Abstract
The loss of muscle mass with age, also known as sarcopenia, is a major scientific and public health problem. Muscle atrophy is associated with the loss of functional capacity and poor health outcomes in elderly men and women. A detailed understanding of this problem in humans can be enhanced by the use of experiments with single muscle fibers. The technique allows the study of maximally activated muscle fibers in the absence of the influence of the nervous system. It is likely that both muscle atrophy and a decrease in muscle fiber quality contribute to muscle dysfunction among elderly. Changes in myofibrillar protein structure and function contribute to the loss of fiber quality and several physiological systems, such as the endocrine and immune systems participate in this process. A better understanding of sarcopenia at the single fiber level may lead to the design of more effective rehabilitative interventions.
Keywords: aging, sarcopenia, skeletal muscle fiber, muscle atrophy, contractility
Introduction
Sarcopenia (sarx=flesh and penia=loss), the loss of muscle mass, has become a major subject of scientific research as well as a public health problem of significant dimensions. Although an operational definition that is universally acceptable is not available, researchers agree that the incidence of sarcopenia increases with advanced adult age, in both sexes but particularly in men. Further, preliminary studies suggest that the costs associated with sarcopenia in the United States may exceed US$20 billion (1). Thus, understanding sarcopenia and developing therapeutic and rehabilitative interventions to slow down its progress or partially reverse its effects is an important scientific and health care and policy goal.
The loss of muscle mass seen in older men and women has significant physiological, functional, and health consequences. Muscle weakness, a decrease in muscle power, a reduction in walking ability, and an increase in hospitalizations are all associated with sarcopenia (2). It is important to note that several additional physiological changes are associated with aging and sarcopenia influencing muscle function even though these changes do not occur inside the muscle fibers and are not known to alter the basic myofilament protein structure and function. Examples of these changes are an increased adipose tissue accumulation around and between muscle fibers and a decrease in the anabolic influence of the endocrine system. Further examples are age-related changes in the nervous system such as the loss of motor neurons, the remodeling of motor units through collateral re-innervation, and the impairment of neuromuscular activation manifested as a decreased maximal firing rate of motor units.
The study of skeletal muscle function in humans (patients or healthy volunteers) has advanced significantly in the last three decades. However, many of tests of skeletal muscle function, particularly those used in the clinical environment, reflect the integrated actions of several physiological systems. Although the integrated response measured as muscle strength or power is of clinical interest, the specific contribution of each of the systems is very difficult to isolate. For example, in vivo measurements of muscle strength and power reflect the integrated action of muscle fibers, tendons, the neuromuscular junctions, peripheral axons, and the activating central nervous system. Clinical testing protocols may identify the presence of weakness and dysfunction but cannot identify the specific level of the impairment is happening and cannot explain the underlying cellular mechanism.
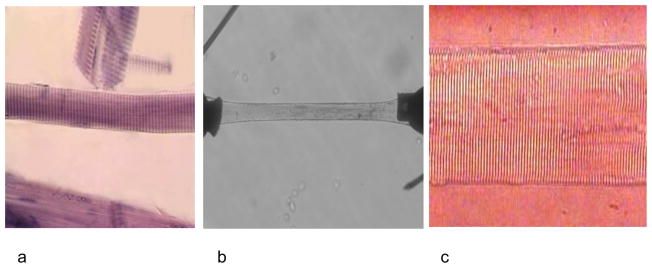
The first experiments with normal single human muscle fibers were published in 1975 (3). Since that time several researchers have used various experimental techniques to isolate and activate segments of single human muscle fibers obtained with the percutaneous muscle biopsy needle (Figure 1). These techniques have been applied to research in healthy volunteers, elite athletes, patients with various diseases of the neuromuscular system, and patients with spinal cord injuries, among others. The first application of this technique to the aging (sarcopenia) problem was published in 1997 (4). This approach allows the investigator to examine the performance of the actin-myosin cross bridges and muscle regulatory proteins in the absence of the influence of the nervous or endocrine systems. Further, because the technique includes the biochemical identification of the type of myosin heavy chain expressed in individual fibers, it is possible to study muscle fiber physiology without the confounding effect of the fiber type heterogeneity that is typical of the intact human neuromuscular system. Fiber are made permeable and then segments are activated maximally with high calcium concentrations. Due to the permeability of fibers there is no (or very little) sarcolemma or sarcoplasmic reticulum that could interfere with the calcium ion movements. Thus, the level of activation (or voluntary drive) is eliminated as a confounder. Finally, the absence of the tendon and mechanical leverage system permits the measurement of force generation directly from the fiber and its myofilament structure and not at a distance from the force generator.
