Abstract
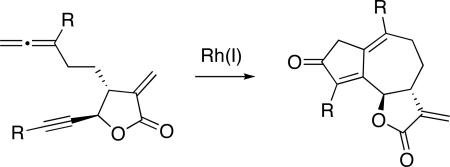
Cyclocarbonylation of α-methylene butyrolactone-containing allene-ynes affords 6,12-guaianolide ring systems. Incorporation of the α-methylene butyrolactone early in a synthetic sequence is rare for reactivity reasons; however, this moiety proves to be beneficial to the allenic Pauson−Khand reaction. The three double bonds and the ketone in the resulting 5-7-5 ring system bear significant differences in their reactivity and are ideally positioned for synthetic application to 6,12-guaianolides and analogs.
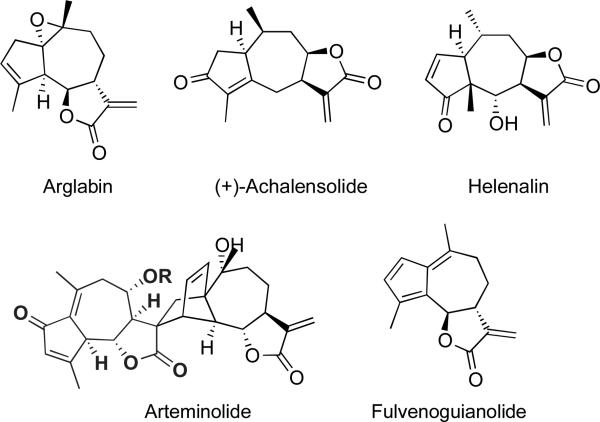
Guaianolides constitute the largest class of sesquiterpene lactones, many of which possess an α-methylene butyrolactone–a privileged moiety represented in 10% of all natural products.1 The skeleton of this class of compounds is represented by a 5-7-5 fused ring system and can be further classified into four subgroups: 6,12- and 8,12-guaianolides, pseudo- and dimeric-guaianolides, each subgroup is represented in Figure 1 by arglabin, (+)-achalensolide, helenalin, and arteminolide, respectively. Many guaianolides are densely oxygenated and rich in stereocenters as is the case with arteminolide. Alternatively, these compounds may be relatively devoid of oxygenated functional groups, an example being fulvenoguaianolide.2
Figure 1.
Representative Naturally Occurring Guaianolides
Surrounding the guaianolides is a wealth of biological activity.3 Bioactivity investigations are ongoing, but one certainty is that in embodiments of the guaianolides with an α-methylene butyrolactone, the cyclopentenone and acrylic ester endows this molecule with not only bioactivity, but also selectivity.4 Recently, guaianolides have captured the attention of medicinal chemists as the importance of controlled, target specific covalent modification as a potential therapeutic option is revisited.5 Moreover, guaianolides are an example of an abundant scaffold in natural products, but are sparsely populated by the bioactive medicinal chemistry compounds.6
The number of synthetic strategies used to access guaianolides is not commensurate with the wealth and range of biological activity associated with these compounds.7 While several reviews summarizing the synthetic chemistry and the bioactivities associated with this class of molecules are available, suffice it to say that the most common approach relies on a structurally advanced natural product, α-santonin possessing a 6-6-5 ring system, that undergoes a skeletal reorganization process to give the 5-7-5 ring system when subjected to light.8 Other than these hemisyntheses, the most common strategy used to form the cyclopentenone moiety starts with a Favorskii rearrangement of a derivative of (S)-carvone.9 While this multi-step process is high yielding, limitations arise when applying this protocol to access a structurally diverse array of natural products. The most common strategies for forming the seven membered ring include: ring expansion of [3.2.0] ring systems,10 