Abstract
During cerebral neocortical development, excitatory neurons are generated from radial glial cells in the ventricular zone (VZ) or from secondary progenitor cells in the subventricular zone (SVZ); these neurons then migrate toward the pial surface. We have observed that post-mitotic neurons generated directly in the VZ accumulated just above the VZ with a multipolar morphology, while secondary progenitor cells having a long ascending process left the VZ faster than the post-mitotic neurons. Recent observations of human developing neocortex have revealed the existence of radial glia-like progenitors (oRG cells) in the SVZ. This type of progenitor was first thought to be human specific; however, similar cells have also been found in mouse neocortex, and the morphology of these cells resembled that of some of the secondary progenitor cells that we had previously observed, suggesting the existence of a common architecture for the developing neocortex among mammals. In this review, we discuss the nature of the SVZ and its similarities and differences between humans and mice.
Keywords: Cerebral neocortex, Radial glia, Electroporation, Basal progenitor, Subventricular zone, Evolution
Introduction
The mammalian cerebral neocortex consists of six layers comprised of morphologically and functionally distinct neurons. Approximately 70–80% of the neurons in the cerebral neocortex are glutamatergic excitatory neurons; the remainder are GABAergic inhibitory neurons. During development, excitatory neurons are generated in the pallial ventricular zone (VZ) facing the lateral ventricle or in the adjacent subventricular zone (SVZ). They then migrate radially toward the brain surface, passing through predecessors generated during earlier stages and stopping their migration at the top of the developing cortical plate. Because the excitatory neurons of the cerebral neocortex are generated and migrate sequentially during the developmental period, early-born neurons are eventually located in the deep layers and late-born neurons are located in the superficial layers in an “inside-out” manner. On the other hand, GABAergic neurons are generated in the ganglionic eminences located in the ventral part of the telencephalon and migrate tangentially to enter the pallium. In this review, we focus on the production of glutamatergic neurons in the VZ and SVZ. Recently, neuron production in the SVZ has attracted much attention because the SVZ becomes enormously thick in primates and the expansion of the SVZ is strongly correlated with the acquisition of huge brains during primate evolution. The progenitors in the SVZ are generated from stem cells in the VZ. Therefore, the molecular mechanisms for producing more secondary proliferative cells from the VZ and for maintaining their self-renewal proliferative activity in the SVZ are thought to be keys to understand brain evolution in humans. Here, we summarize the cytoarchitectural similarities and differences of the SVZ between humans and mice and discuss the molecular mechanisms for the expansion of the SVZ during primate evolution.
Production of neurons and neuronally committed secondary proliferative cells through asymmetric cell divisions of radial glia
Radial glia are the neural stem cells in the VZ (Miyata et al. 2001; Noctor et al. 2001). The long ascending processes, or radial fibers, of these cells reach the basement membrane lying just beneath the pia mater and are anchored by short thick processes, known as the apical endfeet, to the ventricular (apical) surface. The radial fibers function as a scaffold for migrating neurons in the locomotion mode, a fast migration mode of radial migration (Rakic 1972; Nadarajah et al. 2001). As development proceeds, the radial glial cells switch from self-renewal divisions to neurogenic divisions, in which one radial glial cell produces one radial glial cell and one post-mitotic neuron (Takahashi et al. 1996) or a neuronally committed secondary progenitor called a basal progenitor (Noctor et al. 2004). Fate decisions regarding whether the daughter cell remains as a radial glia or acquires a more differentiated state are thought to occur during this asymmetric cell division. During the cell cycle, a radial glial cell undergoes interkinetic