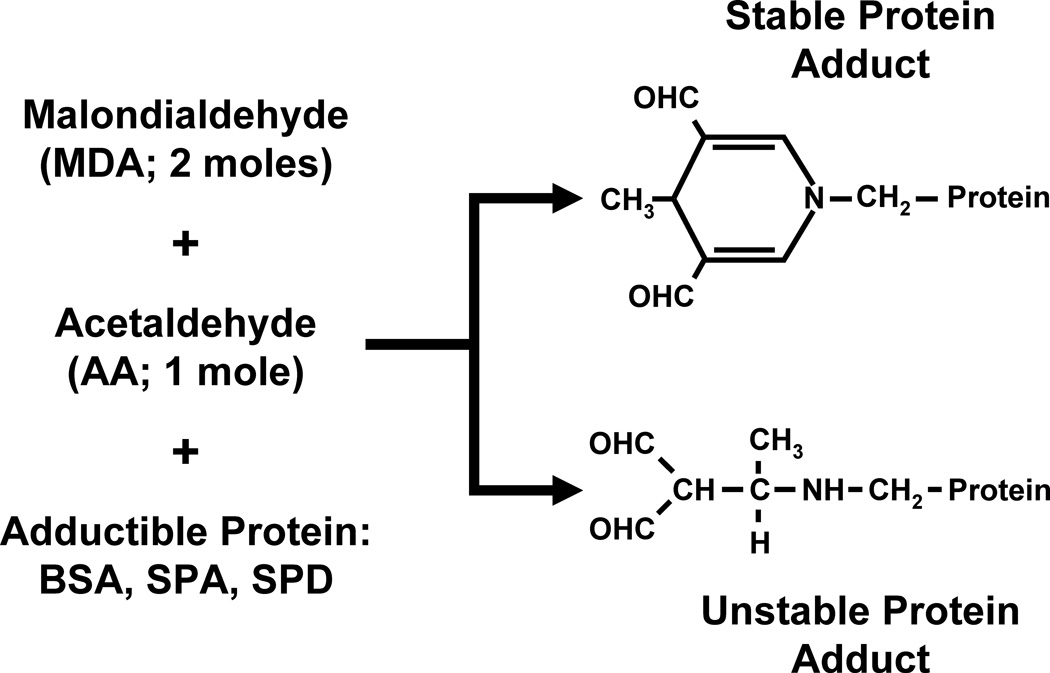
The adduction of AA or MDA individually to proteins results in an unstable protein adduct with a short half-life. However, the formation of a hybrid protein adduct consisting of 2 moles of MDA and 1 mole of AA results in a very stable protein adduct known as the malondialdehyde-acetaldehyde protein adduct (MAA adduct; Figure 1). Because cigarette smoke contains such high concentrations of AA while alcohol consumption leads to elevated MDA levels in the lung, the lungs of a smoker who drinks alcohol are the ideal milieu for the formation of MAA adducts.
Figure 1.
Formation of MAA-adducted proteins. Two moles of malondialdehyde react with 1 mole of acetaldehyde to form a stable hybrid malondialdehyde-acetaldehyde protein adduct. The linear adduct is unstable and is rapidly degraded.
Previously, we have demonstrated in a mouse model of combined cigarette smoke and alcohol exposure that changes in the regulation of ciliary beating and pro-inflammatory cytokine release are enhanced under conditions of alcohol and cigarette smoke co-exposure as compared to individual exposures (Elliott et al., 2007). In addition, we have found that only alcohol and cigarette smoke co-exposure results in AA and MDA concentrations sufficient for and leading to MAA adduct formation in lung (McCaskill et al., 2011). Furthermore, we identified surfactant protein D (SPD) to be one such adducted protein formed in the lung in response to dual smoke and alcohol exposure (McCaskill et al., 2011). Direct exposure of purified MAA-adducted protein to bronchial epithelial cells in vitro results in a protein kinase C epsilon-dependent release of pro-inflammatory cytokines (Wyatt et al., 2001). We therefore hypothesized that purified MAA-adducted protein would produce inflammatory injury in and of itself when directly instilled into the lungs of mice.
Methods
Preparation of MAA-adducted protein
Human lung surfactant proteins were purified from human pulmonary alveolar proteinosis fluid as previously reported (Strong et al., 1998). The MAA-adducted proteins were generated by incubating 1 mg each of BSA (Fraction V, fatty acid free, low endotoxin, Sigma, St. Louis, MO), SP-D or SP-A with an equimolar (1 mmol/L) solution of MDA and acetaldehyde (Aldrich Chemical Co, Milwaukee, WI) for 3 days at 37°C in a sealed polypropylene vessel in a non-oxidizing atmosphere as detailed in our earlier publication (Tuma et al., 1996). At the end of the 72 h incubation, the reaction mixture was exhaustively dialyzed for 24 h at 4°C. The amount of the specific fluorescent 2:1 adduct formed during MAA adduct generation of the proteins was quantified in the post-dialysis retentate using an in-house synthetic analog of MAA, 1-hexyl-4-methyl-1,4-dihydro-3,5-pyridinedicarboxaldehyde (hexyl-MAA) (Xu et al., 1997) as a standard. The measurement of the fluorescence (excitation at 398 nm and emission maximum at 460) was done using a Perkin Elmer Luminescence Spectrophotometer LS 50B (Norwalk, CT) and the data were expressed as nmol of fluorescent MAA equivalents per mg protein.
Mouse lung slice model
Precision-cut mouse lung slices were prepared as previously reported (Perez and Sanderson, 2005) using naïve C57Bl/6 mice. Mice were euthanized with 0.3 mL (12 mL/kg) of 50 mg/mL pentobarbital. After pulling the fur away from the abdomen and exposing the trachea, a 20-guage cannula was inserted just below the larynx and secured by suture. The chest cavity was opened and the lung tested for integrity by inflating with air. After the air was removed, a solution of 2% low melting point agarose (Invitrogen, Carlsbad, CA) in sterile Hanks Balanced Salt Solution (HBSS; pH 7.4) maintained at 37°C was slowly pushed into the lungs until full distention achieved. The abdomen was then covered with a sterile cotton-ball saturated with HBSS (4°C) followed by packing the body with ice for up to 45 min to solidify the agarose. The lungs were removed and the lobes separated and placed in ice-cold HBSS for another 45 minutes. Lung lobes were then trimmed, glued to a vibrating microtome platform (model EMS-4000, Electron Microscope Sciences, Hatfield, PA), and sliced into 150 µm thick slices. The slices were then placed in M-199 media and incubated at 37°C in a 5% CO2 incubator. Before any experimental treatments began, slices were incubated for a minimum of 5 d with daily media change at which time minimal media lactate dehydrogenase release was detected.
