Abstract
Pregnancy induces priming of the maternal cellular and humoral immune system. The paternally-inherited fetal antigens that influence maternal T and B cells include both major and minor histocompatibility antigens – the same antigens that are problematic in allotransplantation. Animal models have facilitated our understanding of the lymphocyte responses to fetal antigens, and our appreciation of the parallel response in pregnant women is increasing. The physiologic properties of the placenta as well as trafficking of cells between mother and fetus allow ample opportunity for sampling of fetal proteins by the maternal immune system. Here, the current state of knowledge of fetal antigen-specific lymphocyte responses in pregnancy is reviewed.
1. Maternal T cells respond to paternally inherited fetal antigens in pregnancy
Pregnancy represents a physiological condition in which the female is confronted with an antigenically foreign body that flourishes despite her own competent immune system. By any standard, the hemochorial placentation of humans, nonhuman primates and rodents, would seem to pose an especially daunting immunological problem because of the vast interface between the trophectoderm-derived syncytiotrophoblast and the maternal blood. Indeed, in these species there is ample exposure of maternal immune cells to trophoblast cells, fetal cells, and their antigens.
Although the mother is immunologically naïve to paternally-derived antigens, she acquires a state of tolerance to them during pregnancy. Whether this tolerance is long-lived (1) or transient (2) can be debated, but most investigators agree that the selective repression of placental genes that could activate maternal immune cells together with activation of genes that suppress immune cells is key to maternal-fetal tolerance (3). The former group of genes includes the highly polymorphic MHC antigens, in particular, class I HLA-A and HLA-B together with class II HLA genes in humans; indeed, the failure of these genes to be expressed at the surface of trophoblast cells seems to fit with early ideas that the maternal immune system remains unaware of the foreignness of the fetus (4).
Today, however, there is no longer any doubt that maternal T and B cells are aware of fetal antigens, that they respond vigorously to the presence of the fetus, and that under normal circumstances they are tolerant to these antigens. This phenomenon has been variously shown in the mouse by a number of investigators using experimental systems in which T and B cells responses to natural or model antigens are tracked in vivo and in vitro (5, 6). Furthermore, the presence of minor and major histocompatibility antigen reactive T lymphocytes and antibodies in parous women is well-documented (1, 7–10). In this article, we briefly review a model of maternal T cell reactivity to paternally-inherited fetal antigens and what we have learned from published experiments using similar models. We go on to discuss the present state of knowledge of fetal antigen-specific lymphocytes in parous women. Finally, some aspects of maternal-fetal reactivity that might be clinically relevant are considered.
2. Ovalbumin as a model fetal minor histocompatibility antigen
Alloantigens can be classified into major histocompatibility complex (MHC) antigens and minor histocompatibility antigens. MHC antigens are highly polymorphic between individuals, and if mismatched between graft donor and recipient, can cause of acute rejection due to direct recognition by host T cells. Minor antigens have a limited number of alleles in the population but pose a significant clinical barrier to transplantation because some alleles are presented in the context of certain class I and class II MHC molecules. These antigens can be problematic in transplantation settings, even when the donor and recipient are matched for HLA; indeed, minor antigens can cause acute and chronic allograft rejection, as well as graft versus host disease (11).
The availability of mice that transgenically express T cell receptors specific for defined model and natural antigens allows the study of fetal antigen-specific lymphocytes during pregnancy in fine detail. We have employed a mouse model similar to that established by Erlebacher et al. (12) to study fetal antigen-specific T cells. This model utilizes chicken ovalbumin (OVA) to represent a fetal mHAg antigen to which the mother is immunologically naïve. OVA is widely used as a model antigen to understand the mechanisms of lymphocyte tolerance, and is a powerful tool because of the many specific reagents that have been developed for it. In the mouse, OVA-derived peptides can be presented in the context of both MHC class I and class II molecules. Specifically, the OVA-derived peptide SIINFEKL (OVA257-264) is presented by the mouse class I molecule H-2Kb, and OVA-derived ISQAVHAAHAEINEAGR peptide (OVA323-339) is presented by the class II molecule, I-Ab (Figure 1). Because the C57Bl/6 strain of mouse possesses the H-2b haplotype, this strain will present these ovalbumin peptides in the context of its own MHC, whether the ova is expressed endogenously as a self antigen or received exogenously as a foreign antigen. Transgenic ACT-mOVA mice constitutively and ubiquitously express a membrane-associated form of chicken ovalbumin under the control of the beta-actin promoter (13).
