Abstract
Sepsis, severe injury, and cancer are associated with loss of muscle mass. Muscle wasting in these conditions is mainly caused by increased proteolysis, at least in part regulated by NF-kB. Despite recent progress in the understanding of mediators and mechanisms involved in muscle wasting, effective and universally accepted treatments by which muscle atrophy can be prevented or reversed are still lacking. Here, we review recent evidence suggesting that curcumin (diferuloylmethane), a component of the spice turmeric, may prevent loss of muscle mass during sepsis and endotoxemia and may stimulate muscle regeneration after traumatic injury. Curcumin has been part of the traditional Asian medicine for centuries, mainly because of its anti-inflammatory properties. Studies suggest that inhibition of NF-kB is one of the mechanisms by which curcumin exerts its ant-inflammatory effects. Curcumin is easily accessible, inexpensive, and non-toxic even at high doses, and may therefore offer an important treatment modality in muscle wasting and injury. It should be noted, however, that the muscle-sparing effects of curcumin are not universally accepted, and more studies are therefore needed to further test the role of curcumin in the prevention and treatment of muscle wasting.
Keywords: Muscle atrophy, proteolysis, NF-kB, catabolic
Introduction
Curcumin (diferuloylmethane), a component of the spice turmeric (Curcuma longa) and responsible for the yellow color of curry, has been part of the traditional Asian medicine for centuries [1,2]. The list of ailments for which curcumin has been used is long and includes respiratory conditions, liver disorders, anorexia, rheumatism, common colds and sinusitis [2,3]. An area in which the use of curcumin has been particularly prevalent is promotion of wound healing [1], but it has also been used as an anti-inflammatory and anti-cancer treatment [2,3].
Several recent studies have focused on mechanisms by which curcumin may exert its beneficial effects. In particular, mechanisms accounting for the anti-inflammatory effects of curcumin have been examined. Such mechanisms include inhibition of NF-kB activity, at least in part reflecting inhibition of IkB kinase (IKK) activity [4,5]. Inhibition of NF-kB activity is of particular interest for the potential use of curcumin in the treatement of muscle wasting since NF-kB activation is an important mechanism for loss of muscle mass [6–10]. Other mechanisms by which curcumin may exert anti-inflammatory effects include activation of the heat shock response [11], inhibition of p38 kinase activity [12,13] and oxygen free radical formation [14], and prevention of cytokine production and release [5].
The growing interest for curcumin in Western medicine is illustrated by several ongoing clinical trials, the majority of which are being conducted in the US. In a recent review of traditional medicines [2], seven current clinical trials were listed testing the effects of curcumin in the treatment of colon and pancreatic cancer, Alzheimer’s disease, chemotherapy-induced mucositis, multiple myeoloma, psoriasis, and cystic fibrosis. In addition, a clinical trial investigating the use of curcumin in patients with familial adenomatous polyposis is also being conducted [1].
The purpose of this review is to discuss the potential use of curcumin in prevention and treatment of muscle wasting, in particular sepsis-induced muscle wasting. Aspects on beneficial effects of curcumin in sepsis, other than prevention of loss of muscle mass, were reviewed recently elsewhere [15].
Muscle wasting in sepsis and other catabolic conditions
Under normal conditions, muscle mass is maintained by a balance between protein synthesis and degradation and muscle wasting can occur when this balance is perturbed. Muscle wasting continues to be a significant clinical problem in patients with various catabolic conditions, including sepsis, AIDS, severe injury, uremia, heart failure, cancer, and starvation [16]. There is evidence that muscle atrophy in these conditions mainly reflects increased breakdown of myofibrillar proteins [17,18] although inhibited protein synthesis may also contribute to the loss of muscle proteins [19]. Increased protein breakdown in atrophying muscle reflects upregulated expression and activity of multiple proteolytic pathways, including lysosomal, calcium-calpain- and ubiquitin-proteasome-dependent mechanisms [16–18]. Among these mechanisms, activation of the ubiquitin-proteasome system is particularly important and is accompanied by a substantial increase in the expression of the muscle-specific ubiquitin ligases atrogin-1 and MuRF1 [20–22].
