Abstract
Background
Given previous evidence of familial predisposition for longevity, we hypothesized that siblings and parents of supercentenarians (age ≥ 110 years) were predisposed to survival to very old age and that, relative to their birth cohorts, their relative survival probabilities (RSPs) are even higher than what has been observed for the siblings of centenarians.
Methods
Mean age at death conditional upon survival to ages 20 and 50 and survival probabilities from ages 20 and 50 to higher ages were determined for 50 male and 56 female siblings and 54 parents of 29 supercentenarians. These estimates were contrasted with comparable estimates based on birth cohort-specific mortality experience for the United States and Sweden.
Results
Conditional on survival to age 20 years, mean age at death of supercentenarians’ siblings was ~81 years for men and women. Compared with respective Swedish and U.S. birth cohorts, these estimates were 17%–20% (12–14 years) higher for the brothers and 11%–14% (8–10 years) higher for the sisters. Sisters had a 2.9 times greater probability and brothers had a 4.3 times greater probability of survival from age 20 to age 90. Mothers of supercentenarians had a 5.8 times greater probability of surviving from age 50 to age 90. Fathers also experienced an increased survival probability from age 50 to age 90 of 2.7, but it failed to attain statistical significance.
Conclusions
The RSPs of siblings and mothers of supercentenarians revealed a substantial survival advantage and were most pronounced at the oldest ages. The RSP to age 90 for siblings of supercentenarians was approximately the same as that reported for siblings of centenarians. It is possible that greater RSPs are observed for reaching even higher ages such as 100 years, but a larger sample of supercentenarians and their siblings and parents is needed to investigate this possibility.
A substantial degree of exceptional longevity has been noted to be familial (1–6). A study of Mormon genealogies based on individuals who lived at least to age 65 years, revealed that siblings of probands who achieved the 97th percentile of their cohort in terms of age (95 years for men and 97 years for women) experienced a mean excess longevity of 14.8 years, representing a relative risk of recurrence (λs) of 2.30 (95% confidence interval [CI], 2.08–2.56) compared to 5000 randomly selected individuals from the sample studied (7). Perls and colleagues (8) analyzed the survival experience of the siblings of centenarians in The New England Centenarian Study (NECS) relative to that of the U.S. 1900 birth cohort. They found that male and female siblings of centenarians had a 16.95 (95% CI, 10.84–23.07) and 8.22 (95% CI, 6.55–9.90) times greater chance, respectively, of living to age 100 compared with the 1900 U.S. birth cohorts (8). The Okinawa Centenarian Study recently reproduced findings similar to those of the NECS study for survival to age 90 (9). In both studies, relative survival probabilities (RSPs) increased with age of the centenarians’ siblings. Relative risk estimates beyond age 100 are not available, as centenarians and especially supercentenarians represent a small group of rare individuals.
Centenarians are rare in the United States, with a prevalence of about 0.7–1 per 10,000 (10). Living to age 110 is even less common, although the number of supercentenarians worldwide has increased steadily at least since the 1980s (11). The exact number of living supercentenarians in the United States is unknown. The 2000 U.S. census listed 1400 supercentenarians, about 1 per 200,000 (12). This is undoubtedly a gross overestimate of the size of the super-centenarian population in the United States (13). Based on age-validated cumulative data on supercentenarians from seven European countries up to 2000, it is estimated that there are roughly 0.8 cumulative supercentenarians per million inhabitants (11). The number of supercentenarians who are alive at any one time is likely to be even smaller. A list of 45 age-validated supercentenarians known to be alive at some point in 2003 in the United States was recently published (14). Kestenbaum and Ferguson (10) reported, based on Medicare data, that there were 105 supercentenarians in the United States in 2000, representing 0.3% of centenarians in the United States. In 2002, there were 139 supercentenarian social security recipients of whom a sizable fraction was thought to be < 110 years old, bringing the true number closer to 75–100 (10).
In this article, we compare the survival experience of siblings and parents of 29 age-validated U.S. supercentenarians to that of their respective U.S. and Swedish birth cohorts. Because individuals who have achieved extreme old age represent a group of select survivors who appear to have fewer genetic and/or environmental exposures associated with premature mortality (15), and based on previous evidence of familial concentration of longevity (7–9,16–18), we hypothesize that (a) both the siblings and the parents of supercentenarians are predisposed to survival to exceptionally old age and (b) that their survival probabilities to very old age, relative to their birth cohorts, will be higher than what has been observed for the siblings of centenarians.
Methods
Participants
Between 1997 and 2005, supercentenarians (individuals 110 years old or older) were identified through the media, the Internet, family members, and other interested individuals who contacted the NECS. All participants were Caucasian and lived in the United States. These individuals comprised a subgroup of supercentenarians included in a phenotyping study (n = 32), for whom pedigree data were available (19). The individuals included in this study were enrolled in the NECS using protocols approved by the Beth Israel Deaconess Medical Center (1997–2002) and Boston University Medical Center (2002 to present) Institutional Review Boards. Among the age-validated supercentenarians enrolled in the NECS, pedigree data were available for 29 participants, aged 110–119 years, who were born between 1880 and 1895. Of the 29 supercentenarians, 24 were born in the United States, 2 each in Australia and Germany, and 1 in Ireland.