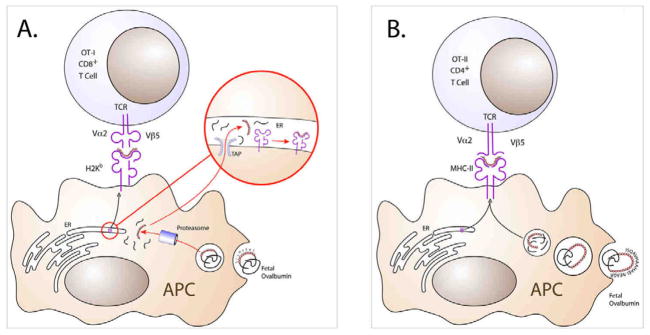
Figure 1. OVA can be presented in the context of murine class I or class II MHC molecules.
MHC class I and class II molecules can present internalized protein antigens derived from exogenous sources. (A) The canonical MHC class I pathway derives antigens from intracellular sources. However, class I MHC can present antigens exogenous sources in a process termed cross-presentation. Here, following internalization, proteins exit the endosome by an unknown mechanism and are broken down in the cytoplasm by the proteasome. Peptides are then transported into the endoplasmic reticulum via TAP (Transporter Associated with antigen Presentation) and then complexed with the class I molecule. The MHC-peptide complex is then transported to the cell surface. OVA derived from the fetus is internalized by maternal antigen presenting cells for cross-presentation by the class I H-2Kb molecule, which is possessed by mice of the H-2b MHC haplotype such as C57Bl/6. This process occurs in a TAP-dependent manner (14). (B) The MHC class II pathway derives protein/peptides from phagocytosis followed by breakdown by lysosomal proteases in the acidic endosomal environment. This is followed by fusion of the endosome with a specialized vesicle related to the late endosome/lysosome called the MIIC (MHC class II-associated compartment), wherein the MHC class II molecule awaits loading of peptide. The class II/peptide complex is then carried to the cell surface. Shown, OVA323-339 peptide (ISQAVHAAHAEINEAGR) associates with the class II molecule, I-Ab. The complex is then transported to the cell surface.
To detect the T cell responses to presented OVA antigen, OVA-specific OT-I and OT-II T cell receptor (TCR) transgenic mice can be used. OT-I transgenic mice express a Vα2/Vβ5 TCR on their CD8+ T lymphocytes that is specific for the H-2Kb/OVA257-264 (class I) complex; likewise, OT-II mice express a Vα2/Vβ5 TCR on their CD4+ T cells that recognizes the I-Ab/OVA323-339 (class II) complex (Figure 1). The vast majority (>95%) of the CD8+ and CD4+ T cells that develop in OT-I and OT-II mice, respectively, monoclonally express these transgenic TCR. If these mice are bred to a recombinase activating gene (RAG)-negative background strain, such that recombination and expression of endogenous TCR are blocked, the proportion of monoclonal OT-I or OT-II T cells approaches 100%. OT-I and OT-II mice exist on the C57Bl/6 background strain, which possesses the H-2b MHC haplotype, such that the T cells recognize the respective OVA peptides in the context of their own MHC molecules.
Using ACT-mOVA male mice in combination with OT-I or OT-II T cells, maternal lymphocyte responses to fetal antigen can be monitored. In our lab, this is done in either of two ways (Figure 2). In one model, wild-type (WT) C57Bl/6 females are bred to ACT-mOVA males to establish experimental pregnancies, or alternatively, to WT C57Bl/6 males to establish control pregnancies. Comparisons made between the WT-bred and OVA-bred females will allow detection of T cell responses that are elicited by paternally inherited fetal antigen.
Figure 2. Two models of how ovalbumin and T cell receptor transgenic mice are used to study maternal-fetal tolerance.
