Abstract
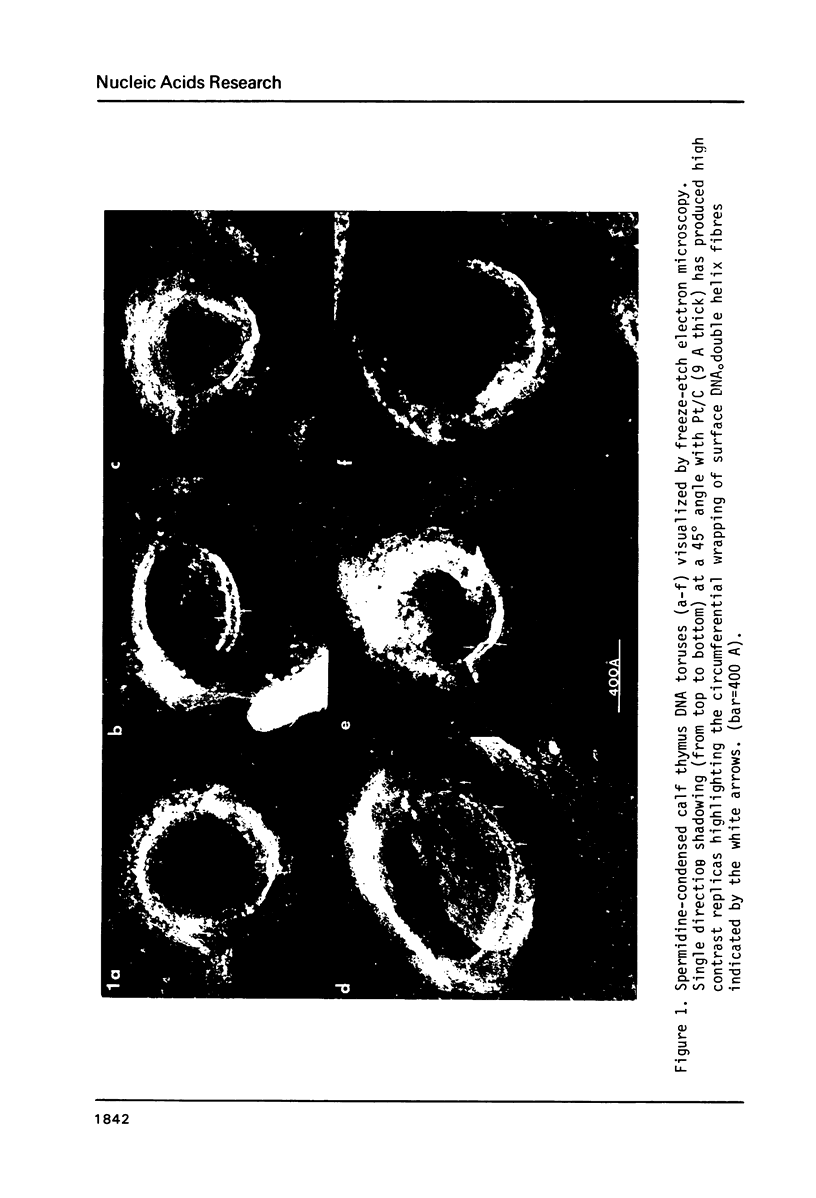
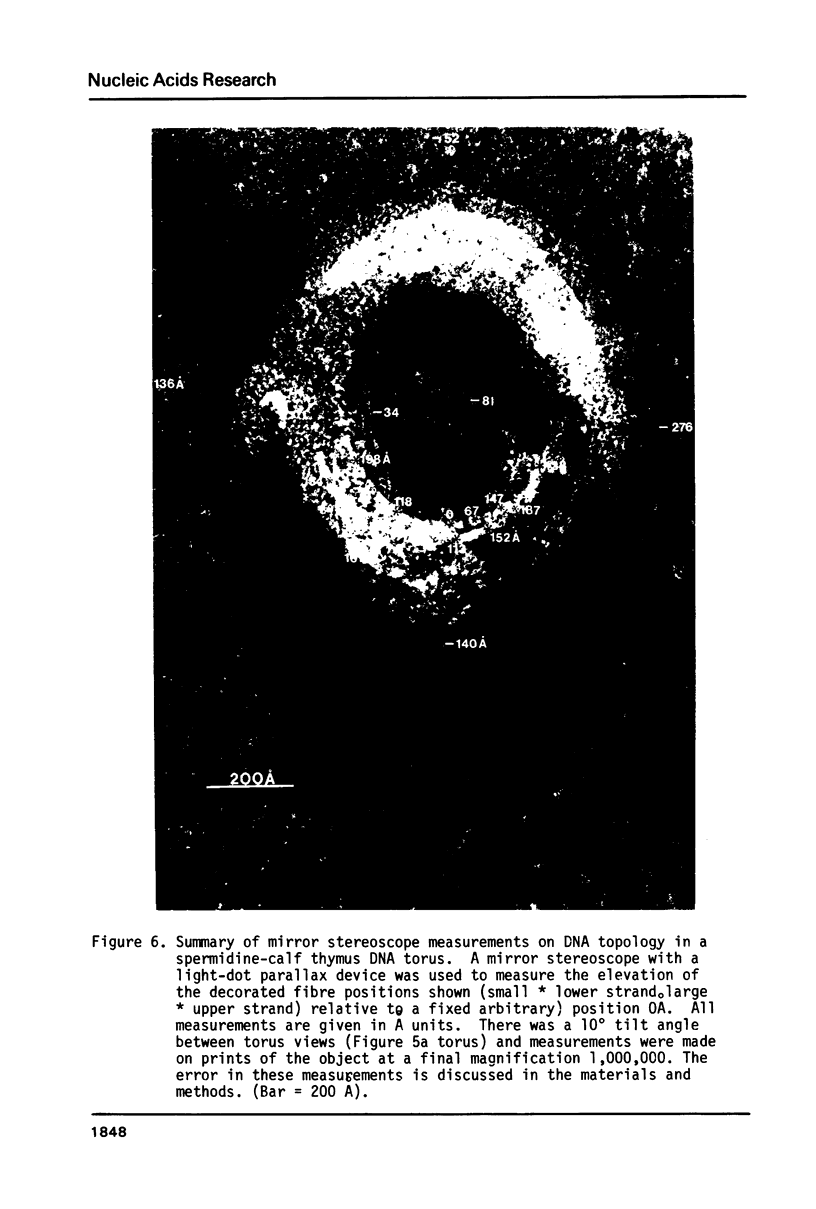
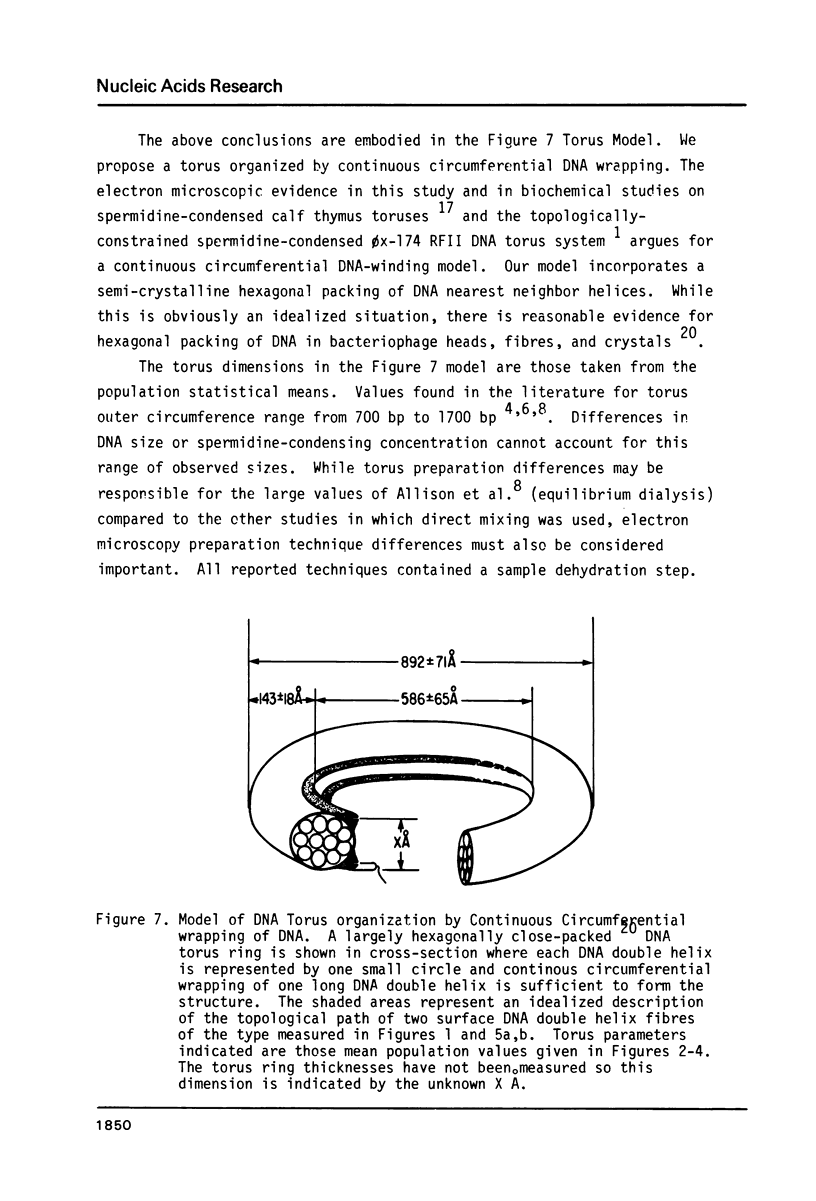
In spermidine-condensed calf thymus DNA preparations, torus-shaped condensates were shown by transmission electron microscopy to exist under the hydrated conditions of the freeze fracture experiment. Using extremely low Pt metal deposition levels (9 A Pt/C) high-contrast replicas of the spermidine-DNA toruses were obtained that showed circumferential wrapping of single DNA double helix-size surface fibres. Stereoscopic analysis of high magnification stereomicrographs established some details of the three-dimensional organization of two DNA double helix sections winding circumferentially on the inner surface of one such torus. These measurements demonstrate the usefulness of stereoscopic analysis of these high macromolecular organization magnification. Measurements on a number of torus-shaped complexes (n = 16) yielded these average dimensions: inner circumference (1840 +/- 204 A) outer circumference (2800 +/- 222 A), torus ring thickness (143 +/- 18 A). These data support a continuous circumferential DNA-winding model of torus organization proposed by Marx & Reynolds.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison S. A., Herr J. C., Schurr J. M. Structure of viral phi 29 DNA condensed by simple triamines: a light-scattering and electron-microscopy study. Biopolymers. 