Pedigree information on the supercentenarians’ parents and siblings was obtained either from the supercentenarian and/or his or her next-of-kin, typically a child or a grandchild. Many families also shared documented family trees with the research staff. The pedigree information was verified whenever possible with publicly available census manuscript records, pedigrees available through www.ancestry.com and cemetery listings. Complete age information was available for 54 parents of supercentenarians born between 1843 and 1877. Of the parents, 36 were born in the United States, 8 in Germany, 2 each in Denmark, Great Britain and Ireland, and 1 each in Australia and Norway; place of birth was unknown for 2 parents. Age information was missing for both parents of one supercentenarian and one parent each of two supercentenarians.
The 29 supercentenarians reported 144 full siblings and 5 half siblings; the 5 half siblings were excluded from the analysis. We further excluded 15 siblings (10%) who had died prior to age 20 (11 of the 15 siblings had died prior to age 5), and thus their deaths were unlikely to be due to age-related diseases that would impact on longevity. In addition, we excluded 11 male and 12 female siblings (23 or 16%) for whom birth, death, and/or age data were incomplete. Most of these siblings were born prior to 1900, and most were reported by informants as having died in childhood. Thus, our analytic sample consists of 106 full siblings of the supercentenarians known to have survived to age 20 of whom 50 (47%) were men and 56 (53%) were women. These siblings were born between 1874 and 1912 and were associated with 26 supercentenarians; three supercentenarians either had no siblings, the siblings’ data were incomplete, or the siblings had died prior to age 20. Eighty-five percent of the siblings (90 individuals) were known to have been born in the United States. Of the remainder, eight were born in Ireland, four in Australia, and one in Germany; place of birth was unknown for three siblings.
Because of our interest in longevity, we examined the survival experience of siblings conditional on survival to age 20. We chose age 20 as a cutoff because siblings who died at younger ages probably did so because of stochastic, nonheritable factors (e.g., infectious diseases, accidents, violence) and because individuals who died at such a young age might be forgotten by currently alive family members (20). By treating the birth cohort controls in the same manner, we attempted to minimize the bias that would result from unreported sibling deaths in childhood and adolescence. The reported number of siblings ranged from 1 to 9 with a mean of 4 siblings.
Because age misreporting is common among the oldest old (21), considerable care was taken to verify the accuracy of the reported dates of birth and death for individuals included in these analyses. In accordance with the recommendations of the National Institute on Aging Panel on the Characterization of Participants in Studies of Exceptional Survival in Humans, age verification began with the individual’s birth certificate when available (22). In the absence of a birth certificate and in the case of rare and potentially sensational ages, such as claims of being 110 years old or older, additional documents for proof of age for different times in the person’s life were obtained. These other sources included census records, school report cards with age, military records, marriage licenses, employment records, old passports, and/or parental age on a child’s birth certificate. Dates of death for those individuals who had died were confirmed using death certificates, the Social Security Death Index or, when these were unavailable, cemetery or funeral home records. Familial reconstruction methods were also used to determine if the ages of parents, grandparents, siblings, and children were reasonable in relation to that of the supercentenarian (23).
Table 1 summarizes the documents that were used to validate the ages of the supercentenarians, their siblings, and their parents. As seen in Table 1, a birth certificate was available for very few participants, the supercentenarians being the exception.
Table 1.
Age Validation Documents for the 29 Supercentenarians, Their Parents, Siblings, and Offspring
| Document | Supercentenarians | Parents | Siblings | Offspring |
|---|---|---|---|---|
| Birth certificate | 13 | 0 | 2 | 1 |
| Census record | 27 | 50 | 118 | 58 |
| Baptismal record | 3 | 1 | 0 | 0 |
| Marriage certificate/License | 10 | 0 | 0 | 0 |
| Other | 4 | 0 | 1 | 0 |
| Driver’s license | 3 | 0 | 2 | 0 |
| Military record | 0 | 0 | 4 | 2 |
| Social Security Death Index | 14 | 1 | 60 | 15 |
| Tombstone | 0 | 11 | 13 | 2 |
| Obituary | 1 | 2 | 3 | 1 |
| Death certificate | 0 | 1 | 14 | 2 |
Note: For several participants, more than one document was used to verify age; thus the number of documents used does not necessarily correspond to the number of participants included in the analysis.
Although birth registration in New England dates back to the late 19th century, the U.S. Birth Registration Area nationwide was not complete until 1935 (24). In the absence of a birth record, early-life census records are commonly used to verify age or as a proof of age to qualify for social security benefits (13,25). Census records were also the most common source of information used to validate age of the participants in this study.
