Abstract
Background and Purpose
The exercise guidelines for people with diabetes mellitus and peripheral neuropathy (DM+PN) have recently changed to allow moderate-intensity weight-bearing exercise, but there are few reports in the literature describing appropriate weight-bearing exercise for those with DM+PN. This case report describes a successful and safe progressive exercise program for an individual with DM+PN.
Case Description
The patient was a 76-year-old man with a 30-year history of DM+PN. He participated in a 12-week, moderate-intensity, progressive exercise program (heart rate approximately 75% of maximum heart rate; rate of perceived exertion=11–13; 3 times per week) involving walking on a treadmill, balance exercises, and strengthening exercises for the lower extremities using body weight resistance.
Outcomes
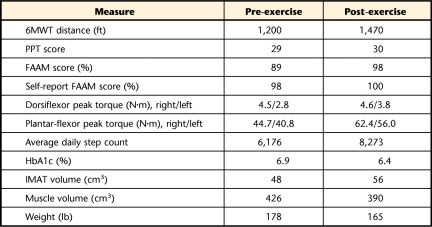
Measurements were taken before and after the 12 weeks of exercise. The patient's Six-Minute Walk Test distance increased from 1,200 to 1,470 ft. His Physical Performance Test score did not change. His Foot and Ankle Ability Measure questionnaire score improved from 89 to 98. Dorsiflexor and plantar-flexor peak torque increased (dorsiflexor peak torque: right side=4.5–4.6 N·m, left side=2.8–3.8 N·m; plantar-flexor peak torque: right side=44.7–62.4 N·m, left side=40.8–56.0 N·m), as did his average daily step count (6,176–8,273 steps/day). Close monitoring of the plantar surface of the feet indicated that the exercise program was well tolerated and there were no adverse events.
Discussion and Conclusions
This case report describes a moderate-intensity exercise program that was successful in increasing some measures of muscle strength, physical function, and activity without causing injury in an individual with DM+PN.
Individuals with diabetes mellitus and peripheral neuropathy (DM+PN) lack the critical level of sensory feedback to protect their feet from injury. Historically, their inability to monitor their own skin condition led the American Diabetes Association (ADA) and the American College of Sports Medicine (ACSM) to recommend exercise that was restricted to non–weight-bearing activities (2006 guidelines).1 The exercise guidelines have recently changed, however, to allow individuals with DM+PN and without acute ulceration to participate in moderate weight-bearing exercise.2 Weight-bearing exercise is important for this population to help maintain mobility, bone health, and general fitness. Although other non–weight-bearing exercises may be beneficial, weight-bearing exercise (walking in particular) does not require equipment or access to a gym, which can be barriers to continuing an exercise program.
People with DM+PN have lower-extremity impairments, including reduced ankle muscle strength and volume.3–5 Evidence suggests that the lower-extremity muscles in people with DM+PN have substantial intermuscular adipose tissue (IMAT) (the visible extracellular adipose tissue that is located beneath the muscle fascia and between and within muscle groups6) and muscle atrophy compared with their peers.7
These muscle changes likely contribute to the observed limitations in physical mobility in people with DM+PN. Adults with DM have an odds ratio of 2.0 for having mobility limitations compared with those without DM.8 The incidence of impaired mobility increases with peripheral artery disease or PN; individuals with DM and lower-extremity diseases have an almost 3-fold increase in risk of impaired mobility compared with those having neither.8 An inability to walk a quarter mile and inability to walk up 10 steps without resting are the most frequently reported mobility limitations.7 Little evidence exists to determine whether these impairments in muscle strength and volume and in mobility can improve with exercise.
The recent “Feet First” randomized controlled clinical trial9 demonstrated that people with DM+PN were able to participate in a low-intensity walking program and show a slight improvement in daily walking activity without increasing their rate of ulceration. This finding led, in part, to the change in exercise guidelines to allow people with DM+PN to participate in moderate-intensity exercise. Despite the benefits of the Feet First study, no lower-extremity strength changes were observed in the low-intensity, community-based exercise program,10 and a Cochrane review of the literature concluded that there is inadequate evidence to evaluate the effect of strengthening exercise for people with DM+PN.11 Because the intervention intensity was quite low in the previous reports, it is unclear whether people with DM+PN are able to increase lower-extremity strength or reduce mobility impairments with an appropriately prescribed moderate-intensity exercise program. The Feet First study helped to change the exercise guidelines for people with DM+PN to allow more weight-bearing activity, but their exercise program did not result in any changes in strength or function. It remains unclear whether a supervised, moderate-intensity exercise program would have improved results in changing muscle strength and physical function in people with DM+PN.
