Abstract
Lycopene, the red pigment of tomatoes, is hypothesized to reduce prostate cancer risk, a disease strongly dependent upon testosterone. In this study, mice lacking the expression of carotene-15,15’-monooxygenase (CMO-I−/−) or wild-type mice were fed either a 10% tomato powder (TP), lycopene-containing (248 nmol/g diet), or their respective control diets for 4 days, after which serum testosterone was measured. A significant diet × genotype interaction (P=0.02) suggests that the TP reduces serum testosterone concentrations in CMO-I−/− mice but not in wild-type mice. Similarly, testicular testosterone was lowered in TP-fed CMO-I−/− mice (P=0.01), suggesting that testosterone synthesis may be inhibited in this group. A similar pattern was also observed for lycopene fed mice. Interestingly, the CMO-I−/− mice showed a greater expression of the gene encoding the CMO-II enzyme responsible for eccentric oxidative carotenoid cleavage in the testes. Therefore, we hypothesize that serum testosterone is reduced by lycopene metabolic products of oxidative cleavage by CMO-II in the testes. Overall, these findings suggest that genetic polymorphisms impacting CMO-I expression and its interaction with CMO-II, coupled with variations in dietary lycopene, may modulate testosterone synthesis and serum concentrations. Furthermore, carefully controlled studies with tomato products and lycopene in genetically defined murine models may elucidate important diet × genetic interactions that may impact prostate cancer risk.
Keywords: tomato, carotenoid, testosterone, prostate cancer, carotene-monooxygenase
Introduction
Epidemiological (1) and laboratory evidence (2) support a hypothesis that consumption of tomato products, including lycopene and its precursors (3), may impact risk of prostate cancer. Potential interactions between tomato phytochemicals and androgenic hormonal status may underlie this relationship, as testosterone is the critical hormone that impacts prostate development, biology, and promotes prostate carcinogenesis (2). We previously observed that changes in testosterone impacted lycopene metabolism, with castration increasing hepatic lycopene and higher testosterone leading to reduced lycopene accumulation (4). More recently, studies suggest that greater intake of tomato phytochemicals and higher serum lycopene may reduce serum testosterone. For example, in a phase II randomized-controlled trial, tomato extract supplementation reduced serum free testosterone in men with clinically localized prostate cancer (5). Additionally, we previously found that serum testosterone concentrations were decreased in rats fed tomato powder or the tomato carotenoids lycopene or phytofluene for 4 days (6). Most critically, lycopene-fed rats exhibit alterations in steroid hormone metabolism in the prostate, including reduced 5-α-reductase gene expression, a critical step in the activation of testosterone to dihydrotestosterone, the most potent ligand for the androgen receptor (7). The pharmacologic agents, finasteride and dutasteride, that target 5-α-reductase, are the only drugs to have demonstrated efficacy as chemopreventive agents in prospective human prostate cancer trials (8, 9). Taken together, these observations, suggest that tomato phytochemicals, including lycopene, impact testosterone production or signaling and may in part explain the inverse association between tomato consumption and prostate cancer risk.
Although most of the attention has focused upon lycopene as the predominant bioactive phytochemical in tomatoes, the carotenoids phytoene and phytofluene should be considered (3), along with polyphenols (10) and other components (11). A new consideration regarding the lycopene-prostate cancer relationship has emerged as a result of recent rapid advances in the field of carotenoid metabolism (10, 11). Indeed, we now postulate that cleavage products of lycopene, phytoene, and/or phytofluene may mediate some of the observed anti-cancer activities (3). Pro-vitamin A carotenoids like β-carotene are clearly metabolized byβ,β-carotene-15,15’-monooxygenase I (CMO-I) through central chain cleavage to form vitamin A, and further metabolism results in the formation of retinoic acid and other retinoids (12). The carotene-9’, 10’-monooxygenase (CMO-II) is responsible for eccentric cleavage of acyclic carotenoids, including lycopene, to form aldehyde metabolites (13). Since these genes show polymorphisms in humans and are inducible by dietary variables (14), we can now postulate a genetic component into the relationship between tomato carotenoid, androgen metabolism and prostate cancer risk.
