Abstract
Aims
Lipoprotein-associated phospholipase A2 (Lp-PLA2) generates proinflammatory and proatherogenic compounds in the arterial vascular wall and is a potential therapeutic target in coronary heart disease (CHD). We searched for genetic loci related to Lp-PLA2 mass or activity by a genome-wide association study as part of the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium.
Methods and results
In meta-analyses of findings from five population-based studies, comprising 13 664 subjects, variants at two loci (PLA2G7, CETP) were associated with Lp-PLA2 mass. The strongest signal was at rs1805017 in PLA2G7 [P = 2.4 × 10−23, log Lp-PLA2 difference per allele (beta): 0.043]. Variants at six loci were associated with Lp-PLA2 activity (PLA2G7, APOC1, CELSR2, LDL, ZNF259, SCARB1), among which the strongest signals were at rs4420638, near the APOE–APOC1–APOC4–APOC2 cluster [P = 4.9 × 10−30; log Lp-PLA2 difference per allele (beta): −0.054]. There were no significant gene–environment interactions between these eight polymorphisms associated with Lp-PLA2 mass or activity and age, sex, body mass index, or smoking status. Four of the polymorphisms (in APOC1, CELSR2, SCARB1, ZNF259), but not PLA2G7, were significantly associated with CHD in a second study.
Conclusion
Levels of Lp-PLA2 mass and activity were associated with PLA2G7, the gene coding for this protein. Lipoprotein-associated phospholipase A2 activity was also strongly associated with genetic variants related to low-density lipoprotein cholesterol levels.
Keywords: Genome-wide association, Inflammation, Lipoprotein-associated phospholipase A2
Introduction
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a 45.4-kDa calcium-independent member of the phospholipase A2 family, which is secreted by leucocytes1 and has been detected in rabbit and human atherosclerotic lesions.2 In the bloodstream, two-thirds of Lp-PLA2 circulates primarily bound to LDL; the remaining third is distributed between HDL and VLDL. Circulating Lp-PLA2 can be measured by different assays ascertaining mass or activity of Lp-PLA2. However, there is only moderate correlation between mass-based and activity-based measurements of Lp-PLA2 (r = 0.51),3 and the independent role of these two measures in cardiovascular disease (CVD) is unclear.
Several lines of evidence suggest that Lp-PLA2 is associated with the development of atherosclerotic disease. Lipoprotein-associated phospholipase A2 generates pro-inflammatory and pro-atherogenic compounds in the arterial vascular wall. A large meta-analysis with almost 80 000 participants in 32 prospective studies showed that high levels of Lp-PLA2 mass and activity were associated with the risk for coronary heart disease (CHD), stroke, and cardiovascular mortality.3 Therefore, Lp-PLA2 might represent an emerging biomarker for improved cardiovascular risk assessment in clinical practice and a potential therapeutic target for primary or secondary prevention of CVD.
Although familial factors explain about one-half and one-quarter of the variance in Lp-PLA2 activity and mass, respectively,4,5 few genetic determinants of Lp-PLA2 have been identified so far.6 Therefore, to better understand genetic control of Lp-PLA2, we investigated genetic loci related to Lp-PLA2 mass or activity by conducting genome-wide association (GWA) analyses in five population-based studies, as part of the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium.7
Methods
For more detailed information on the Methods, see Supplementary material online.
Study data for meta-analysis
We used data from five community-based cohorts in the USA and Europe: Atherosclerosis Risk in Communities (ARIC) Study, Cardiovascular Health Study (CHS), Framingham Heart Study (FHS), Rotterdam Study (RS), and Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA)/Cooperative Health Research in the Region of Augsburg Study (KORA). Baseline clinical and demographic characteristics were assessed at the time of cohort entry for CHS, KORA, and RS and at the time of biomarker collection for ARIC and FHS. All participants gave written informed consent, including consent for genetic studies. All studies received approval from local ethical oversight committees.