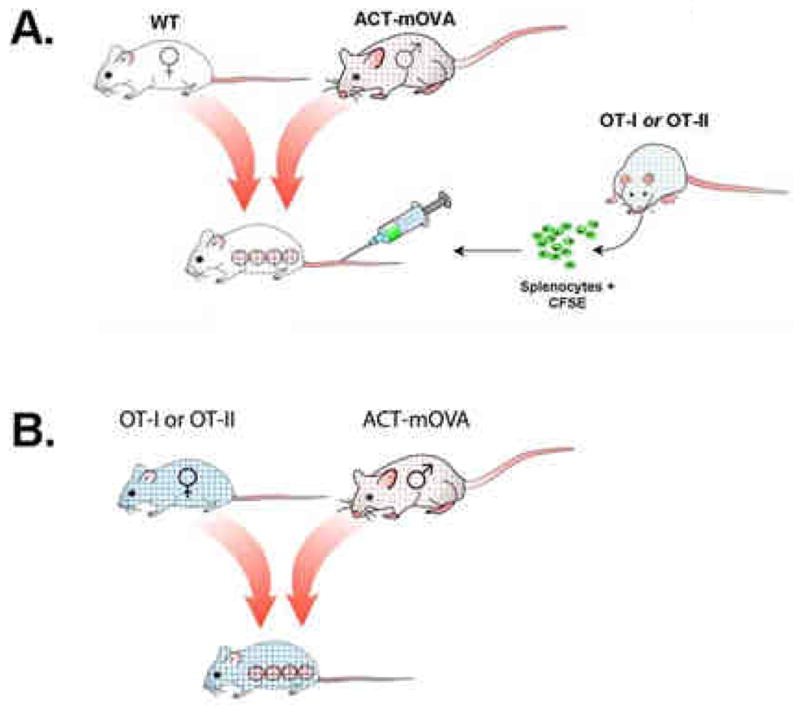
(A) Wild-type C57Bl/6 female mice are bred to ACT-mOVA male mice, which express ovalbumin under the control of the actin promoter. Fetuses inherit and express paternal ovalbumin; if the sire is hemizygous for ovalbumin, approximately half of the fetuses will express ovalbumin and if homozygous, all will express it. Negative controls include wild-type bred females. Lymphocytes from OT-I or OT-II transgenic mice are adoptively transferred into the tail vein of pregnant recipients; these cells can be pre-labeled with fluorescent dye to facilitate detection and evaluation of proliferation. At appropriate time points, the pregnant dams are sacrificed and lymphocytes from relevant lymphoid organs are analyzed by flow cytometry. (B) An alternative model is to breed female OT-I or OT-II mice directly to ACT-mOVA (or wild type; not shown) males. Fetuses inheriting paternal ovalbumin induce reactivity in the dams’ lymphocytes, which are similarly analyzed by flow cytometry.
Detection of the dam’s response to fetal OVA is facilitated by the use of OT-I and OT-II transgenic mice. At mid-gestation (or indeed at any point in pregnancy; (12, 14)), lymphocytes from the spleens or lymph nodes of virgin OT-I or OT-II females are adoptively transferred to the virgin, WT- and ACT-mOVA-bred dams, and subsequently tracked in vivo. There are a number of ways to track the transferred cells; for example, prior to adoptive transfer, cells can be labeled with the fluorescent dye CFSE (carboxyfluorescein succinimidyl ester), which allows both detection of the cells and assessment of their proliferation. In addition, congenic markers such as allelic variations of CD90 or CD45 can be used. Days later, the recipient dam’s blood, spleen and/or lymph node cells are harvested and analyzed by multiparameter flow cytometry. By gating on CD8+/CD4+ cells together with the appropriate congenic marker or TCR, many properties of the transferred lymphocytes can be assessed.
A second model can be used in which males (WT or ACT-mOVA transgenic) are bred directly to OT-I or OT-II TCR transgenic females to detect maternal responses to fetal antigen presented by class I and class II MHC, respectively. Virgin females also serve as additional important controls to distinguish the effects of fetal antigen and pregnancy alone. Lymphoid organs are harvested at various times during pregnancy and the OVA-specific T cells can be assessed. This model has the advantage of detecting responses of T cells that are present throughout the continuum of pregnancy.