nuclear movement, with the nucleus becoming positioned at the apical surface of the cerebral wall during mitosis (M phase of the cell cycle). After telophase, the apical plasma membrane, which faces the lateral ventricle and is enclosed by adherens junction components, is segregated into two daughter cells. The radial fiber becomes very thin during the M phase, rather than disappearing, and is inherited by one of the daughter cells (Miyata et al. 2001; Noctor et al. 2001). Intensive observations have revealed that the apical membrane is indeed segregated unequally into the daughter cells during neurogenic divisions (Kosodo et al. 2004). In this case, the apical daughter inherits a larger proportion of the apical membrane, while the basal daughter inherits the radial fiber and a smaller proportion of the apical membrane. In cell division with a horizontal cleavage plane induced by the overexpression of mInsc, most of the apical daughters differentiate into neurons, while the basal daughters maintain a more undifferentiated state (Konno et al. 2008). These observations suggest the existence of some factors for neuronal differentiation in the apical membrane or some inhibitory factor in the basal compartment. The Par-complex proteins, Par3, Par6, and aPKC, are well-known determinants of cell polarity and have been most extensively studied in invertebrates, such as Drosophila (Yu et al. 2006). During the development of the sensory organ precursor (SOP) or neuroblast in Drosophila, the Par-complex proteins are enriched in the apical membrane domain and are inherited by the apical daughter after cell division. This asymmetric segregation of the Par-complex is important for the apical daughter to acquire a progenitor fate. As in the Drosophila neuroblast, the Par-complex is located at the apical membrane of the radial glia in mice (Costa et al. 2008). However, unlike in Drosophila, the Par3 protein is re-distributed from the apical membrane to the side of the cell cortex closest to the cleavage plane during mitosis and is differentially inherited by the daughter cells (Bultje et al. 2009). The Par3-inherited daughter apparently maintains a radial glial state through the inactivation of Numb, a Notch negative regulator (Bultje et al. 2009). The relation between the distribution of Par3 and the inheritance of the basal process and/or apical membrane is intriguing.
As mentioned above, the basal daughters preferentially assume the stem cell (radial glial) fate after asymmetric cell division. In some cases, however, the basal daughters become post-mitotic neurons and the apical daughters maintain the proliferative activity (Miyata et al. 2001). This fact indicates that the fate decisions arising in asymmetric cell divisions are not completely decisive. We suppose that the tendency for the basal daughter to maintain the proliferative activity may depend on the developmental stages. During the early stages, the cleavage plane is almost always 90° to the ventricular surface, but this angle relaxes progressively as development proceeds (Konno et al. 2008). Thus, the determinants may be unequally segregated especially during the late stages of neocortical development.
Basal progenitors in the SVZ
During the development of cerebral neocortex, neurons are generated not only in the VZ, but also in the SVZ (Haubensak et al. 2004; Miyata et al. 2004; Noctor et al. 2004; Wu et al. 2005). Importantly, cell division in the SVZ of rodents is, in most cases, accomplished through terminal division, producing two neurons (Noctor et al. 2004). The proliferative cells in the SVZ are called “non-surface progenitors” or “basal progenitors” because of their abventricular location, while the VZ cells are called “apical progenitors”. Because the basal progenitors are generated from the apical progenitors as transit-amplifying cells, they are also called “intermediate (neuronal) progenitors”. Since the basal progenitors are already committed to a neuronal fate, they express an early neuronal marker, Tbr2 (Englund et al. 2005). On the other hand, apical progenitors are capable of self-renewal; consequently, they express stem cell markers such as Hes1, Pax6, phospho-vimentin, and Sox2 (Kamei et al. 1998; Zappone et al. 2000; Shimojo et al. 2008).