To answer our main research question—did the parents and siblings of the supercentenarians live longer than expected—we compared the parents’ and siblings’ survival experience with that of their respective U.S. and Swedish birth cohorts, an approach similar to that adopted in previous studies (8,9,26). We used Swedish cohort life tables in addition to the U.S. tables for three reasons. First, the U.S. data for birth cohorts born prior to 1900 were not of sufficient detail to allow comparisons of cohort survival probabilities. Second, Swedish cohort life tables are considered to be of high quality due to highly accurate age reporting dating back to the 1860s (27,29). Third, mortality in Sweden was lower than in the United States during the 19th and early part of the 20th centuries, and thus a comparison with the Swedish experience provides a more rigorous test of the distinctiveness of the familial concentration of longevity than had we used the U.S. data alone.
We first estimated the mean age at death of the siblings and parents of the supercentenarians conditional on survival to age 20 and/or on survival to age 50. Because our focus was on the survival experience of their siblings and parents, the supercentenarians were excluded from the analysis. All parents and male siblings of the supercentenarians had died, and thus their survival experience was complete. Two of the 56 female siblings were alive at the time of the data collection in 2004. The women were born in 1906 and 1907 and were 97 and 98 years old, respectively. We estimated their age at death using cohort life table values for their birth cohorts. Life expectancy at age 97 for the 1907 female birth cohort was approximately 3.1 years, and the life expectancy at age 98 for the 1906 female birth cohort was about 2.9 years. Thus, we estimated the age at death for the two women to be slightly more than 100 years. Second, we calculated survival probabilities (px) for the siblings of the supercentenarians from age 20 to older ages and for the parents of the supercentenarians from age 50 (the end of the reproductive life span for women) to older ages. CI values for these probabilities were based on the assumption of binomial variability.
We then matched each participant by year of birth and sex with their respective U.S. and Swedish birth cohorts to obtain life expectancies conditional on survival to ages 20 and 50 and survival probabilities from age 20 and age 50 to older ages. The weighted average of these cohort-specific estimates was then compared to the corresponding estimates obtained for the siblings and parents of the supercentenarians. Estimates of cohort life expectancies for the United States came from Pope (30), who published sex-specific cohort life expectancies at ages 20, 30, and 50 based on family histories for cohorts born between 1760–1769 and 1880–1889. We used Pope’s estimates for birth cohorts born in 1840–1849, 1850–1859, 1860–1869, 1870–1879, and 1880–1889. We extended the U.S. data series with cohort life tables available from Bell and Miller (31) for 1900, 1910, and 1920. Linear interpolation was used to obtain e20, e30, and e50 for the years between 1890, 1900, 1910, and 1920. Swedish cohort life tables were obtained from the Human Mortality Database (32).
Results
Table 2 shows the results for comparisons of mean ages at death of the siblings and parents of the supercentenarians with the corresponding estimates for U.S. and Swedish birth cohorts conditional on survival to ages 20 and 50. The mean age at death of the supercentenarians’ siblings, who survived to age 20, was about 81 years for both men and women. These estimates were substantially higher than the corresponding estimates for the respective U.S. and Swedish birth cohorts. For male siblings, the mean age at death was about 20% higher (13.7 years) when compared with the U.S. birth cohorts and about 17% higher (11.5 years) when compared with the Swedish birth cohorts. The survival advantage was also substantial for women, although somewhat less pronounced than for men. Conditional on survival to age 20, female siblings’ mean age at death was about 14% higher (9.9 years) when compared to the U.S. birth cohorts and 11% higher (8.1 years) when compared to the Swedish birth cohorts.
Table 2.
Comparisons of Mean Ages at Death Conditional on Survival to Ages 20 and 50 of Supercentenarians’ Siblings and to Age 50 of Supercentenarians’ Parents With the Respective U.S. and Swedish Birth Cohorts, Birth Years 1843–1912
| Siblings of Supercentenarians† | Mean Age at Death by Sex Conditional on Survival to Age x | U.S. Cohort Life Tables* | Swedish Cohort Life Tables* | Excess Years Based on U.S. Tables | Excess Years Based on Swedish Tables |
|---|---|---|---|---|---|
| Age 20 | |||||
| Men | 80.6 (3.90)‡ | 66.9 | 69.1 | 13.7 | 11.5 |
| Women | 80.7 (4.38) | 70.8 | 72.6 | 9.9 | 8.1 |
| Age 50 | |||||
| Men | 82.8 (3.28) | 73.3 | 75.4 | 9.5 | 7.4 |
| Women | 85.2 (3.62) | 77.3 | 78.6 | 7.9 | 6.6 |
| Parents of supercentenarians† | |||||
| Age 50 | |||||
| Men | 76.4 (3.49) | 72.4 | 73.7 | 4.0 | 2.7 |
| Women | 82.4 (3.70) | 74.0 | 75.1 | 8.4 | 7.3 |
Notes: Source: Pope (30). Human Mortality Database: www.mortality.org; calculations by the authors.