Several other models that, in principle, parallel the above-described model have been used to study both T and B cell responses in pregnancy; details of these models have been reviewed recently (5, 15). Responses to both model mHAgs antigens, such as OVA, or natural mHAgs, such as the Y-chromosome encoded “male” antigens have been examined using TCR transgenic mice specific for these antigens (12, 14, 16–18). Importantly, natural responses to these antigens are also evident in the mouse, and can be assessed either by flow cytometry using MHC-peptide pentameric complexes (1), clonotypic antibodies (16), or in vitro by simply stimulating lymphocytes with relevant peptide (19). Furthermore, lymphocyte responses to paternally inherited fetal MHC antigen can be modeled; these studies have been performed to examine the response to naturally expressed fetal MHC (2) or alternatively, to examine the consequences of ectopically driving paternal MHC expression to high levels in the placenta (20, 21). The latter study focused not on T cells but B cells, revealing an additional, as yet underexplored area of maternal lymphocyte responses to the fetus.
3. What have we learned from murine models?
Although maternal ignorance of fetal antigen was initially postulated to explain maternal fetal tolerance (4), there is now unequivocal evidence showing that in semiallogeneic pregnancy, maternal lymphocytes vigorously respond to specific fetal antigens. This holds true for both murine and human pregnancy, major and minor histocompatibility antigens, and B and T lymphocytes. Observations from murine pregnancy include:
Anti-paternal MHC antibodies are observed in multiparous mice (22);
Fetal MHC-specific B cells are deleted during pregnancy (21, 23);
Maternal T cell exposure to paternal/fetal antigen can occur as early as insemination, and is associated with the establishment of a fully functional placenta with a maternal blood supply, but, interestingly, not implantation (12, 14) (Perchellet and Petroff, manuscript in preparation);
Fetal MHC and mHAg-specific CD4+ and CD8+ T cells undergo both proliferation and clonal deletion during pregnancy (2, 12, 14, 16, 20);
Fetal mHAg-specific CD8+ T cells possess an activated/memory phenotype (1, 12, 14);
Accumulation of fetal mHAg-specific CD8+ T cells is controlled by the inhibitory receptor, PD-1(18);
Exposure of fetal antigen-specific maternal T cells results in strong upregulation of multiple T cell inhibitory receptors, which is associated with successful pregnancy (Perchellet and Petroff, manuscript in preparation);
Fetal mHAg-specific CD4+ T cells can assume a regulatory T cell phenotype that is essential for pregnancy success (19, 24).
Murine trophoblast express class I MHC during the latter part of pregnancy (25), and it was therefore postulated that these cells directly present peptide antigen to maternal lymphocytes. However, using MHC- deficient mice, it was shown that maternal antigen presenting cells cross-present fetal antigen, and further, that this process requires TAP (Transporter Associated with antigen Processing) (12, 14). The properties of the cells that cross-present fetal antigen are unknown, but it is reasonable to expect that the acquisition of antigen through trophoblast (or even fetus) -derived cells would induce a tolerogenic phenotype in these antigen presenting cells, in turn leading to tolerance of the cognate maternal CD4+ and CD8+ T cells.
4. Fetal antigen exposure in pregnant women
In human pregnancy, fetal antigens elicit both the cellular and humoral maternal immune systems (26). Maternal T cells against paternally-inherited fetal minor antigens expand in some women when a minor antigen mismatch exists, provided that she possesses the HLA that can present the antigen; these T cells can persist for decades (1, 9, 27, 28). In addition, T cells and antibodies against fetal HLA can be generated, and are particularly prone to arise when mother and fetus are mismatched for certain HLAs (29). Whether continual exposure to fetal antigen is needed to maintain the presence of these T cells is an intriguing question in light of parallel observations that fetal microchimeric cells survive in the mother equally long (30). Indeed, in the reverse situation of maternal microchimerism in the fetus, the persistence of T cells specific for non-inherited maternal antigens is strongly correlated with persistent maternal microchimerism (31).