Mouse lung slices were used to determine if MAA-adducted surfactant proteins are biologically active on lung tissues. Mouse lung slices were treated with BSA-MAA, SPA-MAA and SPD-MAA for 1–24 hours at various concentrations ranging from 50–200 µg/mL. Each data point represents n=3 or more different mice using multiple lung slices from different lobes of the lung.
Nasal instillation model
Male C57BL/6 mice (6–8 weeks old) were obtained from Charles River (Wilmington, MA), housed in group cages, and fed commercial rodent chow and water ad libitum for 1 week. The mice were randomly assigned to a treatment group (sham, non-adducted protein control, or MAA-adducted protein instillation exposures). All mice were weighed weekly throughout the instillation time course. All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center. The nasal instillation was based on a previously established model using cigarette smoke extract (Miller et al., 2002). Prior to initiating the instillation protocol, mice were anesthetized by isofluorane inhalation. Each mouse was positioned with the head back so the nasal pathway was vertical and then 40 µl of treatment solution (sterile PBS or 50 µg/mL MAA-adducted protein) was placed at the nasal openings using a pipette tip. After the instillation was delivered, the mouse remained vertically held to ensure complete inhalation, thus preventing any oral discharge as consciousness was regained after anesthesia. The mice were monitored until awake and moving around normally following the treatment. Mice were intranasally instilled with 1, 10, 50, and 100 µg/mL BSA-MAA (40 µL) daily for 3 wk. For comparison, sham groups of mice were given 40 µl of sterile BSA or SPD (50 µg/mL) in PBS only and control mice were anesthetized, but not nasally instilled. None of the mice exhibited respiratory distress. Whole lung homogenates revealed that the delivered MAA adducted protein to be in the range of 100–1000 ng/mL after intranasal instillation with 50 µg/mL MAA. This compares favorably to the levels of MAA adducted protein detected in mouse lung in vivo exposed to the combination of cigarette smoke and alcohol (McCaskill et al., 2011).
Bronchoalveolar lavage (BAL)
After the instillation period, mice were euthanized by intraperitoneal injection of 50 mg/kg body weight of Nembutal (Abbott Labs, Chicago, IL). The trachea was exposed and a cannula inserted just below the larynx. The proximal end of the trachea was held around the cannula with forceps while 1.0 ml of sterile PBS (Gibco, Grand Island, NY) was instilled into the lungs and recovered by aspiration. A total of 3.0 ml was introduced to the lungs. The BAL fluid was centrifuged at 250g to collect cells. The supernatant from the first mL of BAL fluid recovered was frozen at −80°C to later test for cytokines. Cells from all 3 mL were resuspended, pooled and spun onto slides with a Cytopro Cytocentrifuge (Wescor Inc. Logan, UT) and stained with DiffQuik (Dade Behring, Newark, DE). Phase microscopy counts of the cells performed by two different scorers determined the differential ratio of cell types in 200 cells per slide per mouse. Tracheae were collected after BAL and placed in 50mM Tris-HCl (pH 7.4) lysis buffer with protease inhibitors previously described (Wyatt et al., 1998). These samples were flash frozen in liquid nitrogen and stored at −80°C until assayed for PKC activity.
Protein kinase C activity assay
Epithelial cell lysates from frozen trachea were sonicated and centrifuged at 10,000g for 30 minutes at 4°C. The supernatant was removed (cytosolic fraction) and the pellet was resuspended in cell lysis buffer containing 0.01% Triton X-100 and sonicated again (particulate fraction). PKC isoform activity was determined in crude whole cell cytosolic and particulate fractions similar to methods described previously (Wyatt et al., 2000; Wyatt et al., 2003). To measure PKC isoform activity specifically, 24 µg/mL phorbol-myristate acetate (PMA), 30 mM dithiotreitol, 150 µM ATP, 45 mM Mg-acetate, PKCε isoform-specific substrate peptide (Calbiochem) and 10 µCi/mL [γ-32P]-ATP were mixed in a Tris-HCl buffer (pH 7.5). Chilled (4°C) cell lysate (cytosolic or particulate) samples (20 µL) were added to 40 µL of the reaction mix and incubated for 15 minutes at 30°C. This mixture (60 µL) was then spotted onto P-81 phosphocellulose papers (Whatman, Clinton, NJ) to halt incubation, and papers were subsequently washed 5 times in 75 mM phosphoric acid for 5 minutes, washed once in 100% ethanol for 1 minute, dried, and counted in nonaqueous scintillant (National Diagnostics, Atlanta GA). PKC activity was expressed in relation to the total amount of cellular protein assayed as picomoles of phosphate incorporated per minute per milligram.
Lung histology and inflammatory score
Lungs were harvested from the mouse and tied via the trachea to the cannula. Once removed from the thoracic cavity the lungs were inflated with 0.8 ml of 10% formalin (Sigma, St. Louis, MO). The cannula was then suspended at the end of syringe fixed under 15 cm of pressure overnight so that the lung was submerged in 10% formalin. The following day the lung was then transferred to a 50 ml conical tube and allowed to rest in formalin for 24–48 hours. Lung tissue was arranged in the cassettes, which were processed in an automatic tissue processor to dehydrate tissues that were then embedded in paraffin. Sections (5 µM) were cut, stained with hematoxylin and eosin (H&E), microscopically reviewed, and semi-quantitatively assessed for the degree of inflammation as well as the distribution of the inflammation utilizing a previously published scoring system (Poole et al., 2009).
Chemokine ELISA
Culture supernatants collected from precision-cut lung slices and BAL fluids were also assayed for keratinocyte chemokine (KC) and macrophage inflammatory protein (MIP-2), utilizing the SearchLight array (Thermo Scientific, Rockford, IL), a multiplexed sandwich ELISA.
Statistical analysis
All quantitative experiments were performed in triplicate. All data were analyzed using GraphPad Prism (version 4.00 for Windows, GraphPad Software, San Diego CA) and represented as mean ± standard error. Data were analyzed for statistical significance using one-way ANOVA followed by Bonferroni post-hoc testing between each condition group. Significance was accepted at the 95% confidence interval.