As predicted, CMO-II−/− mice accumulate lycopene in the liver and serum (13), clearly supporting the hypothesis that this gene is critical in lycopene cleavage. Somewhat surprisingly, knockout of CMO-I, an enzyme that does not appear to directly metabolize lycopene, alters tissue lycopene accumulation (15). We have reported that CMO I−/− mice fed lycopene accumulate significantly less hepatic lycopene but more lycopene in the prostate, seminal vesicles, and testes compared with wild-type mice (15). Perhaps, compensatory changes in other enzyme systems in CMO-I−/− mice may impact lycopene metabolism.
The above evidence supports a complex interrelationship between dietary tomato products and lycopene with androgen metabolism and action, a process potentially mediated by genetic variation in genes impacting carotenoid metabolism. Thus, we examined the impact of CMO-I genotype (wild type or CMO-I−/−) in combination with dietary intake of tomato powder or lycopene on specific aspects of testosterone production in mice.
Material and Methods
Mouse husbandry and diets
The generation of CMO-I−/− mice was previously described (16). C57BL/6 × 129/SvJ (F1) mice (wild-type) were purchased from The Jackson Laboratory (Bar Harbor, ME). Genomic DNA from mouse tail biopsies and the Sigma Extract-N-Amp Tissue PCR Kit (St. Louis, MO) were used to confirm genotype. Mice were housed in shoebox cages with free access to water and weighed and handled daily. Mice consumed an AIN-93G diet for 3 weeks prior to randomization onto experimental diets. Fresh diet was provided every 48 hours, new diet was made monthly and was stored in the dark at 4 °C.
Nine- to twelve-wk-old CMO-I−/− or wild-type male mice were randomly assigned to one of two experimental carotenoid-containing diets: 10% tomato powder (FutureCeuticals, Momence, IL) (TP), or lycopene (DSM, Basel Switzerland, 10% water-soluble lycopene beadlet) (LYC). For statistical analysis, the TP group was compared to an AIN-93G dietary group while the LYC group was compared to a placebo beadlet (DSM, 10% water-soluble lycopene beadlet) (PB) dietary group; n = 25 per genotype/diet. Mice consumed the experimental diets for 4 days, ad libitum. At the conclusion of the study, mice were fasted for 3 hours, asphyxiated by CO2, and blood was collected by cardiac puncture. Cohorts of mice were killed daily in the afternoon within a 3 hour time frame to avoid diurnal alterations in serum testosterone. Testes were collected, snap frozen in liquid nitrogen and stored at −80 °C. All animal procedures were approved by the University of Illinois Institutional Animal Care and Use Committee.
The TP diet contained 204 nmol lycopene/g diet while the LYC diet provided 248 nmol lycopene/g diet. The TP diet also contained additional carotenoids: 10.1 nmol phytoene, 2.6 nmol phytofluene, and 0.8 nmol β-carotene/g diet. All diets were balanced for total fiber, nitrogen, and calories (13). Additionally, vitamin A levels were reduced in all diets to 1500 IU retinyl palmitate per kg diet to ensure adequate absorption of carotenoids without resulting in a vitamin A deficiency (15).
Serum and testicular testosterone
Testicular testosterone was extracted as previously described, with minor modifications (17). Briefly, half of a testis (20–60 mg) was homogenized in PBS (125 μL) at room temperature for 20 seconds. Diethyl ether (2.5 mL) was added, the sample was vortexed for 2 minutes, and then centrifuged at 183 × g for 1 minute. The upper phase was removed and saved. Another 1.4 mL diethyl ether was added; the sample was vortexed and placed on dry ice for 10 minutes. The upper phase was again removed and combined with the previous extract. Samples were allowed to evaporate at room temperature and stored at −80 °C until use. Samples were diluted 1:10 in PBS before quantification. Serum and testicular testosterone were quantified with a radioimmunoassaykit (DSL-4000 ACTIVE Testosterone Coated-TubeRadioimmunoassay Kits; Diagnostic Systems Laboratories) as per the manufacturer’s instructions.
