Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy (ARVD/C) is an inherited cardiomyopathy characterized by fibrofatty replacement of the ventricular myocardium, right ventricular (RV) dysfunction, and ventricular arrhythmias. The Johns Hopkins ARVD Program Registry (http://ARVD.com), which was established in 1995 to provide clinical care for patients with ARVD/C and to study this disease, currently consists of over 1000 patients, family members, and borderline phenotypes. From this registry, we sought to define the clinical characteristics, pre-transplant course, indications for, and outcomes of those who underwent cardiac transplantation.
All subjects (or surrogate) gave written informed consent to participate in this study, which was approved by the Johns Hopkins Institutional Review Board. The diagnosis of ARVD/C was based on the presence of major and minor diagnostic criteria according to the 2010 Revised Task Force Criteria(1). A modified version of the American College of Cardiology/American Heart Association HF staging system was used(2) as the original staging system accounts for only left ventricular (LV) abnormalities. We include RV abnormalities and divided Stage B into two separate sub-stages: B1 (asymptomatic ventricular dilatation without dysfunction) and B2 (asymptomatic ventricular dysfunction). Patients who required transplantation for HF were classified as RV failure only (left ventricular ejection fraction ((LVEF) ≥ 55%), predominately RV failure (LVEF ≥ 40% but ≤ 50%), or BiV failure ((LVEF) < 40%). For LVEF 50–55%, patients were classified as predominately RV if the imaging conclusion stated LV dysfunction was present or if there was evidence of LV involvement on pathologic assessment of the transplanted heart. Patients were classified as predominately VT based on their transplant physician’s reason for listing in the clinical record. Genetic testing of PKP2, DSG2, DSP, DSC2, and JUP was performed for those patients whose DNA sample was available.
Twenty-one patients were identified in the Johns Hopkins ARVD/C Registry who either underwent successful cardiac transplantation (n=20) or died as status 1A on the transplant waiting list (n=1) from 1995–2009. Three were excluded after pathological assessment of the explanted heart was consistent with an alternative diagnosis (two with cardiac sarcoidosis), leaving 18 patients who met definite criteria for ARVD/C after transplant. The cohort was 61% male. The average age of first ARVD/C symptoms was 24 ± 13 years (median age 18 years). The two most common clinical reasons for initial presentation were HF in five patients (28%) and sustained VT in five patients (28%). The average age at cardiac transplant was 40 ± 14 years (median age 44 years). Thirteen patients were transplanted predominately as a result of HF symptoms (BiV failure in four patients, predominately RV failure in four patients, RV failure only in five patients) and five were transplanted for predominately VT. RV dysfunction was present in 91% (n=11 with available data) of patients at the time of HF diagnosis and all patients (n=18) at the time of transplant. LV dysfunction was present in 43% (n=14) at the time of HF diagnosis and in 61% (n=18) at time of transplant.