tropone or tropylium cation alkylation,11 olefin metathesis,12 intramolecular oxidative or ene cyclizations,13 and 3,3-sigmatropic rearrangements of 3,3-divinyl cyclopropanes.14 Finally, the α-methylene butyrolactone moiety is nearly always constructed in the final steps of a synthetic sequence via a multi-step process involving a series of homologations, oxidations, reductions, protections and/or deprotection steps. In some cases, late introduction of this moiety comes at a high price to step economy.12b, 14a, 15
Nature's synthetic pathway inspires one to imagine benefits to including the α-methylene butyrolactone earlier in a synthetic sequence. 16 For example, the lactone could potentially serve as a means for limiting conformational mobility, providing a stereocontrol element. One major concern of this approach is the electrophilicity of the α-methylene butyrolactone; but interestingly, studies have shown this group to be a selective thiol-alkylator, not reacting with oxygen or nitrogen containing nucleophiles in vivo.17
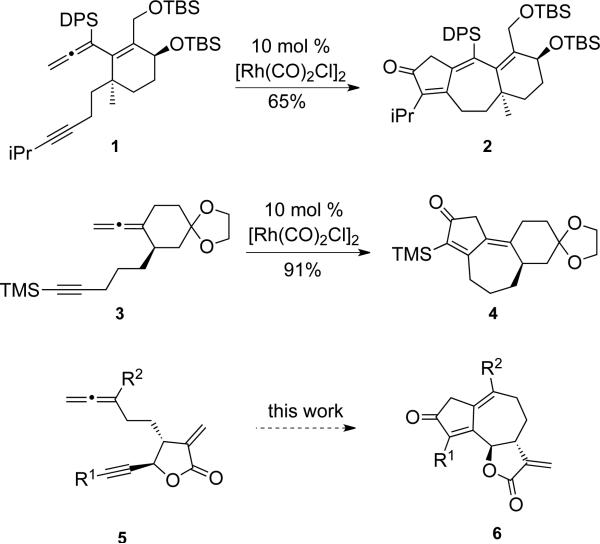
Our group has demonstrated that a number of angular and linear 6-7-5 ring systems can be prepared using an allenic Pauson−Khand reaction (APKR). Incorporation of a five- or six-membered ring in the tether of the reacting allene-yne facilitates and increases the efficiency of the APKR. The synthetic potential of this strategy has been demonstrated by the representative examples below, where allene-ynes 1 and 3 were converted to the linearly fused and angularly fused 6-7-5 ring systems 2 and 4, respectively (Scheme 1).18 By analogy, synthetic entry to the guaianolides by tethering an allene and an alkyne to an α-methylene butyrolactone 5 seemed appealing due to rapid entry into a molecularly complex skeleton 6; yet risky, because of its purported reactivity. Only rare examples incorporating this electrophilic α-methylene butyrolactone early in a synthesis have been reported.19 Furthering our enthusiasm for this synthetic approach is an elegant protocol for preparing functionalized α-methylene butyrolactones via an allylboration/lactonization sequence described by Hall and Kennedy.20
Scheme 1.
An APKR Approach to a Guaianolide Skeleton
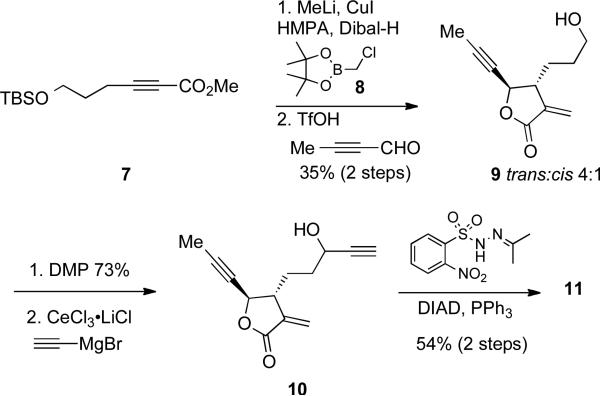
A representative example of our protocol used for rapid assembly of an allene-yne containing α-methylene butyrolactone is depicted in Scheme 2. Methyl 6-(tert-butyldimethylsilyloxy)hex-2-ynoate (7) was prepared in 70% yield in two steps from commercially available 4-pentyn-1-ol. Conjugate addition of a hydride to the acetylenic ester of 7 followed by trapping the alkenylalane intermediate with 2-(chloromethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (8) afforded the corresponding allyl boronate in a Z : E ratio of 1.5 : 1.21 The boronate isomers are not easily separated so they were taken on directly to the allylboration/lactonization step by reacting with 2-butynal and triflic acid to afford lactone 9 as a 4 : 1 mixture of trans : cis isomers.