The true nature of fetal antigen-specific T cells is unclear. On one hand, these cells express memory T cell markers and can be induced to exhibit recall cytokine and cytotoxic responses upon re-exposure to cognate antigen in vitro (9, 28). The observations that parous women make poor bone marrow donors due to increased incidence of graft versus host disease has sparked the hypothesis that these cells might be the culprit; however, it seems unlikely that these activities reflect their function in vivo during pregnancy. Indeed, other experiments suggest that anti-fetal T cells cannot act as killers, but instead are highly tolerant to fetal antigen upon re-exposure in vivo, outside the context of pregnancy (1). Recently, Burlingham and colleagues identified distinct populations of minor alloantigen specific CD8+ T cells with either cytolytic/effector function or suppressive/regulatory function in renal transplant recipients and mother/son pairs (27, 32). The latter (regulatory) subset of cells possesses low avidity T cell receptors, appear to use inhibitory molecules such as CTLA-4, TGF-β and/or IL-10 to exert their regulatory function, and can suppress the cytolytic responses of high-affinity T cells recognizing the same fetal antigens. These studies may reveal a subset of regulatory CD8+ T cells that is important in preventing damaging anti-fetal responses in pregnancy.
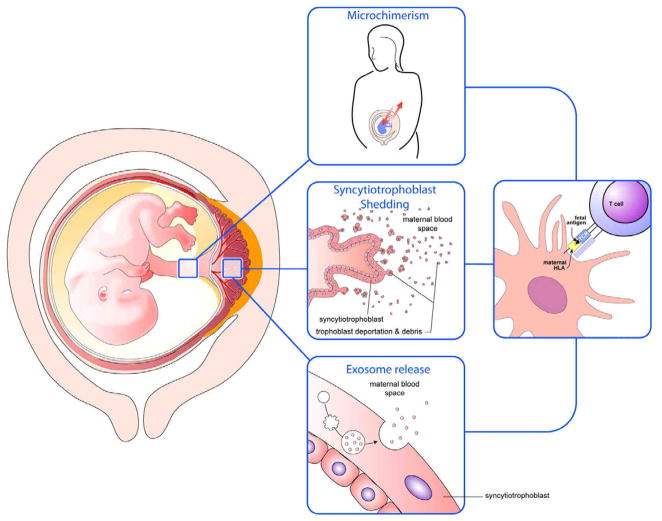
How does fetal antigen access the maternal immune system? The placenta is a probable source of fetal mHAg. These antigens are known to be widely transcribed, and our data strongly suggest that trophoblast cells and placental macrophages express many mHAg at the level of protein (Linscheid and Petroff, unpublished). Furthermore, the human placenta interfaces with maternal blood, shedding large amounts of trophoblast cells, microparticles, and exosomes directly into the circulation (33–35). Any of these could be sources of antigens for uptake by antigen presenting cells (Figure 3).
Figure 3. Models of exposure of fetal antigens and cells to the maternal immune system.
Microchimerism, shedding of trophoblastic cells and debris, and release of exosomes by the syncytiotrophoblast could each lead to ingestion of fetal antigens by maternal antigen presenting cells and subsequent interaction between fetal antigen/MHC with maternal T cells.
Fetal cells, which can actively traffic into and reside within the mother for long periods, is often cited to be the source of antigen that elicits maternal T cell and antibody responses. Fetal blood cells yield positive RT-PCR reaction products and immunohistochemical staining, suggesting that these cells, too, express the proteins (Linscheid and Petroff, unpublished). Fetal lymphocytes and hematopoietic precursor cells are known to traffic into the mother (36–39), and if APC acquire their proteins, either as a result of their death or through “nibbling” (40, 41) by dendritic cells, this too could result in cross presentation of fetal antigen presentation to maternal T cells (Figure 3).
Both of the above scenarios suggest that expansion of anti-fetal T cells and antibodies occurs during pregnancy. Indeed there is much reason to believe that this is the case. First, human trophoblast and cell debris transfers directly into maternal blood throughout pregnancy; second, fetal microchimerism occurs in human pregnancy prior to parturition, even as early as the first trimester; third, in primiparous mice with no possibility of prior fetal antigen exposure, fetal antigens appear in maternal lymphoid organs and elicit lymphocyte responses. On the other hand, it is possible that exposure of women to some fetal antigens also occurs only as a result of parturition. Parturition results in hemorrhage of both mother and fetus as a result of placental detachment, resulting in intermixing of each others’ blood. Long-known, unfortunate evidence that this occurs is the sensitization of of Rh- women bearing Rh+ children.