Figure 1.
Images of dissected single human muscle fiber (a), attached to force transducer and servomotor (b), and preserved sarcomere pattern at higher magnification (c).
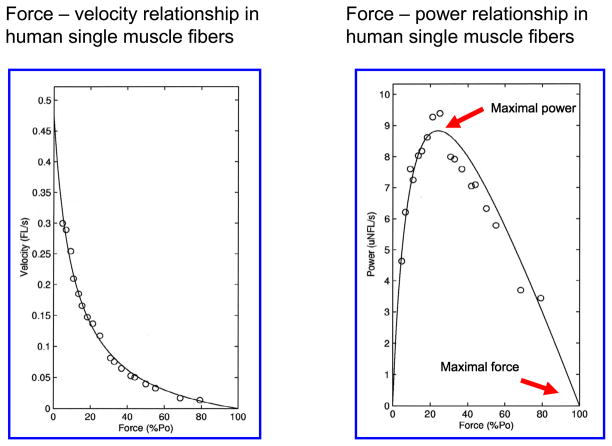
The single muscle fiber technique allows the measurement and/or calculation of several important morphological/physiological, mechanical (Figure 2), and biochemical variables (see table 1):
Figure 2.
Force-velocity and power-velocity curves from human single muscle fiber segments.
Table 1.
Variables obtained from experiments with single muscle fibers
| Morphological | Physiological/mechanical | Biochemical |
|---|---|---|
| sarcomere length | force | myosin heavy chain expression |
| Depth | shortening velocity | myosin light chain expression |
| Length | power | regulatory proteins (i.e., tropomyosin) |
| cross-sectional area | force-velocity curves | structural protein (i.e., titin) |
| Diameter | power-velocity curves | |
| elasticity, stiffness | ||
| specific force (force/size) |
Whole muscle weakness
The presence of muscle weakness is a very frequent clinical observation among elderly men and women. It contributes to the loss of functional capacity and independence and is associated with a higher mortality in this population. Cross-sectional studies show a significant difference in muscle strength between young and old men and women in several muscle groups of both upper and lower limbs. This observation has been confirmed in longitudinal studies showing a larger annual (1.0–1.5%) decrement in strength when compared to cross-sectional observations (5,6). The loss of muscle strength with age is a highly variable process, as expected. For example, longitudinal studies of relatively healthy volunteers also show that women do not lose strength of the upper limbs over a ten-year period. Further, a relatively large percentage of men and women maintain the strength of the lower limbs over the same period of time. The reason for these observations is not clear although it is likely that the level of physical and/or occupational activity plays a role in the preservation of strength in some individuals.
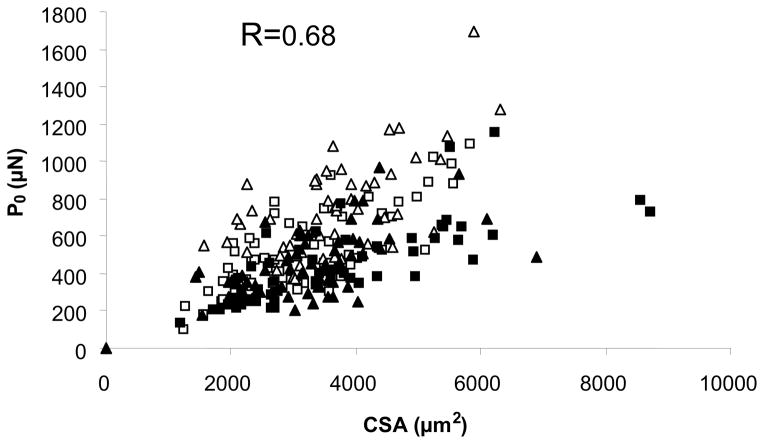
Because of the significant correlation between muscle size and strength, it has been assumed that weakness is mainly due to the loss of muscle mass (or volume), i.e. sarcopenia. However, the association between size and force is far from perfect, as illustrated in Figure 3, and we now know that factors other than size influence muscle strength (and weakness), even in the isolated fiber preparation. Experiments with single muscle fibers have allowed us to study muscle fiber quality (defined as muscle force adjusted for muscle size) and showed very clearly that sarcopenia contributes to, but is not the only explanation of, muscle weakness. In other words, aging and sarcopenia are accompanied by reductions in muscle fiber quality (7). It appears that several changes at the cellular and molecular levels contribute to impaired muscle protein function.
