This editorial refers to ‘Determinants of magnetic resonance imaging detected carotid plaque components: the Rotterdam Study’†, by Q.J.A. van den Bouwhuijsen et al., on page 221
Atherosclerosis and its clinical sequelae are responsible for coronary artery disease, cerebrovascular complications, and peripheral arterial disease, a leading cause of mortality and morbidity in society.1 At the heart of the atherosclerotic disease cascade is the atherosclerotic plaque. For many years, pathology and post-mortem studies were the only method to evaluate the manifestation of atherosclerosis. The vulnerability of atherosclerotic plaques to rupture and cause cardiovascular and cerebrovascular events has long been associated with the presence of a thin fibrous cap with a large lipid core, endothelial denudation with platelet aggregation, presence of active inflammation, calcification, intraplaque haemorrhage, endothelial dysfunction, outward remodelling, and the presence of fissures.2 It has been well established that conventional cardiovascular disease risk factors and the use of the Framingham risk score do not fully explain the level of coronary or cerebrovascular events in asymptomatic individuals.3 Therefore, current research has focused on improving risk stratification using new methodologies to identify subjects at higher risk that can benefit from aggressive treatment.
Over the last decade or two, atherosclerotic plaque imaging using magnetic resonance (MR) has made significant strides and is quickly becoming the preferred methodology for non-invasive evaluation of atherosclerotic plaques in vivo.4 It allows classification of carotid plaques into high-risk and low-risk lesion types (I–VIII).5 MR plaque imaging typically involves acquisition of several contrasts, i.e. black blood proton density-weighted, T1-weighted, and T2-weighted for vessel wall evaluation, along with an angiography sequence for accurate lumen delineation. Based on signal intensities on the various imaging contrasts acquired, plaque components are characterized. A contrast-enhanced T1-weighted scan is often used to better delineate the fibrous cap and define a lipid-rich necrotic core (LRNC).6 Calcification is typically hypointense on all acquisitions, fresh hemorrhage is bright on T1, whereas lipid pools are also bright on T1 and proton density images, but lose signal on T2 sequences, and haemorrhage-related methaemoglobin remains bright on T2 images.
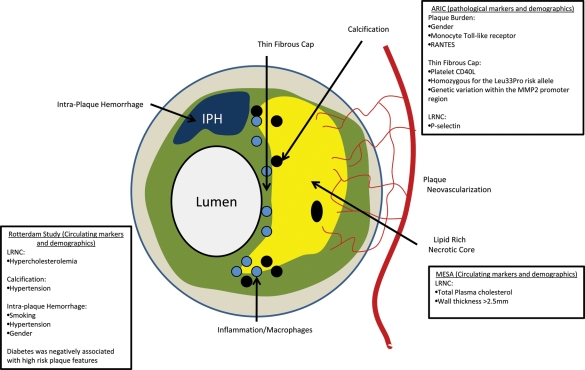
The Multi-Ethnic Study of Atherosclerosis (MESA) was the first epidemiology study that used MR imaging (Figure 1) to evaluate relationships between imaging and cardiovascular events.7 In a substudy of MESA involving carotid MRI in individuals with the highest carotid intima media thickness (IMT), total cholesterol was associated with the presence of LRNCs.8 Other studies in literature, such as the atherosclerosis risk in communities (ARIC) carotid MRI study9 (Figure 1), have also observed a strong correlation between carotid wall thickness and the presence of plaque features such as LRNCs and intraplaque haemorrhage.
Figure 1.
Risk factors associated with plaque characteristics seen on MRI in various trials.
van Bouwhuijsen et al. have now examined the determinants of MRI-detected components of plaque (Figure 1)as part of the Rotterdam Study in a large cohort of >1000 patients.10They investigate associations between cardiovascular risk factors and atherosclerotic lesion composition determined by MRI in asymptomatic individuals with ultrasound-derived carotid wall thickness >2.5mm. In agreement with the MESA study,8 their results indicate that individuals with hypercholesterolaemia were more likely to exhibit LRNCs. They also found that patients with hypertension were more likely to exhibit calcification and intraplaque haemorrhage; and smoking was correlated with intraplaque haemorrhage. Interestingly, and contradicting previous studies,11 they found that diabetes was not associated with high-risk lesion types based on composition. Gender was also an important determinant of plaque size as well as prevalence of intraplaque haemorrhage. Limitations of this study include the fact that the methodologies used for plaque classification were not standard and hence only partially validated previously. Additionally, reliability of detection of LRNCs was limited as no contrast-enhanced imaging was used. Furthermore, only the prevalence of high-risk plaque features was examined, and no quantification was performed.