5. Clinical perspectives
Whether adverse clinical consequences resulting from maternal cellular and/or humoral responses to fetal antigens occur is uncertain. Indeed, anti-paternal HLA antibodies and anti-fetal T cells characterize many normal pregnancies (7–10, 42). However, there are two scenarios in which an abnormal immune response to fetal antigens might be clinically relevant. Villitis of unknown etiology (VUE) is an inflammatory lesion of the placental villi characterized by infiltration of maternal CD4+ and CD8+ T cells. This situation is surprisingly common, occurring in 5–15% of all placentas in the third trimester (43). VUE is has been associated with poor fetal outcome including growth restriction (44) and neurologic impairments (45, 46). Redline (43) speculated that allogeneic major and minor antigens could contribute to the pathogenesis of VUE, and further, that the increased probability of recurrent VUE supports a “role for sensitization” of the mother to these antigens. Placental macrophages appear to participate in VUE, these cells might display fetal minor and/or major alloantigens to maternal T cells (47).
A second situation has become apparent through studies performed in a Danish population of women experiencing secondary recurrent miscarriage. Secondary recurrent miscarriage (SRM) can be defined as idiopathic recurrent pregnancy loss subsequent to a normal pregnancy. These investigators found an association between SRM and male sex of a firstborn (48). The association was even more strongly associated when the mother carried the class II HLA (but, interestingly, not class I HLA) that restricts an HY mHAg (49). These researchers concluded that “immune responses against fetal HY antigens initiated in the pregnancy prior to the secondary recurrent miscarriage may play a causal role in secondary recurrent miscarriage”.
In conclusion, while fetal/maternal major and minor histocompatibility antigen mismatch, followed by cognate maternal lymphocyte recognition of fetal antigen and unfavorable sequelae is clearly not the norm, whether this could occur in some instances remains to be seen. It will thus be quite interesting and important to discover, in the future, whether parallel population studies confirm the above findings, and also whether animal models of maternal-fetal immune tolerance support these conclusions.
Acknowledgments
The author thanks Antoine Perchellet and all of the members of her laboratory for the many discussions and insights related to this topic. Images were constructed by Stanton Fernald (University of Kansas Interdisciplinary center for Male Contraceptive Research & Drug Development Imaging Core). Research support for projects related to this work is provided by NIH grant R01 045611 (M.G.P.), P20 RR16475 (J.S. Hunt) and funds from the University of Kansas Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.James E, Chai JG, Dewchand H, Macchiarulo E, Dazzi F, Simpson E. Multiparity induces priming to male-specific minor histocompatibility antigen, HY, in mice and humans. Blood. 2003;102:388–93. doi: 10.1182/blood-2002-10-3170. [DOI] [PubMed] [Google Scholar]
- 2.Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270:630–3. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 3.Petroff MG. Immune interactions at the maternal-fetal interface. J Reprod Immunol. 2005;68:1–13. doi: 10.1016/j.jri.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Medawar P. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;7:320–38. [Google Scholar]
- 5.Taglauer ES, Adams Waldorf KM, Petroff MG. The hidden maternal-fetal interface: events involving the lymphoid organs in maternal-fetal tolerance. Int J Dev Biol. 2010;54:421–30. doi: 10.1387/ijdb.082800et. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moldenhauer LM, Hayball JD, Robertson SA. Utilising T cell receptor transgenic mice to define mechanisms of maternal T cell tolerance in pregnancy. J Reprod Immunol. 2010;87:1–13. doi: 10.1016/j.jri.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Payne R, Rolfs MR. Fetomaternal leukocyte incompatibility. J Clin Invest. 1958;37:1756–63. doi: 10.1172/JCI103768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regan L, Braude PR, Hill DP. A prospective study of the incidence, time of appearance and significance of anti-paternal lymphocytotoxic antibodies in human pregnancy. Hum Reprod. 1991;6:294–8. doi: 10.1093/oxfordjournals.humrep.a137325. [DOI] [PubMed] [Google Scholar]