Loss of muscle mass in catabolic patients results in weakness, fatigue, and delayed ambulation during sickness with increased risk for thromboembolic and pulmonary complications. Prolonged bed rest in itself accelerates the degradation of muscle proteins, thus creating a vicious cycle [23]. The molecular regulation of muscle protein turnover in atrophying muscle as well as the clinical consequences of muscle wasting have been reviewed in recent papers [16–19].
A widely accepted treatment of muscle wasting is lacking
Despite substantial progress during the last two decades in our understanding of mediators and mechanisms involved in muscle atrophy, we still do not have an effective, universally accepted treatment by which muscle wasting can be prevented or reversed in critically ill patients. The lack of a generally accepted treatment of muscle wasting is reflected by the large number of different regimens that have been proposed in recent studies, including administration of anabolic hormones [24–26], creatine [27], branched-chain amino acids [28], and factors counteracting myostatin [29,30]. Many of these treatments may be costly, are still experimental, and will have to await clinical testing.
Curcumin is easily available, inexpensive and has proven non-toxic even when administered at high doses [1,31–35]. One potential problem with curcumin is its relatively low bioavailability, requiring high doses which may be associated with bad taste and odor [33,34]. Ongoing research aimed at overcoming this hurdle includes development of “nanocurcumin”, i.e., administration of curcumin at high doses in nanoparticles that are hydrophilic on the exterior and hydrophobic on the interior [1]. The hydrophilic exterior allows nanocurcumin to be soluble in water which makes it possible for the particles to enter the blood stream where the biodegradable polymer nanoparticles break down and release the drug. Interestingly, nanocurcumin has been reported to kill cultured pancreatic cancer cells in the laboratory setting [1]. Another method to increase the bioavailability of curcumin is to add piperine (found in black pepper) which increases the uptake of curcumin by 2,000% in humans. This approach is used in the development of curcumin-based drugs for the treatment of malaria [1]. Considering the growing interest for curcumin in Western medicine and an increasing list of potential indications for its use, it can be predicted that there will be a rapid development of new and innovative delivery systems for the drug. Because such systems can be patented (as opposed to the use of the drug itself), more resources from pharmaceutical companies may become available in the future for the development of curcumin formulations.
Role of NF-kB activation in muscle wasting
The molecular regulation of muscle wasting is complex and involves activation of various transcription factors and nuclear cofactors that regulate genes in different proteolytic pathways [reviewed in 36,37]. Among transcription factors that are activated in atrophying muscle, NF-kB is particularly important with several lines of evidence supporting a role of NF-kB in muscle wasting. We reported previously that NF-kB DNA binding activity was increased in skeletal muscle of septic rats [6]. Interestingly, the response to sepsis of NF-kB was biphasic with an early activation and subsequent inhibition of NF-kB activity, possibly reflecting the influence of different mediators of muscle wasting (such as cytokines and glucocorticoids). In addition to sepsis, other catabolic conditions as well are associated with NF-kB activation in muscle, including cancer [10], disuse atrophy [38], and muscle denervation [39]. In other studies, treatment of cultured myotubes with TNFα and IFNγ resulted in a biphasic NF-kB activation [8], and cytokine-induced protein degradation in cultured muscle cells was NF-kB-dependent [7]. Recent experiments, using a pharmacological inhibitor of NF-kB, suggest that NF-kB is also involved in muscle wasting caused by muscular dystrophy [40].
In recent experiments, genetic evidence was provided for a role of NF-kB in the development of muscle wasting [9]. In those experiments, transgenic mice with muscle-specific over-expression of activated IKK displayed increased NF-kB activity and a substantial loss of muscle mass. When these mice were crossed with transgenic mice with muscle-specific over-expression of an IkBα “super-repressor” (IkBα in which Ser 32 was mutated, preventing phosphorylation and degradation of IkBα), NF-kB activation and loss of muscle mass were prevented. In addition, over-expression of the IkBα super-repressor prevented cancer- and denervation-induced muscle wasting, and the same effect was seen after pharmacological inhibition of NF-kB with salicylate [9].
