Abstract
Stem cells can either differentiate into more specialized cells or undergo self-renewal. Several lines of evidence from different organisms suggest that these processes depend on the post-transcriptional regulation of gene expression. The presence of the PUF [Pumilio/FBF (fem-3 binding factor)] domain defines a conserved family of RNA binding proteins involved in repressing gene expression. It has been suggested that a conserved function of PUF proteins is to repress differentiation and sustain the mitotic proliferation of stem cells. In humans, Pumilio-2 (PUM2) is expressed in embryonic stem cells and adult germ cells. Here we show that PUM2 is expressed in a subpopulation of adipose-derived stem cell (ASC) cultures, with a granular pattern of staining in the cytoplasm. Protein levels of PUM2 showed no changes during the differentiation of ASCs into adipocytes. Moreover, RNAi knockdown of pum2 did not alter the rate of adipogenic differentiation compared with wild-type control cells. A ribonomic approach was used to identify PUM2-associated mRNAs. Microarray analysis showed that PUM2-bound mRNAs are part of gene networks involved in cell proliferation and gene expression control. We studied pum2 expression in cell cultures with low or very high levels of proliferation and found that changes in pum2 production were dependent on the proliferation status of the cell. Transient knockdown of pum2 expression by RNAi impaired proliferation of ASCs in vitro. Our results suggest that PUM2 does not repress differentiation of ASCs but rather is involved in the positive control of ASCs division and proliferation.
Introduction
Stem cells can either differentiate into more specialized cells or renew themselves. Self-renewal ensures the maintenance and supply of an adult population of undifferentiated stem cells. Many adult tissues contain populations of stem cells that can renew themselves and repopulate damaged tissues after trauma, disease, or aging [1]. Mesenchymal stem cells (MSCs) are present in many tissues and represent a candidate population for cell-based treatments of injured tissues [2]. However, MSCs account for only a very small fraction of the total population of cells present in adult tissues, and therefore in vitro expansion is required before cell therapy [3]. Unfortunately, stem cells senesce during culture and with increasing numbers of passages, and lose their potential to differentiate under these conditions [2,4]. Large numbers of passages also have an adverse affect on MSC activation and cardio protection [5]. An understanding of the biological basis of self-renewal is essential to determine the mechanisms that maintain and control the propagation of MSCs in an undifferentiated state with no loss of differentiation potential [6].
Gene expression is regulated at various complementary levels, to gain tight control of transcript abundance and translation. Several lines of evidence from different organisms suggest that stem cell self-renewal also depends on post-transcriptional mechanisms of protein translational control [7,8]. This post-transcriptional regulation is mediated by various molecules, including noncoding RNAs and RNA-binding proteins (RBPs) [9]. RBPs can recognize and bind sequences or structural elements present mostly in the untranslated regions (UTRs) of the mRNA and may be classified into families on the basis of their RNA recognition domains [10].
The presence of the PUF (Pumilio/FBF [fem-3 binding factor]) domain defines a family of conserved proteins found in all eukaryotes. The proteins of the PUF family are characterized by the presence of a highly conserved C-terminal RNA-binding domain, composed of 8 Puf repeats. PUF family proteins are not only structurally related but also bind to related sequence motifs in the 3′UTR of the mRNA, thereby modulating mRNA expression in various eukaryotic species. PUF proteins control a wide variety of biological processes, either by enhancing mRNA decay or by repressing translation [11]. PUF proteins were first described in Drosophila as repressors of translation involved in the posterior patterning of embryos, and have since been shown to regulate mRNA decay by recruiting RNA deadenylase complexes via an evolutionarily conserved mechanism [12].
There is evidence to suggest that PUF proteins play a key, conserved role in maintaining the mitotic proliferation of stem cells [13]. In Caenorhabditis elegans, the FBF proteins at least partially control the development of germline stem cells by repressing gld-1, the expression of which promotes commitment to the meiotic cell cycle [14,15]. Pumilio controls the migration of germline stem cells and mitotic divisions of these cells in female Drosophila [16], whereas DjPum is essential to sustain self-renewal of planarian stem cells [17]. Each of these lines of evidence strongly suggests that PUF proteins may mediate a widespread and ancient mechanism for repressing differentiation and maintaining the self-renewal of stem cells.
The function of PUF proteins in vertebrates is unclear, though there is increasing evidence to suggest a role in stem cell self-renewal. Two PUF proteins, Pumilio-1 (PUM1) and Pumilio-2 (PUM2), are present in humans and are often coexpressed in different cell types [18]. Human PUM2 is expressed in embryonic stem cells and germ cells and interacts with Deleted in Azoospermia (DAZ) and DAZ-like proteins [19]. The translational regulator, Dazl, is involved in the maintenance of pluripotency and genetic and epigenetic differentiation programs in mouse primordial germ cells in vivo and in vitro [9]. We investigated whether PUM2 was present in human MSCs and investigated if its function was related to the repression of differentiation and the promotion of self-renewal.
We aimed to characterize the pattern of PUM2 production in MSCs from different tissues and during cell differentiation. We found that PUM2 was produced throughout differentiation into adipocytes and changes in its production were dependent on the proliferation status of the cells. Identification of PUM2-associated mRNAs revealed that the protein may regulate cell proliferation networks. This result was confirmed by RNAi assays, where partial ablation of PUM2 resulted in impaired cell proliferation.
Materials and Methods
Isolation, culture, and differentiation of MSCs and adipose-derived stem cells
Stem cells were obtained from bone marrow (BM), umbilical cord blood (UCB), and adipose-derived stem cells (ASCs) and were cultured and characterized as described previously [20]. All samples were collected after informed consent had been obtained in accordance with guidelines for research involving human subjects, and with the approval of the Ethics Committee of Fundação Oswaldo Cruz, Brazil (approval number 419/07). All tests were performed with cell cultures at passages 3–5.