Associations between significant single nucleotide polymorphisms (SNPs) and CHD/coronary artery disease (CAD) were assessed in the Coronary Artery Disease Genome-Wide Replication and Meta-Analysis (CARDIoGRAM) consortium data. CARDIoGRAM includes over 22 000 cases with CAD, MI, or both and over 60 000 controls from all published and several unpublished genome-wide association studies (GWAS) in individuals of European descent.8
Lipoprotein-associated phospholipase A2 mass concentrations were measured in ARIC, CHS, FHS, and KORA using a commercially available sandwich enzyme immunoassay (PLAC® test; diaDexus, Inc., San Francisco, CA, USA). Lipoprotein-associated phospholipase A2 activity was measured in CHS, FHS, and RS on microtiter plates by colourimetric or radioactive substrate methods (diaDexus CAM Kit, diaDexus, Inc., San Francisco, CA, USA or Perkin Elmer Life Sciences, Inc., Waltham, MA, USA).
Genotyping was performed in each cohort using high-density SNP marker platforms. Genotypes were imputed to ∼2.5 million HapMap SNPs (platform and genetic analysis details provided in Supplementary material online).
Statistical analyses
Associations between genotype and Lp-PLA2 mass and activity were analysed separately within each cohort, using linear regression of natural log-transformed phenotype on number (or imputed dose) of reference alleles, i.e. an additive model (for more details, see Supplementary material online). All analyses were adjusted for age, sex, and if applicable, recruitment site. To assess whether classical cardiovascular risk factors confounded the associations, analyses were additionally adjusted for diabetes, lipid-lowering medication, antihypertensive treatment, aspirin intake ≥3 per week, current smoking, hormone replacement therapy (HRT; coded men, women without HRT, women with HRT), body mass index (BMI), systolic blood pressure, diastolic blood pressure, triglycerides, waist circumference, HDL-C, LDL-C, and prevalent CVD. For the most significant SNPs, we also analysed interactions between genetic variant and age, sex, BMI, or smoking status.
To combine results across cohorts, we performed an inverse variance–weighted meta-analysis using the software METAL,9 which was specifically developed for meta-analysing GWAS results. A meta-analysis is a statistical method that combines analyses from different independent studies to give an overall effect measure. In the case of meta-analysing different GWAS, this approach increases the power to detect significant genetic variants compared with analysing each GWAS alone.10 Cohort-specific standard errors were adjusted using genomic control.11 In GWAS, we chose P = 5 × 10−8 as the threshold for significance12 (see Supplementary material online). To determine whether the multiple SNPs associated in the respective region are due to linkage disequilibrium (LD) with the top SNP or if there are multiple independent signals, we performed a meta-analysis based on models adjusted additionally for the SNPs with the smallest P-values.
Results
Sample size, demographics, and laboratory characteristics of each cohort are presented in Table 1. Median values for Lp-PLA2 mass (241.8 ng/mL in FHS to 413.0 ng/mL in ARIC) and activity (38.2 nmol/min/mL in CHS to 153.9 nmol/min/mL in FHS) varied substantially across cohorts, but much of these differences may be explained by the different assays used.
Table 1.