Weighted average of life expectancy at birth conditional on survival to age 20 or age 50 for respective birth cohorts. U.S. and Swedish cohort life table estimates were assumed to have zero variance.
There were 50 male and 56 female siblings and 27 male and 27 female parents of supercentenarians.
Standard errors in parentheses.
After age 50, the advantage of the siblings of the supercentenarians was also substantial. Conditional on survival to age 50, the male siblings of supercentenarians lived 13% (9.5 years) longer on average than their U.S. birth cohort counterparts and about 9.8% (7.4 years) longer than their Swedish counterparts. The respective figures for female siblings were 10% (7.9 years) and 8% (6.6 years). Also of note is that there was essentially no sex difference in the mean age at death among the siblings of the supercentenarians conditional on survival to age 20. The female advantage over their male siblings did not become evident until after age 50; after this age, sisters lived just over 2 years longer than brothers.
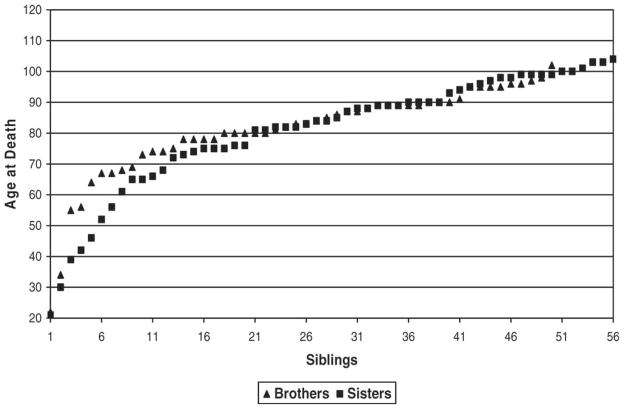
The above comparisons for the siblings are conditional on survival to age 20 to minimize bias caused by the omission of brothers and sisters who died at young ages. It is, however, possible that informants may have also been less likely to recall siblings who died in young adulthood, i.e., older than 20 years but younger than 50 years. To explore this possibility, we plotted the ages at death of the 106 siblings included in our analysis by gender (Figure 1). Relatively few siblings, two brothers (4%) and five sisters (9%), were reported to have died between these ages. Thus it is possible that siblings who died in this age interval may have been omitted. The extent of this potential bias is, however, unknown.
Figure 1.
Age at death of the male (n = 50) and female (n = 56) siblings of the 29 supercentenarians.
Table 2 also shows the mean age at death for the parents of the supercentenarians conditional on survival to age 50, near the end of the reproductive life span for women. These results also point to a survival advantage for the parents compared to their respective Swedish or U.S. birth cohorts. The mean age at death for fathers was 4%–6% (2.7–4.0 years) higher than that of the Swedish or U.S. birth cohorts, and it was 10%–11% (7.3–8.4 years) higher for the mothers.
Table 3 presents the survival probabilities, including their 95% CI values, from age 20 to older ages for the siblings of supercentenarians relative to their respective Swedish birth cohorts (no comparable data were available for the United States). The number of individuals achieving very old age allowed for reasonably valid results up to age 90. Beyond age 90, the small number of survivors makes the estimates unstable and produces large CI values.
Table 3.
Comparisons of Estimated Survival Probabilities of Supercentenarians’ Siblings From Age 20 to Higher Ages With the Respective Swedish Birth Cohorts, Birth Years 1874–1912
| Probability of Survival From Age 20 to Age… | Women |
Men |
||||
|---|---|---|---|---|---|---|
| Siblings of Supercentenarians (95% CI) | Swedish Cohort Life Tables* | Relative Survival Probability (95% CI) | Siblings of Supercentenarians (95% CI) | Swedish Cohort Life Tables* | Relative Survival Probability (95% CI) | |
| 40 | 0.95 (0.85–0.99) | 0.91 | 1.04 (0.93–1.09) | 0.96 (0.86–1.00) | 0.90 | 1.07 (0.96–1.11) |
| 60 | 0.88 (0.76–0.95) | 0.80 | 1.10 (0.95–1.19) | 0.92 (0.81–0.98) | 0.77 | 1.19 (1.05–1.27) |
| 80 | 0.64 (0.50–0.77) | 0.43 | 1.49 (1.16–1.79) | 0.66 (0.51–0.79) | 0.31 | 2.13 (1.65–2.55) |
| 90 | 0.38 (0.25–0.52) | 0.13 | 2.92 (1.92–4.00) | 0.26 (0.15–0.40) | 0.06 | 4.33 (2.50–6.67) |
| 100 | 0.14 (0.06–0.26) | 0.01 | 14.00 (6.00–26.00) | 0.02 (0.001–0.11) | 0.002 | 10.00 (0.50–55.00) |
| N | 56 | 50 | ||||
Notes: Source: Calculations by the authors; Human Mortality Database: www.mortality.org
Weighted average of survival probability from age 20 to age x (px) for respective Swedish birth cohorts. The Swedish cohort life table estimates are treated as having zero variance.