Whether the basal progenitors produce different types of neurons compared with the neurons derived directly from the apical progenitors is important. Tarabykin et al. identified an SVZ-specific transcript, Svet1 (Tarabykin et al. 2001). Based on the expression profiles of Svet1, including those in several mutant mice, they proposed a model in which the basal progenitors and the apical progenitors mainly produce neurons in the superficial layers and the deep layers, respectively. Many other studies have followed, describing the correlated expression patterns in the embryonic SVZ and the postnatal superficial layers as well as coincidental deficiencies in these structures in several knockout mice (Sugitani et al. 2002; Land and Monaghan 2003; Nieto et al. 2004; Zimmer et al. 2004; Arnold et al. 2008; Cubelos et al. 2008), supporting this model. However, several controversial observations have also been reported. Tbr2 is expressed in the basal progenitors (Englund et al. 2005) and is widely used as their marker. Sessa et al. (2008) generated Tbr2-conditional knockout mice, in which Tbr2 was deleted in the cerebral neocortex, and observed a reduction in all layers, rather than a specific reduction in the superficial layers. In addition, Tbr2-positive cells and abventicular dividing cells were found not only during the late stages, but also during the early stages of neocortical development, when the deep-layer neurons are generated (Noctor et al. 2008). These observations led to another model in which the basal progenitors are not a specific source of the superficial-layer neurons but contribute to neuronal production in all layers (Kowalczyk et al. 2009).
These controversies seem to have been caused, at least in part, by confusion regarding the identity of the SVZ, since the word “SVZ” appears to be used differently among different investigators and no reliable basal progenitor markers are available. Tbr2 is widely used to identify basal progenitors, but whether Tbr2 is not expressed in the direct progeny of apical progenitors remains unclear. Englund et al. mentioned this possibility (Englund et al. 2005). Moreover, recent time-lapse observations have demonstrated that multipolar cells produced directly from apical progenitors express Tbr2 (Shitamukai et al. 2011), suggesting that Tbr2 is a very early marker of cells committed to a neuronal fate irrespective of whether they are basal progenitors or post-mitotic cells derived from apical progenitors.
Svet1 is also frequently used to identify basal progenitors. Svet1 was first thought to be a non-coding RNA (Tarabykin et al. 2001), but our subsequent study clarified that the Svet1 sequence is located in the first intron of the unc5d gene and is a part of the primary transcript of this gene, which encodes a repulsive axon-guidance receptor for Netrin (Sasaki et al. 2008). We found that the Svet1 sequence is spliced out before the mRNA is transported to the cytoplasm. Unc5D protein was mainly found on the cell surface of multipolar cells in the SVZ, most of which were post-mitotic (Tabata et al. 2009). Similar to the reported expression profile of Svet1 RNA (Tarabykin et al. 2001), some of the Unc5D-expressing cells were indeed positive for Ki67, a marker of mitotic cells, although the proportion was only about 10%. We therefore concluded that Svet1 is mainly expressed in post-mitotic multipolar cells in the SVZ (Sasaki et al. 2008).
Therefore, to estimate the contribution of basal progenitors to the production of neocortical neurons in mice, a more accurate method is needed.
Different migratory profiles between the direct progeny of apical progenitors and basal progenitors
We have established an in utero electroporation system (Tabata and Nakajima 2001) that enables us to introduce any given plasmid DNAs into the VZ cells. Using this system, we visualized the VZ-derived cells with a green fluorescent protein (GFP) and noticed that two distinct populations migrate from the VZ during late cortical plate development (Tabata et al. 2009) (Fig. 1). One population finishes their final cell division in the VZ and stays in the VZ for about 10 h after the division. During this stay time, they assume a typical pin-like morphology, extending the apical foot process to the ventricular surface with no or a short basal process. They then transform into multipolar cells without further cell division and accumulate just above the VZ, an area that we have named the “multipolar cell accumulation zone (MAZ)” (Tabata et al. 2009). This accumulation is typically observed 36 h after electroporation on embryonic day (E) 14.5 in mice (Fig. 1) (Tabata et al. 2009). By 60 h after electroporation, the cells have further transformed into bipolar cells to enter the cortical plate. The other population migrates out of the VZ faster than the former population. They assume the morphology of somal-translocation cells and become widely distributed in the intermediate zone (IZ) (Fig. 1a, b). They then often retract their basal process and undergo cell division in the IZ. Since these two populations differ from each other in the timing of VZ-exit, we have named them the slowly exiting population (SEP) and the rapidly exiting population (REP), respectively. SEP cells are the direct progeny of apical progenitors, and REP cells are mainly basal progenitors, possibly including glial progenitors. The fact that SEP cells have apical endfeet and that REP cells have basal processes is perfectly consistent with the recent observation of fate decisions during asymmetric cell divisions.