- 9.Piper KP, McLarnon A, Arrazi J, Horlock C, Ainsworth J, Kilby MD, et al. Functional HY-specific CD8+ T cells are found in a high proportion of women following pregnancy with a male fetus. Biol Reprod. 2007;76:96–101. doi: 10.1095/biolreprod.106.055426. [DOI] [PubMed] [Google Scholar]
- 10.van Kampen CA, Maarschalk M, Langerak-Langerak J, Roelen DL, Claas FHJ. Kinetics of the pregnancy-induced humoral and cellular immune response against the paternal HLA class I antigens of the child. Hum Immunol. 2002;63:452–8. doi: 10.1016/s0198-8859(02)00396-8. [DOI] [PubMed] [Google Scholar]
- 11.Dierselhuis M, Goulmy E. The relevance of minor histocompatibility antigens in solid organ transplantation. Curr Opin Organ Transplant. 2009;14:419–25. doi: 10.1097/MOT.0b013e32832d399c. [DOI] [PubMed] [Google Scholar]
- 12.Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117:1399–411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–62. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 14.Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, Robertson SA. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol. 2009;182:8080–93. doi: 10.4049/jimmunol.0804018. [DOI] [PubMed] [Google Scholar]
- 15.Moldenhauer LM, Hayball JD, Robertson SA. Utilising T cell receptor transgenic mice to define mechanisms of maternal T cell tolerance in pregnancy. J Reprod Immunol. doi: 10.1016/j.jri.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Jiang SP, Vacchio MS. Multiple mechanisms of peripheral T cell tolerance to the fetal “allograft”. J Immunol. 1998;160:3086–90. [PubMed] [Google Scholar]
- 17.Norton MT, Fortner KA, Oppenheimer KH, Bonney EA. Evidence that CD8 T-cell homeostasis and function remain intact during murine pregnancy. Immunology. 2010;131:426–37. doi: 10.1111/j.1365-2567.2010.03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taglauer ES, Yankee TM, Petroff MG. Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J Reprod Immunol. 2009;80:12–21. doi: 10.1016/j.jri.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci U S A. 2010;107:9299–304. doi: 10.1073/pnas.1003909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou M, Mellor AL. Expanded cohorts of maternal CD8+ T-cells specific for paternal MHC class I accumulate during pregnancy. J Reprod Immunol. 1998;40:47–62. doi: 10.1016/s0165-0378(98)00030-8. [DOI] [PubMed] [Google Scholar]
- 21.Ait-Azzouzene D, Caucheteux S, Tchang F, Wantyghem J, Moutier R, Langkopf A, et al. Transgenic major histocompatibility complex class I antigen expressed in mouse trophoblast affects maternal immature B cells. Biol Reprod. 2001;65:337–44. doi: 10.1095/biolreprod65.2.337. [DOI] [PubMed] [Google Scholar]
- 22.Herzenberg LA, Gonzales B. Appearance of H-2 agglutinins in outcrossed female mice. Proc Natl Acad Sci USA. 1962;48:570–3. doi: 10.1073/pnas.48.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ait-Azzouzene D, Gendron MC, Houdayer M, Langkopf A, Burki K, Nemazee D, et al. Maternal B lymphocytes specific for paternal histocompatibility antigens are partially deleted during pregnancy. J Immunol. 1998;161:2677–83. [PubMed] [Google Scholar]
- 24.Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, et al. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol. 2010;85:121–9. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Redline RW, Lu CY. Localization of fetal major histocompatibility complex antigens and maternal leukocytes in murine placenta. Implications for maternal-fetal immunological relationship. Lab Invest. 1989;61:27–36. [PubMed] [Google Scholar]
- 26.van Rood JJ, Eernisse JG, van Leeuwen A. Leucocyte antibodies in sera from pregnant women. Nature. 1958;181:1735–6. doi: 10.1038/1811735a0. [DOI] [PubMed] [Google Scholar]
- 27.van Halteren AG, Jankowska-Gan E, Joosten A, Blokland E, Pool J, Brand A, et al. Naturally acquired tolerance and sensitization to minor histocompatibility antigens in healthy family members. Blood. 2009;114:2263–72. doi: 10.1182/blood-2009-01-200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verdijk RM, Kloosterman A, Pool J, van de Keur M, Naipal AM, van Halteren AG, et al. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood. 2004;103:1961–4. doi: 10.1182/blood-2003-05-1625. [DOI] [PubMed] [Google Scholar]