The purpose of this case report is to describe the effects of a moderate-intensity, 12-week, progressive walking and resistance exercise program that reflects recently changed guidelines for exercise for people with DM+PN2 on physical performance (Six-Minute Walk Test [6MWT], Physical Performance Test [PPT], and Foot and Ankle Ability Measure [FAAM]), calf muscle performance (dorsiflexor and plantar-flexor peak torque), and calf muscle structure and composition (IMAT, muscle volume) in a single individual with DM+PN.
Case Description
Background
The case reported here is that of an individual who participated in an ongoing randomized controlled trial comparing weight-bearing exercise, non–weight-bearing exercise, and a nonexercising condition for people who have DM+PN. The overall goal of the trial is to determine whether people with DM+PN are able to perform moderate-intensity weight-bearing exercise to increase activity level and physical mobility without increasing the incidence of foot ulcers or skin breakdown. This case report is based on the patient's participation in the 12-week exercise program as part of the clinical trial. The patient provided written informed consent, and the study was approved by the Human Research Protection Office at Washington University, St Louis, Missouri.
Patient
The patient was a 76-year-old Caucasian man with a body mass index (BMI) of 27.1 kg/m2 and a 30-year history of type 2 diabetes mellitus. He lived in the community and reported independence in all of his activities of daily living, although he no longer drove. He also reported that he avoids taking the stairs due to a fear of falling. His comorbid conditions included a history of myocardial infarction, coronary artery bypass graft, and hypertension that was controlled with medication. His blood sugar was controlled through oral medication. His goals for the exercise program and physical therapy interventions included improving his walking ability and endurance and having an exercise program that he could continue on his own.
Clinical Impression 1
The patient did not have any medical history that prohibited him from participating in an exercise program,2 and his primary physician approved his participation in the exercise program. He ambulated independently without an assistive device and had been stable on his medications for longer than 6 months. Based on this clinical impression, we decided to test his level of sensation, especially on weight-bearing surfaces, to determine his risk of unperceived injury during exercise activities. We also wanted to take baseline measurements of his muscle performance and functional activities to help determine the appropriate level of intervention intensity and provide a comparison for change.
Examination
Peripheral Neuropathy
The patient had severe peripheral neuropathy based on his inability to feel a 5.07 or 6.10 Semmes-Weinstein monofilament on the plantar surface of the foot and based on his vibration perception threshold of 50 V as measured with a biothesiometer (threshold for neuropathy is 25 V; 50 V is the maximum value of the machine).12,13 This level of severe neuropathy placed him at risk for unnoticed trauma, so we provided safety guidelines, as described in the “Intervention” section.
Six-Minute Walk Test
The patient performed the 6MWT14 as a measure of physical function and walking endurance, with a meaningful change indicated by a change in distance walked of greater than 72 ft (1 ft=0.3048 m).15 The patient walked back and forth in a hallway between 2 cones that were placed 100 ft apart. He was told that the goal was to walk as far as possible in 6 minutes. The 6MWT distance was recorded as total distance walked in feet. He was able to walk 1,200 ft in his initial 6MWT.
Physical Performance Test
The modified 9-item PPT was used to assess the patient's physical function. This test is designed to mimic activities of daily living and includes items such as stair climbing and the sit-to-stand maneuver. Scores on the 9-item PPT have been shown to correlate well with disability and frailty.16 Each of the items is scored on a scale of 0 to 4 based on the time it takes to complete the task. Each task is performed twice, and the average time is used to determine the score. The patient scored 29 on his initial PPT. A maximum score is 36, and a minimum score is 0. Higher scores indicate higher levels of physical function.
Foot and Ankle Ability Measure
The FAAM is a reliable, responsive, and valid self-report measure of physical function for individuals with a broad range of musculoskeletal disorders of the lower leg, foot, and ankle.17 The questions investigate the individual's perception of 26 activities of daily living (eg, walking on even ground, walking up hills, stepping up and down curbs, home responsibilities, recreational activities). The FAAM was found to be more responsive to changes in functional status compared with the 36-Item Short-Form Health Survey questionnaire (SF-36) and has a high test-retest reliability value of .89, as well as a minimal detectable change of 5.7 points and a minimal clinically important change of 8 points.17 A higher score on the FAAM indicates better physical function, with a maximum score of 100% and a minimum score of 0%. This patient's initial FAAM score was 89%.