Scheme 2.
Synthesis of Allene-yne containing Butyrolactone 11
Concomitant removal of the TBS ether occured to give 9 in 35% overall yield for this two-step process. The α-methylene butyrolactones isomers were separated by column chromatography and the relative stereochemistry for each lactone isomer was assigned by comparing our results with that of Hall and later unambiguously confirmed by an X-ray crystal structure (vide infra). The resulting hydroxyl group of 9 was oxidized to an aldehyde using Dess−Martin periodinane conditions in 73% yield. Next, addition of cerium acetylide to the aldehyde afforded the corresponding propargyl alcohol that was subjected to N-isopropylidene-N’-2-nitrobenzenesulfonyl hydrazine (IPNBSH), affording allene-yne containing α-methylene butyrolactone 11 (depicted in Table 1) in 54% yield for the two steps.22,23
Table 1.
APK Reaction of Monosubstituted Allenes (R2 = H)
 | |||
|---|---|---|---|
| entry | substrate | product | yield time |
| 1 |
|
|
90% 30 min |
| 2 |
|
|
90% 30 min |
| 3 |
|
|
81% 30 min |
| 4 |
|
|
92% 20 min |
| 5 |
|
|
67% 150 min |
| 6 |
|
|
51% 30 min |
| 7 |
|
|
67% 30 min |
| 8 |
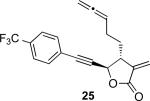
|
|
87% 30 min |
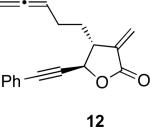
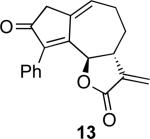
With an α-methylene butyrolactone-containing alleneyne in hand, we set out to test the feasibility of the allenic Pauson−Khand reaction. To our delight, the reaction of allene-yne 12 with 10 mol % of rhodium biscarbonylchloride dimer in toluene at 90 °C afforded the cyclocarbonylation product 13 in 90% yield in 20 minutes (entry 1, Table 1). The efficiency and ease of this allenic Pauson−Khand reaction warrants some comment. Prior to actually carrying out this reaction, there were concerns about the stability of not only the α-methylene butyrolactone but also the embedded propargylic ester, which is known to undergo metal catalyzed depropargylation.24 These concerns appear to be unfounded and in fact, this functionally rich substrate represents one of the best allenic Pauson−Khand reactions we have observed.
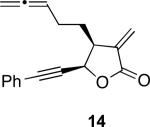
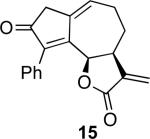
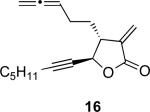
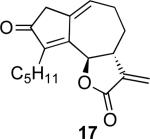
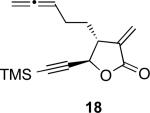
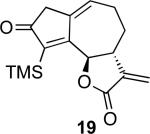
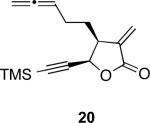
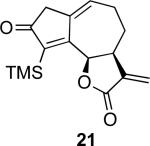
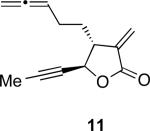
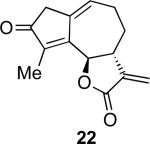
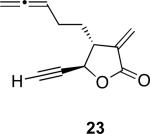
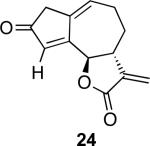
With the feasibility of this reaction established, we set out to explore the scope and limitations of the α-methylene butyrolactone-containing APKR for the preparation of other guaianolide-type skeletons. The allene-yne 14 possessing the cis-stereochemistry at the lactone also gave the cyclocarbonylation product in 90% yield with a reaction time of 30 minutes (entry 2). Alkyl substitution of the alkyne terminus of allene-yne 16 with a pentyl group gave cyclopentenone 17 in 81% yield (entry 3). A TMS group on the alkyne afforded the Pauson−Khand trans and cis adducts 19 and 21 in 92% and 67% yield, respectively (entries 4 and 5). Introduction of a methyl group on the alkyne furnished the cyclocarbonylation product 22 in 51% yield (entry 6). The reaction was also compatible with terminal alkynes as depicted in entry 7 for which allene-yne 23 lead to the desired product 24 in 67% yield. When the terminus of the alkyne was substituted with a phenyl group bearing an electron-withdrawing group such as a trifluoromethyl group, the APKR product 26 was obtained in 87% yield (entry 8). Moreover, except for entry 5 (reaction time was 150 minutes in that case), all of the reactions reached completion in less than 30 minutes.