CI = confidence interval.
These estimates show that the gap in survival at advanced ages between the siblings and their corresponding Swedish birth cohorts was substantial. As seen in Table 3, the estimate for the Swedish birth cohorts falls within the 95% CI of the sibling estimates from age 20 to age 40 and to age 60 for women and from age 20 to age 40 for men, but by age 90 the differences in survival were substantial. For example, female siblings of supercentenarians were nearly three times and male siblings were four times more likely to survive to age 90 when compared with their respective Swedish birth cohorts. At age 100, the estimates continue to show a substantial survival advantage for women.
Table 4 shows that the mothers of the supercentenarians were 1.4 times more likely to survive from age 50 to age 80 and 5.8 times more likely to survive to age 90 when compared with their corresponding Swedish birth cohorts, with the latter estimate suggesting a statistically significant survival advantage. Although the fathers of supercentenarians had a 1.7 times greater probability of surviving from age 50 to age 80 and a 2.7 times greater probability of surviving to age 90, neither estimate reached statistical significance.
Table 4.
Comparisons of Estimated Survival Probabilities of Supercentenarians’ Parents From Age 50 to Ages 80 and 90 With the Respective Swedish Birth Cohorts, Birth Years 1843–1877
| Probability of Survival From Age 50 to Age… | Women |
Men |
||||
|---|---|---|---|---|---|---|
| Mothers of Supercentenarians (95% CI) | Swedish Cohort Life Tables* | Relative Survival Probability (95% CI) | Fathers of Supercentenarians (95% CI) | Swedish Cohort Life Tables* | Relative Survival Probability (95% CI) | |
| 80 | 0.50 (0.30–0.70) | 0.36 | 1.39 (0.83–1.94) | 0.52 (0.31–0.72) | 0.31 | 1.68 (1.00–2.32) |
| 90 | 0.35 (0.17–0.56) | 0.06 | 5.83 (2.83–9.33) | 0.12 (0.03–0.31) | 0.05 | 2.67 (0.60–6.20) |
| N | 26 | 25 | ||||
Notes: Source: Calculations by the authors; Human Mortality Database: www.mortality.org
Weighted average of survival probability from age 50 to age x (px) for respective Swedish birth cohorts. The Swedish cohort life table estimates are treated as having zero variance.
CI = confidence interval.
Discussion
A substantial survival advantage was observed among both the parents and the siblings of the supercentenarians compared to members of their respective birth cohorts. These results contribute to the growing body of evidence that exceptional longevity aggregates strongly in specific families. The RSPs for the siblings of the supercentenarians living to at least age 90 were similar to the NECS’s previous findings regarding the mortality experience of 2092 siblings of centenarian probands (mean age = 101 years). Male siblings of these centenarians were about 4 times and female siblings were about 2.5 times more likely to survive to age 90 than the members of the 1900 U.S. birth cohort (8). We hypothesized that the RSPs of siblings of supercentenarian probands would be even higher. However, at least for survival to age 90, this did not appear to be the case. It would thus appear that familial influences on longevity, to at least age 90, are similar for siblings of centenarians and for those of supercentenarians. The sample size of this study does not allow for a statistically valid assessment of the RSPs for siblings surviving to even older ages, although the results suggest that the RSP is higher for survival to age 100 and the results are significant for women. These findings point to the importance of building a large enough database of supercentenarians and their kin to determine survival advantage beyond age 90.
At age 20, no sex difference in life expectancy was noted among the siblings of the supercentenarians. The female advantage over their male siblings did not become evident until after age 50, the end of their reproduction; after this age, sisters lived just over 2 years longer than their brothers. One possible explanation for the women at younger ages having similar mortality rates to the men is that maternal mortality during reproductive ages was substantial in the latter half of the 19th and early part of the 20th century, which elevated female mortality relative to male mortality (33). Another possibility is that gender-independent factors are playing predominant roles in survival through early to midadulthood.
The parents of the supercentenarians also exhibited a survival advantage when compared with the Swedish and U.S. birth cohorts, although this advantage was more pronounced for the mothers. The RSP at age 90 for mothers was 5.8. The survival advantage of the fathers was not significantly different from the survival of Swedish birth cohorts, although the results suggest that fathers too may have enjoyed longer life spans.