1981 Mar;20(3):469–488. doi: 10.1002/bip.1981.360200305. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Gosule L. C., Schellman A. DNA condensation with polyamines. II. Electron microscopic studies. J Mol Biol. 1978 May 25;121(3):327–337. doi: 10.1016/0022-2836(78)90367-4. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Samson S., Dickerson R. E. Structure of a B-DNA dodecamer at 16 K. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4040–4044. doi: 10.1073/pnas.79.13.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Casjens S. R. DNA packaging by the double-stranded DNA bacteriophages. Cell. 1980 Sep;21(2):319–331. doi: 10.1016/0092-8674(80)90468-7. [DOI] [PubMed] [Google Scholar]
- Eickbush T. H., Moudrianakis E. N. The compaction of DNA helices into either continuous supercoils or folded-fiber rods and toroids. Cell. 1978 Feb;13(2):295–306. doi: 10.1016/0092-8674(78)90198-8. [DOI] [PubMed] [Google Scholar]
- Furlong D., Swift H., Roizman B. Arrangement of herpesvirus deoxyribonucleic acid in the core. J Virol. 1972 Nov;10(5):1071–1074. doi: 10.1128/jvi.10.5.1071-1074.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard B., Warren R. A. Reactivity of the alpha-putrescinylthymine amino groups in phi W-14 deoxyribonucleic acid. Biochemistry. 1982 Oct 26;21(22):5458–5462. doi: 10.1021/bi00265a012. [DOI] [PubMed] [Google Scholar]
- Gosule L. C., Schellman J. A. Compact form of DNA induced by spermidine. Nature. 1976 Jan 29;259(5541):333–335. doi: 10.1038/259333a0. [DOI] [PubMed] [Google Scholar]