Characteristics of study participants (n = 13 664)
| Study sample |
|||||
|---|---|---|---|---|---|
| ARIC | CHS | FHS | RS | KORA | |
| Number | 798 | 3217 | 6909 | 1538 | 1202 |
| Age (years) | 58.6 ± 5.5 | 72.3 ± 5.3 | 49.3 ± 13.8 | 69.1 ± 9.0 | 53.5 ± 8.9 |
| Male (%) | 62 | 38.5 | 46.9 | 39.5 | 52.6 |
| Current smoking (%) | 24.2 | 11.3 | 15.7 | 23.5 | 17.7 |
| BMI (kg/m2) | 28.0 ± 4.8 | 26.3 ± 4.5 | 27.4 ± 5.4 | 26.2 ± 3.6 | 27.4 ± 4.1 |
| Waist (cm) | 99.8 ± 13.4 | 93.1 ± 12.9 | 96.0 ± 15.0 | 90.2 ± 11.0 | 91.0 ± 12.7 |
| Systolic blood pressure (mmHg) | 125 ± 19 | 135 ± 21 | 121 ± 17 | 138 ± 22 | 134 ± 19 |
| Diastolic blood pressure (mmHg) | 73 ± 10 | 70 ± 11 | 75 ± 10 | 73 ± 11 | 82 ± 11 |
| Hypertension treatment (%) | 34.2 | 30.2 | 19.3 | 31.1 | 17.5 |
| Lipid-lowering medication (%) | 9.4 | 4.4 | 13.3 | 2.2 | 4.6 |
| Aspirin ≥3 per week (%) | — | 28.1 | 18.5 | — | 5.1 |
| Total cholesterol (mg/dL) | 214 ± 42 | 213 ± 39 | 194 ± 37 | 256 ± 50 | 238 ± 44 |
| HDL-C (mg/dL) | 44 ± 15 | 55 ± 16 | 54 ± 17 | 52 ± 15 | 54 ± 16 |
| LDL-C (mg/dL) | 138 ± 36 | 130 ± 35 | 115 ± 32 | — | 147 ± 41 |
| Total/HDL-C ratio | 5.3 (2.2) | 4.1 ± 1.2 | 3.9 ± 1.4 | 5.3 ± 1.6 | 4.8 ± 1.8 |
| Triglycerides (mg/dL) | 159 ± 130 | — | 125 ± 90 | — | 187 ± 150 |
| Prevalent diabetes (%) | 18.6 | 11.7 | 7.5 | 9.9 | 4.7 |
| Prevalent CVDa (%) | 0 | 0 | 6.2 | 7.4 | 1.9 |
| Hormone-replacement therapy (in women) (%) | 29.6 | 8.6 | 16.4 | 21.3 | 28.6 |
| Lp-PLA2 mass (ng/mL) | 413.0 (328.5/513.5) | 329.9 (261.4/407.4) | 241.8 (208.0/299.3) | — | 269.0 (214.0/330.5) |
| Lp-PLA2 activity (nmol/mL/min) | — | 38.2 (30.9/47.0) | 153.9 (129.2/181.2) | 44.5 (36.3/51.3) | — |
Mean ± SD for continuous, per cent for categorical variables, median (25th/75th percentile) for Lp-PLA2 concentrations.
aHistory of myocardial infarction, angina, coronary revascularization, stroke, or transient ischemic attack.
Genome-wide association of lipoprotein-associated phospholipase A2 mass and activity
The meta-analysis included 2 661 766 SNPs from one or more studies. Genomic control (λgc) parameters were small (all λgc≤ 1.058), suggesting negligible inflation in the type-I error rate.
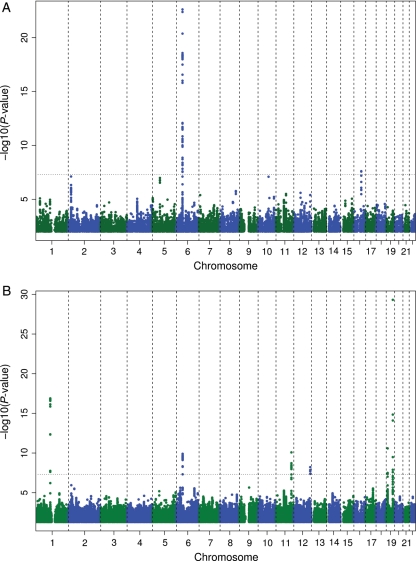
Figure 1A and B illustrates the primary findings from the meta-analysis; details are in Table 2. In total, 49 SNPs were significantly associated with Lp-PLA2 mass and 59 with Lp-PLA2 activity. For Lp-PLA2 mass, these signals clustered at two loci on chromosomes 6 and 16. For activity, the SNPs clustered at loci on chromosomes 1, 6, 11, 12, and (two clusters) 19 (Supplementary material online, Tables S1 and S2).
Figure 1.
Association of log-transformed lipoprotein-associated phospholipase A2 mass (A) and lipoprotein-associated phospholipase A2 activity (B) concentrations and 2 661 766 single nucleotide polymorphisms displayed per chromosome and region. The dashed line indicates the significance threshold of P < 5 × 10−8.
Table 2.