The observed familial aggregation for exceptional longevity is likely due to important environmental, behavioral, and genetic factors that family members have in common. The results of this study cannot identify what proportion of this aggregation is genetic versus environmental and/or behavioral. The results from twin studies have suggested that about 25% of the variance of age at death is genetic and 75% is environmental and/or behavioral (34,35). However, the oldest participants in most twin studies were in their early to mid-80s. Perhaps a more appropriate interpretation is that 25% of the variation in who lives to an average life expectancy can be explained by genetic variation and the remainder by variation in environmental factors and behaviors (36,37). Hjelmborg and colleagues (38) recently analyzed survival data from Danish, Finnish, and Swedish twins born between 1870 and 1910 and found that the relative recurrence risk of reaching age 92—that is, the ratio of the chance of one twin reaching a certain age given that the co-twin had reached that age to the prevalence of reaching that age in the entire twin population—was 4.8 for monozygotic men compared to 1.8 for dizygotic men and 2.5 for monozygotic women versus 1.6 for dizygotic women. On the basis of these findings, the authors concluded that there may be an increasingly greater genetic component to survival to extreme old age. The substantial familial component to longevity noted here among the siblings and the parents of the supercentenarians is at least consistent with this observation.
There has been much speculation about what genes and what environmental factors make important contributions to exceptional longevity. In humans, numerous cardiovascular genes have been noted to be associated with exceptional longevity. This finding should not be a surprise given that cardiovascular diseases are the primary causes of death among elderly persons. However, except perhaps for apolipoprotein E among Caucasians, most studies have demonstrated relatively modest differences in allelic frequencies for these genes between centenarians and controls (39,40). It may be that centenarians, or for that matter supercentenarians, are rare not because of a few rare factors, but rather because of rare combinations of common genetic and environmental exposures (37).
There are several limitations to studying supercentenarians and their families. Only nine countries have been identified as having the ability to find nearly all supercentenarians, and not surprisingly the United States is not among them (11). Thus, it is unclear what proportion of the entire supercentenarian population alive between 1997 and 2005 we have captured in our data and whether our results are generalizable to all families of supercentenarians. It is possible that larger families with multiple siblings surviving to very old age are more readily identified, thus potentially biasing our results towards families with larger sibships and increased longevity. However, we did not ascertain the supercentenarians in this study via their relationship with any of their siblings. Rather, they were found by way of their mention in the media because of their exceptional age.
Another limitation is that the family history data collected relies upon the memories of supercentenarians and their family members. As noted above, siblings who died at young ages may be inadvertently omitted. We have based our comparisons of sibling survival conditional on survival to age 20 to minimize the bias resulting from unreported sibling deaths in childhood and adolescence. It is possible, however, that siblings who died in their 20s and 30s could also be missed. In addition, we also based our comparisons of parent survival conditional on survival to age 50, near the end of reproduction for women.
We compared the survival of siblings and parents to estimates for U.S. and Swedish birth cohorts matched to our participants by year of birth and gender, i.e., to the average survival experience of the entire birth cohort. The only exception is our use of cohort life-table estimates for the United States prior to 1900, which are based on family histories for cohorts born between 1760 and 1769 and between 1880 and 1889 (30). Our approach is consistent with several previous studies that have compared the survival of family members to that of the general population (8,9,26) Thus, this approach simply answers the question of whether the parents and siblings of supercentenarians lived longer than the average member of their birth cohorts. It does not speak to what factors—biological, environmental, or behavioral—may contribute to the observed familial concentration of longevity, including characteristics associated with fecundity and marriage.
Because there are concerns about the quality of the historical mortality data in the United States, we chose to compare the survival experience of our participants not only to cohort mortality in the United States but also to that in Sweden, where mortality estimates even at the oldest ages are known to be reliable dating back to the mid-19th century (27–29).
Age validation is a critical component in studies of exceptional longevity. We have attempted to address this issue as thoroughly as possible by using multiple records following the guidelines recommended by the National Institute on Aging Panel on the Characterization of Participants in Studies of Exceptional Survival in Humans and by others (22). In contrast to European countries, the absence of birth certificates for a sizable proportion of the oldest old in the United States poses a serious problem, as noted earlier and demonstrated in Table 1. Instead, researchers have to rely on alternative documents for age verification, such as early life census records, which is also the most common method of age verification in this study (13). The International Database on Longevity (IDL), a multidemographic study center effort based at the Max Planck Institute for Demographic Research, aims to obtain complete and valid supercentenarian mortality data for low mortality countries (United States, Canada, Japan, and European countries; http://www.supercentenarians.org). Complete data such as these are critical if one is to minimize or avoid the ascertainment bias that, as discussed above, may be an issue in a case series such as described here (11,42).
Acknowledgments
This work was supported by the National Institute on Aging (U01-AG-023755: TP, IE, IK; K24-AG25727: TP; K08AG22785, K23 AG026754 [Paul Beeson Physician Faculty Scholar in Aging Award]: DT, TP).
We thank Paola Sebastiani and Doug Ewbank for their statistical advice and Hans-Peter Kohler and Samuel H. Preston for their helpful comments and suggestions.