Fig. 1.
Two distinct populations migrating from the VZ. Model for the initial phase of migrations from the neocortical VZ during late cortical plate development (a). Two populations migrate from the VZ. One population, the SEP, finishes its final cell division in the VZ and remains there for about 10 h; this population then stays just above the VZ, which is called the MAZ. The SEP cells further transform into locomotion cells to enter the CP. The other population, REP, migrates faster than the SEP and divides in the IZ. The morphologies and positions of the GFP-positive cells at 12 h (b) and 36 h (c) after electroporation at E14.5 are shown. Scale bar 30 μm
As mentioned above, basal progenitors are thought to be the major source of layer II/III neurons. Our labeling method using GFP and bromodeoxyuridine (BrdU) revealed that the SEP differentiated into pyramidal neurons in layers II/III during the adult stage. We estimated the contribution of the SEP to the GFP-labeled layer II/III neurons, which had been transfected on E14.5, as 50.2% in the dorsomedial neocortex (Tabata et al. 2009), suggesting that the contribution of the direct progeny of radial glia is considerable, even for the production of superficial-layer neurons.
Histological relationship between MAZ and SVZ
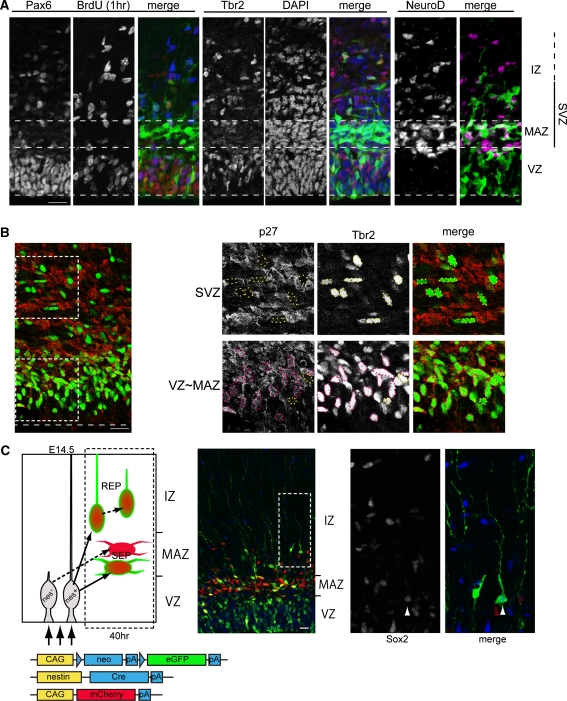
Post-mitotic multipolar cells accumulate in the MAZ, the definition of which is clearly different from that for the SVZ. According to the definitions of the Boulder Committee, the SVZ is the region where the mitotic cells exist outside the VZ. The mitotic cells in the SVZ do not undergo interkinetic nuclear movement (Boulder-Committee 1970). Here, we show the distribution of early neuronal markers and mitotic cell markers on the same or adjacent sections (Fig. 2). We introduced a CAG-promoter-driven enhanced GFP (EGFP)-expressing vector into the ventricular surface of the lateral neocortex of an E15 mouse embryo using in utero electroporation and fixed the brain 30 h later. BrdU was applied once at 1 h before fixation. The multipolar cells visualized by EGFP had formed the MAZ at this time point (Fig. 2a). The early post-mitotic neuronal marker, NeuroD, was highly correlated with the MAZ, since the post-mitotic multipolar cells in the MAZ are positive for NeuroD (Tabata et al. 2009). This region can also be recognized histologically as a “high cell-density region” adjacent to the VZ, as it is densely filled with multipolar cells (Fig. 2a, DAPI stain). This “high cell-density region” above the VZ is sometimes referred to as the “SVZ”. However, based on the original definition of the SVZ by the (Boulder-Committee 1970), this high cell-density region above the VZ is not identical to the Boulder Committee’s SVZ, because this region (MAZ) is mostly composed of “post-mitotic” multipolar cells (Tabata et al. 2009).