For adipogenic or osteogenic differentiation, ASCs were seeded onto glass coverslips (Sarstedt) in 24-well plates (TPP, Trasadingen, Switzerland) and were treated with poietics differentiation basal medium adipogenic or osteogenic (Cambrex Bioscience) supplemented with human MSC adipogenic or osteogenic SingleQuots (Cambrex Bioscience) in accordance with the manufacturer's instructions. To determine the degree of adipogenic differentiation and lipid accumulation, the accumulation of cytoplasmic triglycerides in the cells was detected by staining with Oil Red O (Sigma-Aldrich). Briefly, the cells were washed in phosphate-buffered saline (PBS), then fixed with 4% paraformaldehyde for 30 min, and finally stained for 30 min with 0.5% Oil Red O solution. The stained cells were examined under a Nikon eclipse E-600 microscope and photographed with a Nikon Coolpix 995 camera. After the Oil Red O retained in the cells was extracted with isopropanol, the absorbance was spectrophotometrically determined at 550 nm in an EL×800 ELISA reader (Bio-Tek) using BioTek's Gen5™ Data Analysis Software. We also performed reverse transcription–polymerase chain reaction (RT-PCR) to estimate the amounts of adipocyte-specific fatty acid-binding protein 4 (FABP4) mRNA in induced and noninduced (negative control) cultures. To determine the degree of osteogenic differentiation and calcium deposition, the culture was observed by Alizarin red staining (Fluka Chemie). Briefly, the cells were washed in PBS, then fixed with 4% paraformaldehyde for 30 min, and finally stained for 3 min with 2% Alizarin red solution. The stained cells were examined and photographed. Alizarin red retained in the differentiating cells was extracted with 10% cetylpyridiniumchloride, and the absorbance was spectrophotometrically determined at 570 nm in an EL×800 ELISA reader (Bio-Tek) using BioTek's Gen5™ Data Analysis Software.
Flow cytometry: intracellular staining
ASCs were first permeabilized with CytoFix/cytoPerm staining kit (Becton Dickinson), in accordance with the manufacturer's instructions. Cells were incubated with anti-PUM2 antibody (1/50) (Santa Cruz Biotechnology). They were then washed and stained with a secondary FITC-conjugated rabbit anti-goat IgG (Sigma-Aldrich). Controls for the flow cytometry procedure included cells incubated with PE- and FITC-conjugated isotype antibodies. Cytometric evaluation was performed in a FACSCalibur machine (Becton Dickinson). Data were analyzed with FlowJo software (Tree Star). The values given are the means of 2 independent assays.
The cell cycles of ASCs were assessed by flow cytometry, checking the incorporation of 50 μg/mL propidium iodide (Sigma) into the cellular DNA after 72 h of transfection with siPUM2 or siNC1. Flow cytometry was performed on a FACSCalibur device (Becton Dickinson). Data files were analyzed with FlowJo software (Treestar) and Dean-Jett-Fox algorithm. To observe apoptotic cells, cells were treated with Alexa Fluor® 488 annexin V and propidium iodide (Molecular Probe), followed by flow cytometric analysis.
Immunocytochemistry
For immunocytochemistry analysis, treated and untreated ASCs were washed with PBS, fixed by incubation with 4% paraformaldehyde for 10 min, and permeabilized by incubation in 0.5% Triton X-100. After blocking with 1% bovine serum albumin, cells were incubated for 1 h at room temperature with primary goat anti-human PUM2 (1/20) antibodies (Santa Cruz Biotechnology). Cells were incubated with rabbit anti-goat AP (Sigma-Aldrich) secondary antibodies for 30 min at a dilution of 1:7,000 following the NBT/BCIP staining step. Slides were examined under a Nikon E-600 microscope. Digital images were captured with a CoolSNAP-PROcf (Media Cybernetics) camera controlled by Image Pro-Plus software from Diagnostic Instruments.
Total RNA extraction and RT-PCR
RNA was extracted using the RNeasy mini kit (Qiagen). RT-PCR and quantitative PCR (qPCR) in real-time were performed as previously described [20]. For a list of primers, see Supplementary Table S1 (Supplementary Data are available online at www.liebertonline.com/scd). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and RNA polymerase II were used as internal controls. Experiments were performed from at least 3 donors, with technical triplicates. Student's t-test was used to assess the significance of differences between the cell populations that were analyzed. P-values ≤0.05 were considered to be statistically significant.
Western blotting
ASCs were incubated in lysis buffer [20 mM HEPES (pH 7.4), 100 mM NaCl, 50 mM NaF, 1% Triton X-100, 10% glycerol, 1 mM dithiothreitol, and protease inhibitors] for 1 h at 4°C. They were then centrifuged at 14,000 g, for 10 min, at 4°C. The supernatant was removed and treated with denaturing buffer (160 mM Tris-HCl pH 6.8, 4% SDS, 10% β-mercaptoethanol, 24% glycerol 24%, and 0.02% bromophenol blue). The goat anti-hPUM2 antibody (1:200; Santa Cruz Biotechnology), rabbit anti-β-actin antibody (1:200; Santa Cruz Biotechnology), and rabbit anti-polymerase (RNA) II (DNA directed) polypeptide B (POLR2B) antibody (1:200; GeneTex) were used for western blotting, following SDS-PAGE separation and protein transfer to nitrocellulose. The signals obtained were quantified with Image Pro-Plus software.
Immunoprecipitations
Immunoprecipitation (IP) reactions were performed with 2 μg of anti-Pum2 antibody (goat polyclonal; Santa Cruz Biotechnology) bound to protein G-agarose beads (Sigma). ASCs were lysed in polysome lysis buffer (Tris-HCl pH 7.4 15 mM, MgCl2 15 mM, NaCl 0.3 M, 1% Triton X-100, 1 mM DTT, 100 U/mL RNase Out, PMSF 1 mM, and E64 10 μM) for 1 h at 4°C. Beads were washed, then buffer and cell lysate were added, and the reaction mixtures were tumbled for 2 h at 4°C. After this incubation, the beads were thoroughly washed again with polysome lysis buffer and then either boiled in denaturing buffer for western experiments or had their RNA extracted for microarray and RT-PCR experiments. Identical IPs were performed with beads precoated with preimmune goat serum as a negative control.
Microarray analysis
RNA was processed for hybridization with GeneChip 3′ IVT Express (Affymetrix), in accordance with the manufacturer's instructions. Briefly, cDNA was synthesized from immunoprecipitated RNA using reverse transcriptase followed by second-strand synthesis to generate double-stranded cDNA. An in vitro transcription reaction was used to generate biotinylated cRNA. After purification and fragmentation, cRNA was hybridized onto GeneChip Affymetrix Human Genome U133 Plus 2.0 arrays. Posthybridization washes were preformed on an Affymetrix GeneChip® Fluidics Station 450. Arrays were scanned on an Affymetrix GeneChip Scanner 3000. Scanned arrays were normalized using GCRMA in Partek software (Partek Incorporated). Ratios of signal intensity were calculated and genes that have fold change (IP-PUM2/IP-negative) ≥2.0 were listed (Supplementary Table S2). The list obtained was used as input to ingenuity pathways analysis (IPA) to obtain an indication of the functional relationship among the genes enriched in association with PUM2 protein. All microarray data were submitted to the gene expression omnibus (GEO) database and can be found under the accession number GSE 26626.