Ankle Dorsiflexor and Plantar-Flexor Peak Torque
Concentric isokinetic ankle dorsiflexor and plantar-flexor peak torque as measures of muscle performance were assessed using a Biodex Multijoint System 3 Pro isokinetic dynamometer (Biodex Medical Systems, Shirley, New York). The tests were performed at an angular velocity of 60°/s. The patient was allowed 3 practice trials to ensure he was comfortable with the tests. The mean values for peak torque were calculated for 3 trials. The minimal detectable change for ankle dorsiflexor and plantar-flexor peak torque in older adults is 0.12 N·m and 5.06 N·m, respectively.18 The patient's initial plantar-flexor peak torque was 44.7 and 40.8 N·m for the right and left sides, respectively, and his dorsiflexor peak torque was 4.5 and 2.8 N·m for the right and left sides, respectively.
Intermuscular Adipose Tissue and Muscle Volumes
Intermuscular adipose tissue and lean muscle volumes were quantified using magnetic resonance imaging (MRI) on the right leg. The MRI scans were performed with the patient in a supine position with a Siemens CP extremity coil placed over the right calf muscle. The MRI measurements were performed with a 3.0-tesla magnet (Siemens Corp, New York, New York) with a pulse sequence of echo time=12 milliseconds, repetition time=1,500 milliseconds, and matrix=256 × 256; both a fat-saturated image and a non-fat-saturated image were collected.7 Thirty transverse slices were collected beginning at the joint space of the knee and proceeding distally. The slices were 7 mm thick with no interslice gap. Nine consecutive slices were selected to calculate muscle and IMAT volumes. Volumes were quantified using a PC workstation and custom-designed Matlab software (MathWorks, Natick, Massachusetts). The software uses voxel brightness to distinguish between muscle and adipose tissues. The subcutaneous adipose tissue was removed from each image by drawing a line along the deep fascial plane surrounding the calf muscle so that only the fat within and between the muscles (IMAT) remained. The software uses edge detection algorithms to assist the user in separating the subcutaneous fat from the muscle as well as separating individual muscles and muscle compartments. The IMAT and muscle volumes are reported in cubic centimeters. Based on test-retest reliability of data obtained for 21 subjects, the error in measuring muscle volume is less than 1% and less than 2% for measuring fat volumes on average in any muscle or compartment. The patient initially had 48 cm3 of IMAT and 426 cm3 of lean muscle in the calf section studied.
Activity Monitoring
The patient was given a StepWatch activity monitor (Orthocare Innovations, Oklahoma City, Oklahoma) and was instructed to wear the monitor around his ankle during all waking hours to determine baseline activity levels. He was given the monitor to wear for at least 9 consecutive days, calculating an average of 7 days. The first and last days of wear were deleted because a full 24 hours would not be recorded. For a day to be included, the monitor had to be worn for at least 8 hours. After the initial baseline activity level was determined, the patient continued to wear the activity monitor for the duration of the study during all waking hours. The device records strides per day, and steps per day were calculated by multiplying strides by 2 (1 stride equals 2 steps). Results of validity tests performed in our laboratory indicate a mean absolute error of 1.8% (SD=2.4%) for individuals with DM+PN.12 Results of the activity monitor were used to prescribe the patient's specific walking distance to start his progressive walking program as described below and to monitor his average daily step count throughout the study. The patient had an initial average daily step count of 6,176 steps/day.
Clinical Impression 2
The patient was determined to be appropriate for participation in this exercise program due to the presence of DM+PN and lack of any severe foot deformities or comorbidities that might limit his ability to tolerate 60 minutes of exercise. He was chosen for this case report from the initial group of study participants due to his enthusiasm and adherence to the exercise program, his severe neuropathy with lack of severe foot deformity, and his exemplary participation and changes in outcome measures during his 12-week participation in the clinical trial.