Fig. 2.
Histological architectures of the SVZ. The distributions of Pax6, Tbr2, and NeuroD-positive cells and the cells with BrdU uptake were compared with the GFP-positive multipolar cells in the MAZ (a). E15 mouse embryos were electroporated with GFP expression vector and fixed at E16.5. BrdU was applied 1 h before fixation. Double immunostaining for Tbr2 and p27 in E15 mouse neocortex (b). Tbr2-positive cells in the SVZ above the MAZ were mostly negative for p27, whereas the cells in the VZ or MAZ were frequently positive (encircled by the magenta dotted line). A subset of REP cells had long basal processes and expressed Sox2, representing the oRG cell-characters (c). Electroporation of the plasmid-mixture composed of the nestin-promoter-driven Cre-recombinase-expressing vector (pNestin-Cre), the Cre-dependent-EGFP-expressing vector (pCLNL-EGFP), and the CAG-promoter-driven mCherry-expressing vector (CAG-mCherry) preferentially labeled REP with GFP and mCherry and labeled SEP with mCherry alone. The mCherry single-positive SEP cells had accumulated in the MAZ as multipolar cells. The GFP-labeled REP cells were located in the IZ/SVZ. Some of these cells had long basal processes and expressed Sox2. Scale bar 20 μm
On the other hand, the Boulder Committee’s SVZ can be identified as the region where the cells incorporate BrdU and express the proliferative cell markers, PCNA or Ki67, or earliest neuronal marker, Tbr2, besides the VZ (Fig. 2a). Using these markers, the SVZ was found to spread in and beyond the MAZ. Therefore, MAZ is overlapped with the lower part of the Boulder Committee’s SVZ. The upper border of the SVZ was not clear, because the cells positive for these markers were gradually reduced in the superficial part. The GFP-positive REP cells were found above the MAZ and were frequently positive for PCNA, compared with the cells in the MAZ (mainly SEP) (Tabata et al. 2009). The dense and strongly Tbr2-positive region was also found in the upper part of the VZ (Englund et al. 2005) (Fig. 2). These cells expressed p27, a negative regulator of the G1 to S phase transition, while those in the IZ were mostly negative (Fig. 2b), suggesting the possibility that the major population of Tbr2-expressing cells in the upper VZ may be undergoing transformation into post-mitotic multipolar cells. In contrast, GFP-positive multipolar cells in the MAZ were negative or only weakly positive for Tbr2 (Tabata et al. 2009), reflecting their differentiated state.
Histological architecture of the human SVZ
During the evolution of humans, the cerebral neocortex has expanded enormously. One of the key events during this neocortical expansion is the expansion of the SVZ. In primate fetal brains, the SVZ is histologically subdivided into two regions, the outer SVZ (OSVZ) and the inner SVZ (ISVZ). The ISVZ lies just above the VZ and is composed of cells oriented in various directions. The OSVZ is located more superficially and represents a columnar structure composed of radially oriented nuclei (Smart et al. 2002). As development proceeds, the OSVZ is dramatically expanded, while the ISVZ remains almost constant through gestational weak (GW) 15.5 and diminishes by GW17 (Hansen et al. 2010). Visualization of the individual human OSVZ cells on slice cultures revealed that they had a long basal process but no apical process, representing a somal-translocation-cell morphology. These cells express various combinations of stem cell markers such as Pax6, Sox2, and Hes1, resembling the profile of VZ cells. Based on these observations, OSVZ cells are regarded as abventricularly located radial glial cells and are thus called OSVZ radial glia-like (oRG) cells (Hansen et al. 2010). In contrast, the number of mitotic cells in the ISVZ is much smaller than that in the OSVZ (Lukaszewicz et al. 2005).