Results
Surfactant proteins can be MAA-adducted
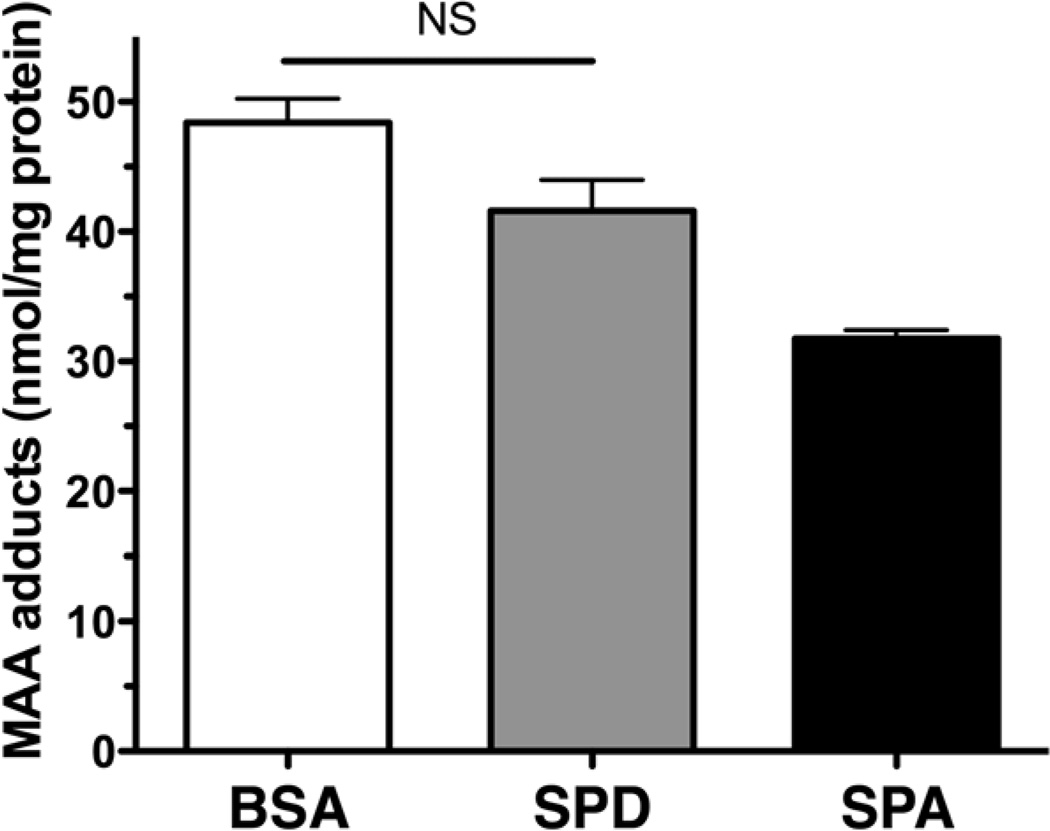
To determine if biologically relevant lung proteins could form protein adducts with malondialdehyde (MDA) and acetaldehyde (AA), purified surfactant proteins A and D (SPA and SPD) were reacted in vitro with 2 moles of MDA and 1 mole of AA (Figure 1). The resulting concentration of MAA-adducted proteins formed was then compared to the amount of MAA that would adduct to bovine serum albumin (BSA). Both BSA and SPD formed MAA adducts (40–50 nmol/mg protein) in equivalent concentrations with no significant differences to each other (Figure 2). Even though the MAA adduction of SPA was not significantly different from that of SPD, it was slightly (P<0.05) less adducted than that of BSA. Therefore, we chose to use SPD-MAA as the optimal adducted protein for our lung studies. These data demonstrate that native resident lung proteins such as surfactant proteins are potential targets for MAA adduction and demonstrate, for the first time, biologically relevant human proteins that are MAA-adducted.
Figure 2.
MAA adduction of human lung surfactant protein in vitro. Human surfactant protein D (SPD) and surfactant protein A (SPA) were purified from whole lung lavage fluid and chemically adducted with malondialdehyde and acetaldehyde as described in Methods. Purified SPD and SPA form hybrid aldehyde adducts similar to MAA-adducted bovine serum albumin (BSA).
MAA-adducted surfactant protein stimulates PKC-dependent chemokine release in vitro
To determine if MAA-adducted surfactant proteins are biologically active on lung tissues, precision-cut mouse lung slices were treated with BSA-MAA, SPA-MAA and SPD-MAA for various times and concentrations. Lung slice tissue was homogenized for PKCε activity assay and the remaining lung slice culture media supernatants assayed for keratinocyte chemokine (KC) release. KC is a functional homologue to human interleukin-8 (IL-8). SPD-MAA (50–200 µg/mL) activated PKCε between 1–2 hours with a subsequent return to baseline PKCε activity levels (media control) from 3–6 hours (data not shown). Concentrations less that 50 µg/mL did not significantly activate PKC, nor did non-adducted proteins (data not shown). PMA (100ng/mL) was used as a positive control to activate PKCε. The magnitude of SPD-MAA stimulated PKCε activity was equivalent to that of BSA-MAA and slightly, but not significantly, larger than SPA-MAA (data not shown).
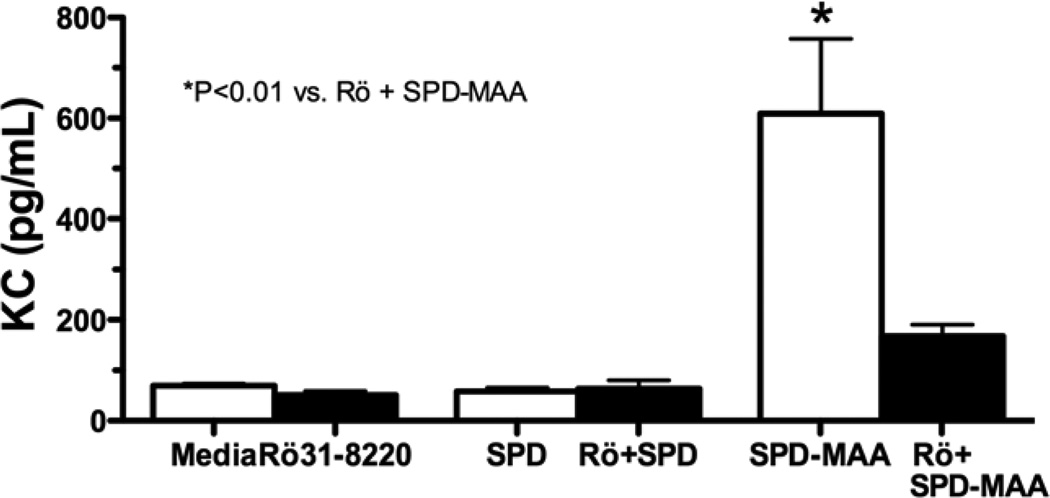
SPD-MAA treatment of mouse lung slices for 24 hr resulted in significant (P<0.01) stimulation of KC release (Figure 3). No stimulation of KC was observed by non-adducted SPD. Pretreating the lung slices for 1 hr with 1 µM Rö 31–8220, a novel PKCε isoform inhibitor, prior to SPD-MAA treatment blocked the stimulated release of KC. Additionally, macrophage inhibitory protein (MIP-2), like KC, also a functional homologue to human IL-8, was elevated after SPD-MAA treatment (data not shown). These data show that SPD-MAA stimulated chemokine release requires PKCε activation in an in vitro lung slice model.