References
- 1.Caselli G, Pozzi L, Vaupel JW, et al. Familial clustering in Sardinian longevity: a genealogical approach. Exp Gerontol. 2006;41:727–736. doi: 10.1016/j.exger.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Gavrilov LA, Gavrilova NS, Olshansky SJ, Carnes BA. Genealogical data and the biodemography of human longevity. Soc Biol. 2002;49:160–173. doi: 10.1080/19485565.2002.9989056. [DOI] [PubMed] [Google Scholar]
- 3.Pearl R, Pearl RD. The Ancestry of the Long-Lived. Baltimore, MD: The Johns Hopkins University Press; 1934. [Google Scholar]
- 4.Robine JM, Allard M, editors. Towards a Genealogical Epidemiology of Longevity. Berlin: Springer-Verlag; 1997. [Google Scholar]
- 5.Swedlund AC, Meindl RS, Nydon J, Gradie MI. Family patterns in longevity and longevity patterns of the family. Hum Biol. 1983;55:115–129. [PubMed] [Google Scholar]
- 6.Vandenbroucke JP, Matroos AW, van der Heide-Wessel C, van der Heide R. Parental survival, and independent predictor of longevity of middle-aged persons. Am J Epidemiol. 1984;119:742–750. doi: 10.1093/oxfordjournals.aje.a113795. [DOI] [PubMed] [Google Scholar]
- 7.Kerber RA, O’Brien E, Smith KR, Cawthon RM. Familial excess longevity in Utah genealogies. J Gerontol Biol Sci. 2001;56A:B130–B139. doi: 10.1093/gerona/56.3.b130. [DOI] [PubMed] [Google Scholar]
- 8.Perls TT, Wilmoth J, Levenson R, et al. Life-long sustained mortality advantage of siblings of centenarians. Proc Natl Acad Sci U S A. 2002;99:8442–8447. doi: 10.1073/pnas.122587599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willcox BJ, Willcox DC, He Q, Curb JD, Suzuki M. Siblings of Okinawan centenarians share lifelong mortality advantages. J Gerontol A Biol Sci Med Sci. 2006;61:345–354. doi: 10.1093/gerona/61.4.345. [DOI] [PubMed] [Google Scholar]
- 10.Kestenbaum B, Ferguson BR. Number of centenarians in the United States 01/01/1990, 01/01/2000, and 01/01/2010 based on improved Medicare data. [Accessed April 29, 2006];Paper presented at: Society of Actuaries. Available at: http://ce.soa.org/living-to-100/6b_papers.pdf.
- 11.Robine JM, Vaupel JW. Emergence of supercentenarians in low-mortality countries. N Am Actuarial J. 2002;6:54–63. [Google Scholar]
- 12.Hustead E. Ending the mortality table. Presented at the Living to 100 and Beyond Symposium, sponsored by the Society of Actuaries; Orlando, FL. January 12–14, 2005. [Google Scholar]
- 13.Rosenwaike I, Stone LF. Verification of ages of supercentenarians in the United States: results of a matching study. Demography. 2003;40:727–739. doi: 10.1353/dem.2003.0038. [DOI] [PubMed] [Google Scholar]
- 14.Coles L. Demography of human supercentenarians. J Gerontol A Biol Sci Med Sci. 2004;59:579–586. doi: 10.1093/gerona/59.6.b579. [DOI] [PubMed] [Google Scholar]
- 15.Vaupel JW, Carey JR, Christensen K, et al. Biodemographic trajectories of longevity. Science. 1998;280:855–860. doi: 10.1126/science.280.5365.855. [DOI] [PubMed] [Google Scholar]
- 16.Perls T, Shea-Drinkwater M, Bowen-Flynn J, et al. Exceptional familial clustering for extreme longevity in humans. J Am Geriatr Soc. 2000;48:1483–1485. [PubMed] [Google Scholar]
- 17.Perls TT, Bubrick E, Wager CG, Vijg J, Kruglyak L. Siblings of centenarians live longer. Lancet. 1998;351:1560. doi: 10.1016/S0140-6736(05)61126-9. [DOI] [PubMed] [Google Scholar]
- 18.Gudmundsson H, Gudbjartsson DF, Frigge M, Gulcher JR, Stefansson K. Inheritance of human longevity in Iceland. Eur J Hum Genet. 2000;8:743–749. doi: 10.1038/sj.ejhg.5200527. [DOI] [PubMed] [Google Scholar]
- 19.Schoenhofen EA, Wyszynski DF, Andersen S, et al. Characteristics of 32 supercentenarians. J Am Geriatr Soc. 2006;54:1237–1240. doi: 10.1111/j.1532-5415.2006.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robine JM, Allard M. Jeanne Calment: validation of the duration of her life. In: Jeune B, Vaupel JW, editors. Validation of Exceptional Longevity. Vol. 6. Odense, Denmark: Odense Monographs on Population Aging, Odense University Press; 1999. pp. 145–172. [Google Scholar]
- 21.Perls TT, Bochen K, Freeman M, Alpert L, Silver MH. Validity of reported age and centenarian prevalence in New England. Age Ageing. 1999;28:193–197. doi: 10.1093/ageing/28.2.193. [DOI] [PubMed] [Google Scholar]