Bromodeoxyuridine proliferation assay
Cells were maintained at 40% and 90% confluence, resulting in different proliferative activities, since this activity is inhibited by cell contact. These cultures were monitored for 7 days (days 1, 3, 5, and 7). About 4×104 cells were placed into 6- or 24-well plates, corresponding to 40% and 90% confluence, respectively. After cell adhesion to the surface, the cultures were incubated with bromodeoxyuridine (BrdU; 100 μM) for 24 h, suspended, and fixed in 4% paraformaldehyde for 30 min at room temperature. After fixation, cells were incubated twice with 2 N HCl at 50°C for 10 min. After incubation with boric buffer (50 mM boric acid and 12 mM borax, pH 8.5) at room temperature for 10 min, cells were permeabilized with PBS-0.3% Triton and blocked with 5% bovine serum albumin at room temperature for 30 min, followed by incubation with an FITC-conjugated anti-BrdU antibody for 1 h at 37°C. Quantitative analyses of BrdU-labeled cells were performed using a FACS Calibur flow cytometer and Flow Jo software (Flow Jo).
RNA interference assays
All chemically synthesized dsRNA sequences for the Dicer-substrate 27-mers used in this study were synthesized and HPLC purified (Integrated DNA Technologies). Nonsilencing dsRNA controls (siNC1) included 27mers (Integrated DNA Technologies). Transfections were performed in 6-well plates using Lipofectamine™2000 reagent (Invitrogen) for delivery of dsRNA into ASCs in accordance with the manufacturer's protocol. The final concentration of 3 PUM2-specific dicer-substrate siRNA mix and NC1 siRNA was 10 nM, and the lipid concentration was 5 μL in 1 mL of media. After 24–72 h post-transfection, mRNA was measured.
Results
PUM2 is expressed in ASCs, UCB, and BM-derived MSCs
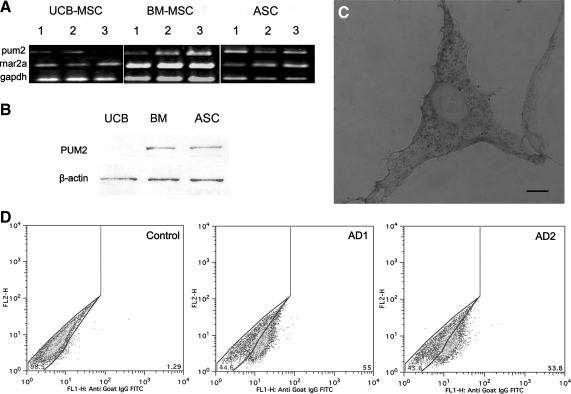
We investigated the expression of PUM2 in MSCs and ASCs by first checking for the presence of the pum2 transcript. We performed RT-PCR on total RNA from MSCs isolated from BM, UCB, and ASCs cultures. We used GAPDH and RNA polymerase IIa mRNAs as controls. An amplicon corresponding to the pum2 transcript was identified in all samples from at least 3 different donors with a similar profile for replicates and biological samples of the same type of MSC, and for all sources. DNA fragments were cloned and sequenced to confirm the identity of the PCR amplicon (Fig. 1A).
FIG. 1.
PUM2 production in ASCs, UCB-, and BM-derived MSCs. (A) RT-PCR analysis of pum2 expression in MSCs from different sources and ASCs. Numbers indicate different donors. Expression of the GAPDH and RNApolII genes was used as a control. (B) Western blot analysis with anti-hPUM2 (114 kDa) antibody on protein extracts from cells derived from 3 sources: UCB-MSC, BM-MSC, and ASC. The β-actin (43 kDa) protein was used as a loading control. (C) Indirect immunocytochemical studies showing the distribution of PUM2 in ASCs. Scale bar=40 μm. (D) Flow cytometry quantification of PUM2+ cells: the control was cells incubated with FITC-conjugated isotype antibodies used to set the gate. A dot plot of the population of ASC from 2 donors (AD1 and AD2) is displayed. Fluorescence intensity (FL2-H channel) is shown on the y-axis on a log (100–104) scale and fluorescence intensity (FL1-H channel) is shown on the x-axis, on a log (100–104) scale. ASCS, adipose-derived stem cells; BM, bone marrow; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MSCs, mesenchymal stem cells; PUM2, Pumilio-2; UCB, umbilical cord blood.
We also checked for the presence of PUM2 protein in MSCs and ASCs. Anti-human PUM2 antibody was used in western blot analysis. A band of ∼114 kDa, corresponding to the expected molecular weight of PUM2, was observed in protein extracts from BM-MSC and ASCs from different donors (Fig. 1B). A single PCR product was clearly amplified from total RNA from human umbilical cord blood-derived mesenchymal stem cell (UCB-MSC), but the band obtained from protein extracts, though present, was very faint (Fig. 1B). PUM2 is generally distributed throughout the cytoplasm in mammalian cells. We investigated the cellular distribution of PUM2 in ASCs, by carrying out indirect immunocytochemical analysis. PUM2 staining displayed a granular pattern in the cell cytoplasm (Fig. 1C). A detailed analysis of the results showed that not all the cells were stained with the specific antibody. We determined the percentage of cells from the ASC population that expressed PUM2 by labeling 3 samples from independent AD donors with the antibody against PUM2 and then performing flow cytometry analysis. We found that about 50% of the ASC population contained PUM2, consistent with the production of this protein by a subpopulation of ASCs (Fig. 1D).