Figure 3.
In vitro stimulation of mouse lung by SPD-MAA adducted protein. Lung slices were treated with SPD or SPD-MAA in the presence or absence of Rö 31-8220 for 24 hr and media supernatants assayed for keratinocyte chemokine (KC). The significant (P<0.01) increase in KC release in response to SPD-MAA was inhibited by a 1 hr pretreatment with Rö 31-8220.
Nasal instillation of MAA-adducted protein produces in vivo lung cellular inflammation
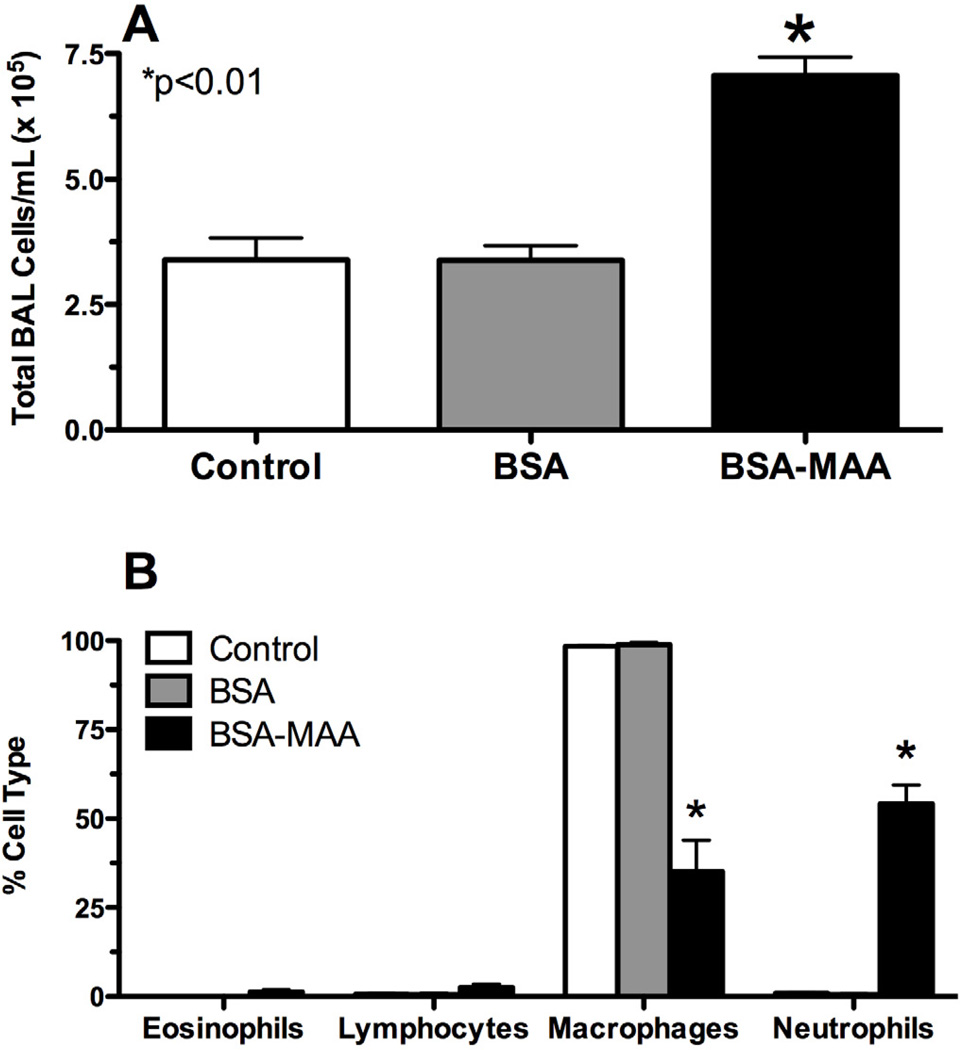
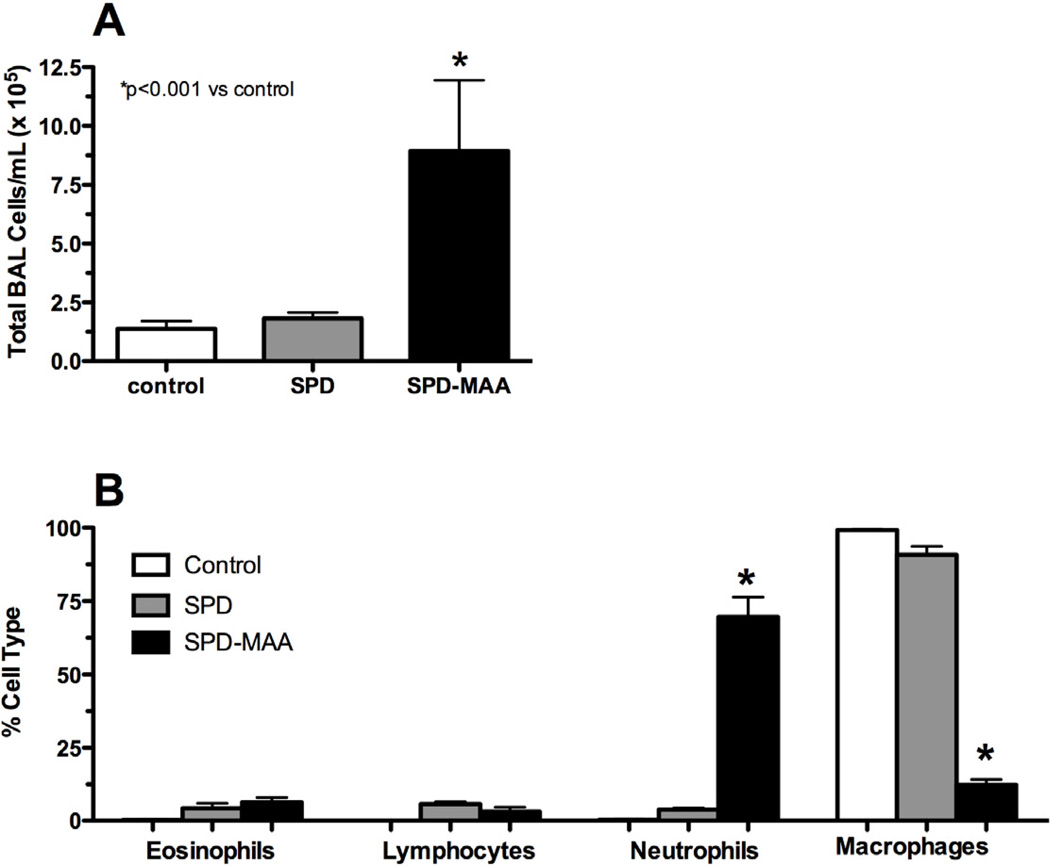
To determine the in vivo effects of MAA-adducted proteins on the lung, mice were nasally instilled with BSA- and SPD-MAA as described in the Methods. No significant changes in weight gain were measured as the mice in all groups gained an average of 1.5 g over the 3 wk period (data not shown). Nasal instillation of BSA-MAA (50 µg/mL) for 3 wk induced a significant (P<0.01) increase in total leukocyte counts in lung BAL fluid compared to either sterile PBS control or non-adducted sterile 50 µg/mL BSA (Figure 4A, N=6 mice per treatment group). This increase in lavage cellularity was predominately due to an increase in BAL neutrophils (Figure 4B). No change in lymphocytes or eosinophils was observed. Importantly, no change in BAL cellularity was observed with the nasal instillation of non-adducted BSA. SPD-MAA inhalation produced comparable effect as BSA-MAA on increasing total BAL cellularity (Figure 5A) and lung neutrophil recruitment (Figure 5B). Collectively, these data demonstrate that the recruitment of inflammatory neutrophils occurs after MAA-adducted protein instillation suggesting that the MAA moiety on the protein adduct is essential for this neutrophil recruitment.