Taken together, previous observations provide strong evidence that NF-kB is involved in muscle wasting during different catabolic conditions and that NF-kB inhibitors may be efficacious in the management of muscle-wasting conditions. Of note, inhibition of NF-kB activity is an important mechanism by which curcumin may exert beneficial effects. Indeed, curcumin has been used as an “NF-kB inhibitor” in previous studies [31].
Sepsis-induced muscle wasting is blocked by curcumin
In a recent study, we tested the effect of curcumin on NF-kB activity and protein breakdown in skeletal muscle during sepsis [41]. In those experiments, treatment of rats in vivo with curcumin blocked the sepsis-induced increase in muscle protein breakdown and reduced the nuclear translocation of the NF-kB subunit p65 as well as p65 DNA binding activity. Because treatment of incubated muscles from septic rats with curcumin reduced p65 activity and protein breakdown, we interpreted the results as indicating that curcumin can reduce sepsis-induced muscle proteolysis and NF-kB activation through a direct effect in skeletal muscle. In addition, because treatment with curcumin of incubated septic muscles in which protein breakdown had already been increased during the septic course resulted in reduced NF-kB activity and protein degradation, it is possible that curcumin can be used both as treatment and prevention of muscle wasting in sepsis.
An interesting observation in our recent study was that although curcumin inhibited sepsis-induced muscle proteolysis, the treatment with curcumin did not influence the gene expression of atrogin-1 and MuRF1 [41]. A similar disconnection between changes in muscle proteolysis and the expression of atrogin-1 and MuRF1 has been reported in other muscle-wasting conditions as well [12,42], suggesting that various proteolytic mechanisms and the gene expression of certain components of the ubiquitin-proteasome pathway may be differentially regulated.
Further support for a role of curcumin in the treatment and prevention of muscle wasting caused by sepsis was provided in a recent study by Jin and Li [12]. In that report, mice were treated for 4 days with curcumin followed by injection of lipopolysaccharide (LPS). The pretreatment with curcumin resulted in a dose-dependent inhibition of LPS-induced loss of muscle weight and protein. Of note, in the study by Jin and Li [12], treatment with curcumin blocked the LPS-induced increase in mRNA levels for atrogin-1 but not for MuRF1, an observation that further supports the concept that there is not an absolute correlation between changes in muscle protein content and the expression of atrogin-1 and MuRF1 in muscle-wasting conditions.
It should be noted that although the present review is focused on the effects of curcumin on sepsis-induced muscle wasting (in part reflecting a long-standing interest in the catabolic response to sepsis in our laboratory), there is evidence that curcumin may inhibit the catabolic response in skeletal muscle during other catabolic conditions as well. For example, Wyke et al [10] reported that treatment of cultured myotubes with curcumin prevented the increase in protein degradation caused by a cachectic factor isolated from experimental tumors in mice. In another study, Thaloor et al [31] found that curcumin stimulated muscle regeneration after traumatic injury, further supporting the concept that curcumin may protect muscle tissue from various injurious influences.
In addition to improving protein balance in skeletal muscle, there is evidence that curcumin may protect other organs and tissues as well during sepsis. For example, in a recent study, curcumin prevented the metabolic derangement in the liver during sepsis induced by CLP in rats [42]. Importantly, in the same study, curcumin also prevented sepsis-induced mortality.
Curcumin may prevent sepsis-induced muscle wasting through multiple mechanisms
Because previous studies provided evidence that NF-kB may be involved in the development of muscle wasting during various catabolic conditions [6–10] and that curcumin can block NF-kB activation [4,5], we hypothesized in our recent study [41] that treatment with curcumin would inhibit NF-kB activity in skeletal muscle during sepsis. Indeed, results in that study [41] were consistent with inhibition of NF-kB activity after treatment of septic rats with curcumin assessed as inhibited nuclear translocation of p65 and reduced p65 DNA binding activity. In other studies, the effects of curcumin on protein degradation in myotubes treated with a tumor-related cachectic factor were interpreted as being caused by inhibition of NF-kB [10].