Mouse REP cells morphologically and histologically resemble primate oRG cells
Although oRG cells were first thought to be a unique cell type in fetal primate cortices, counterparts have also been observed in non-primates, such as ferrets (Fietz et al. 2010) and mice (Shitamukai et al. 2011; Wang et al. 2011). These counterparts have long basal processes resembling those of human oRG cells and express stem cell markers such as Pax6, phosphovimentin, and Ki67. Shitamukai et al. observed oRG-like cells with long basal processes in mouse cortices and named these cells outer VZ (oVZ) cells. They successfully observed sequential self-renewal cell divisions of the oVZ cells. As described above, REP cells are mitotically active and initially have a long basal process (Tabata et al. 2009). This feature of REP cells resembles that of oRG cells. In many cases, the basal process of REP is retracted before or during mitoses and two neurons are produced, suggesting that most of the REP cells are basal progenitors. This fact indicates that the morphological feature of a long basal process is not unique to oRG/oVZ cells, but is common to oRG/oVZ cells and at least a significant proportion of basal progenitors. Considering the fact that Pax6- and Sox2-expressing cells were distributed in the IZ, where the REP cells are distributed, and that some REP cells with a long basal process actually express Sox2 (Fig. 2c) or Pax6, we assumed that oRG cells may comprise a subpopulation of REP. On the other hand, the primate ISVZ is composed of randomly oriented cells, similar to the mouse MAZ (see Fig. 1F of Tabata et al. 2009). The possibility that the histological organization of the developing cerebral neocortex may be common among mammals, with neurons generated directly from the VZ cells forming the MAZ/ISVZ just above the VZ and secondary proliferative cells (basal progenitors or oRG cells) widely distributed outside the VZ, is fascinating. Considering that most REP cells are basal progenitors, we hypothesized that the production and stemness of REP cells may have been greatly enhanced during primate evolution, leading to the formation of a histologically identifiable OSVZ above the ISVZ. As described above, the mouse MAZ is formed by the accumulation of post-mitotic multipolar cells above the VZ. However, few mitotic figures or PCNA-positive cells are found in the mouse MAZ, similar to the ISVZ in humans. These mitotic cells in the mouse MAZ are the REP cells distributed in the SVZ, overlapping with the MAZ. Therefore, primate oRG cells might also be located both in the OSVZ and ISVZ, and mitotic cells in the primate ISVZ may correspond to the oRG cells or basal progenitors. In fact, the cleavage plane of mitotic cells in the ISVZ tends to be horizontal (Fietz et al. 2010), resembling the cleavage plane of oRG cells in the OSVZ (Hansen et al. 2010).
The existence of oRG cells has only been reported recently, and the molecular mechanisms responsible for the production of oRG cells and the maintenance of its stemness are largely unknown. Considering that oRG cells have a basal process but no apical process, the existence of some stem cell property maintenance factor associated with the basal process may be postulated. Fietz et al. demonstrated that the integrin signal evoked by the attachment of the basal process to the basement membrane beneath the pia mater was one such factor. They suggested that integrin signals via the basal process endowed oRG cells with an epithelial cell property and maintained its ability to undergo repeated asymmetric divisions. On the other hand, Shitamukai et al. and Wang et al. demonstrated the importance of Notch signaling for the generation or maintenance of oRG/oVZ cells. Shitamukai et al. observed that oVZ cells expressed Hes1, a marker for Notch signaling, and that after the cell division of oVZ cells, the daughter cells that inherited the basal process selectively expressed Hes1 (Shitamukai et al. 2011). Furthermore, they showed that the overexpression of a dominant-negative Mindbomb-1 (Mib-1), which eliminates the ligand function on signal sending cells, reduced oVZ cells. This effect was also observed when the dominant-negative Mib-1 was expressed clonally. They suggested that the ligand of Notch was provided by the sibling daughter cells after the cell division of the oVZ cells and not by the surrounding cells. How Notch signaling is preferentially activated in basal process-inherited sibling cells would be an interesting topic.