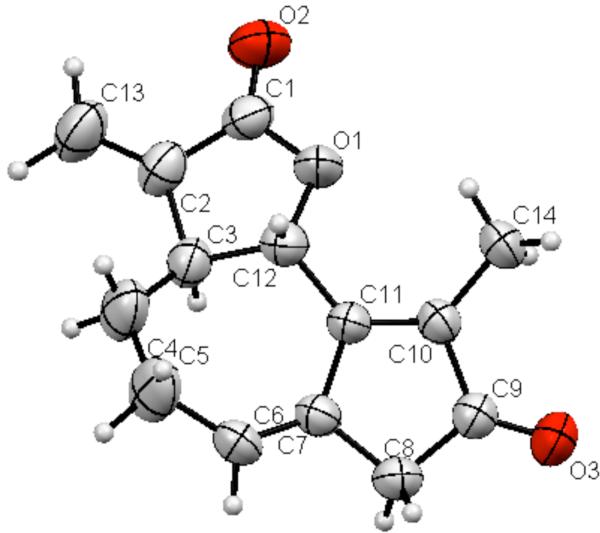
While the structures of the compounds were characterized unambiguously using the NMR data, the fortuitous crystallization of 22 provided additional support corroborating the trans stereochemistry of the lactone. The stereochemistry of the lactone was also established by examining the coupling constant for the proton adjacent to the oxygen of the lactone at the ring fusion; with Jab (δ 5.36) for the trans product 13 at 9.9 Hz and Jab (δ 5.60) for the cis product 15 at 7.5 Hz. Similarly, the respective coupling constants for 17, 19, 21, 22, 24, and 26 are 9.9, 10.0, 7.2, 9.0, 10.0 and 10.0 Hz.
It is important to point out that Mukai has synthesized an 8,12-guaianolide, (+)-achalensolide using a rhodium catalyzed allenic Pauson−Khand reaction.16a In contrast with the present approach, Mukai's synthesis requires 15 steps from isoascorbic acid for the assembly of an alleneyne possessing a protected diol in the tether. Five additional steps afford a cyclopentenone that is structurally related to 13 but lacking the α-methylene moiety on the butyrolactone. To install this moiety for the completion of the synthesis, a reduction, protection, deprotection and oxidation sequence of reactions on the cyclopentenone was required. Further comparisons between their APKR to the results within reveals theirs as high yielding, but higher temperatures and much longer reaction times were required (refluxing toluene, 24 h).
In summary, we have developed a Pauson-Khand reaction of α-methylene butyrolactone-containing allene-ynes to access the 5-7-5 ring system of 6,12-guaianolides. These facile and in many cases high yielding reactions suggest that incorporation of the α-methylene butyrolactone early in a synthetic sequence is a viable synthetic strategy to these targets. It is postulated that this moiety serves as a tether restricting conformational mobility of the allene-yne. Furthermore, the mild conditions of the rhodium catalyzed APKR are documented by the survival of both the 4-alkylidene cyclopentenone and the α-methylene butyrolactone through the reaction. This strategy also showcases the synthetic utility of Hall's allylboration/lactonization protocol and paves the way for biological studies of non-naturally occurring 6,12-guiaianolides.