It should be noted that although curcumin is frequently used as an “NF-kB inhibitor”, the drug can exert other anti-inflammatory effects as well, including inhibition of p38 kinase activity [12,13], oxygen radical scavenging [14], and induction of the heat shock response [11]. Interestingly, in the study by Jin and Li [12], pretreatment of endotoxemic mice with curcumin did not inhibit NF-kB DNA binding activity but instead exerted its protective effects by inhibiting p38 kinase activity. In our recent study [41], results suggested that curcumin reduced p38 kinase activity in septic muscle (in addition to inhibiting NF-kB activity), suggesting that curcumin may prevent sepsis-induced muscle wasting by multiple mechanisms. It should be pointed out that although curcumin-induced inhibition of protein breakdown in septic muscle was associated with reduced NF-kB and p38 kinase activities, additional studies will be needed to test the causative link between these observations. It should also be noted that further studies are needed to determine whether mechanisms other than or in addition to NF-kB and p38 inhibition are involved in the anti-catabolic effects of curcumin during sepsis and endotoxemia.
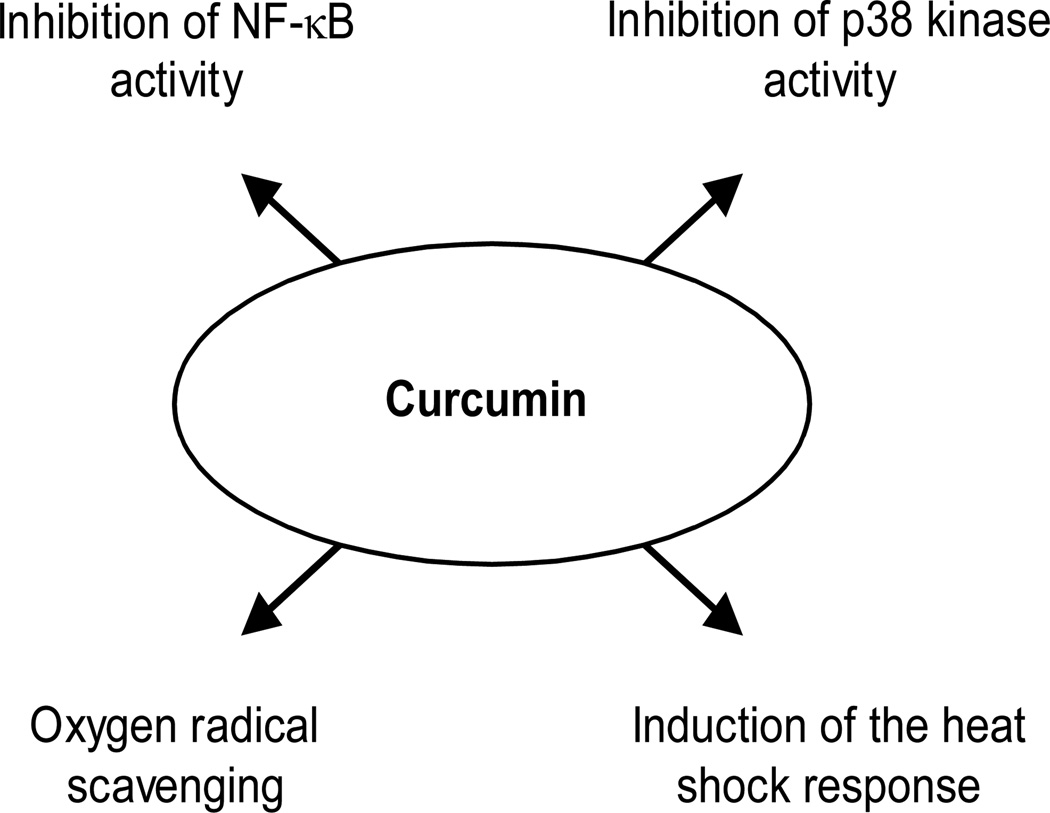
Mechanisms involved in the anti-inflammatory effects of curcumin and that may potentially be involved in the prevention and treatment of muscle wasting are summarized in Fig 1.