Figure 4.
Nasal instillation of MAA-adducted protein causes lung inflammation in mice. Mice instilled with BSA-MAA (50 µg/mL) exhibited enhanced bronchoalveolar lavage (BAL) inflammatory cells consisting primarily of neutrophils. The total number of lung lavage cells significantly increased (P<0.01) with BSA-MAA instillation. No inflammation was observed in mice instilled with the sterile PBS vehicle or with non-adducted BSA.
Figure 5.
Bronchoalveolar lavage (BAL) of mice instilled with SPD-MAA. Mice instilled with SPD-MAA (50 µg/mL) exhibited enhanced lung lavage inflammatory cells consisting primarily of neutrophils. The total number of lung lavage cells significantly increased (P<0.001) with SPD-MAA instillation. No inflammation was observed in mice instilled with the sterile PBS vehicle (control) or with non-adducted SPD.
MAA-adducted protein in vivo stimulates PKCε activation
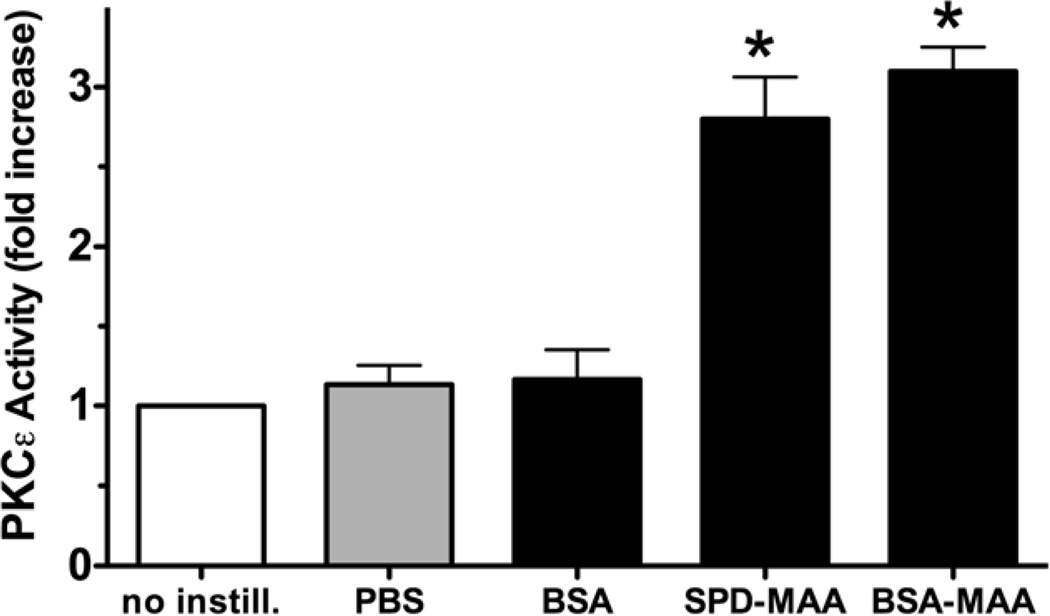
Because the in vitro treatment of mouse lung slices caused a PKCε-dependent release of the neutrophil-recruiting chemokine, KC (Figure 6), we determined if nasal-instilled MAA-adducted protein would in vivo stimulate lung PKCε activity. Mice were instilled with 50 µg/mL of either adducted or non-adducted BSA or SPD daily for 3 weeks and tracheal epithelial cell PKCe activity assayed. Compared to the control with no instillation, nasal instillation with either BSA-MAA or SPD-MAA stimulated a significant (P<0.05) elevation in PKCε activity. Nasal instillation with either sterile PBS or non-adducted BSA produced no observed differences compared with the no-instillation control. Non-adducted SPD did not activate PKCε when nasally instilled (data not shown). These data confirm that MAA-adducted protein activates PKCε in vivo as well as in vitro and suggests that the airway epithelium is a cellular target for this PKCε-driven response.
Figure 6.
MAA adducts activate tracheal epithelial cell PKC epsilon in vivo. Mice were nasally instilled with 50 µg/mL BSA-MAA or surfactant protein D- (SDP) MAA and tracheal epithelial PKCε activity assayed. Both BSA-MAA and SPD-MAA stimulated PKCε activity compared to control PBS or BSA (50 µg/ml), which had no effect (*P<0.05 vs. no instillation control).