Real-Time PCR analysis
Relative testicular gene expression was evaluated by qRT-PCR. Briefly, testicular RNA was isolated using 2.0 ml Trizol (Invitrogen, Carlsbad, CA) per the manufacturer’s instructions, including treatment with DNase I (New England Biolab, Ipswich, MA). The concentrations and quality of mRNA were determined by spectrophotometry and agarose gel electrophoresis. Complimentary DNA was synthesized using Superscript II Reverse Transcriptase (Invitrogen) and random hexamers (Applied Biosystems, Foster City, CA). Primer pairs were selected to measure CMO-II (NM_1332217): Forward-5’-GTTATCTACTTCGAGTTGGACCTGG and Reverse-5’-AAGCAACGCCATTCCATCA and 18s (Internal Control, Forward-5’-GATCCATTGGAGGGCAAGTCT and Reverse-5’ ACTGCAGCAACTTTAATATACGCTATT). Real-time PCR was performed using a 7900HT Fast Real-Time PCR detection system (Applied Biosystems) with the SYBR green fluorescence dye (Invitrogen). Statistical analysis was conducted on the ΔCt value using two-way ANOVA (detailed below) and data is presented as fold change (2-ΔΔCt) ± the standard error of the mean (SEM) of ΔCt for testis tissue of wild-type mice that consumed the control diet (AIN or PBC).
Statistical analysis
A factorial design with 2 genotypes (CMO-I−/−, wild-type) and 2 diets (AIN vs. TP or LYC vs. PBC) was utilized. This design allowed us to investigate the impact of the main effects (diet and genotype) and the interaction between main effects on study outcomes. All parameters were analyzed by two-way analysis of variance (ANOVA) using SAS 9.2 (Cary, NC) with α = 0.05. Although it is possible to test for differences in the levels of main effects by post-hoc analysis, it is not generally advisable if interactions are present. When assumptions for ANOVA were violated, data were natural log transformed. For analysis of serum and testicular testosterone, all data points outside of two standard deviations were considered extreme outliers and were removed from the dataset. Results were expressed as the mean ± (SEM).
Results
Serum & testicular testosterone
Serum testosterone was measured in wild-type and CMO-I−/− mice that consumed a TP or AIN diet for 4 days (Figure 1). Neither diet nor genotype alone significantly impacted serum testosterone, but a significant interaction (P=0.02) suggests that the TP reduced serum testosterone levels in CMO-I−/− mice (Figure 1A). Serum testosterone levels are largely derived by synthesis of testosterone in the testis. To determine if changes in testicular production of testosterone contribute to the observed serum testosterone changes, testicular testosterone was measured. Again, genotype and diet significantly interacted to alter testicular testosterone (P=0.01) (Figure 1C). Although not significant, testicular testosterone appears to be slightly elevated in CMO-I−/− mice, but the LYC and TP diets appeared to modulate testosterone levels. To determine if lycopene specifically contributed to the observed dietary tomato powder by CMO-I genotype interaction effect on serum and testicular testosterone, we examined lycopene-fed wild-type and CMO-I−/− mice. The lycopene diet significantly reduced serum testosterone (P=0.03) (Figure 1B), and the expression of CMO-I and lycopene significantly interacted to alter testicular testosterone (P=0.03) (Figure 1D).
Fig. 1.