Fig 1.
Anti-inflammatory effects of curcumin potentially involved in the prevention and treatment of muscle wasting.
The muscle-sparing effects of curcumin are not universally accepted
Although some of the studies reviewed here suggest that curcumin can exert anti-catabolic and protective effects in skeletal muscle during sepsis, endotoxemia, after injury [12,31,41], and perhaps in cancer-related muscle wasting as well [10], conflicting results have also been reported. For example, treatment of rodents with curcumin did not prevent muscle atrophy caused by unloading [38], experimental cancer [43], or muscular dystrophy [40]. It is possible that some of the apparently contradictory results in previous reports reflect differences in route of administration and dose of curcumin. It is also possible that the varying responses to curcumin reflect differences in disease states causing the catabolic response in skeletal muscle. The specificity with regards to the anti-catabolic effects of curcumin in different conditions causing muscle wasting will be an important subject for future studies.
Conclusions and outlook
Although only relatively few studies have been published on the potential use of curcumin for the prevention and treatment of muscle wasting, the present review is important because it highlights the need of continued efforts to find effective treatment of this debilitating condition. Available results suggest that curcumin may be a potentially useful drug to prevent loss of muscle mass, at least when caused by sepsis. It is likely that the use of this ancient drug will continue to attract increasing interest in Western medicine, particularly as the molecular mechanisms by which the drug acts are clarified and the methods of administering the drug are being refined. It should be noted that this review is not claiming that the use of curcumin to prevent and treat muscle wasting has been proven, but we believe that early and preliminary observations are promising. If continued experiments further support the use of curcumin in the management of muscle wasting, it may open up the possibility to treat muscle wasting with a drug that is easily accessible, inexpensive, and non-toxic even at high doses.
Acknowledgments
Supported in part by NIH grants R01 DK37908 and R01 NR04585
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Singh S. From exotic spice to modern drug? Cell. 2007;130:765–768. doi: 10.1016/j.cell.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell. 2007;130:769–774. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Aggarwal BB. Activation of transcription factor NF-kB is suppressed by curcumin (diferuloylmethane) J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 5.Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, et al. Curcumin blocks cytokine-mediated NF-kB activation and proinflammatory gene expression by inhibiting inhibitory factor IkB kinase activity. J Immunol. 1999;163:3474–3483. [PubMed] [Google Scholar]
- 6.Penner CG, Gang G, Wray C, Fischer JE, Hasselgren PO. The transcription factors NF-kB and AP-1 are differentially regulated in skeletal muscle during sepsis. Biochem Biophys Res Commun. 2001;281:1331–1336. doi: 10.1006/bbrc.2001.4497. [DOI] [PubMed] [Google Scholar]
- 7.Li YP, Reid MB. NF-kB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am J Physiol. 2000;279:R1165–R1170. doi: 10.1152/ajpregu.2000.279.4.R1165. [DOI] [PubMed] [Google Scholar]
- 8.Ladner KJ, Caligiuri MA, Guttridge DC. Tumor necrosis factor-regulated biphasic activation of NF-kB is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem. 2003;278:2294–2303. doi: 10.1074/jbc.M207129200. [DOI] [PubMed] [Google Scholar]