PUM2 production is not altered during cellular differentiation
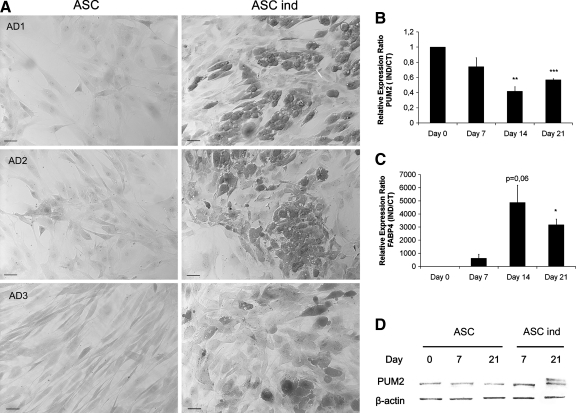
It has been suggested that the ancestral functions of PUF proteins include maintenance of the ability of the cell to renew itself as well as repression of cellular differentiation. We investigated the putative role of PUM2 in cell differentiation by analyzing the levels of PUM2 transcript and protein, and their distribution during the differentiation of ASCs into adipocytes. ASCs from 3 donors were induced to differentiate in the presence or absence of adipogenic medium for 21 days. RNA and protein samples were obtained at 0, 7, 14, and 21 days, and the accumulation of lipid droplets in the cells was observed by staining with Oil Red O (Fig. 2A). Depending on the donor, between 30% and 50% of the total ASC population differentiated into adipocytes. qPCR analysis showed that PUM2 transcript levels halved during differentiation, with the fabp4 adipocyte marker displaying the opposite trend (Fig. 2B, C). We monitored PUM2 protein levels during differentiation using western blotting. Unlike PUM2 transcript levels, PUM2 protein levels remained constant throughout differentiation (Fig. 2D). A second band was observed after 21 days of induction suggesting a putative post-translational modification of PUM2.
FIG. 2.
Production of PUM2 in ASCs during the process of cellular differentiation. ASCs were induced to differentiate in the absence (ASC) or presence (ASC ind) of adipogenic medium for 21 days. (A) The accumulation of lipid droplets in the cells of 3 donors (AD1, AD2, and AD3) was observed by staining with Oil Red O. Scale bar=80 μm. (B) RT-qPCR for pum2 (C) and fabp4 during differentiation. The mean±SEM for 3 independent experiments is shown. GAPDH was used as an internal housekeeping control for expression. (D) Western blot analysis with hPUM2 antibody, on protein extracts from ASCs and ASCs induced to differentiate for 1, 7, and 21 days. β-actin was used as a control. CT, control; IND, induced; RT-qPCR, reverse transcription–quantitative polymerase chain reaction. FABP4, fatty acid-binding protein 4. *P≤0.05, **P≤0.01, ***P≤0.001.
mRNAs associated with PUM2 are involved in the control of cell proliferation
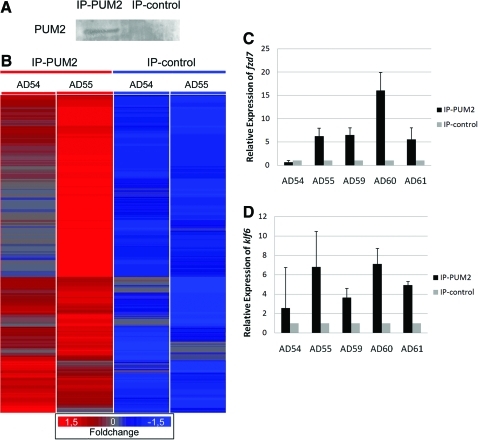
To better understand the role of PUM2, we attempted to identify the mRNAs regulated by the protein in ASCs. A ribonomic approach was used to isolate the PUM2-associated transcripts. We used the RIP-Chip method modified for ASCs [21]. PUM2 containing ribonucleoproteins (RNPs) were immunoprecipitated from ASCs lysates with a specific antibody; the presence of PUM2 in the immunoprecipitates was confirmed by western blot (Fig. 3A). The bound mRNAs were identified by microarray hybridization in a GeneChip Affymetrix Human Genome U133 Plus 2.0 Array (Fig. 3B). An antibody isotype IP was used as a control. We considered signals showing at least a 2-fold increase when compared with the control to be positive. A total of 78 signals showed a 3-fold enrichment, and 300 showed a 2-fold enrichment in the PUM2 eluates (Supplementary Table S2). To validate our microarray results, IP assays were performed in cells from 3 new donors. A total of 10 microarray-positive candidates were confirmed by qPCR in the 3 independent assays (Fig. 3C, D and Supplementary Fig. S1). The PUM2 binding element that is normally present in the 3′UTRs has been described in humans and was present in the UTRs of the 10 transcripts analyzed by qPCR (data not shown).
FIG. 3.
Identification of Pum2-associated mRNAs. (A) IP-western blot of PUM2, probed with anti-PUM2-specific antibody. IP-PUM2 and IP-control (negative control); (B) mRNA associated with PUM2. Rows represent unique transcripts and the color code indicates the degree of enrichment. Two experiments with Pum2 protein (AD54 and AD55) and 2 negative controls are shown. (C, D) RT-qPCR of anti-PUM2 immunoprecipitated mRNAs. Transcript levels in IP-PUM2 versus either negative IP (IP-control), normalized to concentration, are shown. Analysis of enrichment of target (fzd7and klf6) of IP-PUM2 ASC from 5 donors (AD54, AD55, AD59, AD60, and AD61). IP, immunoprecipitation. Color images available online at www.liebertonline.com/scd
We analyzed the functional relationship of PUM2-associated mRNAs by searching for biological functions by gene ontology, and we used the IPA to identify gene networks formed by the transcripts. IPA identified gene networks involved in the control of cell growth, proliferation, and gene expression (Table 1). Among the putative target transcripts, we identified PUM2-coding mRNA, which suggests that PUM2 regulates expression of its own mRNA.
Table 1.
Function and Analysis of Pumilio-2 Targets
| Categorya | Top functions | Enrichment score |
|---|---|---|
| Biological process | Binding | 7.64 |
| Transcription regulator activity | 2.88 | |
| Transporter activity | 2.05 | |
| Component | Biological regulation | 2.44 |
| Establishment of localization | 2.20 | |
| Metabolic process | 2.09 | |
| Cellular process | 2.09 | |
| Molecular function | Protein binding | 12.8 |
| Nucleic acid binding | 2.40 | |
| Chromatin binding | 2.20 |
| Associated network functionsb | Score |
|---|---|
| Cellular growth and proliferation, reproductive system development and function, cancer | 43 |
| Gene expression, endocrine system development and function, endocrine system disorders | 42 |
| Cellular function and maintenance, skeletal and muscular system development and function, cell-mediated immune response | 41 |
GO annotations of PUM2 mRNA targets.
Ingenuity networks: functions associated with the networks regulated by PUM2.
PUM2, Pumilio-2.