- Gosule L. C., Schellman J. A. DNA condensation with polyamines I. Spectroscopic studies. J Mol Biol. 1978 May 25;121(3):311–326. doi: 10.1016/0022-2836(78)90366-2. [DOI] [PubMed] [Google Scholar]
- Hsiang M. W., Cole R. D. Structure of histone H1-DNA complex: effect of histone H1 on DNA condensation. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4852–4856. doi: 10.1073/pnas.74.11.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse Y., Mitsui Y., Iitaka Y., Miyaki K. Preliminary x-ray studies of the interaction of salmon sperm DNA with spermine. J Mol Biol. 1978 Jun 15;122(1):43–53. doi: 10.1016/0022-2836(78)90107-9. [DOI] [PubMed] [Google Scholar]
- Klimenko S. M., Tikchonenko T. I., Andreev V. M. Packing of DNA in the head of bacteriophage T2. J Mol Biol. 1967 Feb 14;23(3):523–533. doi: 10.1016/s0022-2836(67)80122-0. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Bose R. J., Warren R. A. 5-(4-Aminobutylaminomethyl)uracil, an unusual pyrimidine from the deoxyribonucleic acid of bacteriophage phiW-14. Biochemistry. 1973 Jan 2;12(1):151–157. doi: 10.1021/bi00725a025. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Manning G. S. Thermodynamic stability theory for DNA doughnut shapes induced by charge neutralization. Biopolymers. 1980 Jan;19(1):37–59. doi: 10.1002/bip.1980.360190104. [DOI] [PubMed] [Google Scholar]