- 9.Cai D, Frantz JD, Tawa NE, Melendez PA, Lidow HGW, Hasselgren PO, et al. IKKβ/NF-kB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Wyke SM, Russell ST, Tisdale MJ. Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-kB activation. Br J Cancer. 2004;91:1742–1750. doi: 10.1038/sj.bjc.6602165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunsmore KE, Chen PG, Wong HR. Curcumin, a medicinal herbal compound capable of inducing the heat shock response. Crit Care Med. 2001;29:2199–2204. doi: 10.1097/00003246-200111000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Jin B, Li YP. Curcumin prevents lipopolysaccharide-induced atrogin-1/MAFbx upregulation and muscle mass loss. J Cell Biochem. 2007;100:960–969. doi: 10.1002/jcb.21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter Y, Liu G, Yang J, Fier A, Mendez C. Sublethal hemorrhage induces tolerance in animals exposed to cecal ligation and puncture by altering p38, p44/42, and SAPK/JNK MAP kinase activation. Surg Infect. 2003;4:17–27. doi: 10.1089/109629603764655245. [DOI] [PubMed] [Google Scholar]
- 14.Chattopadhyay I, Bandyopadhyay U, Biswas K, Maity P, Banerjee RK. Indomethacin inactivates gastric peroxidase to induce reactive-oxygen-mediated gastric mucosal injury and curcumin protects it by preventing peroxidase inactivation and scavenging reactive oxygen. Free Radic Biol Med. 2006;40:1397–1408. doi: 10.1016/j.freeradbiomed.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Thimermann C. The spice of life: Curcumin reduces the mortality associated with experimental sepsis. Crit Care Med. 2006;34:2009–2011. doi: 10.1097/01.CCM.0000224230.63684.06. [DOI] [PubMed] [Google Scholar]
- 16.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteaosme pathway in normal and disease states. J Am Soc Nephrol. 2006;17:1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- 17.Hasselgren PO, Fischer JE. Muscle cachexia: current concepts of intracellular mechanisms and molecular regulation. Ann Surg. 2001;233:9–17. doi: 10.1097/00000658-200101000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acharyya S, Guttridge DC. Cancer cachexia signaling pathways continue to emerge yet much still points to the proteasome. Clin Cancer Res. 2007;13:1356–1361. doi: 10.1158/1078-0432.CCR-06-2307. [DOI] [PubMed] [Google Scholar]
- 19.Lang CH, Frost RA, Vary CV. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol. 2007;293:E453–E459. doi: 10.1152/ajpendo.00204.2007. [DOI] [PubMed] [Google Scholar]
- 20.Gomes MD, Lecker SH, Jogoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 22.Wray CJ, Mammen JM, Hershko DD, Hasselgren PO. Sepsis upregulates the gene expression of multiple ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol. 2003;35:698–705. doi: 10.1016/s1357-2725(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 23.Fitts RH, Romatowski JG, Peters JR, Paddon-Jones D, Wolfe RR, Ferrando AA. The deleterious effects of bed rest on human skeletal muscle fibers are exacerbated by hypercholesterolemia and ameliorated by dietary supplementation. Am J Physiol. 2007;293:C313–C320. doi: 10.1152/ajpcell.00573.2006. [DOI] [PubMed] [Google Scholar]
- 24.Pereira C, Murphy K, Jeschke M, Herndon DN. Post burn muscle wasting and the effects of treatments. Int J Biochem Cell Biol. 2005;37:1948–1961. doi: 10.1016/j.biocel.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Herndon DN, Ramzy PI, DebRoy MA, Zheng M, Ferrando AA, Chinkes DL, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg. 1999;229:713–720. doi: 10.1097/00000658-199905000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang CH, Li BG, Wray CJ, Hasselgren PO. Insulin-like growth factor-I inhibits lyosomal and proteasome-dependent proteolysis in skeletal muscle after burn injury. J Burn Care Rehabil. 2002;23:318–325. doi: 10.1097/00004630-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Kley RA, Tarnopolsky MA, Vorgerd M. Creatine treatment in muscle disorders: a meta-analysis of randomized controlled trials. J Neurol Neurosurg Psychiatry. 2008;79:366–367. doi: 10.1136/jnnp.2007.127571. [DOI] [PubMed] [Google Scholar]