Supplementary Material
Figure 1.
X-ray crystal structure of 22.
Acknowledgment
We thank The National Institutes of Health for financial support of this project (GM54161).
Footnotes
Supporting Information Available. Experimental procedures as well as 1H and 13C NMR spectra for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a Kitson RRA, Millemaggi A, Taylor RJK. Angew. Chem. Int. Ed. 2009;48:9426–9451. doi: 10.1002/anie.200903108. [DOI] [PubMed] [Google Scholar]; b Hoffmann HMR, Rabe J. Angew. Chem. Int. Ed. 1985;24:94–110. [Google Scholar]
- 2.Wong H-F, Brown GD. J. Nat. Prod. 2002;65:481–486. doi: 10.1021/np0103113. [DOI] [PubMed] [Google Scholar]
- 3.a Christensen S, Skytte D, Denmeade SR, Dionne C, Møller J, Nissen P, Isaacs JT. Anticancer Agents Med. Chem. 2009;9:276–294. doi: 10.2174/1871520610909030276. [DOI] [PubMed] [Google Scholar]; c Wagner S, Hofmann A, Siedle B, Terfloth L, Merfort I, Gasteiger J. J. Med. Chem. 2006;49:2241–2252. doi: 10.1021/jm051125n. [DOI] [PubMed] [Google Scholar]; d Bruno M, Rosselli S, Maggio A, Raccuglia RA, Bastow KF, Lee K. J. Nat. Prod. 2005;68:1042–1046. doi: 10.1021/np0500575. [DOI] [PubMed] [Google Scholar]; e Lee S, Kim H, Seo J, Kang H, Kim K, Son K, Lee H, Kwon B, Shin J, Seo Y. J. Org. Chem. 2002;67:7670–7675. doi: 10.1021/jo020299z. [DOI] [PubMed] [Google Scholar]; f Blanco JG, Gil RR, Bocco JL, Meragelman TL, Genti-Raimondi S, Flury A. J. Pharmacol. Exp. Ther. 2001;297:1099–1105. [PubMed] [Google Scholar]; g Dirsch VM, Stuppner H, Vollmar AM. Cancer Res. 2001;61:5817–5823. [PubMed] [Google Scholar]; h Yuuya S, Hagiwara H, Suzuki T, Ando M, Yamada A, Suda K, Kataoka T, Nagai K. J. Nat. Prod. 1999;62:22–30. doi: 10.1021/np980092u. [DOI] [PubMed] [Google Scholar]; i Giordano OS, Pestchanker MJ, Guerreiro E, Saad JR, Enriz RD, Rodriguez AM, Jauregui EA, Guzman J, Maria AOM, Wendel GH. J. Med. Chem. 1992;35:2452–2458. doi: 10.1021/jm00091a013. [DOI] [PubMed] [Google Scholar]
- 4.Konaklieva MI, Plotkin BJ. Mini Rev. Med. Chem. 2005;5:73–95. doi: 10.2174/1389557053402828. [DOI] [PubMed] [Google Scholar]
- 5.a Singh J, Petter RC, Baillie TA, Whitty A. Nat. Rev. Drug Discovery. 2011;10:307–317. doi: 10.1038/nrd3410. [DOI] [PubMed] [Google Scholar]; b Potashman M, Duggan ME. J. Med. Chem. 2009;52:1231–1246. doi: 10.1021/jm8008597. [DOI] [PubMed] [Google Scholar]
- 6.a Welsch ME, Snyder SA, Stockwell BR. Curr. Opin. Chem. Biol. 2010;14:347–361. doi: 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Rosen J, Gottfries J, Muresan S, Blacklund A, Oprea TI. J. Med. Chem. 2009;52:1953–1962. doi: 10.1021/jm801514w. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Renner S, Otterlo WA, Seoane MD, Mocklinghoff S, Hofmann B, Wetzel S, Schuffenhauer A, Ertl P, Oprea TI, Steinhilber D, Brunseld L, Rauh D, Wadlmann H. Nat. Chem. Biol. 2009;5:585–592. doi: 10.1038/nchembio.188. [DOI] [PubMed] [Google Scholar]; c Kunzmann MH, Staub I, Bottcher T, Sieber SA. Biochemistry. 2011;50:910–916. doi: 10.1021/bi101858g. [DOI] [PubMed] [Google Scholar]; d Elford TG, Ulaczykp-Lesanko A, De Pascale G, Wright GD, Hall DG. J. Comb. Chem. 2009;11:155–168. doi: 10.1021/cc8001487. [DOI] [PubMed] [Google Scholar]
- 7.For a recent review on the synthesis of trans-guaianolides and leading citations, see: Schall A, Reiser O. Eur. J. Org. Chem. 2008:2353–2364.