Association of the top single nucleotide polymorphisms in eight loci with log-transformed lipoprotein-associated phospholipase A2, adjusted for age, sex, and site (if necessary)
| SNP | Mass |
Activity |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ARIC | CHS | FHS | KORA | Meta-analysis | CHS | FHS | RS | Meta-analysis | ||
| rs4420638 (G/A) | Sample size | 798 | 3217 | 6899 | 1202 | 12 116 | 3217 | 6899 | 1538 | 11654 |
| Chromosome: 19 | P-value | 4.3E–04 | 1.9E–01 | 7.9E–01 | 5.1E–02 | 8.6E–02 | 5.4E–06 | 1.6E–19 | 3.9E–10 | 4.9E–30 |
| Location: 50114786 | Beta | −0.075 | −0.023 | 0.002 | −0.034 | −0.009 | −0.071 | −0.048 | −0.099 | −0.054 |
| Gene: APOC1 | Standard error | 0.021 | 0.017 | 0.006 | 0.017 | 0.005 | 0.016 | 0.005 | 0.016 | 0.005 |
| Effect allele: A | r2 | 0.0140 | 0.0005 | 0.0009 | 0.0032 | — | 0.0053 | 0.0162 | 0.0240 | — |
| Effect allele frequency | 0.82 | 0.83 | 0.84 | 0.82 | — | 0.83 | 0.84 | 0.80 | — | |
| I2 | 0.803 | 0.816 | ||||||||
| rs1805017 (C/T) | Sample size | 798 | 3217 | 6896 | 1202 | 12113 | 3217 | 6896 | 1538 | 11 651 |
| Chromosome: 6 | P-value | 2.6E–02 | 9.5E–08 | 5.0E–14 | 1.4E–03 | 2.4E–23 | 1.8E–02 | 1.8E–04 | 7.1E–02 | 2.4E–06 |
| Location: 46792181 | Beta | 0.043 | 0.051 | 0.040 | 0.052 | 0.043 | −0.021 | −0.017 | −0.019 | −0.018 |
| Gene: PLA2G7 | Standard error | 0.019 | 0.010 | 0.005 | 0.016 | 0.004 | 0.009 | 0.004 | 0.010 | 0.004 |
| Effect allele: T | r2 | 0.0056 | 0.0085 | 0.0097 | 0.0085 | — | 0.0016 | 0.0062 | 0.0014 | — |
| Effect allele frequency | 0.25 | 0.27 | 0.26 | 0.23 | — | 0.27 | 0.26 | 0.26 | — | |
| I2 | 0 | 0 | ||||||||
| rs7528419 (G/A) | Sample size | NA | 3217 | 6907 | NA | 10 124 | 3217 | 6907 | 1538 | 11662 |
| Chromosome: 1 | P-value | NA | 2.6E–03 | 4.9E–03 | NA | 7.1E–05 | 1.2E–05 | 9.4E–12 | 2.6E–03 | 1.3E–17 |
| Location: 109618715 | Beta | NA | 0.029 | 0.017 | NA | 0.020 | 0.041 | 0.034 | 0.033 | 0.035 |
| Gene: CELSR2 | Standard error | NA | 0.010 | 0.006 | NA | 0.005 | 0.009 | 0.005 | 0.011 | 0.004 |
| Effect allele: A | r2 | NA | 0.0026 | 0.0022 | NA | — | 0.0054 | 0.0107 | 0.0051 | — |
| Effect allele frequency | NA | 0.78 | 0.79 | NA | — | 0.78 | 0.79 | 0.76 | — | |
| I2 | 0.096 | 0 | ||||||||
| rs6511720 (G/T) | Sample size | 798 | 3217 | 6907 | 1202 | 12 124 | 3217 | 6907 | 1538 | 11662 |
| Chromosome: 19 | P-value | 2.4E–01 | 1.6E–03 | 3.1E–02 | 2.8E–01 | 5.5E–05 | 9.9E–10 | 3.1E–03 | 5.2E–03 | 2.6E–11 |
| Location: 11063306 | Beta | −0.043 | −0.039 | −0.025 | −0.035 | −0.032 | −0.071 | −0.029 | −0.040 | −0.045 |
| Gene: LDLR | Standard error | 0.036 | 0.012 | 0.012 | 0.033 | 0.008 | 0.012 | 0.010 | 0.014 | 0.007 |
| Effect allele: T | r2 | 0.0016 | 0.0029 | 0.0016 | 0.0010 | — | 0.0101 | 0.0045 | 0.0043 | — |