SPD-MAA induces lung inflammation
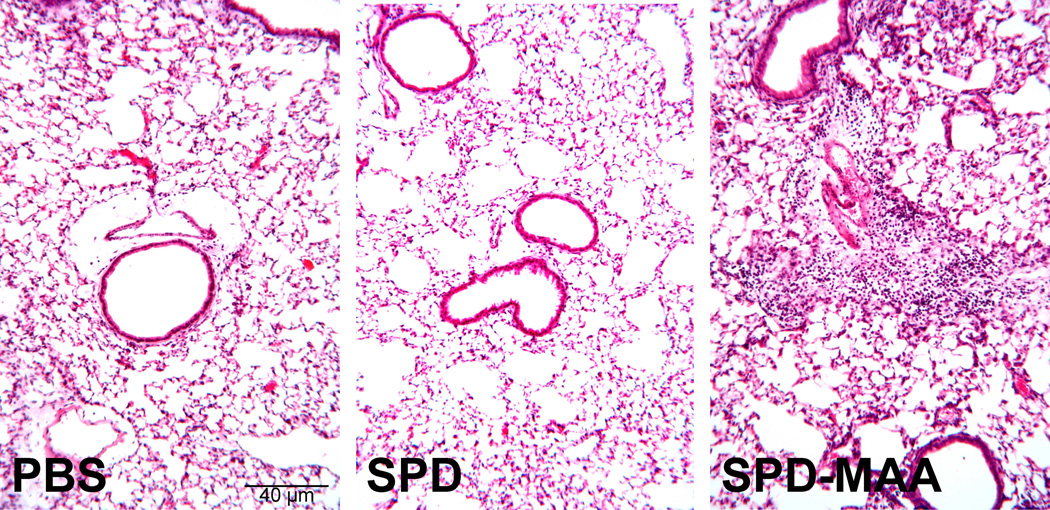
To determine the effect of SPD-MAA instillation on the lung parenchyma, formalin-fixed, paraffin-embedded whole lungs were sectioned and stained with hematoxylin and eosin. The lung histology was notable for increased inflammatory cells within the peribronchiolar region of the small airways of SPD-MAA instilled mice compared to PBS control (Figure 7A). This increase in diffuse peribronchiolar inflammation was not observed in PBS or non-adducted SPD-instilled mice. Although all SPD-MAA instilled mice displayed varying degrees of inflammation, no alveolar anomalies (emphysema) were identified in any of the animals.
Figure 7.
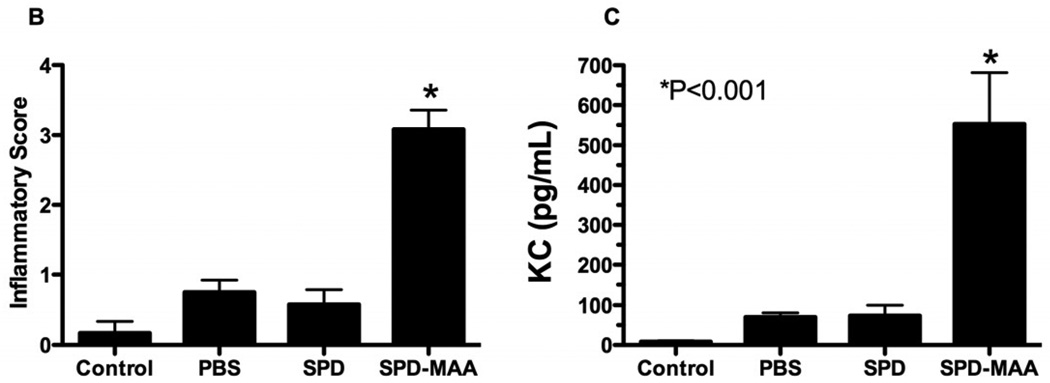
Nasal instillation of SPD-MAA causes lung inflammation in mice. A. Mice instilled with SPD-MAA (50 µg/mL) demonstrated a peribronchiolar inflammation consisting primarily of neutrophils (10× magnification) on H&E stained lung sections. B. Inflammatory score of lungs and BAL KC levels after intranasal inhalation of sterile PBS, SPD, and SPD-MAA (50 µg/mL; 50 µL) after 3 weeks of daily intranasal inhalation exposure in mice (N=6 mice per group). Error bars are SE. *P<0.001 is statistically significant of SPD-MAA versus PBS-treated mice.
Utilizing an established lung pathology inflammatory scoring system (inflammatory score range of 0 to 4) (Poole et al., 2009), the ranges of SPD-MAA-induced histopathologic changes were semi-quantitatively assessed. There was a significant increase in bronchiolar compartment inflammation in the SPD-MAA instilled mice as compared to either PBS or non-adducted SPD-instilled mice (Figure 7B, P<0.001, N=6 mice/group). To characterize this immune inflammatory response with in vivo SPD-MAA instillation, BAL fluid was assayed for the presence of the neutrophil attractant KC (CXCL1). There was a significant increase in KC in response to SPD-MAA vs. no instillation control, sterile PBS only, or non-adducted SPD (Figure 7B; N=6 mice per treatment group; p<0.001). These observations demonstrate a robust inflammatory chemokine response and a significant, semi-quantifiable lung neutrophil infiltration occurring with SPD-MAA adduct instillation.
Discussion
In this study, we demonstrate that exposure to MAA-adducted proteins increases lung inflammation in an in vivo mouse exposure model using direct nasal instillation. As evidenced by histology and bronchoalveolar lavage cell differentiation, this elevated inflammation primarily consisted of a peribronchiolar infiltration of neutrophils in response to nasal instillation with MAA-adducted protein. Mechanistically, this neutrophil recruitment was preceded by MAA-adducted protein stimulation of both epithelial cell PKCε activity and chemokine release into the lung. Ex vivo examination of mouse lung slices exposed to MAA-adducted protein demonstrated that PKCε activation was required for this chemokine release. Similar results were observed irrespective of whether the nasally instilled protein was a native lung surfactant protein or a non-native lung protein such as BSA. These results suggested that the biological response is to the MAA moiety and not to the protein that is adducted. Furthermore, we demonstrate that lung surfactant proteins such as SPA and SPD, which are native to the lung airspaces, can be as effectively MAA-adducted as the previously established and well-characterized BSA-MAA (Wyatt et al., 2001; Xu et al., 1997). Because no significant difference in MAA adduction was detected between BSA and SPD, we chose to focus on using SPD, and not SPA, for our mouse instillation studies.