PUM2 is expressed in proliferating cells
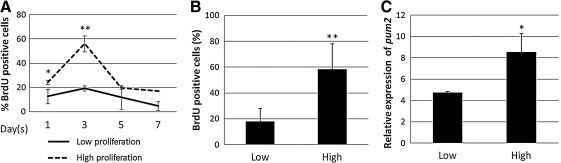
PUF proteins have been reported to regulate the proliferative status of eukaryotic cells by maintaining mitotic division. We studied pum2 expression in ASCs under conditions of high or low proliferation. Cultures were seeded with ASCs at low density (40% confluence) or high density (90% confluence), and were maintained for 7 days. Cell proliferation was measured by counting the cells from 5 donors on days 1, 3, 5, and 7. Proliferation was evaluated by BrdU incorporation. We observed that the main differences in proliferation were after 72 h. The size of the population of cells plated at low density showed an increase in BrdU incorporation of almost 3 times that in the population of cells plated at high density (Fig. 4A). Similar differences were observed when whole cells were counted (Fig. 4B). The overall pattern of change was similar for the various biological samples. Considering this data, the pattern of pum2 transcript production was also determined in cultures of ASCs displaying high and low levels of cell proliferation after 72 h of plating. Expression of pum2 was higher in ASCs displaying high levels of proliferation than in ASCs displaying lower levels of proliferation (Fig. 4C).
FIG. 4.
Expression of pum2 is associated with proliferation. ASCs were used to seed cultures at low density (high rates of proliferation) and high density (low rates of proliferation), which were then incubated for 7 days. (A) The mean numbers BrdU-positive cells for 2 donors at 1, 3, 5, and 7 days after seeding. (B) Mean numbers of BrdU-positive cells at 3 days for both cultures. (C) RT-qPCR analysis of pum2 expression at 3 days for both cultures. GAPDH was used as an internal housekeeping gene control. *P<0.05, **P<0.01. BrdU, bromodeoxyuridine.
RNA interference of PUM2 impairs cell proliferation
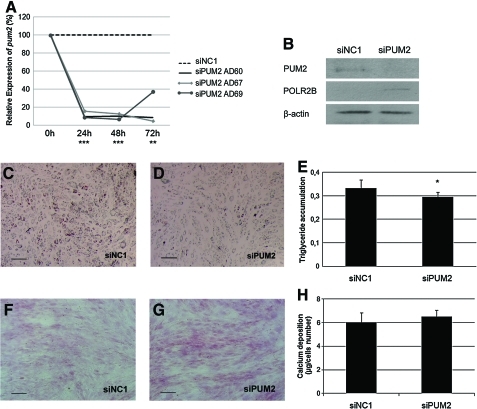
To investigate the role of PUM2 in the control of cell proliferation, we determined whether gene silencing using PUM2-specific siRNA molecules would alter proliferation rates. To screen for gene-specific silencing, ASCs of 3 different donors (AD60, 67, and 69) were transfected with a mix of siRNAs at a concentration of 10 nM. Expression of pum2 was measured after 24, 48, and 72 h of transfection. We confirmed a 62%–95% knockdown of pum2 mRNA levels after 24 h, which remained constant until 72 h after transfection compared with cells transfected with a scrambled control (siNC1) (Fig. 5A). When the expression of PUM2 was analyzed by western blot, a clear reduction of the band corresponding to the protein was observed in the knockdown cells (Fig. 5B). The pum2 knockdown did not significantly affect cell morphology nor did it demonstrate an evident morphological phenotype. We evaluated the cell survival rates of PUM2 knockdown cells by using the annexin V assay (Molecular Probe), which allowed quantifying the percentage of living, apoptotic, or dead cells. There were no significant differences of the PUM2 knockdown cells comparison with the control cells (Supplementary Fig. S2). As PUF proteins are repressors of gene expression, we analyzed the mRNA levels of some PUM2-associated mRNAs (klf6, polr2b, and eif4bp2) in the knockdown cells. There were no significant changes in the mRNA levels of the selected genes compared with control siNC1 in ASCs (Supplementary Fig. S3). However, when we analyzed the protein levels of POLR2B, 1 of the putative PUM2 regulated genes, in the knockdown cells by western blot the expression of POLR2B was clearly increased (Fig. 5B). This result suggests that PUM2 may be negatively regulated the expression of its associated genes at the translational level.
FIG. 5.
Pum2 mRNA knockdown affects cellular differentiation. (A) ASCs (3 donors: AD60, AD67, and AD69) were transfected using 10 nM of the pum2 duplex mix (siPUM2) or 10 nM of the Scrambled-negative control duplex (siNC1). After 24, 48, and 72 h transfection, (A) RNA was isolated and pum2 levels were measured using qRT-PCR. Relative expression was normalized to GAPDH internal control mRNA. (B) Western blot analysis with anti-hPUM2 (upper line, 114 kDa) and anti-hPOLR2B (middle line, 140 kDa) antibodies on protein extracts from pum2 knockdown (siPUM2) or control (siNC1) cells. The β-actin (lower line, 43 kDa) protein was used as a loading control. (C–H) The effects of pum2 knockdown on differentiation. ASCs were transfected with siPUM2 or siNC1 and after 48 h induction of adipocyte differentiation for 7 days (C–E) or osteogenesis for 14 days (F–H). Adipogenesis was observed by Oil Red O staining in control cells (C) and pum2 knockdown cells (D). (E) The stained Oil Red O was extracted with isopropanol. The absorbance of the extracted Oil Red O was spectrophotometrically determined at 550 nm to measure triglyceride accumulation. (F–H) Osteogenesis was observed by Alizarin red staining in (F) control cells and (G) pum2 knockdown cells. (E) The stained Alizarin red was extracted with 10% cetylpyridiniumchloride. The absorbance of the extracted Alizarin red was spectrophotometrically determined at 570 nm to measure calcium deposition accumulation. Scale bar=200 μm. *P≤0.05, **P≤0.01, ***P≤0.001. POLR2B, polymerase (RNA) II (DNA directed) polypeptide B. Color images available online at www.liebertonline.com/scd
PUF proteins have been reported to be repressors of cell differentiation both in embryonic and adult stem cells [13]. We evaluated the expression of pum2 after osteogenic, adipogenic, and chondrogenic differentiation. Expression of pum2 in cells undergoing osteogenesis increased by 7 times compared with the control cells, whereas in the other lineages, the mRNA levels of pum2 were slightly reduced (Supplementary Fig. S4B, C). To assess the role of PUM2 in the control of ASCs differentiation, we performed the adipogenic and osteogenic induction of ASCs in pum2 knockdown cells. ASCs were transfected with pum2 siRNAs as described, and after 48 h adipogenic or osteogenic differentiation was induced. Adipogenesis was allowed for 7 days and the presence of adipose-like cells was observed by Oil Red O staining. A small reduction in the percentage of differentiated cells was observed between pum2 knockdown and control ASCs cells (Fig. 5C–E). Osteogenesis was allowed for 17 days and the presence of calcium deposition was observed by Alizarin red (an early stage marker of matrix mineralization, a crucial step toward the formation of calcified extracellular matrix associated with true bone) staining and quantified by absorbance. There were no significant changes compared to control (Fig. 5F–H).