Figure 3.
Scatter plot of fiber CSA and maximal force (P0) for single muscle fibers from young (□, type I; △, type IIa) and older men (■, type I; ▲, type IIa).
Weakness of single muscle fibers
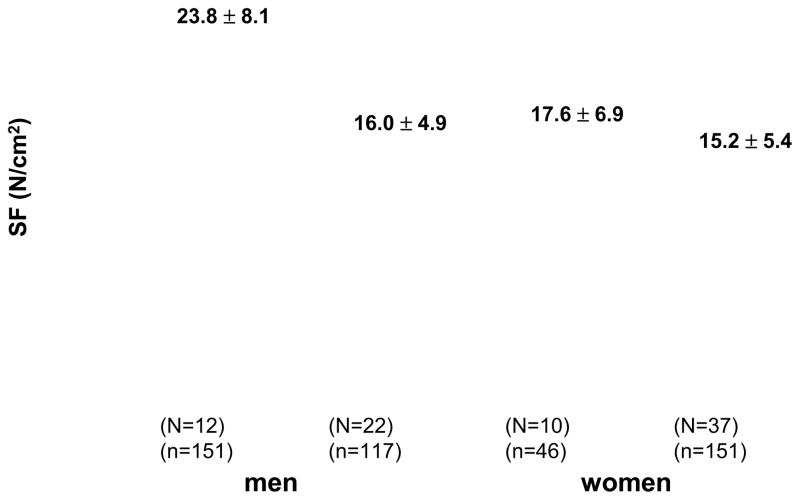
Cross-sectional studies of single muscle fibers obtained from healthy, community-dwelling men and women (4,7) show a reduction in muscle fiber quality in fibers expressing type I or IIA myosin heavy chain (Figure 4). This difference is more noticeable in both fiber types in men and some of the changes observed in women did not reach statistical significance. It is interesting that when some of the same volunteers were tested approximately ten years after baseline (the longitudinal phase of the study), muscle fiber size and quality showed no significant changes over time and the only mechanical characteristic that showed a significant reduction was single muscle fiber peak power (8). One interpretation of these findings is that the fibers that survived and were not lost to the biological effects of aging, compensated for those who were lost with the passage of time, maintaining their normal size and ability to generate force. This compensatory strategy has been observed in other populations such as post-polio survivors.
Figure 4.
Mean specific force (SF) of type IIA fibers (n=465) from m. vastus lateralis in young and older men (N=34) and women (N=47). Values are expressed as mean ± SD. N=number of subjects and n=number of fibers.
In addition to the ability to generate force, another important contractile property, maximum unloaded shortening velocity is influenced by age and gender. For example, it has been reported, although with some differential effects depending on the study, that among men and women shortening velocity was reduced with age in fibers expressing type I or type IIA myosin heavy chain isoforms (9,10). Older women showed a slower shortening velocity than older men in both fiber types. A more recent comparison between older men and women of several contractile properties of single muscle fibers, however, showed no significant differences at the level of single fibers that could explain differences at the whole muscle level in strength and power (11).