Equivalent adduction of a lung surfactant to that of BSA has important ramifications for the lung inflammation observed in response to SPD-MAA instillation. Previously, we demonstrated in submerged monolayers of bronchial epithelial cells that BSA-MAA rapidly activates PKC and results in the stimulated release of the neutrophil chemokine, IL-8 (Wyatt et al., 2001). MAA-stimulated chemokine release was blocked by inhibition of PKCα and by pan-isoform inhibitors of PKC. Since those early studies, we have identified a sequential regulation of cytokine release in airway epithelial cells whereby PKCα activation precedes and is required for subsequent PKCε activation (Wyatt et al., 2010). This pathway was confirmed using dominant-negative PKC isoform cell lines, thus avoiding pharmacologic inhibitor specificity concerns. In our current studies using both in vitro mouse lung slices and in vivo nasal instillation, both BSA-MAA and SPD-MAA significantly elevated PKCε activity and led to release of the mouse neutrophil chemokines, KC and MIP-2, functional homologues to human IL-8. Likewise, a significant increase in neutrophils was observed in the BALF and the peribronchiolar areas of the lung. Surfactant protein adduction may also impact subsequent lung susceptibility to infection as the availability of surfactant protein for innate anti-microbial defense could be impacted by MAA adduction. Indeed, chronic alcohol exposure in animal models has already demonstrated decreased levels of surfactant (Lazic et al., 2007; Sozo et al., 2009). These data strongly indicate that the identity of the adducted protein may be less important than the formation of the stable hybrid aldehyde adduct regardless of the protein it is covalently bound to in terms of chemokine regulation and inflammatory cell recruitment.
The in vivo formation of lung MAA-adducted proteins represents an important consideration for lung pathophysiology beyond the establishment that surfactant proteins can be targets for MAA adduction and that lung exposure to SPD-MAA results in lung inflammation. In our early cellular studies, we observed that very high concentrations (1 mM) of both acetaldehyde and malondialdehyde are required for 2–5 days to produce PKC activation (Wyatt et al., 2001). These conditions parallel the in vitro conditions for MAA-adducting lysine-rich proteins (Tuma et al., 2001). Thus, to achieve MAA adduction of native proteins in the lung, the airways would have to be exposed in vivo to chronically high levels of both acetaldehyde and malondialdehyde. To model this, we co-exposed mice to both alcohol and cigarette smoke for up to 6 wk and demonstrated that the levels of acetaldehyde and malondialdehyde required for the formation of MAA-adducted protein were only reached under co-exposure conditions (McCaskill et al., 2011). Importantly, MAA-adducted proteins were only detected in the lungs of the smoke and alcohol co-exposed mice. A dominant MAA-adducted protein identified by smoke and alcohol co-exposure in this study was SPD (McCaskill et al., 2011). While we demonstrated that airway PKCε activity was elevated under conditions of SPD-MAA formation in the cigarette smoke and alcohol co-exposure model, the functional effects of such MAA formation were not examined. Here, we provide evidence for the first time that directly instilling SPD-MAA into a mouse lung can mimic the type of lung neutrophilia observed in previously reported studies of chronic cigarette smoke and alcohol exposed mice (Elliott et al., 2007).
The intracellular signaling pathway that links the formed SPD-MAA in the lung to PKC-stimulated chemokine release and subsequent neutrophil recruitment remains to be defined. MAA-adducted proteins bind to cells through membrane scavenger receptors (Terpstra et al., 2000; Duryee et al., 2005). Previously, we have shown that airway epithelial cells express several classes of scavenger receptors (Wyatt et al., 2005) including scavenger receptor A (SRA) (Bowdish and Gordon, 2009), also known as macrophage scavenger receptor I (MSR-I). A specific ligand for SRA, fucoidan, was capable of competing with and blocking BSA-MAA from binding to SRA. Similar to MAA-adducted protein, fucoidan treatment also resulted in the activation of PKC, suggesting that SRA ligand binding is one pathway to PKC activation in airway epithelium. Importantly, this study showed that migration of epithelial cells into a wound was delayed under conditions of exposure to BSA-MAA or fucoidan (Wyatt et al., 2005). Thus, the impact of MAA-adducted proteins on airway wound repair may represent another important pathway for chronic inflammatory lung disease/remodeling in addition to the effect of MAA adducts on neutrophil recruitment.
Chronic inflammatory lung diseases now represent the third leading cause of death in the United States affecting over 25 million adults (Mathews et al., 2011). While the vast majority of chronic lung disease is due to cigarette smoking, little has been studied with regard to alcohol and chronic inflammatory lung disease. Because clinical observations have established that alcohol use disorders (AUD) are associated with the co-morbidity of cigarette smoking, the lung of a smoker who drinks would represent the target organ ideally capable of creating an environment of high malondialdehyde and acetaldehyde levels required for the formation of MAA-adducted protein. However, individual differences in the expression of phase I metabolizing enzymes such as aldehyde dehydrogenases could impact the chronic formation of MAA-adducted lung protein. Likewise, potential differences in the expression of lung epithelial cell scavenger receptors could alter responses to MAA adduct-induced lung injury. Indeed, genetic polymorphisms in SR-A expression have been linked to chronic obstructive pulmonary disease (Hersh et al., 2006; Ohar et al., 2010). Future studies translating preclinical mouse data on MAA adduct-induced lung injury to individuals with AUD will be necessary to define a role for MAA adducts in alcohol and cigarette smoke-induced chronic lung disease.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Omaha VA Medical Center, Omaha, NE (Department of Veterans Affairs [VA Merit Review] to TAW.) This work was supported by NIH-NIAAA (R37AA008769) to JHS, NIH-NIAAA (R01AA017993-S1) to TAW, and NIH-NIAAA (R01AA017993) to TAW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bowdish DM, Gordon S. Conserved domains of the class A scavenger receptors: evolution and function. Immunol. Rev. 2009;227:19–31. doi: 10.1111/j.1600-065X.2008.00728.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) MMWR Morb. Mortal. Wkly. Rep. Vol. 59. 2010. Vital signs: current cigarette smoking among adults aged >or=18 years --- United States, 2009; pp. 1135–1140. [PubMed] [Google Scholar]
