Abstract
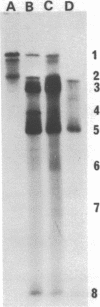
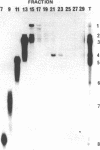
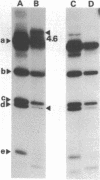
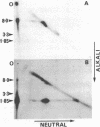
Several different forms of cauliflower mosaic virus (CaMV) DNA were detected in nucleic acid preparations from CaMV-infected turnip leaves. As well as supercoiled and open-circular molecules, various linear DNA structures were identified. The relative amounts of these DNA forms varied in plants infected with different CaMV isolates. Restriction enzyme mapping and one- and two-dimensional gel electrophoresis revealed the presence of linear molecules apparently formed by breaks in the second strand at each of the three discontinuities. Two major linear DNA forms are double-stranded over part of their length and appear to have single-stranded extensions of the -strand of variable length. Since these DNA forms are not produced during extraction and probably exist as unencapsidated or partially encapsidated molecules, they may represent intermediates either in DNA replication or in virion assembly.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Covey S. N., Lomonossoff G. P., Hull R. Characterisation of cauliflower mosaic virus DNA sequences which encode major polyadenylated transcripts. Nucleic Acids Res. 1981 Dec 21;9(24):6735–6747. doi: 10.1093/nar/9.24.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey S. N., Turner D., Mulder G. A small DNA molecule containing covalently-linked ribonucleotides originates from the large intergenic region of the cauliflower mosaic virus genome. Nucleic Acids Res. 1983 Jan 25;11(2):251–264. doi: 10.1093/nar/11.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Franck A., Guilley H., Jonard G., Richards K., Hirth L. Nucleotide sequence of cauliflower mosaic virus DNA. Cell. 1980 Aug;21(1):285–294. doi: 10.1016/0092-8674(80)90136-1. [DOI] [PubMed] [Google Scholar]
- Gardner R. C., Howarth A. J., Hahn P., Brown-Luedi M., Shepherd R. J., Messing J. The complete nucleotide sequence of an infectious clone of cauliflower mosaic virus by M13mp7 shotgun sequencing. Nucleic Acids Res. 1981 Jun 25;9(12):2871–2888. doi: 10.1093/nar/9.12.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R., Howell S. H. Structure of the cauliflower mosaic virus genome. II. Variation in DNA structure and sequence between isolates. Virology. 1978 May 15;86(2):482–493. doi: 10.1016/0042-6822(78)90087-9. [DOI] [PubMed] [Google Scholar]
- Kislev N., Rubenstein I. Utility of ethidium bromide in the extraction from whole plants of high molecular weight maize DNA. Plant Physiol. 1980 Dec;66(6):1140–1143. doi: 10.1104/pp.66.6.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski N., Hagen G., Guilfoyle T. J. A transcriptionally active, covalently closed minichromosome of cauliflower mosaic virus DNA isolated from infected turnip leaves. Cell. 1982 Jun;29(2):395–402. doi: 10.1016/0092-8674(82)90156-8. [DOI] [PubMed] [Google Scholar]
- Richards K. E., Guilley H., Jonard G. Further characterization of the discontinuities in cauliflower mosaic virus DNA. FEBS Lett. 1981 Nov 2;134(1):67–70. doi: 10.1016/0014-5793(81)80552-2. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volovitch M., Drugeon C., Yot P. Studies on the single-stranded discontinuities of the cauliflower mosaic virus genome. Nucleic Acids Res. 1978 Aug;5(8):2913–2925. doi: 10.1093/nar/5.8.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]