Intervention
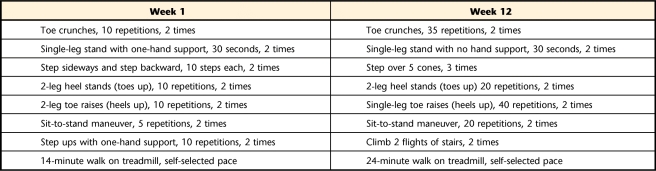
The patient participated in a moderate-intensity weight-bearing exercise program 3 times a week for 12 weeks. The exercises included in this program were adapted from the Feet First intervention9 and from exercises used in prior interventions that reduced falls in frail older adults and those with peripheral neuropathy.19 Where progression for an activity was not described in the published study, we added an appropriate exercise progression to maximize progressive improvements. Activity performance was supervised by a physical therapist. The exercise sessions began with flexibility and stretching exercises. The stretches included: toe stretches aimed at stretching the toe extensor muscles, a supine hamstring muscle stretch, prone knee flexion, rocking on hands and knees, and a standing calf stretch. Each stretch was performed twice with a 30-second hold. The balance exercises followed a progressively challenging program in which external support decreased or the perturbation to a balanced individual increased (eg, more compliant surface, movement of arms and legs). The strengthening exercises were focused on the ankle dorsiflexor and plantar-flexor muscles using body-weight resistance (ie, heel-raises, bilateral progressing to unilateral for the plantar-flexor muscles; stair climbing and sit-to-stand maneuver for the hip, knee, and ankle extensor muscles) rather than machines or weights in an effort to make the exercises easier to continue after the end of the program (Tab. 1).
Table 1.
Exercises Performed During Weeks 1 and 12a
Progression was determined at each exercise session based on the patient's report of an exercise becoming easy and his tolerance for increasing his repetitions of an exercise.
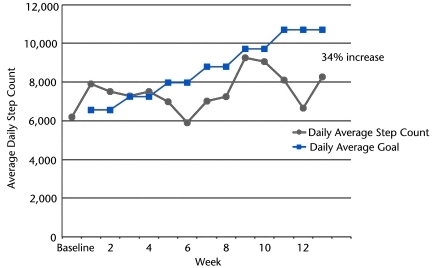
The aerobic intervention included walking on a treadmill for a duration individually calculated for the patient based on his own average step count recorded over 7 days using the StepWatch activity monitor as described above. The goal was for the patient to increase his average step count 10% every 2 weeks by increasing his daily step count 24% on the 3 days that he participated in the exercise program. In this case, he began with a step count of 6,176 steps/day, and the goal was to reach 10,712 steps/day (see the Figure for step count weekly goals). To reach the goal of 10,712 steps/day, the patient was asked to walk 14 minutes on the treadmill at each visit during the first 2 weeks with a goal of gaining an additional 1,400 steps per exercise visit during the time on the treadmill. By maintaining his normal level of activity when not exercising, his average daily step count was expected to increase by 10% over the 2 weeks. The time on the treadmill was increased every 2 weeks to continue to increase average daily step count at a rate of 10% every 2 weeks. The patient wore a heart rate monitor to assist the physical therapist in ensuring that the exercise intensity was within the mild to moderate range based on heart rate (40%–60% of maximum heart rate) and rate of perceived exertion of 11 to 13. Exercise participation was modified, postponed, or stopped based on guidelines established by the ADA and ACSM.2 Exercise would be postponed if: (1) blood glucose level was greater than 300 mg/dL, (2) resting systolic blood pressure was greater than 200 mm Hg, or (3) resting diastolic blood pressure was greater than 110 mm Hg. If blood glucose level was less than 80 to 100 mg/dL, carbohydrates would be ingested. The entire exercise program lasted approximately 60 minutes.
Figure.
Step activity monitor data by week. Patient's actual average daily step count compared with the target goal for average daily step count. Actual daily step count is represented by circles, and average daily goal is represented by squares (goal was increased every 2 weeks). Note that average daily step count increased by 34%, but that the target goal for average daily step count was not achieved.
Precautions to Avoid Skin Injury
Before and after each exercise session, the physical therapist and the patient each performed a visual inspection of the patient's feet and footwear. A digital photograph was taken before and after the exercise session to document the skin condition. Foot skin temperature was recorded using a handheld infrared thermometer (Diabetica Temptouch; Diabetic Solutions Inc, San Antonio, Texas) before and after each session as described previously.20 The temperature was recorded at 7 sites on the plantar surface of the foot where risk for skin breakdown is greatest: the great toe; the first, third, and fifth metatarsal heads; medial and lateral midfoot; and the heel. The temperature measures were used to determine areas that might be at risk for injury and were part of a composite assessment for skin injury that included redness, swelling, and pain. If there was an area of redness, swelling, pain, or increased temperature, activity was limited, and the patient was instructed to closely monitor the area and decrease daily activity.