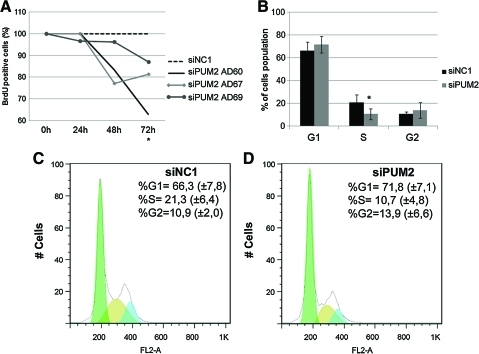
Since we had evidence of pum2 gene silencing in cells after transfection, we next performed analysis of cell proliferation rates in transfected ASCs to determine whether cell proliferation levels were affected by pum2 gene knockdown. BrdU incorporation was measured at 24, 48, and 72 h post-transfection. We observed a 13% to 37% decrease in BrdU incorporation levels 72 h post-transfection in pum2-siRNA-transfected cells from the different donors (Fig. 6A). This indicated that pum2 knockdown clearly impairs ASC proliferation. To determine whether this reduction in the proliferation rates was related to an arrest in some stages of the cell cycle, we performed a FACS-based cell cycle analysis of the knocked-down cells. Cell cycle analysis showed that pum2 gene silencing causes a decrease of cells in the S phase of the cell cycle compared with control cells (P<0.05) (Fig. 6B–D). We analyzed the expression of pum2 in cells cultures at the 4th and 14th passage. Interestingly, the expression of pum2 was significantly reduced in high passage (Supplementary Fig. S4A). These data suggest that with senescence, the cells reduce the proliferation so as the level of expression of pum2.
FIG. 6.
Pum2 mRNA knockdown reduced cell proliferation. (A) Proliferation was assessed by BrdU incorporation 24, 48, and 72 h after transfection with siRNA pum2 or siNC1. (B–D) FACS-based cell cycle analysis demonstrates that pum2 knockdown causes a decrease in the percentages of cells in the S phase of the cell cycle compared with control cells (P<0.05). No significant change was observed in the percentage of the pum2 knockdown cell population in G1 and G2 compared with control cells. (B) Representative graphs of the percentage of control cells (siNC1) and pum2 knockdown groups in various phases of the cell cycle from 2 donors and technical replicates. A representative histogram of control cells (C) and pum2 knockdown cells (D) based on the “Dean-Jett-Fox” model. *P≤0.05. Color images available online at www.liebertonline.com/scd
Discussion
The PUF protein family comprises regulatory RBPs that repress gene expression by inducing mRNA degradation or blocking translation. In humans, PUM2 has a tissue-specific distribution, being found in embryonic stem cells and germline cells in adults [19]. PUF proteins seem to have a conserved function in controlling germline-cell survival and migration [13,22]. We hypothesized that human Pumilio proteins might also play a role in sustaining the adult population of MSCs. We investigated the production of Pum2 in MSCs from various sources. Both transcript and protein were detected in MSCs, consistent with a fundamental role of PUM2 in stem cell biology. In UCB cells, the transcript was present, though only low levels of the Pum2 protein were detected. This suggested that post-transcriptional regulation of Pum2 may downregulate the protein in these cells. PUM2 was produced by about half of the cells present in the culture. It is widely accepted that ASCs are comprised of a mixture of different cell populations. The pattern of PUM2 production is consistent with this idea and it remains to be determined whether the PUM2-positive cells correspond to the functional stem cell population. PUM2 was diffusely distributed in the cytoplasm of ASCs, but concentrated into granule-like structures. In eukaryotic cells, PUM2 has been found associated with stress granules, particularly in neurons [23]. It has also been suggested that PUF proteins interact transiently with P-body-like structures [12]. The granular pattern observed suggests that PUM2 may interact with high-molecular-weight RNP regulatory complexes in ASCs to negatively regulate its associated mRNAs.
It has been proposed that PUF proteins play a key role in repressing the differentiation of the adult stem-cell population. In the absence of Pumilio, germline cells in Drosophila differentiate prematurely and exhaust the stem cell population [24,25]. In C. elegans, the deletion of FBF-1 leads to stem cells entering meiosis and differentiating into gametes [14]. We investigated this putative role of PUM2 in ASCs by studying the production of mRNA and protein during differentiation into adipocytes and osteoblasts. Some downregulation of transcript levels was observed during differentiation to adipocytes, but with no significant change in protein levels. This suggests that positive post-transcriptional mechanisms compensate for the decrease in abundance of the transcript. The continued presence of PUM2 in differentiated cells indicates that the mere presence of this protein is insufficient to repress the differentiation of adult stem cells as previously suggested. Moreover, RNAi knockdown of pum2 did not increase the rate of adipogenic or osteogenic differentiation compared with wild-type control cells. This strengthens the notion that PUM2 is not directly involved in repression of ASC differentiation even when pum2 mRNA is upregulated during osteogenesis.
After 21 days of differentiation, a second PUM2 band was observed in the adipocyte-like protein extracts. This could be the result of a post-translational modification. Recently, it was shown that PUM1 is phosphorylated to enhance its binding activity [26] and Xenopus Pum2 is phosphorylated during oocyte maturation [27]. Human PUM2 has putative phosphorylation sites, so we cannot exclude the possibility of specific phosphorylation to regulate its biological activity.
IP of RBPs followed by microarray analysis has been used to identify the putative regulated mRNAs of RBPs in several eukaryotic cells [28]. In our experimental conditions, we were able to identify about 300 transcripts associated with PUM2 RNPs in ASCs. This number of mRNAs is in agreement with the population of transcripts bound by PUF proteins in other organisms such as C. elegans [29] and human cell lines. The mRNA targets for the human PUM1 and PUM2 proteins have been analyzed in HeLa and HEK 293 cells showing that these proteins bind a large set of transcripts with a large overlap of targets [30–32]. Compared with previous reports, we found differences in the composition of the associated population of transcripts. Although this could be due to technical differences in the assays, we expected these differences because reported PUM2 targets were identified in cancer cell lines. To our knowledge, our study is the first genome-wide characterization of human Pumilio mRNA targets in somatic cells. As these proteins are post-transcriptional regulators, the composition of the associated population of mRNAs is dependent of the cell transcriptome resulting in the observed differences. The pum2 mRNA was highly enriched in the immunoprecipitated population of transcripts that has also been observed for C. elegans FBF proteins [33]. This suggests that PUM2 regulates its own mRNA as has been proposed for several regulators of protein translation [34]. One of the common biological processes related to the target mRNAs observed in many PUF proteins was the presence of transcripts coding for proteins involved in cell proliferation control networks.