- 22.Cawthon R, Cummings S, Curb JD, et al. National Institute on Aging Panel on the Characterization of Participants in Studies of Exceptional Survival in Humans. Bethesda, MD: National Institute on Aging; 2002. [Google Scholar]
- 23.DeJardins B. Validation of Extreme Longevity Cases in the Past: The French-Canadian Experience. Vol. 6. Odense, Denmark: Odense University Press; 1999. [Google Scholar]
- 24.Shapiro S. Development of birth registration and birth statistics in the United States. Popul Stud (Camb) 1950;4:86–111. [Google Scholar]
- 25.Preston SH, Elo IT, Rosenwaike I, Hill M. African-American mortality at older ages: results of a matching study. Demography. 1996;33:193–209. [PubMed] [Google Scholar]
- 26.Schoenmaker M, de Craen AJ, de Meijer PH, et al. Evidence of genetic enrichment for exceptional survival using a family approach: the Leiden Longevity Study. Eur J Hum Genet. 2006;14:79–84. doi: 10.1038/sj.ejhg.5201508. [DOI] [PubMed] [Google Scholar]
- 27.Wilmoth JR, Lundström H. Extreme longevity in five countries: presentation of trends with special attention to issues of data quality. Eur J Popul. 1996;12:63–93. doi: 10.1007/BF01797166. [DOI] [PubMed] [Google Scholar]
- 28.Kannisto V. The Development of Oldest Old Mortality, 1950–1990: Evidence from 28 Developed Countries. Odense, Denmark: Odense University Press; 1994. [Google Scholar]
- 29.Glei D, Lundström H, Wilmoth J. [Accessed May 16, 2007];About Mortality Data for Sweden. Available at: http://www.mortality.org.
- 30.Pope C. Adult mortality in America before 1900. A view from family histories. In: Goldin C, Rockoff H, editors. Strategic Factors in Nineteenth Century American Economic History: A Volume to Honor Robert W. Fogel. Chicago: University of Chicago Press; 1992. pp. 267–296. [Google Scholar]
- 31.Bell FC, Miller ML. Life Tables for the United States Social Security Area 1900–2100. Washington, DC: Office of the Chief Actuary Social Security Administration; 2005. SSA Publ. No. 11-11536. [Google Scholar]
- 32. [Accessed May 16, 2007];University of California Berkeley, Max Planck Institute for Demographic Research Human Mortality Database. Available at: www.mortality.org or www.humanmortality.de.
- 33.Perls T, Fretts R. The evolution of menopause and human life span. Ann Hum Biol. 2001;28:237–245. doi: 10.1080/030144601300119052. [DOI] [PubMed] [Google Scholar]
- 34.Herskind AM, McGue M, Holm NV, Sorensen TI, Harvald B, Vaupel JW. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870–1900. Hum Genet. 1996;97:319–323. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- 35.McGue M, Vaupel JW, Holm N, Harvald B. Longevity is moderately heritable in a sample of Danish twins born 1870–1880. J Gerontol Biol Sci. 1993;48A:B237–B244. doi: 10.1093/geronj/48.6.b237. [DOI] [PubMed] [Google Scholar]
- 36.Cournil A, Kirkwood TB. If you live long, choose your parents well. Trends Genet. 2001;17:233–235. doi: 10.1016/s0168-9525(01)02306-x. [DOI] [PubMed] [Google Scholar]
- 37.Perls T, Terry DF. Understanding the determinants of exceptional longevity. Ann Intern Med. 2003;139:445–449. doi: 10.7326/0003-4819-139-5_part_2-200309021-00013. [DOI] [PubMed] [Google Scholar]
- 38.Hjelmborg J, Iachine I, Skytthe A, et al. Genetic influence on human lifespan and longevity. Hum Genet. 2006;119:312–321. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- 39.Schachter F, Faure-Delanef L, Guenot F, et al. Genetic associations with human longevity at the APOE and ACE loci. Nat Genet. 1994;6:29–32. doi: 10.1038/ng0194-29. [DOI] [PubMed] [Google Scholar]
- 40.Barzilai N, Atzmon G, Schechter C, et al. Unique lipoprotein phenotype and genotype in humans with exceptional longevity. JAMA. 2003;290:2030–2040. doi: 10.1001/jama.290.15.2030. [DOI] [PubMed] [Google Scholar]
- 41.Jeune B, Vaupel JW. Exceptional Longevity: From Prehistory to the Present, Monograph on Population Aging, No. 2. Odense, Denmark: Odense University Press; 1995. [Google Scholar]
- 42.Robine JM, Vaupel JW. Supercentenarians: slower ageing individuals or senile elderly? Exp Gerontol. 2001;36:915–930. doi: 10.1016/s0531-5565(00)00250-3. [DOI] [PubMed] [Google Scholar]