Adherence to Exercise
All exercise sessions were monitored by a physical therapist. In order to assist with transportation costs, the patient received $10.00 for each exercise visit he attended. The patient attended 86% of the scheduled exercise classes. He missed 5 exercise sessions due to illness and conflict with previously scheduled physician's appointments.
Outcomes
Data for outcome measures were obtained before and after the 12-week moderate-intensity exercise program (Tab. 2). The postintervention measurements were collected within 72 hours of the final exercise session. The patient was able to progress on all of the prescribed exercises and was able to increase his time on the treadmill at his self-selected pace of 2.0 mph from 14 minutes to 24 minutes. He was able to tolerate a moderate-intensity exercise program based on a measured heart rate range of 80 to 109 bpm during his treadmill walking (60%–70% of his age-predicted maximum heart rate is 86–101) and based on a rate of perceived exertion of 11 to 13 on a 6 to 20 scale, which is consistent with fairly light to moderate-intensity exercise. The patient improved his 6MWT distance from 1,200 to 1,470 ft. There was no change in PPT score, but his FAAM score improved from 89% to 98%. The patient's average daily step count increased from 6,176 to 8,273 steps/day. His glucose control level improved as indicated by a decrease in glycosylated hemoglobin (HbA1c) from 6.9% to 6.4%.
Table 2.
Outcome Measuresa
6MWT=Six-Minute Walk Test, PPT=Physical Performance Test, FAAM=Foot and Ankle Ability Measure, HbA1c=glycosylated hemoglobin, IMAT=intermuscular adipose tissue.
Dorsiflexor and plantar-flexor peak torque increased (right dorsiflexor peak torque=4.5–4.6 N·m, left dorsiflexor peak torque=2.8–3.8 N·m, right plantar-flexor peak torque=44.7–62.4 N·m, and left plantar-flexor peak torque=40.8–56.0 N·m). Surprisingly, IMAT volume increased (48–56 cm3) and calf muscle volume decreased (426–390 cm3). He also lost 5.9 kg (13 lb) over the 12-week intervention.
The patient was able to tolerate the 12-week exercise program without complications or skin breakdown. His only injury came from trimming his own toenails, and this injury resolved in less than 1 week. We monitored the patient's blood glucose level before and after each exercise session, and he consistently had blood glucose levels greater than 100 mg/dL and less than 300 mg/dL, so no carbohydrates were ingested before, during, or after exercise. Based on temperature monitoring on the plantar surface of the feet, there were several instances where the patient's temperature difference was greater than 4 degrees between the right and left feet. Initially, we instructed the patient to limit his activity and did not allow him to exercise on 2 occasions. Because he continued to show temperatures greater than 4 degrees between left and right feet at the same location and did not have negative consequences, we changed our criteria to include that there must be a temperature difference of greater than 4 degrees as well as redness, swelling, or pain in that area to require activity limitation. The patient showed side-to-side temperature differences 6 times but had no instances of skin injury or skin breakdown after these revised temperature guidelines were in place.
Discussion
The guidelines from the ADA and ACSM have recently changed to indicate that people with DM+PN may participate in moderate weight-bearing exercise. Prior to this change in guidelines, it was recommended that people with DM+PN limit weight-bearing activity due to concerns about increased risk for skin injury. This case report provides an example of a moderate-intensity weight-bearing exercise program that was successful in safely increasing 6MWT distance, average daily step count, and ankle strength in a person with DM+PN.
The patient presented in this case report was able to increase his 6MWT distance from 1,200 to 1,470 feet (22%) after the 12-week exercise program. The 6MWT has been shown to be a measure of overall mobility and physical function,21,22 and a change of 270 ft is consistent with a substantial change, as reported by Perera et al.15 It seems reasonable that the 6MWT distance improved, as the patient participated in a duration-based walking program. Even though he did not increase his walking speed during treadmill training, he was able to increase his walking speed during the 6MWT. Essentially, he was training to walk, and he was successful in improving his ability to walk.