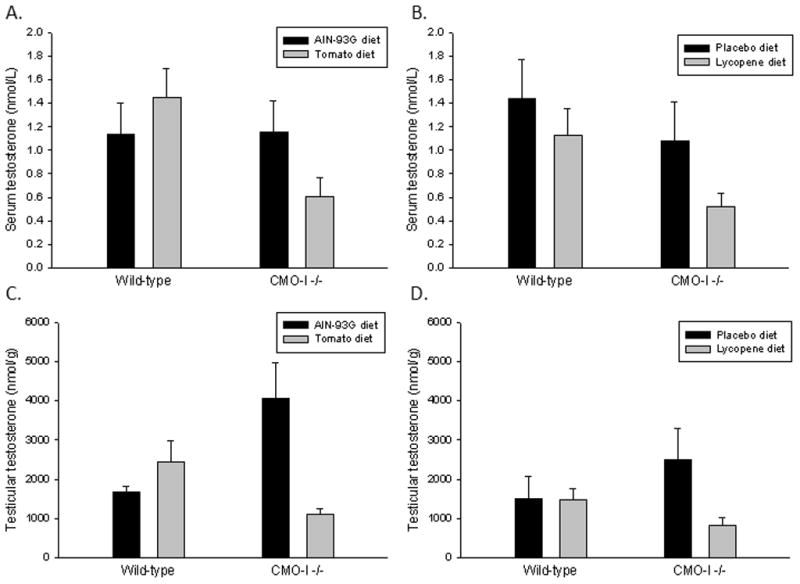
CMO-I−/− mice and wild-type mice were fed AIN-93G diet or diets supplemented with tomato powder, water-soluble lycopene beadlets, or placebo beadlets for 4 days. Serum (A, B) and testicular (C, D) testosterone were quantified with a radioimmunoassaykit (DSL-4000 ACTIVE Testosterone Coated-Tube Radioimmunoassay Kits; Diagnostic Systems Laboratories). Bars represent means ± SEM; serum n = 10–15, testis n = 8–10. P<0.05 was considered statistically significant.
A: Genotype, P=0.08; Diet, P=0.68; Interaction, P=0.02.
B: Gentoype, P=0.37; Diet, P=0.03; Interaction, P=0.35.
C: Genotype, P=0.85; Diet, P=0.04; Interaction, P=0.01.
D: Genotype, P=0.77; Diet, P=0.45; Interaction, P=0.02.
Testis RNA expression
We hypothesized that the second carotenoid oxygenase enzyme, CMO-II, may have played a role in altering testosterone levels through the production of bioactive lycopene metabolites (13, 14, 18). The expression of CMO-II was significantly upregulated in testicular tissues of CMO-I−/− compared to wild-type mice (Figure 2). The lycopene or tomato powder diets did not directly impact the expression of CMO-II in mouse testes. The mRNA expression of testicular CMO-I was not altered in wild-type mice by the TP or LYC diets compared their respective controls (data not shown).
Fig. 2.
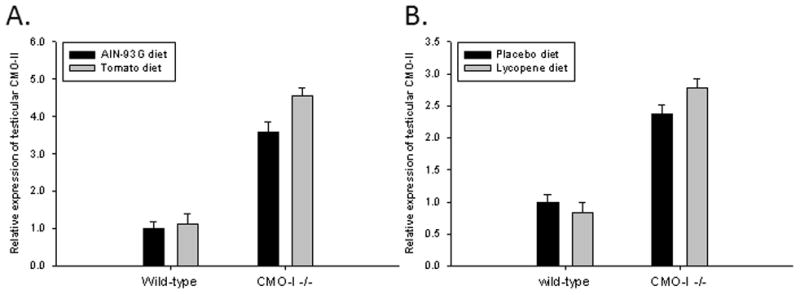
The relative mRNA expression of testicular CMO-II compared to 18s expression of wild-type and CMO-I−/− mice fed an (A) AIN-93G, tomato powder, (B) placebo beadlet or lycopene diet. Statistical analysis was conducted on the ΔCt value using 2-way ANOVA and data is presented as fold change (2-ΔΔCt) ± SEM of ΔCt for testis tissue of wild-type mice that consumed the control diet (AIN-93G or placebo beadlet). Bars represent means ± SEM; n = 9–11. P<0.05 was considered statistically significant.
A) Genotype, P=0.02; Diet, P=0.09; Interaction, P=0.68.
B) Genotype, P<0.01; Diet, P=0.26; Interaction, P=0.73.
Tissue carotenoid concentrations
Testicular lycopene accumulation was not altered by genotype in this short term feeding study (data not shown). Phytoene and phytofluene did not accumulate in detectable levels in testicular tissue of either wild-type or CMO-I−/− mice (data not shown). As predicted, β-carotene significantly accumulated in testicular tissue of CMO-I−/− mice compared to wild-type mice (P<0.0001) (data not shown), confirming earlier reports (15, 16). The tomato powder diet contained very little beta-carotene and testis tissue accumulated approximately 10,000 times more lycopene than beta-carotene.