| Effect allele frequency | 0.10 | 0.13 | 0.10 | 0.08 | — | 0.13 | 0.10 | 0.12 | — | |
| I2 | 0 | 0.739 | ||||||||
| rs964184 (G/C) | Sample size | 798 | 3217 | 6907 | 1202 | 12 124 | 3217 | 6907 | 1538 | 11662 |
| Chromosome: 11 | P-value | 2.7E–01 | 1.6E–02 | 1.3E–01 | 2.8E–01 | 5.3E–03 | 1.4E–03 | 6.3E–08 | 4.1E–02 | 8.4E-11 |
| Location: 116154127 | Beta | −0.026 | −0.034 | −0.010 | −0.020 | −0.016 | −0.044 | −0.030 | −0.029 | −0.032 |
| Gene: ZNF259 | Standard error | 0.023 | 0.014 | 0.007 | 0.019 | 0.006 | 0.014 | 0.006 | 0.014 | 0.005 |
| Effect allele: C | r2 | 0.0014 | 0.0017 | 0.0013 | 0.0010 | — | 0.0030 | 0.0088 | 0.0020 | — |
| Effect allele frequency | 0.85 | 0.87 | 0.86 | 0.87 | — | 0.87 | 0.86 | 0.87 | — | |
| I2 | 0 | 0 | ||||||||
| rs7756935 (C/A) | Sample size | 798 | 3217 | 6907 | 1202 | 12 124 | 3217 | 6907 | 1538 | 11662 |
| Chromosome: 6 | P-value | 2.8E–01 | 9.2E–02 | 1.5E–01 | 3.3E–01 | 7.2E–01 | 1.5E–03 | 4.9E–06 | 2.1E–04 | 1.3E–10 |
| Location: 46782984 | Beta | 0.021 | 0.017 | −0.009 | 0.016 | −0.002 | −0.030 | −0.023 | −0.043 | −0.027 |
| Gene: PLA2G7 | Standard error | 0.020 | 0.010 | 0.006 | 0.016 | 0.005 | 0.010 | 0.005 | 0.012 | 0.004 |
| Effect allele: A | r2 | 0.0013 | 0.0009 | 0.0012 | 0.0008 | — | 0.0030 | 0.0072 | 0.0081 | — |
| Effect allele frequency | 0.78 | 0.79 | 0.81 | 0.79 | — | 0.79 | 0.81 | 0.8 | — | |
| I2 | 0.568 | 0.255 | ||||||||
| rs10846744 (G/C) | Sample size | NA | NA | 6904 | 1202 | 8106 | NA | 6904 | 1538 | 8442 |
| Chromosome: 12 | P-value | NA | NA | 3.3E–05 | 7.1E–01 | 4.9E–05 | NA | 2.0E–08 | 9.9E–02 | 6.1E–09 |
| Location: 123878378 | Beta | NA | NA | 0.026 | 0.007 | 0.025 | NA | 0.030 | 0.023 | 0.029 |
| Gene: SCARB1 | Standard error | NA | NA | 0.006 | 0.020 | 0.006 | NA | 0.005 | 0.014 | 0.005 |
| Effect allele: C | r2 | NA | NA | 0.0041 | 0.0001 | — | NA | 0.0086 | 0.0011 | — |
| Effect allele frequency | NA | NA | 0.16 | 0.16 | — | NA | 0.16 | 0.14 | — | |
| I2 | 0 | 0.066 | ||||||||
| rs247616 (C/T) | Sample size | 798 | 3217 | 6907 | 1202 | 12124 | 3217 | 6907 | 1538 | 11 662 |
| Chromosome: 16 | P-value | 3.3E–01 | 3.1E–01 | 6.0E–07 | 6.1E–04 | 2.5E–08 | 6.4E–01 | 8.4E–01 | 2.1E–01 | 4.2E–01 |
| Location: 55547091 | Beta | 0.022 | 0.008 | 0.026 | 0.059 | 0.023 | −0.004 | −0.001 | −0.013 | −0.003 |
| Gene: CETP | Standard error | 0.022 | 0.008 | 0.005 | 0.017 | 0.004 | 0.008 | 0.004 | 0.010 | 0.004 |
| Effect allele: T | r2 | 0.0011 | 0.0003 | 0.0043 | 0.0098 | — | 0.0001 | 0.0034 | 0.0004 | — |
| Effect allele frequency | 0.33 | 0.34 | 0.32 | 0.34 | — | 0.34 | 0.32 | 0.33 | — | |
| I2 | 0.618 | 0 | ||||||||
Significant SNPs in the meta-analysis with respective data from each cohort are marked bold.