In the case of this patient, PPT scores did not change (29–30). However, his FAAM score did improve by 9 points, which is beyond the minimal detectable change (5.7 points) and minimal clinically important change (8 points) of the test.17 The patient reported improvements on the FAAM in walking uphill, climbing stairs, going up and down curbs, squatting, and doing heavy work (eg, pushing and pulling, climbing, carrying) and in recreational activities. The FAAM is a measure of physical function that is specific to activities involving the foot, ankle, and lower-extremity, whereas the PPT incorporates upper-extremity activities as well. The exercise program reported here is focused entirely on the lower extremity, so it is possible that the FAAM is a more sensitive measure of improvement than the PPT in this case. In addition, the FAAM is a self-report measure, whereas the PPT is a performance-based measure. It is possible that this intervention did not improve performance but may have improved self-efficacy or what this patient believes he is able to do.
Dorsiflexor and plantar-flexor strength measures (peak torque) responded differently from each other in this intervention. The dorsiflexor peak torque values did not change as much as the plantar-flexor peak torque values, which increased by 37% to 40% (0.1–1.0 N·m versus 15.2–17.7 N·m total change). There are several reasons that could account for this difference in response. First, more of the exercises in this program were focused on increasing strength in the plantar flexors. The primary exercise for the dorsiflexors was heel stands (toes up), whereas toe raises (heels up), stair climbing, and the sit-to-stand maneuver were performed with the plantar flexors. It has been reported previously that dorsiflexion is impaired in people with DM+PN.5 This patient's preintervention plantar-flexor peak torque values were consistent with plantar-flexor peak torque (right/left) values reported for community-dwelling older adults (44.7/40.8 N·m versus 37.7 N·m reported), but his dorsiflexor peak torque (right/left) values were lower than those reported in the literature (4.5/2.8 N·m versus 9.5 N·m reported).18 It is possible that due to the process of diabetes mellitus and peripheral neuropathy, the dorsiflexors may be more difficult to strengthen or less able to adapt than the plantar flexors.
The Feet First study9 described a low-intensity intervention for people with DM+PN aimed at increasing walking activity and improving strength, but the authors reported no strength changes in their exercise program. The intervention that we describe here is more intensive than the Feet First study, based on our goal of increasing the step count by 10% every 2 weeks. This patient was able to tolerate this more aggressive program without injury or complications and was able to increase his average daily step count from 6,176 to 8,273 steps/day (34% increase) (Figure). However, he did not actually meet the target goal of increasing his step count by 10% every 2 weeks (goal of 10,712 steps/day). Because he was walking at his self-selected pace and we based the walking program on a predetermined walking time, his actual steps taken during the treadmill walk were less than the number required for the 10% increase to occur. During weeks 10 to 12, there was a decline in the patient's average daily step count (Figure). No exercise sessions were missed during this time, but the patient decreased his activity outside of the exercise classes due to high outside temperatures (heat advisory warning people to stay inside).
Although the patient was able to increase his average daily activity, ankle muscle strength, and 6MWT distance, he lost weight overall and he lost muscle volume and gained IMAT in the calf. A previous study of a walking program in older adults23 indicated that an exercise program can attenuate strength loss and IMAT gain in the thigh. Although a recent study showed that lower levels of physical activity are associated with increased IMAT in the calf in people with DM+PN,24 it is unknown whether neuropathic calf muscle is able to adapt or what the intensity of the stimulus should be to elicit change in muscle structure. Based on this case report, it appears that changes in calf muscle volume and calf IMAT volume are not directly related to change in muscle performance or physical function, but this conclusion is in conflict with the results of a previous study.7 Future studies should focus on investigating the relationship between IMAT and muscle function and their response to exercise.
This patient lost 5.9 kg during the 12 weeks of exercise intervention, but there was no targeted dietary intervention in the program. It is unknown whether the patient altered his diet in any way in an effort to make healthy lifestyle changes. We speculate that the muscle loss in the calf was related to his overall weight loss, but we were surprised at the increase in the calf IMAT volume and the magnitude of the calf muscle loss. Better control or information regarding diet and its relationship to muscle changes would be useful in future studies.
Although there was a decrease in lean muscle volume of the calf in this patient, this volume measure was not capturing the entire calf muscle, so it is possible that there were changes to the calf muscles that occurred proximally or distally to the region we captured in the MRI. Future studies would benefit from imaging the entire lower extremity rather than a small region of interest to determine any changes to muscles with various neuropathic involvements that occur from an exercise program in this population.