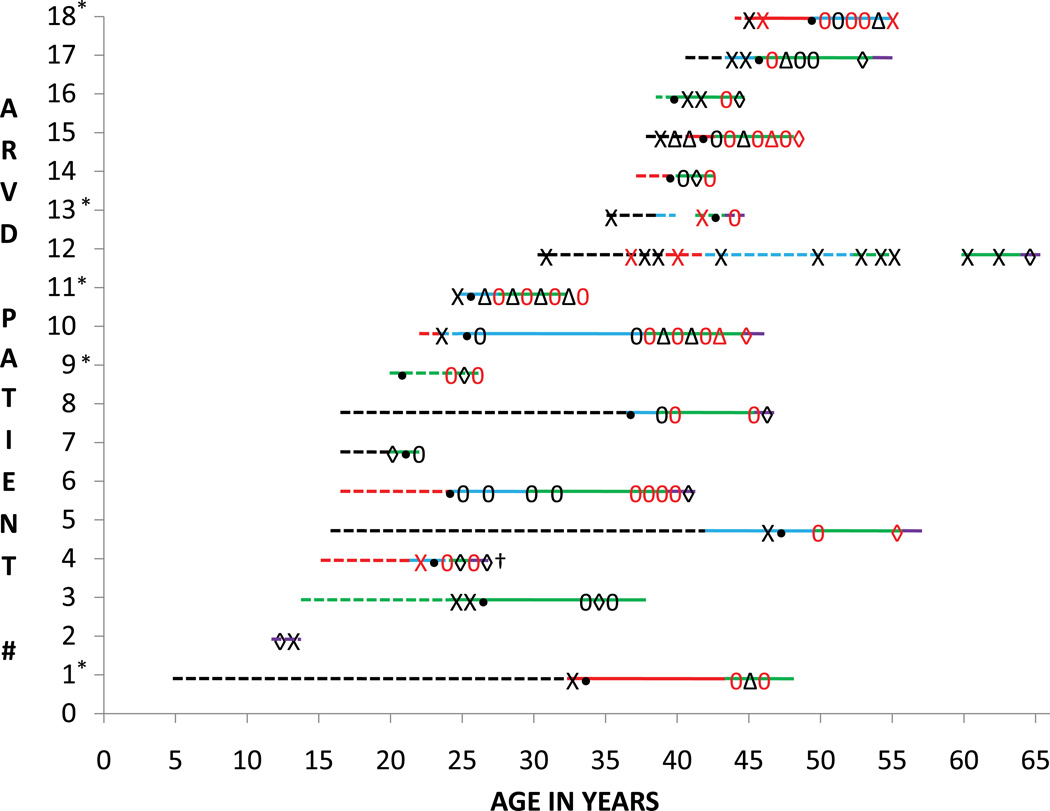
For patients transplanted for HF, most had a prolonged clinical course from ARVD/C and HF symptom onset (17.6±13.3 and 7.2±6.4 years, respectively), (Figure 1). One patient had a fulminant HF course, requiring transplant only three months after first symptoms. At the time of transplant, eight of the 13 patients had evidence of BiV involvement, and eight had reached stage D HF. Patients transplanted for HF also had a significant burden of VT. No patients transplanted for primarily for HF received a left ventricular assist device (LVAD).
Figure 1. Clinical timelines.
Each patient is represented as a line numbered 1 through 18. * = transplanted for primarily VT. Each line starts at the age of symptom onset. The line is dashed until a diagnosis of ARVD/C is established and then the line becomes solid. Each line (dashed or solid) ends at the age at which cardiac transplantation was performed. The lines are colored based on the stage of HF: black line = stage A HF; red line stage = B1 HF; blue line = stage B2 HF; green line = stage C HF; purple line = Stage D HF. X = sustained VT or ventricular fibrillation (VF); red X = three or more episodes of VT/VF; 0 = appropriate ICD therapy for VT/VF; red 0 = 3 or more appropriate ICD therapies delivered in 1 year; • = ICD implantation; △ = catheter ablation for VT; red △ = 2 or more catheter ablations for VT in 1 year; ◊ = HF hospitalization; red ◊ = 2 or more HF hospitalizations; † indicates death while awaiting transplant.
For patients transplanted for predominately VT, the clinical course from ARVD/C symptom onset and first episode of VT to transplant (14.7±15.9 and 7.9±4.4 years, respectively) was also prolonged. Three patients had undergone at least one prior VT ablation procedure. The other two patients were not ablation candidates because of multiple VT morphologies. All were tried on multiple anti-arrhythmic therapies including amiodarone. At the time of transplant, four patients had symptomatic HF but only one had reached stage D HF. Three patients had BiV involvement, and one received a LVAD for refractory VT.
ARVD/C-associated pathogenic mutations were found in 47% of patients who underwent genetic evaluation, including six plakophilin-2 mutations (PKP2) (five heterozygous and one homozygous) and two compound heterozygous DSG2 mutations in a single patient. Five of the seven patients were transplanted for HF and all had evidence of BiV involvement. The patient with a fulminant heart failure course had a heterozygous PKP2 mutation in addition to a DSC2 variant of unknown significance (found in 1/400 controls).