Finally, studies with human single fibers show that aging is associated with changes in fiber elasticity in both type I and IIA fibers (12). A greater instantaneous stiffness per force unit was measured in fibers from older men. These findings at the cellular level may explain why the whole muscle-tendon unit of older men has been found to be stiffer. This increase in stiffness may be due to an increase in the number and proportion of actin-myosin cross-bridges in a low force state or alterations in the compliance of structures in series in muscle fibers. It is interesting that, in these experiments, the commonly seen increase in force after a quick stretch is preserved in older men. Thus, it could be speculated that the relative preservation of eccentric strength in the elderly may have a cellular basis.
Physiological, cellular, and molecular contributors to sarcopenia and weakness
From a physiological point of view, muscle fiber weakness can be explained by the interaction of several age-related alterations. The loss of anabolic stimuli is a characteristic of aging partly due to the decline in the concentration of testosterone (and other anabolic hormones). Moreover, both epidemiologic and clinical studies, suggest that elderly have a sub-clinical level of inflammation that may increase muscle catabolism. For example, higher concentrations of the messenger RNA and protein of inflammatory markers such as TNF-α have been reported in frail elderly (13). This sub-clinical inflammation appears amenable to rehabilitation since it is partially reversed with exercise training. Research in the last decade (14) demonstrates a reduction in satellite cell number and of activation in older volunteers. The loss of satellite cells appears to be more significant in the population of cells associated with type IIA fibers. It is reasonable to suggest that this decline may result in the loss of the regenerative capacity of muscle fibers and an inability to compensate for the loss of fibers known to occur with aging. A final mechanism contributing to muscle loss in older men and women is the reported increase in the levels of myostatin in this population. Because myostatin is a negative regulator of muscle mass, an increase in circulating levels may lead to muscle atrophy. It is likely that no single mechanism can explain muscle dysfunction in elderly and that all of the above contribute to some degree to the development of sarcopenia.
Some research studies have looked at potential molecular contributors to sarcopenia and muscle dysfunction in elderly. The concentration of myosin, the most important motor protein, has been shown to be reduced in fibers from old subjects expressing type I or IIA myosin heavy chain isoforms. This may suggest that old muscle fibers have fewer cross-bridges per muscle fiber area (15) and therefore a lower capacity to generate force per area of contractile tissue. It is important to note that immobilization, commonly present in this population, further reduces the concentration of myosin in human single fibers. Thus the combination of aging and immobilization may have very deleterious effects on muscle function. Because the level of physical activity declines with adult age, the functional and clinical consequences of this synergistic effect should be obvious to the reader (16). Another important change at the molecular level is the chemical alteration of the myosin molecule. It is possible that post-translational chemical modifications such as protein methylation, glycosylation, and/or oxidation alter the function of the myosin filament (17). In fact it has been shown that the velocity of filament sliding measured in vitro is impaired although the immediate consequences for human muscle function of this observation have not been studied. Finally, at least two recent studies have demonstrated that sarcopenia is associated with a typical genetic pattern (a molecular model) that includes the upregulation of a set of genes and the simultaneous downregulation of another set of genes (18). This genetic signature is accompanied by a protein profile that appears to distinguish old and young skeletal muscle (19). The specific contribution of this genetic signature to sarcopenia and the various expressions of muscle dysfunction in elderly remains to be determined.