r2: increase in r2 after adding the SNP to a linear regression model with log Lp-PLA2 as outcome and age and sex (+site, if necessary) in the model.
I2: measure of heterogeneity.
Lipoprotein-associated phospholipase A2 mass meta-analysis
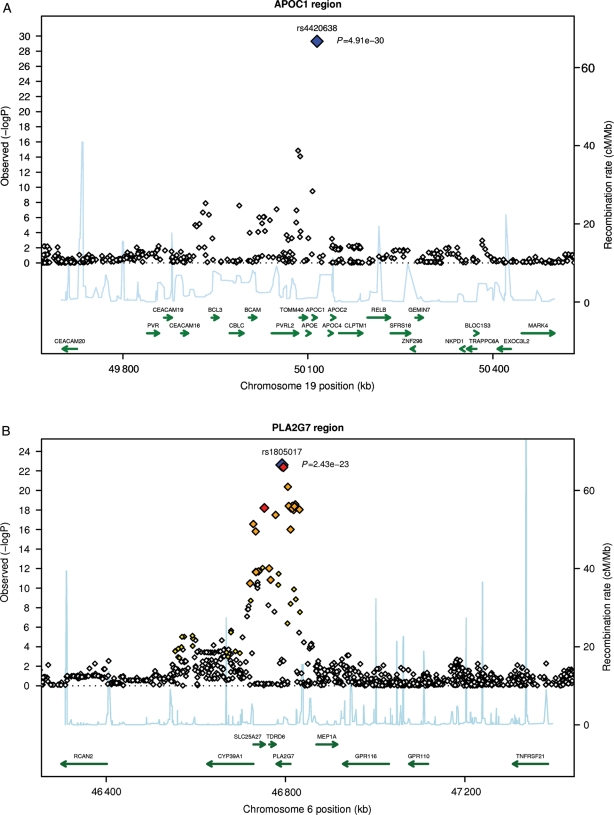
Of the 49 SNPs significantly associated with Lp-PLA2 mass, 47 were within the Lp-PLA2 gene (PLA2G7), at chromosome 6p21.2-p12. The strongest association [P = 2.4 × 10−23; log Lp-PLA2 difference per allele (beta): 0.043] was for rs1805017 (Figure 2A), which is located within the coding region of PLA2G7 in exon 4 and leads to an arginine-to-histidine substitution at position 92 (Arg92His). Supplementary material online, Table S3 shows unadjusted median values of Lp-PLA2 mass by rs1805017 genotype for each cohort.