- Duryee MJ, Freeman TL, Willis MS, Hunter CD, Hamilton BC, 3rd, Suzuki H, Tuma DJ, Klassen LW, Thiele GM. Scavenger receptors on sinusoidal liver endothelial cells are involved in the uptake of aldehyde-modified proteins. Mol. Pharmacol. 2005;68:1423–1430. doi: 10.1124/mol.105.016121. [DOI] [PubMed] [Google Scholar]
- Elliott MK, Sisson JH, Wyatt TA. Effects of cigarette smoke and alcohol on ciliated tracheal epithelium and inflammatory cell recruitment. Am. J. Respir. Cell Mol. Biol. 2007;36:452–459. doi: 10.1165/rcmb.2005-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding JJ. Nonenzymatic covalent posttranslational modification of proteins in vivo. Adv. Protein Chem. 1985;37:247–334. doi: 10.1016/s0065-3233(08)60066-2. [DOI] [PubMed] [Google Scholar]
- Hersh CP, DeMeo DL, Raby BA, Litonjua AA, Sylvia JS, Sparrow D, Reilly JJ, Silverman EK. Genetic linkage and association analysis of COPD-related traits on chromosome 8p. COPD. 2006;3:189–194. doi: 10.1080/15412550601009321. [DOI] [PubMed] [Google Scholar]
- Lazic T, Wyatt TA, Matic M, Meyerholz DK, Grubor B, Gallup JM, Kersting KW, Imerman PM, Almeida-DeMacedo M, Ackermann MR. Maternal alcohol ingestion reduces surfactant protein A expression by preterm fetal lung epithelia. Alcohol. 2007;41:347. doi: 10.1016/j.alcohol.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews TJ, Minino AM, Osterman MJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2008. Pediatrics. 2011;127:146–157. doi: 10.1542/peds.2010-3175. [DOI] [PubMed] [Google Scholar]
- McCaskill ML, Kharbanda KK, Tuma DJ, Reynolds J, DeVasure J, Sisson JH, Wyatt TA. Hybrid malondialdehyde and acetaldehyde protein adducts form in the lungs of mice exposed to alcohol and cigarette smoke. Alcohol. Clin. Exp. Res. 2011;35:1. doi: 10.1111/j.1530-0277.2011.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LM, Foster WM, Dambach DM, Doebler D, McKinnon M, Killar L, Longphre M. A murine model of cigarette smoke-induced pulmonary inflammation using intranasally administered smoke-conditioned medium. Exp. Lung. Res. 2002;28:435–455. doi: 10.1080/01902140290096728. [DOI] [PubMed] [Google Scholar]
- Miller NS, Gold MS. Comorbid cigarette and alcohol addiction: epidemiology and treatment. J. Addict. Dis. 1998;17:55–66. doi: 10.1300/J069v17n01_06. [DOI] [PubMed] [Google Scholar]
- Ohar JA, Hamilton RF, Jr, Zheng S, Sadeghnejad A, Sterling DA, Xu J, Meyers DA, Bleecker ER, Holian A. COPD is associated with a macrophage scavenger receptor-1 gene sequence variation. Chest. 2010;137:1098–1107. doi: 10.1378/chest.09-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten CA, Martin JE, Owen N. Can psychiatric and chemical dependency treatment units be smoke free? J. Subst. Abuse Treat. 1996;13:107–118. doi: 10.1016/0740-5472(96)00040-2. [DOI] [PubMed] [Google Scholar]
- Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J. Gen. Physiol. 2005;125:535–553. doi: 10.1085/jgp.200409216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, Von Essen SG, Romberger DJ. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L1085–L1095. doi: 10.1152/ajplung.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samokhvalov AV, Irving HM, Rehm J. Alcohol consumption as a risk factor for pneumonia: a systematic review and meta-analysis. Epidemiol. Infect. 2010;138:1789–1795. doi: 10.1017/S0950268810000774. [DOI] [PubMed] [Google Scholar]
- Sisson JH. Alcohol and airways function in health and disease. Alcohol. 2007;41:293–307. doi: 10.1016/j.alcohol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozo F, O’Day L, Martiz G, Kenna K, Stacy V, Brew N, Walker D, Bocking A, Brien J, Harding R. Repeated ethanol exposure during late gestation alters the maturation and innate immune status of the ovine fetal lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;296:L510–L518. doi: 10.1152/ajplung.90532.2008. [DOI] [PubMed] [Google Scholar]
- Strong P, Kishore U, Morgan C, Lopez Bernal A, Singh M, Reid KB. A novel method of purifying lung surfactant proteins A and D from the lung lavage of alveolar proteinosis patients and from pooled amniotic fluid. J. Immunol. Methods. 1998;220:139–149. doi: 10.1016/s0022-1759(98)00160-4. [DOI] [PubMed] [Google Scholar]
- Terpstra V, van Amersfoort ES, van Velzen AG, Kuiper J, van Berkel TJ. Hepatic and extrahepatic scavenger receptors: function in relation to disease. Arterioscler. Thromb. Vasc. Biol. 2000;20:1860–1872. doi: 10.1161/01.atv.20.8.1860. [DOI] [PubMed] [Google Scholar]
- Tuma DJ, Kearley ML, Thiele GM, Worrall S, Haver A, Klassen LW, Sorrell MF. Elucidation of reaction scheme describing malondialdehyde-acetaldehyde-protein adduct formation. Chem. Res. Toxicol. 2001;14:822–832. doi: 10.1021/tx000222a. [DOI] [PubMed] [Google Scholar]
- Tuma DJ, Thiele GM, Xu D, Klassen LW, Sorrell MF. Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long-term ethanol administration. Hepatology. 1996;23:872–880. doi: 10.1002/hep.510230431. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Forget MA, Sisson JH. Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am. J. Pathol. 2003;163:1157–1166. doi: 10.1016/S0002-9440(10)63475-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TA, Kharbanda KK, Tuma DJ, Sisson JH. Malondialdehyde-acetaldehyde-adducted bovine serum albumin activates protein kinase C and stimulates interleukin-8 release in bovine bronchial epithelial cells. Alcohol. 2001;25:159–166. doi: 10.1016/s0741-8329(01)00177-x. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Kharbanda KK, Tuma DJ, Sisson JH, Spurzem JR. Malondialdehyde-acetaldehyde adducts decrease bronchial epithelial wound repair. Alcohol. 2005;36:31–40. doi: 10.1016/j.alcohol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Schmidt SC, Rennard SI, Sisson JH. Acetaldehyde-stimulated PKC activity in airway epithelial cells treated with smoke extract from normal and smokeless cigarettes. Proc. Soc. Exp. Biol. Med. 2000;225:91–97. doi: 10.1046/j.1525-1373.2000.22511.x. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Slager RE, Heires AJ, Devasure JM, Vonessen SG, Poole JA, Romberger DJ. Sequential activation of protein kinase C isoforms by organic dust is mediated by tumor necrosis factor. Am. J. Respir. Cell Mol. Biol. 2010;42:706–715. doi: 10.1165/rcmb.2009-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TA, Spurzem JR, May K, Sisson JH. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am. J. Physiol. 1998;275:L827–L835. doi: 10.1152/ajplung.1998.275.4.L827. [DOI] [PubMed] [Google Scholar]
- Xu D, Thiele GM, Kearley ML, Haugen MD, Klassen LW, Sorrell MF, Tuma DJ. Epitope characterization of malondialdehyde-acetaldehyde adducts using an enzyme-linked immunosorbent assay. Chem. Res. Toxicol. 1997;10:978–986. doi: 10.1021/tx970069t. [DOI] [PubMed] [Google Scholar]