Discussion
Human and experimental studies suggest that consuming tomato products reduces serum testosterone action, which is positively associated with prostate cancer risk (1, 5–7, 19). Additionally, evidence suggests testosterone status impacts lycopene metabolism and that the CMO-I and CMO-II genes are involved in lycopene homeostasis. In the present study, we found that serum testosterone levels were influenced by the expression of CMO-I and the intake of tomato carotenoids. The results of this study suggest that an interaction between these variables determines the resulting testosterone level. The significant genotype × diet interactions suggest that dietary TP reduces serum testosterone in CMO-I−/− mice. In parallel, dietary TP and LYC reduce testicular testosterone concentrations in CMO-I−/− mice but have no effect in wild-type mice. Data from the LYC-fed mice suggest that lycopene is largely responsible for the dietary tomato powder’s effects on testosterone levels. Additionally, because the testis is the primary site of testosterone production, alterations in testicular production of testosterone are likely responsible for the changes in serum testosterone.
At present, the CMO-I gene appears to encode the enzyme primarily responsible for central cleavage of β-carotene, providing two molecules of vitamin A, which can then interface with its receptor to signal critical downstream events essential for development and health. Lycopene does not appear to be a substrate for CMO-I, yet is clearly cleaved by CMO-II (13, 14). Interestingly, the CMO-I−/− mice show changes in lycopene metabolism (15) suggesting an indirect effect. For reasons currently unknown, the CMO-II gene mRNA expression is upregulated in testes of CMO-I−/− mice (Figure 2). The fact that CMO-II gene expression is increased may in turn result in the production of various lycopene metabolites. It is probable that CMO-II action is a key step in the degradation and excretion of lycopene. However, we postulate that lycopene metabolites may be agonists or antagonists to retinoic acid receptors, peroxisome proliferator-activated receptors, or other members of the steroid receptor family that are key regulators of cell biology (20).
Our findings, comparing wild type mice to CMO-I−/− mice, also suggest that polymorphisms in the CMO-I or CMO-II genes may impact the relationship between dietary tomato products, lycopene, and prostate cancer risk. Indeed, there is a person to person heterogeneity in serum carotenoid concentrations, even when fed similar dietary concentrations in controlled studies (21). Thus, polymorphisms in CMO-I and CMO-II may account for the inconsistent results and conclusions drawn from various epidemiologic studies. Recently, two single-nucleotide polymorphisms (SNPs) existing in the human CMO-I gene were reported. These SNPs are found in high frequency within the European, Chinese, and Japanese populations (22). Several SNPs have also been identified in the coding region of the CMO-I gene (23). Certainly genetic variations impacting carotenoid bioavailability and metabolism may occur at many steps in the process. When genetic variation is coupled to the profound impact of diet composition and inherent measurement error in many diet assessment tools, the poor correlation between estimated intake and serum or tissue concentrations of tomato carotenoids is not surprising.
Thus far, there are no published human studies examining the impact of CMO-II gene SNPs on tissue or serum lycopene concentrations. Alternatively, mutations in CMO-II result in altered carotenoid tissue deposition in domestic livestock (24, 25). We propose that alterations in the function of the CMO-I and CMO-II enzymes, due to SNPs or epigenetic regulation, may explain the effects of tomato carotenoids on endocrine processes related to prostate cancer risk. Future studies are required to unravel these intriguing interrelationships.
2 brief statements describing the novelty and impact of the paper.
The mechanism of action for dietary tomato carotenoids for prostate cancer prevention is unclear. Mice lacking the central carotenoid cleavage enzyme (CMO-I) were utilized to demonstrate that dietary tomato carotenoids act via the CMO-I enzyme to alter serum testosterone, an important risk factor for prostate cancer. Furthermore, the carotenoid lycopene may contribute to the tomato powder feeding effect on androgens.
Significant interactions between CMO-I and dietary tomato carotenoid intake as well as alterations in the expression of the eccentric carotenoid cleavage enzyme (CMO-II) suggest that tomato carotenoid metabolites may mediate changes in serum and testicular testosterone concentrations.