- 8.a Zhang W, Luo S, Fang F, Chen Q, Hu H, Jia X, Zhai H. J. Am. Chem. Soc. 2005;127:18–19. doi: 10.1021/ja0439219. [DOI] [PubMed] [Google Scholar]; b Blay G, Bargues V, Cardona L, Collado AM, Garcia B, Munoz MC, Pedro JR. J. Org. Chem. 2000;65:2138–2144. doi: 10.1021/jo991756n. [DOI] [PubMed] [Google Scholar]; c Greene AE. J. Am. Chem. Soc. 1980;102:5337–5343. [Google Scholar]
- 9.For selected examples, see: Ball M, Andrews SP, Wierschem F. Org. Lett. 2007;9:663–666. doi: 10.1021/ol062947x.Andrews SP, Ball M, Cleator E, Oliver S, Högenauer K, Simic O, Antonello A, Hünger U, Smith MD, Ley SV. Chem. Eur. J. 2007;13:5688–5712. doi: 10.1002/chem.200700302.Ley SV, Antonello A, Balskus EP, Booth DT, Christensen SB, Cleator E, Gold H, Högenauer K, Hünger U, Myers RM, Oliver SF, Simic O, Smith MD, Søhoel H, Woolford AJA. Proc. Natl. Acad. Sci. U.S.A. 2004;101:12073–12078. doi: 10.1073/pnas.0403300101.Oliver SF, Högenauer K, Simic O, Antonello A, Smith MD, Ley SV. Angew. Chem. Int. Ed. 2003;42:5996–6000. doi: 10.1002/anie.200353140.
- 10.a Devreese AA, Demuynck M, De Clercq PJ, Vanderwalle M. Tetrahedron. 1983;39:3039–3048. [Google Scholar]; b Termone D, De Clercq P, De Keukeleire D, Vanderwalle M. Synthesis. 1977:46–48. [Google Scholar]
- 11.a Carret S, Deprés J-P. Angew. Chem. Int. Ed. 2007;46:6870–6873. doi: 10.1002/anie.200702031. [DOI] [PubMed] [Google Scholar]; b Rigby JH, Senanayake C. J. Am. Chem. Soc. 1987;109:3147–3149. [Google Scholar]
- 12.Kalindindi S, Jeong WB, Schall A. Angew. Chem. Int. Ed. 2007;46:6361–6363. doi: 10.1002/anie.200701584. [DOI] [PubMed] [Google Scholar]
- 13.a Yang H, Qiao X, Li F, Ma H, Xie L, Xu X. Tetrahedron Lett. 2009;50:1110–1112. [Google Scholar]; b Kuroda C, Kobayashi K, Koito A, Anzai S. Bull Chem. Soc. Jpn. 2001;74:1947–1961. [Google Scholar]; c Gonzales AG, Galindo A, Afonso MM, Mansilla H, Palenzuela JA. Tetrahedron. 1988;44:4575–4584. [Google Scholar]
- 14.Gone JR, Wallock NJ, Lindeman S. Tetrahedron Lett. 2009;50:1023–1025. [Google Scholar]
- 15.a Hirose T, Miyakoshi N, Mukai C. J. Org. Chem. 2008;73:1061–1066. doi: 10.1021/jo702330y. [DOI] [PubMed] [Google Scholar]; b Lee E, Lim JW, Yoon CH, Sung Y-S, Kim YK. J. Am. Chem. Soc. 1997;119:8391–8392. [Google Scholar]; c Posner GH, Babiak KA, Loomis GL, Frazee WJ, Mittal RD, Karie IL. J. Am. Chem. Soc. 1980;102:7498–7505. [Google Scholar]