- Marx K. A., Reynolds T. C. Spermidine-condensed phi X174 DNA cleavage by micrococcal nuclease: torus cleavage model and evidence for unidirectional circumferential DNA wrapping. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6484–6488. doi: 10.1073/pnas.79.21.6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards K. E., Williams R. C., Calendar R. Mode of DNA packing within bacteriophage heads. J Mol Biol. 1973 Aug 5;78(2):255–259. doi: 10.1016/0022-2836(73)90114-9. [DOI] [PubMed] [Google Scholar]
- Ruben G. C., Telford J. N. Dimensions of active cytochrome c oxidase in reconstituted liposomes using a gold ball shadow width standard: a freeze-etch electron microscopy study. J Microsc. 1980 Feb;118(2):191–216. doi: 10.1111/j.1365-2818.1980.tb00262.x. [DOI] [PubMed] [Google Scholar]
- Skuridin S. G., Kadykov V. A., Shashkov V. S., Evdokimov Iu M., Varshavskii Ia M. Obrazovanie kompaktnoi formy DNK v rastvore pri vzaimodeistvii so spermidinom. Mol Biol (Mosk) 1978 Mar-Apr;12(2):413–420. [PubMed] [Google Scholar]
- Widom J., Baldwin R. L. Cation-induced toroidal condensation of DNA studies with Co3+(NH3)6. J Mol Biol. 1980 Dec 25;144(4):431–453. doi: 10.1016/0022-2836(80)90330-7. [DOI] [PubMed] [Google Scholar]
- Wilson R. W., Bloomfield V. A. Counterion-induced condesation of deoxyribonucleic acid. a light-scattering study. Biochemistry. 1979 May 29;18(11):2192–2196. doi: 10.1021/bi00578a009. [DOI] [PubMed] [Google Scholar]