- 29.Dankers MKA, Roelen DL, Korfage N, de Lange P, Witvliet M, Sandkuijl L, et al. Differential immunogenicity of paternal HLA Class I antigens in pregnant women. Hum Immunol. 2003;64:600–6. doi: 10.1016/s0198-8859(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 30.Adams KM, Nelson JL. Bi-directional cell trafficking during pregnancy: long-term consequenses for human health. In: Mor G, editor. Immunology of Pregnancy. Georgetown, TX: Landes Bioscience; 2004. [Google Scholar]
- 31.Dutta P, Molitor-Dart M, Bobadilla JL, Roenneburg DA, Yan Z, Torrealba JR, et al. Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice. Blood. 2009;114:3578–87. doi: 10.1182/blood-2009-03-213561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burlingham WJ, Goulmy E. Human CD8+ T-regulatory cells with low-avidity T-cell receptor specific for minor histocompatibility antigens. Hum Immunol. doi: 10.1016/j.humimm.2008.08.289. In Press, Uncorrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burton GJ, Jones CJP. Syncytial knots, sprouts, apoptosis, and trophoblast deportation from the human placenta. Taiwan J Obstet Gynecol. 2009;48:28–37. doi: 10.1016/S1028-4559(09)60032-2. [DOI] [PubMed] [Google Scholar]
- 34.Redman CWG, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 2008;29:73–7. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176:1534–42. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 36.Adams KM, Lambert NC, Heimfeld S, Tylee TS, Pang JM, Erickson TD, et al. Male DNA in female donor apheresis and CD34-enriched products. Blood. 2003;102:3845–7. doi: 10.1182/blood-2003-05-1570. [DOI] [PubMed] [Google Scholar]
- 37.Bianchi DW. Fetal cells in the maternal circulation: feasibility for prenatal diagnosis. Br J Haematol. 1999;105:574–83. doi: 10.1046/j.1365-2141.1999.01383.x. [DOI] [PubMed] [Google Scholar]
- 38.Evans PC, Lambert N, Maloney S, Furst DE, Moore JM, Nelson JL. Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood. 1999;93:2033–7. [PubMed] [Google Scholar]
- 39.Osada H, Doi S, Fukushima T, Nakauchi H, Seki K, Sekiya S. Detection of fetal HPCs in maternal circulation after delivery. Transfusion. 2001;41:499–503. doi: 10.1046/j.1537-2995.2001.41040499.x. [DOI] [PubMed] [Google Scholar]
- 40.Harshyne LA, Zimmer MI, Watkins SC, Barratt-Boyes SM. A role for class A scavenger receptor in dendritic cell nibbling from live cells. J Immunol. 2003;170:2302–9. doi: 10.4049/jimmunol.170.5.2302. [DOI] [PubMed] [Google Scholar]
- 41.Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717–23. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 42.Hunt JS, Pace JL, Morales PJ, Ober C. Immunogenicity of the soluble isoforms of HLA-G. Mol Hum Reprod. 2003;9:729–35. doi: 10.1093/molehr/gag087. [DOI] [PubMed] [Google Scholar]
- 43.Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol. 2007;38:1439–46. doi: 10.1016/j.humpath.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 44.Becroft DM, Thompson JM, Mitchell EA. Placental villitis of unknown origin: Epidemiologic associations. Am J Obstet Gynecol. 2005;192:264–71. doi: 10.1016/j.ajog.2004.06.062. [DOI] [PubMed] [Google Scholar]
- 45.Redline RW, O’Riordan MA. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch Pathol Lab Med. 2000;124:1785–91. doi: 10.5858/2000-124-1785-PLAWCP. [DOI] [PubMed] [Google Scholar]
- 46.Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol. 2005;192:452–7. doi: 10.1016/j.ajog.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 47.Kim J-S, Romero R, Kim MR, Kim YM, Friel L, Espinoza J, et al. Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology. 2008;52:457–64. doi: 10.1111/j.1365-2559.2008.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svarre Nielsen H, Nybo Andersen A-M, Kolte AM, Christiansen OB. A firstborn boy is suggestive of a strong prognostic factor in secondary recurrent miscarriage: a confirmatory study. Fertil Steril. 2008;89:907–11. doi: 10.1016/j.fertnstert.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 49.Nielsen HS, Steffensen R, Varming K, Van Halteren AG, Spierings E, Ryder LP, et al. Association of HY-restricting HLA class II alleles with pregnancy outcome in patients with recurrent miscarriage subsequent to a firstborn boy. Hum Mol Genet. 2009;18:1684–91. doi: 10.1093/hmg/ddp077. [DOI] [PubMed] [Google Scholar]