Here, we propose a model in which the basic organization of the SVZ is the same between humans and rodents. The post-mitotic cells generated from the VZ form the MAZ in mice and the ISVZ in primates, whereas the secondary proliferative cells are distributed more widely in the SVZ. Particularly in primates, the stemness of secondary proliferative cells is greatly enhanced, and these cells form the OSVZ. Further investigation of the molecular mechanisms involved in the maintenance of the stem cell properties of oRG/oVZ cells will likely be essential for understanding human brain evolution and the pathology of developmental disorders.
Acknowledgments
We thank J. Miyazaki for the CAG promoter, and U. Lendahl for the nestin promoter. This research was supported by the Strategic Research Program for Brain Sciences (“Understanding of molecular and environmental bases for brain health”), Grant-in-Aid for Scientific Research, and the Global COE Program of the Ministry of Education, Culture, Sports, and Science and Technology of Japan, the Japan Society for the Promotion of Science, the Promotion and Mutual Aid Corporation for Private Schools of Japan, and Keio University Special Grant-in-Aid for Innovative Collaborative Research Projects.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Hidenori Tabata, Phone: +81-3-53633743, FAX: +81-3-53791977, Email: tabata@a8.keio.jp.
Kazunori Nakajima, Phone: +81-3-53633743, FAX: +81-3-53791977, Email: kazunori@z6.keio.jp.
References
- Arnold SJ, Huang GJ, Cheung AF, Era T, Nishikawa S, Bikoff EK, Molnar Z, Robertson EJ, Groszer M. The T-box transcription factor Eomes/Tbr2 regulates neurogenesis in the cortical subventricular zone. Genes Dev. 2008;22:2479–2484. doi: 10.1101/gad.475408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulder-Committee Embryonic vertebrate central nervous system: revised terminology. Anat Rec. 1970;166:257–261. doi: 10.1002/ar.1091660214. [DOI] [PubMed] [Google Scholar]
- Bultje RS, Castaneda-Castellanos DR, Jan LY, Jan YN, Kriegstein AR, Shi SH. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron. 2009;63:189–202. doi: 10.1016/j.neuron.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MR, Wen G, Lepier A, Schroeder T, Gotz M. Par-complex proteins promote proliferative progenitor divisions in the developing mouse cerebral cortex. Development. 2008;135:11–22. doi: 10.1242/dev.009951. [DOI] [PubMed] [Google Scholar]
- Cubelos B, Sebastian-Serrano A, Kim S, Redondo JM, Walsh C, Nieto M. Cux-1 and Cux-2 control the development of Reelin expressing cortical interneurons. Dev Neurobiol. 2008;68:917–925. doi: 10.1002/dneu.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietz SA, Kelava I, Vogt J, Wilsch-Brauninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, Huttner WB. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010;13:690–699. doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci USA. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y, Inagaki N, Nishizawa M, Tsutsumi O, Taketani Y, Inagaki M. Visualization of mitotic radial glial lineage cells in the developing rat brain by Cdc2 kinase-phosphorylated vimentin. Glia. 1998;23:191–199. doi: 10.1002/(SICI)1098-1136(199807)23:3<191::AID-GLIA2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, Matsuzaki F. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- Kosodo Y, Roper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004;23:2314–2324. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk T, Pontious A, Englund C, Daza RA, Bedogni F, Hodge R, Attardo A, Bell C, Huttner WB, Hevner RF. Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb Cortex. 2009;19:2439–2450. doi: 10.1093/cercor/bhn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land PW, Monaghan AP. Expression of the transcription factor, tailless, is required for formation of superficial cortical layers. Cereb Cortex. 2003;13:921–931. doi: 10.1093/cercor/13.9.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewicz A, Savatier P, Cortay V, Giroud P, Huissoud C, Berland M, Kennedy H, Dehay C. G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron. 2005;47:353–364. doi: 10.1016/j.neuron.