Countermeasures
It is not the purpose of this manuscript to discuss in detail the possible benefits of various countermeasures that have been studied to slow down or reverse sarcopenia. Nevertheless, it is important to keep in mind that some of these have been shown to be effective and that it may be possible to reverse, at least partially, the age-related muscle loss and dysfunction. The only strategy that has been shown to be safe and effective is some form of resistance (strength or power) exercise training; this is true even in very old individuals. Positive changes can be induced by exercise training at the whole muscle and single fiber levels. Some research indicates that dietary supplementation, particularly in the form of protein, may add to the benefits of exercise training. Finally, some studies have evaluated the potential benefits of hormonal strategies; particularly testosterone and growth hormone. Although some, but not all, short term studies may suggest a positive benefit, it remains to be determined whether such an intervention is effective and/or safe in the long-term.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Janssen I, Shepard DS, Katzmatzyk, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 2.Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiou C-F, Anthony MS, et al. For the Health, Aging and Body Composition Study. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57:1411–1419. doi: 10.1111/j.1532-5415.2009.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood DS, Zollman J, Reuben JP, Brandt PW. Human skeletal muscle: properties of the “chemically skinned” fiber. Science. 1975;187:1075–1076. doi: 10.1126/science.187.4181.1075. [DOI] [PubMed] [Google Scholar]
- 4.Larsson L, Li X, Frontera WR. Effects of ageing on shortening velocity and myosin isoform composition in single skeletal muscle cells from man. Am J Physiol (Cell Physiol) 1997;272:C638–49. doi: 10.1152/ajpcell.1997.272.2.C638. [DOI] [PubMed] [Google Scholar]
- 5.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12 yr longitudinal study. J Appl Physiol. 2000;88:1321–6. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 6.Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone M. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity and health. J Gerontol (Biol Sci) 2001;56A:B209–17. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- 7.Frontera WR, Krivickas L, Suh D, Hughes VA, Goldstein R, Roubenoff R. Skeletal muscle fiber quality in older men and women. Am J Physiol. 2000;279:C611–8. doi: 10.1152/ajpcell.2000.279.3.C611. [DOI] [PubMed] [Google Scholar]
- 8.Frontera WR, Reid KF, Phillips EM, Krivickas LS, Hughes VA, Roubenoff R, Fielding RA. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol. 2008;105:637–642. doi: 10.1152/japplphysiol.90332.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krivickas L, Suh D, Wilkins J, Hughes VA, Roubenoff R, Frontera WR. Age and sex related differences in maximum shortening velocity of skeletal muscle fibers. Am J Phys Med Rehabil. 2001;80:447–55. doi: 10.1097/00002060-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Yu F, Hedström M, Cristea A, Dalén N, Larsson L. Effects of aging and gender on contractile properties in human skeletal muscle and single fibres. Acta Physiol. 2007;190:229–241. doi: 10.1111/j.1748-1716.2007.01699.x. [DOI] [PubMed] [Google Scholar]
- 11.Krivickas LS, Fielding RA, Murray A, Callahan D, Johansson A, Dorer D, Frontera WR. Sex differences in single muscle power in older adults. Med Sci Sports Exerc. 2006;38:57–63. doi: 10.1249/01.mss.0000180357.58329.b1. [DOI] [PubMed] [Google Scholar]
- 12.Ochala J, Frontera WR, Krivickas LS. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol Biol Sci. 2007;62A:375–381. doi: 10.1093/gerona/62.4.375. [DOI] [PubMed] [Google Scholar]
- 13.Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. resistance exercise decreases skeletal muscle tumor necrosis factor α in frail elderly humans. FASEB J. 2001;15:475–482. doi: 10.1096/fj.00-0274com. [DOI] [PubMed] [Google Scholar]
- 14.Kadi F, Ponsot E. The biology of satellite cells and telomeres in human skeletal muscle: effect of aging and physical activity. Scand J Med Sci Sports. 2010;20:39–48. doi: 10.1111/j.1600-0838.2009.00966.x. [DOI] [PubMed] [Google Scholar]
- 15.D’Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003;552:499–511. doi: 10.1113/jphysiol.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 297:1772–1774. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 17.Clarke S. Aging as war between chemical and biochemical processes: Protein methylation and the recognition of age-damaged proteins for repair. Ageing Res Rev. 2003;2:263–285. doi: 10.1016/s1568-1637(03)00011-4. [DOI] [PubMed] [Google Scholar]
- 18.Giresi PG, Stevenson EJ, Theilhaber J, Koncarevic A, Parkington J, Fielding RA, kandarian SC. Identification of a molecular signature of sarcopenia. Physiol Genomics. 2005;21:253–263. doi: 10.1152/physiolgenomics.00249.2004. [DOI] [PubMed] [Google Scholar]
- 19.Gelfi C, Vigano A, Ripamonti M, Pontoglio A, Begum S, Pellegrino MA, Grassi B, Bottinelli R, Wait R, Cerretelli P. The human muscle proteome in aging. J Prot Res. 2006;5:1344–1353. doi: 10.1021/pr050414x. [DOI] [PubMed] [Google Scholar]