As plaque imaging gains popularity and clinical trials that use imaging as an endpoint12 become more frequent, the need to select individuals with certain plaque characteristics as study subjects will exist. This study provides valuable data with regard to the traditional risk factors that determine plaque features that may be the target for intervention in such future clinical trials. Results of this study will help screen patients as candidates for early therapies and clinical trials that use imaging as an endpoint.13
Funding
Supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health R01 HL071021 and R01 HL078667 (to Z.A.F.).
Conflict of interest: none declared.
References
- 1.Fuster V, Kelly BB, Vedanthan R. Promoting global cardiovascular health: moving forward. Circulation. 2011;123:1671–1678. doi: 10.1161/CIRCULATIONAHA.110.009522. [DOI] [PubMed] [Google Scholar]
- 2.Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 3.Law MR, Wald NJ, Morris JK. The performance of blood pressure and other cardiovascular risk factors as screening tests for ischaemic heart disease and stroke. J Med Screen. 2004;11:3–7. doi: 10.1177/096914130301100102. [DOI] [PubMed] [Google Scholar]
- 4.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 5.Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106:1368–1373. doi: 10.1161/01.cir.0000028591.44554.f9. [DOI] [PubMed] [Google Scholar]
- 6.Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, Takaya N, Polissar NL, Yuan C. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437–3444. doi: 10.1161/CIRCULATIONAHA.104.528174. [DOI] [PubMed] [Google Scholar]
- 7.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 8.Wasserman BA, Sharrett AR, Lai S, Gomes AS, Cushman M, Folsom AR, Bild DE, Kronmal RA, Sinha S, Bluemke DA. Risk factor associations with the presence of a lipid core in carotid plaque of asymptomatic individuals using high-resolution MRI: the multi-ethnic study of atherosclerosis (MESA) Stroke. 2008;39:329–335. doi: 10.1161/STROKEAHA.107.498634. [DOI] [PubMed] [Google Scholar]
- 9.Wagenknecht L, Wasserman B, Chambless L, Coresh J, Folsom A, Mosley T, Ballantyne C, Sharrett R, Boerwinkle E. Correlates of carotid plaque presence and composition as measured by MRI: The atherosclerosis risk in communities study. Circ Cardiovasc Imaging. 2009;2:314–322. doi: 10.1161/CIRCIMAGING.108.823922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Bouwhuijsen QJA, Vernooij M, Hofman A, Krestin GP, van der Lugt A, Witteman JCM. Determinants of magnetic resonance imaging detected carotid plaque components: the Rotterdam Study. Eur Heart J. 2012;33:221–229. doi: 10.1093/eurheartj/ehr227. First published on 6 August 2011. doi:10.1093/eurheartj/ehr243. [DOI] [PubMed] [Google Scholar]
- 11.Esposito L, Saam T, Heider P, Bockelbrink A, Pelisek J, Sepp D, Feuer R, Winkler C, Liebig T, Holzer K, Pauly O, Sadikovic S, Hemmer B, Poppert H. MRI plaque imaging reveals high-risk carotid plaques especially in diabetic patients irrespective of the degree of stenosis. BMC Med Imaging. 2010;10:27. doi: 10.1186/1471-2342-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muntendam P, McCall C, Sanz J, Falk E, Fuster V High-Risk Plaque Initiative. The BioImage Study: novel approaches to risk assessment in the primary prevention of atherosclerotic cardiovascular disease—study design and objectives. Am Heart J. 2010;160:49–57. doi: 10.1016/j.ahj.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]