Other investigators9 have indicated that muscle quality (peak torque/muscle volume) may be a key component in muscle function. In this case report, strength increased despite a decrease in muscle volume, which would indicate an improvement in muscle quality as defined by peak torque/muscle volume. It is possible that the underlying contractile properties of the muscle were changed, which could account for the increase in peak torque independent of a change in muscle volume. It also is possible that the improvement in muscle strength was due to a change in the nervous system rather than a change in the muscle structure. We do not have measures that answer these questions, but they should be investigated in future studies.
Of note, this patient's HbA1c dropped by 0.5% from 6.9% to 6.4%, indicating an improvement in glucose control over the 12 weeks of exercise. Other exercise studies have shown HbA1c reductions of 0.6% to 1.6% with combined aerobic and resistance exercise programs of 8 weeks to 24 months.25–29 In all of these studies, the exercise programs were of higher intensity than the one we report here, and further study is needed to determine whether a higher-intensity exercise program is safe and can result in larger drops in HbA1c in people with DM+PN. We can only speculate that even with this moderate-intensity exercise program, the muscles may have improved insulin sensitivity, which could have been responsible for the improvement in HbA1c. More research is needed to determine whether this speculation is accurate.
Although this case report provides important information for clinicians, there are limitations to be considered. First, this is a case report of a single patient, and it is unclear whether these findings are generalizable to the larger population with DM+PN. This person did not have severe foot deformity or a history of plantar ulcer, and studies are needed to determine the safety of this type of exercise for people with these diabetic complications. We believe that our precautions of a detailed foot examination before and after exercise are important components of safe exercising by people with DM+PN. We also used temperature measurements from the plantar surface of the foot as a means of identifying areas that might be at risk for skin breakdown.20 However, there were multiple false positives (cases where the temperature difference was greater than 4° between the right and left feet without other signs or symptoms indicating tissue damage). For this patient, we concluded that a detailed visual foot examination before and after exercise was adequate to prevent injury. Additional research is needed to investigate the usefulness of temperature readings in populations at risk for skin injury.
This case report provides an example of a weight-bearing exercise program that was successfully implemented for a person with DM+PN. He was able to complete the program without injury and increase his 6MWT distance, plantar-flexor peak torque, and average daily step count. More work is needed to determine the appropriate intensity of exercise to elicit changes in muscle structure and to determine the safety of high-intensity resistance and aerobic exercise in people with DM+PN.
Footnotes
All authors provided concept/idea/project design. Dr Tuttle and Dr Hastings provided writing and data collection. Dr Tuttle provided data analysis. Dr Mueller provided fund procurement, facilities/equipment, and institutional liaisons. Dr Hastings and Dr Mueller provided consultation (including review of manuscript before submission).
This work was completed as part of Dr Tuttle's dissertation work in the Movement Science Program, Washington University, St Louis, Missouri.
The authors report no conflict of interest present in this work.
This work was supported by grant funding from NIH NCMRR R21 HD058938 (Dr Mueller), T32 HD007434-14 (Dr Mueller, Dr Tuttle), NSMRC R24HD650837, NIH UL1 RR024992, and scholarships from the Foundation for Physical Therapy (Dr Tuttle).
References
- 1. Sigal RJ, Kenny GP, Wasserman DH, et al. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1433–1438 [DOI] [PubMed] [Google Scholar]
- 2. Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes—the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33:e147–e167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salsich GB, Brown M, Mueller MJ. Relationships between plantar flexor muscle stiffness, strength, and range of motion in subjects with diabetes-peripheral neuropathy compared to age-matched controls. J Orthop Sports Phys Ther. 2000;30:473–483 [DOI] [PubMed] [Google Scholar]
- 4. Park SW, Goodpaster BH, Strotmeyer ES, et al. ; for the Health, Aging and Body Composition Study Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30:1507–1512 [DOI] [PubMed] [Google Scholar]