Acknowledgments
This work was funded by National Institutes of Health grant PHS-1-RO1 CA125384.The Pelotonia Fellowship program supported Dr. Nancy Engelmann Moran. We thank FutureCeuticals for donating the tomato powder and DSM for providing the lycopene and placebo beadlets. We thank Drs. Adrian Wyss at DSM Nutritional Products and Johannes von Lintig at Case Western Reserve University for providing the original breeding pairs of CMO-I−/− and CMO-II−/− mice for our studies.
Abbreviations
- TP
tomato powder diet
- AIN
AIN-93G diet
- LYC
lycopene beadlet diet
- PBC
placebo beadlet control diet
- CMO-I
carotene-15,15’-monooxygenase
- CMO-II
carotene-9’10’-monooxygenase
- PBS
phosphate buffered saline
- SNP
single nucleotide polymorphism
References
- 1.Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995 Dec 6;87(23):1767–76. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- 2.Erdman JW, Jr, Ford NA, Lindshield BL. Are the health attributes of lycopene related to its antioxidant function? Arch Biochem Biophys. 2009 Mar 15;483(2):229–35. doi: 10.1016/j.abb.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelmann N, Clinton SK, Erdman JW., Jr Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Adv Nutr. 2011;2:51–61. doi: 10.3945/an.110.000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boileau TW, Clinton SK, Zaripheh S, Monaco MH, Donovan SM, Erdman JW., Jr Testosterone and food restriction modulate hepatic lycopene isomer concentrations in male F344 rats. J Nutr. 2001 Jun;131(6):1746–52. doi: 10.1093/jn/131.6.1746. [DOI] [PubMed] [Google Scholar]
- 5.Kumar NB, Besterman-Dahan K, Kang L, Pow-Sang J, Xu P, Allen K, Riccardi D, Krischer JP. Results of a randomized clinical trial of the action of several doses of lycopene in localized prostate cancer: Administration prior to radical prostatectomy. Clin Med Urol. 2008 Apr 16;1:1–14. doi: 10.4137/cmu.s718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell JK, Stroud CK, Nakamura MT, Lila MA, Erdman JW., Jr Serum testosterone is reduced following short-term phytofluene, lycopene, or tomato powder consumption in F344 rats. J Nutr. 2006 Nov;136(11):2813–9. doi: 10.1093/jn/136.11.2813. [DOI] [PubMed] [Google Scholar]
- 7.Herzog A, Siler U, Spitzer V, Seifert N, Denelavas A, Hunziker PB, Hunziker W, Goralczyk R, Wertz K. Lycopene reduced gene expression of steroid targets and inflammatory markers in normal rat prostate. FASEB J. 2005 Feb;19(2):272–4. doi: 10.1096/fj.04-1905fje. [DOI] [PubMed] [Google Scholar]
- 8.Akaza H, Kanetake H, Tsukamoto T, Miyanaga N, Sakai H, Masumori N, Nakatsu H, Sagiyama K, Sakamoto S, Endo Y, Yamanouchi T REDUCE Study Group. Efficacy and safety of dutasteride on prostate cancer risk reduction in asian men: The results from the REDUCE study. Jpn J Clin Oncol. 2011 Mar;41(3):417–23. doi: 10.1093/jjco/hyq221. [DOI] [PubMed] [Google Scholar]
- 9.Unger JM, Thompson IM, Jr, LeBlanc M, Crowley JJ, Goodman PJ, Ford LG, Coltman CA., Jr Estimated impact of the prostate cancer prevention trial on population mortality. Cancer. 2005 Apr 1;103(7):1375–80. doi: 10.1002/cncr.20919. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, DeGroff VL, Clinton SK. Tomato and soy polyphenols reduce insulin-like growth factor-I-stimulated rat prostate cancer cell proliferation and apoptotic resistance in vitro via inhibition of intracellular signaling pathways involving tyrosine kinase. J Nutr. 2003 Jul;133(7):2367–76. doi: 10.1093/jn/133.7.2367. [DOI] [PubMed] [Google Scholar]
- 11.Canene-Adams K, Campbell JK, Zaripheh S, Jeffery EH, Erdman JW., Jr The tomato as a functional food. J Nutr. 2005 May;135(5):1226–30. doi: 10.1093/jn/135.5.1226. [DOI] [PubMed] [Google Scholar]