Several studies have suggested that one of the functions of PUF proteins conserved throughout evolution is the maintenance of mitotic division, resulting in the self-renewal of stem cells [13]. PUF proteins are also involved in controlling mitotic division in lower eukaryotes. In Saccharomyces cereviseae, cells with a knockout of the PUF5 gene proliferate less strongly than wild-type cells, whereas overproduction of this protein results in higher rates of division and proliferation in the mutated strain [35]. C. elegans PUF proteins are somehow involved in control of cell proliferation. FBF-1 and PUF8 have redundant functions in controlling the germline sex determination pathway [36]. FBF-2 suppresses expression of the gene encoding GLD-1, which promotes entry into meiosis [14]. Mitotic germline cells contain particularly high levels of PUF8, whereas PUF8-negative mutants contain fewer germ cells. PUF8 does not abolish differentiation or block entry into meiosis; it instead promotes mitotic proliferation [15]. Consistent with these data, our results show that PUM2 is produced preferentially in cells displaying high levels of proliferation. Knockdown of pum2 in ASCs resulted in a reduction of cell proliferation, which strongly suggests a role in the positive regulation of proliferation. Reduction of pum2 expression did not completely inhibit cell proliferation. This could be due to a positive effect of the remnant pum2 expression or the result of a redundant control of proliferation by other factors. We hypothesize that the latter could be the more plausible explanation revealing a robust control of ASC proliferation. Analysis of the knocked-down cells showed that these cells were affected in their ability to progress through the S phase of their cell cycle. Several transcripts coding for cell cycle-related proteins were found to be associated with PUF proteins in different organisms [29–32], suggesting that PUF proteins could be directly involved in the control of progression through the cell cycle. Among the target mRNAs, we found several transcripts coding for proteins involved in the negative regulation of proliferation. PUF proteins have been described as regulators of regulators [30]. PUF proteins negatively control the expression of their associated mRNAs by promoting deadenylation and repression of translation of target transcripts. Our results showed that in knocked down cells the expression level of POLR2B was increased, strongly suggesting that PUM2 repress translation of its target mRNAs as it has been previously described. In this case, we could imagine PUM2 acting as a repressor of repressors, hence resulting in the maintenance of mitotic divisions. Further work is necessary to understand in detail the underlying mechanisms involved in the PUM2-control of cell proliferation and the establishment of the resulting gene regulatory networks.
Conclusion
In conclusion, we observed the production of PUM2 mRNA and protein in ASCs from various tissues. This pattern of production was maintained during differentiation, suggesting that PUM2 is insufficient for the repression of differentiation. Knockdown of pum2 did not promote cell differentiation nor did it result in increased ASCs differentiation rates. PUM2 production was found to be dependent on the proliferation status of ASCs. Knockdown of pum2 expression resulted in impaired proliferation of ASCs, with cells accumulating in the S phase of the cell cycle. Our results suggest that the role of PUM2 in ASCs may be related to the promotion and maintenance of cell proliferation.
Supplementary Material
Acknowledgments
We thank Marco Stimamiglio and Alexandra C. Senegaglia for scientific advice and helpful discussions. This work was supported by grants from Ministério da Saúde and Conselho Nacional de Desenvolvimento Cientifico e Tecnológico—CNPq (CT-Saúde/MS/SCTIE/DECIT/MCT/CNPq No. 17/2008). This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HSN261200800001E. The content of this article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. M.A.K., S.G., and B.D. received fellowships from CNPq and P.S. from CAPES.
Author Disclosure Statement
The authors indicate no potential conflicts of interest.
References
- 1.Baksh D. Song L. Tuan R. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry FP. Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Derubeis AR. Cancedda R. Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann Biomed Eng. 2004;32:160–165. doi: 10.1023/b:abme.0000007800.89194.95. [DOI] [PubMed] [Google Scholar]
- 5.Crisostomo PR. Wang M. Wairiuko GM. Morrell ED. Terrell AM. Seshadri P. Nam UH. Meldrum DR. High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock. 2006;26:575–580. doi: 10.1097/01.shk.0000235087.45798.93. [DOI] [PubMed] [Google Scholar]
- 6.Chen L. Daley GQ. Molecular basis of pluripotency. Hum Mol Genet. 2008;17:R23–R27. doi: 10.1093/hmg/ddn050. [DOI] [PubMed] [Google Scholar]
- 7.Kohlmaier A. Edgar BA. Proliferative control in Drosophila stem cells. Curr Opin Cell Biol. 2008;20:699–706. doi: 10.1016/j.ceb.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampath P. Pritchard DK. Pabon L. Reinecke H. Schwartz SM. Morris DR. Murry CE. A hierarchical network controls protein translation during murine embryonic stem cell self-renewal and differentiation. Cell Stem Cell. 2008;2:448–460. doi: 10.1016/j.stem.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Haston KM. Tung JY. Reijo Pera RA. Dazl functions in maintenance of pluripotency and genetic and epigenetic programs of differentiation in mouse primordial germ cells in vivo and in vitro. PLoS One. 2009;4:e5654. doi: 10.1371/journal.pone.0005654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lunde BM. Moore C. Varani G. RNA-binding proteins: modular design for efficient function. Nat Rev Mol Cell Biol. 2007;8:479–490. doi: 10.1038/nrm2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wharton RP. Aggarwal AK. mRNA regulation by Puf domain proteins. Sci STKE. 2006;2006:pe37. doi: 10.1126/stke.3542006pe37. [DOI] [PubMed] [Google Scholar]