- 5. Andreassen CS, Jakobsen J, Andersen H. Muscle weakness: a progressive late complication in diabetic distal symmetric polyneuropathy. Diabetes. 2006;55:806–812 [DOI] [PubMed] [Google Scholar]
- 6. Ruan XY, Gallagher D, Harris T, et al. Estimating whole body intermuscular adipose tissue from single cross-sectional magnetic resonance images. J Appl Physiol. 2007;102:748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hilton TN, Tuttle LJ, Bohnert KL, et al. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. 2008;88:1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mobility limitation among persons aged > or = 40 years with and without diagnosed diabetes and lower extremity disease: United States, 1999–2002. MMWR Morb Mortal Wkly Rep. 2005;54:1183–1186 [PubMed] [Google Scholar]
- 9. LeMaster JW, Mueller MJ, Reiber GE, et al. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: Feet First randomized controlled trial. Phys Ther. 2008;88:1385–1398 [DOI] [PubMed] [Google Scholar]
- 10. Kruse RL, LeMaster JW, Madsen RW. Fall and balance outcomes after an intervention to promote leg strength, balance and walking in people with diabetic peripheral neuropathy: “Feet First” randomized controlled trial. Phys Ther. 2010;90:1568–1579 [DOI] [PubMed] [Google Scholar]
- 11. White CM, Pritchard J, Turner-Stokes L. Exercise for people with peripheral neuropathy. Cochrane Database Syst Rev. 2004;(4):CD003904 [DOI] [PubMed] [Google Scholar]
- 12. Maluf KS, Mueller MJ. Comparison of physical activity and cumulative plantar tissue stress among subjects with and without diabetes mellitus and a history of recurrent plantar ulcers. Clin Biomech. 2003;18:567–575 [DOI] [PubMed] [Google Scholar]
- 13. Diamond JE, Mueller MJ, Delitto A, Sinacore DR. Reliability of a diabetic foot evaluation. Phys Ther. 1989;69:797–802 [DOI] [PubMed] [Google Scholar]
- 14. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med. 2002;166:111–117 [DOI] [PubMed] [Google Scholar]
- 15. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. Geriatr Soc. 2006;54:743–749 [DOI] [PubMed] [Google Scholar]
- 16. Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci. 2000;55:M350–M355 [DOI] [PubMed] [Google Scholar]
- 17. Martin RL, Irrgang JJ, Burdett RG, et al. Evidence of validity for the Foot and Ankle Ability Measure (FAAM). Foot Ankle Int. 2005;26:968–983 [DOI] [PubMed] [Google Scholar]
- 18. Hartmann A, Knols R, Murer K, de Bruin ED. Reproducibility of an isokinetic strength-testing protocol of the knee and ankle in older adults. Gerontology. 2009;55:259–268 [DOI] [PubMed] [Google Scholar]
- 19. Richardson JK, Sandman D, Vela S. A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Arch Phys Med Rehabil. 2001;82:205–209 [DOI] [PubMed] [Google Scholar]
- 20. Lavery LA, Higgins KR, Lanctot DR, et al. Home monitoring of foot skin temperatures to-prevent ulceration. Diabetes Care. 2004;27:2642–2647 [DOI] [PubMed] [Google Scholar]
- 21. Lord SR, Menz HB. Physiologic, psychologic, and health predictors of 6-minute walk performance in older people. Arch Phys Med Rehabil. 2002;83:907–911 [DOI] [PubMed] [Google Scholar]
- 22. Harada ND, Chiu V, Stewart AL. Mobility related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80:837–841 [DOI] [PubMed] [Google Scholar]
- 23. Goodpaster BH, Chomentowski P, Ward BK, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol. 2008;105:1498–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tuttle LJ, Sinacore DR, Cade WT, Mueller MJ. Lower physical activity is associated with higher intermuscular adipose tissue in people with type 2 diabetes and peripheral neuropathy. Phys Ther. 2011;91:923–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bweir S, Al-Jarrah M, Almalty AM, et al. Resistance exercise training lowers HbA1c more than aerobic training in adults with type 2 diabetes. Diabetol Metab Syndr. December 2009:1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sigal RJ, Kenny GP, Boule N, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357–369 [DOI] [PubMed] [Google Scholar]
- 27. Maiorana A, O'Driscoll G, Cheetham C, et al. The effect of combined aerobic and resistance exercise on vascular function in type 2 diabetes. J Am Coll Cardiol. 2001;38:860–866 [DOI] [PubMed] [Google Scholar]
- 28. Tokmakidis S, Zois C, Volaklis K, et al. The effects of a combined strength and aerobic exercise program on glucose control and insulin action in women with type 2 diabetes. Eur J Appl Physiol. 2004;92:437–442 [DOI] [PubMed] [Google Scholar]
- 29. Loimaala A, Groundstroem K, Rinne M, et al. Effect of long-term endurance and strength training on metabolic control and arterial elasticity in patients with type 2 diabetes mellitus. Am J Cardiol. 2009;103:972–977 [DOI] [PubMed] [Google Scholar]