- 12.Wyss A, Wirtz GM, Woggon WD, Brugger R, Wyss M, Friedlein A, Riss G, Bachmann H, Hunziker W. Expression pattern and localization of beta,beta-carotene 15,15’-dioxygenase in different tissues. Biochem J. 2001 Mar 15;354(Pt 3):521–9. doi: 10.1042/0264-6021:3540521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford NA, Clinton SK, von Lintig J, Wyss A, Erdman JW., Jr Loss of carotene-9’,10’-monooxygenase expression increases serum and tissue lycopene concentrations in lycopene-fed mice. J Nutr. 2010 Dec;140(12):2134–8. doi: 10.3945/jn.110.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD. The biochemical characterization of ferret carotene-9’,10’-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem. 2006 Jul 14;281(28):19327–38. doi: 10.1074/jbc.M512095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindshield BL, King JL, Wyss A, Goralczyk R, Lu CH, Ford NA, Erdman JW., Jr Lycopene biodistribution is altered in 15,15’-carotenoid monooxygenase knockout mice. J Nutr. 2008 Dec;138(12):2367–71. doi: 10.3945/jn.108.099663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, Wyss A. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem. 2007 Nov 16;282(46):33553–61. doi: 10.1074/jbc.M706763200. [DOI] [PubMed] [Google Scholar]
- 17.Jeyaraj DA, Grossman G, Petrusz P. Altered bioavailability of testosterone in androgen-binding protein-transgenic mice. Steroids. 2005 Sep;70(10):704–14. doi: 10.1016/j.steroids.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Ford NA, Elsen AC, Zuniga K, Lindshield BL, Erdman JW. Lycopene and apo-12’-lycopenal reduce cell proliferation and alter cell cycle progression in human prostate cancer cells. Nutr Cancer. 2011 Feb;63(2):256–63. doi: 10.1080/01635581.2011.523494. [DOI] [PubMed] [Google Scholar]
- 19.Wertz K, Siler U, Goralczyk R. Lycopene: Modes of action to promote prostate health. Arch Biochem Biophys. 2004 Oct 1;430(1):127–34. doi: 10.1016/j.abb.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Sharoni Y, Danilenko M, Dubi N, Ben-Dor A, Levy J. Carotenoids and transcription. Arch Biochem Biophys. 2004 Oct 1;430(1):89–96. doi: 10.1016/j.abb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Allen CM, Schwartz SJ, Craft NE, Giovannucci EL, De Groff VL, Clinton SK. Changes in plasma and oral mucosal lycopene isomer concentrations in healthy adults consuming standard servings of processed tomato products. Nutr Cancer. 2003;47(1):48–56. doi: 10.1207/s15327914nc4701_6. [DOI] [PubMed] [Google Scholar]
- 22.Leung WC, Hessel S, Meplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G. Two common single nucleotide polymorphisms in the gene encoding beta-carotene 15,15’-monoxygenase alter beta-carotene metabolism in female volunteers. FASEB J. 2009 Apr;23(4):1041–53. doi: 10.1096/fj.08-121962. [DOI] [PubMed] [Google Scholar]
- 23.Tourniaire F, Leung W, Meplan C, Minihane A, Hessel S, von Lintig J, Flint J, Gilbert H, Hesketh J, Lietz G. Consequenes of genetic variations in beta-carotene cleavage enzymes in female volunteers. Carotenoid Sciences. 2008 Jun;12:57. ABSTRACT. [Google Scholar]
- 24.Berry SD, Davis SR, Beattie EM, Thomas NL, Burrett AK, Ward HE, Stanfield AM, Biswas M, Ankersmit-Udy AE, Oxley PE, Barnett JL, Pearson JF, et al. Mutation in bovine beta-carotene oxygenase 2 affects milk color. Genetics. 2009 Jul;182(3):923–6. doi: 10.1534/genetics.109.101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vage DI, Boman IA. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (ovis aries) BMC Genet. 2010 Feb 2;11:10. doi: 10.1186/1471-2156-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