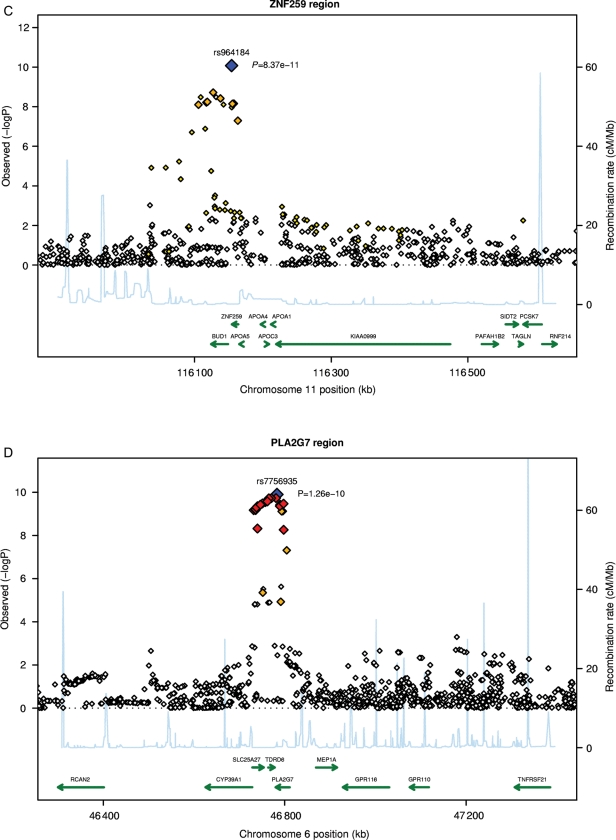
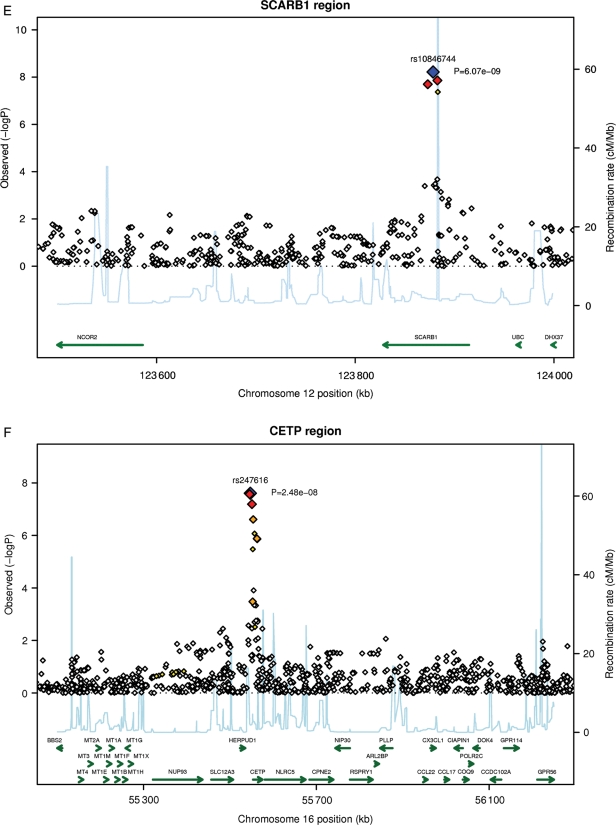
Figure 2.
Continued.
Figure 2.
Continued.
Figure 2.
Regional plots for top single nucleotide polymorphisms rs4420638 (A), rs1805017 (B), rs964184 (C), rs7756935 (D), rs10846744 (E), and rs247616 (F). LD: red: r2 (with primary SNP) >0.8; orange: r2 between 0.5 and 0.8; yellow: r2 between 0.2 and 0.5; white: r2 < 0.2.
Based on pairwise LD (based on HapMap CEU) coefficients, 27 of the 47 signals with PLA2G7 lie within the same highly preserved haplotype block (Lewontin's D′ > 0.99). The remaining 21 associated SNPs in this region were correlated with rs1805017 (r² < 0.83).
Lp-PLA2 mass was also associated with SNPs on chromosome 16 within the cholesteryl ester transfer protein (CETP) gene (CETP; Figure 2F): rs247616 (P = 2.5 × 10−8; beta: 0.023).
There were no significant interactions between age, sex, BMI, or smoking and the two most significant SNPs in each region (rs1805017 and rs247616) in relation to Lp-PLA2 mass (Supplementary material online, Table S4). The association with Lp-PLA2 remained significant for these two SNPs after multivariableadjustment with similar effect sizes and P-values (Supplementary material online, Table S5).