- 28.Eley HL, Russell ST, Tisdale MJ. Effect of branched-chain amino acids on muscle atrophy in cancer cachexia. Biochem J. 2007;407:113–120. doi: 10.1042/BJ20070651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogdanovich S, McNally EM, Khurana TS. Myostatin blockade improves function but not histopathology in a murine model of limb-girdle muscular dystrophy 2C. Muscle Nerve. 2008;37:308–316. doi: 10.1002/mus.20920. [DOI] [PubMed] [Google Scholar]
- 30.Liu CM, Yang Z, Liu CW, Wang R, Tien P, Dale R, et al. Myostatin antisense RNA-mediated muscle growth in normal and cancer cachexia mice. Gene Therapy. 2008;15:155–160. doi: 10.1038/sj.gt.3303016. [DOI] [PubMed] [Google Scholar]
- 31.Thaloor D, Miller KJ, Gephart J, Mitchell PO, Pavlath GK. Systemic administration of the NF-kB inhibitor curcumin stimulates muscle regeneration after traumatic injury. Am J Physiol. 1999;277:C320–C329. doi: 10.1152/ajpcell.1999.277.2.C320. [DOI] [PubMed] [Google Scholar]
- 32.Ravindranath V, Chandrasekhara N. Metabolism of curcumin. Studies with [3H]curcumin. Toxicology. 1982;22:337–344. doi: 10.1016/0300-483x(81)90027-5. [DOI] [PubMed] [Google Scholar]
- 33.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 34.Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10–13. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: A component of turmeric (Curcuma longa) J Altern Complement Med. 2003;9:161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 36.Hasselgren PO. Transcription factors and nuclear cofactors in muscle wasting. In: Vincent JL, editor. Year Book of Intensive Care and Emergency Medicine. Heidelberg, Germany: Springer-Verlag; 2007. pp. 229–237. [Google Scholar]
- 37.Hasselgren PO. Ubiquitination, phsophorylation, and acetylation – triple threat in muscle wasting. J Cell Physiol. 2007;213:679–689. doi: 10.1002/jcp.21190. [DOI] [PubMed] [Google Scholar]
- 38.Farid M, Reid MB, Li YP, Gerken E, Durham WJ. Effects of dietary curcumin and N-acetylcysteine on NF-kB activity and contractile performance in ambulatory and unloaded murine soleus. Nutr Metab. 2005;2:20. doi: 10.1186/1743-7075-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mourkiati F, Kratsios P, Luedde T, Song YH, Delafontaine P, Adami R, et al. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass and promotes regeneration. J Clin Invest. 2006;116:2866–2868. doi: 10.1172/JCI28721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durham WJ, Arbogast S, Gerken E, Li YP, Reid MB. Progressive nuclear factor-kB activation resistant to inhibition by contraction and curcumin in mdx mice. Muscle Nerve. 2006;34:298–303. doi: 10.1002/mus.20579. [DOI] [PubMed] [Google Scholar]
- 41.Poylin V, Fareed MU, O’Neal P, Alamdari N, Reilly N, Menconi M, et al. The NF-kB inhibitor curcumin blocks sepsis-induced muscle proteolysis. Mediat Inflam. 2008:1–13. doi: 10.1155/2008/317851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siddiqui A, Cui X, Wu R, Dong W, Zhou M, Hu M, Simms HH, Wang P. The anti-inflammtory effect of curcumin in an experimental model of sepsis is mediated by upregulation of peroxisome proliferator-activated receptor-[gamma] Crit Care Med. 2006;34:1874–1882. doi: 10.1097/01.CCM.0000221921.71300.BF. [DOI] [PubMed] [Google Scholar]
- 43.Busquets S, Carbo N, Almendro V, Quiles MT, Lopez-Soriano FJ, Argiles JM. Curcumin, a natural product present in turmeric, decreases tumor growth but does not behave as an anticachectic compound in a rat model. Cancer Lett. 2001;167:33–38. doi: 10.1016/s0304-3835(01)00456-6. [DOI] [PubMed] [Google Scholar]