- 16.Bouwmeester HJ, Kodde J, Verstappen FWA, Altug IG, De Kraker J-W, Wallaart TE. Plant Physiol. 2002;129:134–144. doi: 10.1104/pp.001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a Drahl C, Cravatt BF, Sorensen EJ. Angew. Chem. Int. Ed. 2005;44:5788–5809. doi: 10.1002/anie.200500900. [DOI] [PubMed] [Google Scholar]; b Hall IH, Lee K-H, Mar EC, Starns CO. J. Med. Chem. 1977;20:376–378. doi: 10.1021/jm00213a003. [DOI] [PubMed] [Google Scholar]; c Kupchan SM, Fessler DC, Eakin MA, Giacobbe TJ. Science. 1970;168:376–378. doi: 10.1126/science.168.3929.376. [DOI] [PubMed] [Google Scholar]
- 18.a Brummond KM, Chen D, Davis MM. J. Org. Chem. 2008;73:5064–5068. doi: 10.1021/jo8007258. [DOI] [PubMed] [Google Scholar]; b Brummond KM, Gao D. Org. Lett. 2003;5:3491–3494. doi: 10.1021/ol035322x. [DOI] [PubMed] [Google Scholar]
- 19.For an example where the α-methylene butyrolactone moiety is incorporated early in the synthesis see: Elford G, Hall DG. J. Am. Chem. Soc. 2010;132:1488–1489. doi: 10.1021/ja9104478.
- 20.a Hall DG. Synlett. 2007:1644–1655. [Google Scholar]; b Rauniyar V, Hall DG. Angew. Chem. Int. Ed. 2006;45:2426–2428. doi: 10.1002/anie.200504432. [DOI] [PubMed] [Google Scholar]; c Kennedy JWJ, Hall DG. J. Org. Chem. 2004;69:4412–4428. doi: 10.1021/jo049773m. [DOI] [PubMed] [Google Scholar]; d Kennedy JWJ, Hall DG. J. Am. Chem. Soc. 2002;124:898–899. doi: 10.1021/ja016391e. [DOI] [PubMed] [Google Scholar]; e Kennedy JWJ, Hall DG. J. Am. Chem. Soc. 2002;124:11586–11587. doi: 10.1021/ja027453j. [DOI] [PubMed] [Google Scholar]
- 21.Chataigner I, Zammattio F, Lebreton J, Villiéras J. Tetrahedron. 2008;64:2441–2455. [Google Scholar]
- 22.a Krasovskiy A, Kopp F, Knochel P. Angew. Chem. Int. Ed. 2006;45:497–500. doi: 10.1002/anie.200502485. [DOI] [PubMed] [Google Scholar]; b Imamoto T, Takiyama N, Nakamura K. Tetrahedron Lett. 1985;26:4763–4236. [Google Scholar]; c Imamoto T, Sugiura Y, Takiyama N. Tetrahedron Lett. 1984;25:4233–4236. [Google Scholar]
- 23.Movassaghi M, Ahamd OK. J. Org. Chem. 2007;72:1838–1841. doi: 10.1021/jo062325p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitasev B. Ph.D. Thesis. University of Pittsburgh; USA: 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.