- 12.Goldstrohm AC. Hook BA. Seay DJ. Wickens M. PUF proteins bind Pop2p to regulate messenger RNAs. Nat Struct Mol Biol. 2006;13:533–539. doi: 10.1038/nsmb1100. [DOI] [PubMed] [Google Scholar]
- 13.Wickens M. Bernstein DS. Kimble J. Parker R. A PUF family portrait: 3′UTR regulation as a way of life. Trends Genet. 2002;18:150–157. doi: 10.1016/s0168-9525(01)02616-6. [DOI] [PubMed] [Google Scholar]
- 14.Crittenden SL. Bernstein DS. Bachorik JL. Thompson BE. Gallegos M. Petcherski AG. Moulder G. Barstead R. Wickens M. Kimble J. A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature. 2002;417:660–663. doi: 10.1038/nature754. [DOI] [PubMed] [Google Scholar]
- 15.Ariz M. Mainpal R. Subramaniam K. C. elegans RNA-binding proteins PUF-8 and MEX-3 function redundantly to promote germline stem cell mitosis. Dev Biol. 2009;326:295–304. doi: 10.1016/j.ydbio.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parisi M. Lin H. The Drosophila pumilio gene encodes two functional protein isoforms that play multiple roles in germline development, gonadogenesis, oogenesis and embryogenesis. Genetics. 1999;153:235–250. doi: 10.1093/genetics/153.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvetti A. Rossi L. Lena A. Batistoni R. Deri P. Rainaldi G. Locci MT. Evangelista M. Gremigni V. DjPum, a homologue of Drosophila Pumilio, is essential to planarian stem cell maintenance. Development. 2005;132:1863–1874. doi: 10.1242/dev.01785. [DOI] [PubMed] [Google Scholar]
- 18.Spassov DS. Jurecic R. Cloning and comparative sequence analysis of PUM1 and PUM2 genes, human members of the Pumilio family of RNA-binding proteins. Gene. 2002;299:195–204. doi: 10.1016/s0378-1119(02)01060-0. [DOI] [PubMed] [Google Scholar]
- 19.Moore FL. Jaruzelska J. Fox MS. Urano J. Firpo MT. Turek PJ. Dorfman DM. Pera RA. Human Pumilio-2 is expressed in embryonic stem cells and germ cells and interacts with DAZ (Deleted in AZoospermia) and DAZ-like proteins. Proc Natl Acad Sci U S A. 2003;100:538–543. doi: 10.1073/pnas.0234478100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rebelatto CK. Aguiar AM. Moretão MP. Senegaglia AC. Hansen P. Barchiki F. Oliveira J. Martins J. Kuligovski C, et al. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood) 2008;233:901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- 21.Tenenbaum SA. Lager PJ. Carson CC. Keene JD. Ribonomics: identifying mRNA subsets in mRNP complexes using antibodies to RNA-binding proteins and genomic arrays. Methods. 2002;26:191–198. doi: 10.1016/S1046-2023(02)00022-1. [DOI] [PubMed] [Google Scholar]
- 22.Subramaniam K. Seydoux G. Dedifferentiation of primary spermatocytes into germ cell tumors in C. elegans lacking the pumilio-like protein PUF-8. Curr Biol. 2003;13:134–139. doi: 10.1016/s0960-9822(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 23.Vessey JP. Vaccani A. Xie Y. Dahm R. Karra D. Kiebler MA. Macchi P. Dendritic localization of the translational repressor Pumilio 2 and its contribution to dendritic stress granules. J Neurosci. 2006;26:6496–6508. doi: 10.1523/JNEUROSCI.0649-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forbes A. Lehmann R. Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development. 1998;125:679–690. doi: 10.1242/dev.125.4.679. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z. Lin H. Nanos maintains germline stem cell self-renewal by preventing differentiation. Science. 2004;303:2016–2019. doi: 10.1126/science.1093983. [DOI] [PubMed] [Google Scholar]
- 26.Kedde M. van Kouwenhove M. Zwart W. Oude Vrielink JA. Elkon R. Agami R. A Pumilio-induced RNA structure switch in p27-3′ UTR controls miR-221 and miR-222 accessibility. Nat Cell Biol. 2010;12:1014–1020. doi: 10.1038/ncb2105. [DOI] [PubMed] [Google Scholar]
- 27.Ota R. Kotani T. Yamashita M. Biochemical chacacterization of pumilio 1 and pumilio2 in Xenopus oocytes. J Biol Chem. 2010;286:2853–2863. doi: 10.1074/jbc.M110.155523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez-Diaz P. Penalva LO. Post-transcription meets post-genomic: the saga of RNA binding proteins in a new era. RNA Biol. 2006;3:101–109. doi: 10.4161/rna.3.3.3373. [DOI] [PubMed] [Google Scholar]
- 29.Kershner AM. Kimble J. Genome-wide analysis of mRNA targets for Caenorhabditis elegans FBF, a conserved stem cell regulator. Proc Natl Acad Sci U S A. 2010;107:3936–3941. doi: 10.1073/pnas.1000495107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris AR. Mukherjee N. Keene JD. Ribonomic analysis of human Pum1 reveals cis-trans conservation across species despite evolution of diverse mRNA target sets. Mol Cell Biol. 2008;28:4093–4103. doi: 10.1128/MCB.00155-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galgano A. Forrer M. Jaskiewicz L. Kanitz A. Zavolan M. Gerber AP. Comparative analysis of mRNA targets for human PUF-family proteins suggests extensive interaction with the miRNA regulatory system. PLoS One. 2008;3:e3164. doi: 10.1371/journal.pone.0003164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hafner M. Landthaler M. Burger L. Khorshid M. Hausser J. Berninger P. Rothballer A. Ascano M. Jungkamp AC, et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamont LB. Crittenden SL. Bernstein D. Wickens M. Kimble J. FBF-1 and FBF-2 regulate the size of the mitotic region in the C. elegans germline. Dev Cell. 2004;7:697–707. doi: 10.1016/j.devcel.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Pullmann R. Kim HH. Abdelmohsen K. Lal A. Martindale JL. Yang X. Gorospe M. Analysis of turnover and translation regulatory RNA-binding protein expression through binding to cognate mRNAs. Mol Cell Biol. 2007;27:6265–6278. doi: 10.1128/MCB.00500-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennedy BK. Gotta M. Sinclair DA. Mills K. McNabb DS. Murthy M. Pak SM. Laroche T. Gasser SM. Guarente L. Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae. Cell. 1997;89:381–391. doi: 10.1016/s0092-8674(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 36.Bachorik JL. Kimble J. Redundant control of the Caenorhabditis elegans sperm/oocyte switch by PUF-8 and FBF-1, two distinct PUF RNA-binding proteins. Proc Natl Acad Sci U S A. 2005;102:10893–10897. doi: 10.1073/pnas.0504593102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.