In the largest assembled cohort of ARVD/C patients receiving cardiac transplantation, we found patients often presented at a relatively young age, significantly younger than the average age of onset among our ARVD/C registry participants(3), and most commonly with VT or HF symptoms. In contrast to most published case reports, the clinical course of these patients was generally prolonged. LV dysfunction was a common finding at both at presentation and at transplant. The most common indication for cardiac transplantation was HF with less than one third of patients for control of ventricular arrhythmias. The frequency of desmosomal mutations was similar to other large ARVD/C registries(4). One year post-transplant survival was 94% and 88% were alive at an average post-transplant follow-up of 6.2±4.8 years (median 4.5 years).
Acknowledgments
Financial Support: The authors wish to acknowledge funding from the National Heart, Lung, and Blood Institute (K23HL093350 to HT), the St. Jude Medical Foundation, Medtronic Inc., and Boston Scientific Corp. The Johns Hopkins ARVD Program (www.ARVD.com) is supported by the Bogle Foundation, the Healing Hearts Foundation, the Campanella family, and Wilmerding Endowments, and the Dr. Francis P. Chiaramonte Private Foundation.
Dr Calkins receives research support from Boston Scientific, Medtronic, and St Jude Medical and is a consultant for Medtronic. Dr. Tedford received a travel grant from Medtronic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The other authors have no conflicts of interest to disclose.
Contributor Information
Ryan J. Tedford, Division of Cardiology, Department of Medicine, The Johns Hopkins Hospital.
Cynthia James, Division of Cardiology, Department of Medicine, The Johns Hopkins Hospital.
Daniel P. Judge, Division of Cardiology, Department of Medicine, The Johns Hopkins Hospital.
Crystal Tichnell, Division of Cardiology, Department of Medicine, The Johns Hopkins Hospital.
Brittney Murray, Division of Cardiology, Department of Medicine, The Johns Hopkins Hospital.
Aditya Bhonsale, Division of Cardiology, Department of Medicine, The Johns Hopkins Hospital.
Binu Philips, Division of Cardiology, Department of Medicine, The Johns Hopkins Hospital.
Theodore Abraham, Division of Cardiology, Department of Medicine, The Johns Hopkins Hospital.
Darshan Dalal, Division of Cardiology, Department of Medicine, The Johns Hopkins Hospital.
Marc K. Halushka, Department of Pathology, The Johns Hopkins Hospital.
Harikrishna Tandri, Division of Cardiology, Department of Medicine, The Johns Hopkins Hospital.
Hugh Calkins, Division of Cardiology, Department of Medicine, The Johns Hopkins Hospital.
Stuart D. Russell, Division of Cardiology, Department of Medicine, The Johns Hopkins Hospital.
References
- 1.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular Cardiomyopathy/Dysplasia: Proposed modification of the task force criteria. Circulation. 2010 April 6;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: Executive summary A report of the american college of Cardiology/American heart association task force on practice guidelines (committee to revise the 1995 guidelines for the evaluation and management of heart failure) Circulation. 2001 December 11;104:2996–3007. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 3.Dalal D, Nasir K, Bomma C, et al. Arrhythmogenic right ventricular dysplasia: A united states experience. Circulation. 2005 December 20;112:3823–3832. doi: 10.1161/CIRCULATIONAHA.105.542266. [DOI] [PubMed] [Google Scholar]
- 4.den Haan AD, Tan BY, Zikusoka MN, et al. Comprehensive desmosome mutation analysis in north americans with arrhythmogenic right ventricular Dysplasia/Cardiomyopathy / CLINICAL PERSPECTIVE. Circulation: Cardiovascular Genetics. 2009 October 01;2:428–435. doi: 10.1161/CIRCGENETICS.109.858217. [DOI] [PMC free article] [PubMed] [Google Scholar]