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/S0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–3145. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4:143–150. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- Nieto M, Monuki ES, Tang H, Imitola J, Haubst N, Khoury SJ, Cunningham J, Gotz M, Walsh CA. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J Comp Neurol. 2004;479:168–180. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Kriegstein AR. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J Comp Neurol. 2008;508:28–44. doi: 10.1002/cne.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Tabata H, Tachikawa K, Nakajima K. The cortical subventricular zone-specific molecule Svet1 is part of the nuclear RNA coded by the putative Netrin receptor gene Unc5d and is expressed in multipolar migrating cells. Mol Cell Neurosci. 2008;38:474–483. doi: 10.1016/j.mcn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Sessa A, Mao CA, Hadjantonakis AK, Klein WH, Broccoli V. Tbr2 directs conversion of radial glia into basal precursors and guides neuronal amplification by indirect neurogenesis in the developing neocortex. Neuron. 2008;60:56–69. doi: 10.1016/j.neuron.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Shitamukai A, Konno D, Matsuzaki F. Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J Neurosci. 2011;31:3683–3695. doi: 10.1523/JNEUROSCI.4773-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart IH, Dehay C, Giroud P, Berland M, Kennedy H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugitani Y, Nakai S, Minowa O, Nishi M, Jishage K, Kawano H, Mori K, Ogawa M, Noda T. Brn-1 and Brn-2 share crucial roles in the production and positioning of mouse neocortical neurons. Genes Dev. 2002;16:1760–1765. doi: 10.1101/gad.978002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 2001;103:865–872. doi: 10.1016/S0306-4522(01)00016-1. [DOI] [PubMed] [Google Scholar]
- Tabata H, Kanatani S, Nakajima K. Differences of migratory behavior between direct progeny of apical progenitors and basal progenitors in the developing cerebral cortex. Cereb Cortex. 2009;19:2092–2105. doi: 10.1093/cercor/bhn227. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr The leaving or Q fraction of the murine cerebral proliferative epithelium: a general model of neocortical neuronogenesis. J Neurosci. 1996;16:6183–6196. doi: 10.1523/JNEUROSCI.16-19-06183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabykin V, Stoykova A, Usman N, Gruss P. Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene expression. Development. 2001;128:1983–1993. doi: 10.1242/dev.128.11.1983. [DOI] [PubMed] [Google Scholar]
- Wang X, Tsai JW, Lamonica B, Kriegstein AR. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat Neurosci. 2011;14:555–561. doi: 10.1038/nn.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SX, Goebbels S, Nakamura K, Kometani K, Minato N, Kaneko T, Nave KA, Tamamaki N. Pyramidal neurons of upper cortical layers generated by NEX-positive progenitor cells in the subventricular zone. Proc Natl Acad Sci USA. 2005;102:17172–17177. doi: 10.1073/pnas.0508560102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Kuo CT, Jan YN. Drosophila neuroblast asymmetric cell division: recent advances and implications for stem cell biology. Neuron. 2006;51:13–20. doi: 10.1016/j.neuron.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Zappone MV, Galli R, Catena R, Meani N, De Biasi S, Mattei E, Tiveron C, Vescovi AL, Lovell-Badge R, Ottolenghi S, Nicolis SK. Sox2 regulatory sequences direct expression of a (beta)-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development. 2000;127:2367–2382. doi: 10.1242/dev.127.11.2367. [DOI] [PubMed] [Google Scholar]
- Zimmer C, Tiveron MC, Bodmer R, Cremer H. Dynamics of Cux2 expression suggests that an early pool of SVZ precursors is fated to become upper cortical layer neurons. Cereb Cortex. 2004;14:1408–1420. doi: 10.1093/cercor/bhh102. [DOI] [PubMed] [Google Scholar]