Lipoprotein-associated phospholipase A2 activity meta-analysis
The smallest P-value for association with Lp-PLA2 activity (P = 4.9 × 10−30; beta: −0.054) was for rs4420638 (Figure 2B), located on chromosome 19 near the APOE–APOC1–APOC4–APOC2 cluster and ∼11 kb proximal to the APOE-ɛ4 allele. The association was similar in all three studies, with beta coefficients of −0.099 to −0.048 (Table 2). Other SNPs with significant associations were rs7528419 (CELSR2), rs6511720 (LDLR), rs964184 (ZNF259), and rs10846744 (SCARB1) (Table 2). Lipoprotein-associated phospholipase A2 activity was also associated with PLA2G7. The strongest association was for rs7756935 (P = 1.3 × 10−10; beta: −0.027), which was in perfect LD with another functional SNP within the PLA2G7 gene, rs1051931 in exon 11 (Ala379Val).
There were no significant gene–environment interactions of age, sex, BMI, and smoking status with the top SNPs in six loci in relation to log-transformed Lp-PLA2 activity (Supplementary material online, Table S4).
Association of top single nucleotide polymorphisms with prevalent coronary heart disease/coronary artery disease
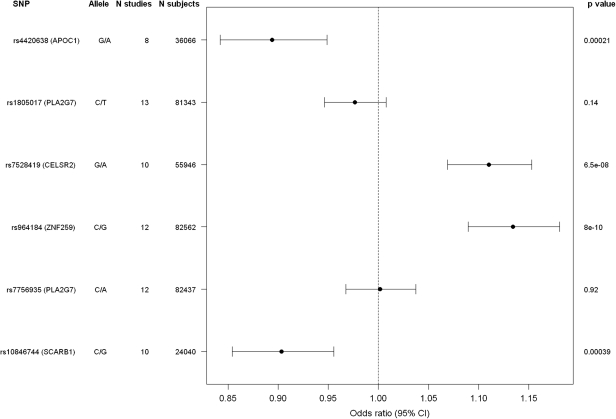
For all but two (rs247616, CETP; rs6511720, LDLR) of the top SNPs, the association to CHD/CAD could be assessed in the CARDIoGRAM project. Four of the top SNPs related to Lp-PLA2 activity [rs964184 (ZNF259), rs4420638 (APOC1), rs7528419 (CELSR2), rs10846744 (SCARB1)] were significantly associated with prevalent CHD/CAD (Figure 3). The strongest association was found for rs964184 with a P = 8.0 × 10−10, with an odds ratio of 1.13 per G allele (95% confidence interval 1.09–1.18). The two SNPs in the PLA2G7 gene most significantly associated with Lp-PLA2 mass or activity were not significantly associated with prevalent CHD/CAD in CARDIoGRAM. The estimated changes in Lp-PLA2 mass or activity per risk allele revealed in our meta-analysis were combined with the CHD risk estimations related to Lp-PLA2 mass or activity drawn from a published meta-analysis3 to estimate the expected CHD risk changes per allele. For SD values (necessary to calculate the expected risks), we chose the respective data from FHS, as of the five cohorts FHS had the largest number of participants. This approach revealed CHD risk increases between 0.8 and 2.1% per allele and were found to be mostly smaller than that found in the CARDIoGRAM data. The CARDIoGRAM study had ≥70% power to detect CHD risk changes of 3.5% or more (Supplementary material online, Table S6).
Figure 3.
Association of the top single nucleotide polymorphisms in six loci with prevalent CHD/CAD in the CARDIoGRAM consortium.
Discussion
Meta-analysis of GWAS from five population-based studies comprising 13 664 subjects suggests that genes influencing Lp-PLA2 mass and activity differ. While enzyme concentration was regulated mainly by the gene coding for Lp-PLA2 (PLA2G7), genetic variants involved in lipid metabolism were strongly associated with Lp-PLA2 activity.
Lipoprotein-associated phospholipase A2 mass
Lipoprotein-associated phospholipase A2 mass was dependent on variants within PLA2G7. Previous studies identified numerous SNPs within PLA2G7: variants in exon 9 (Val279Phe; rs45619133), exon 11 (Val379Ala; rs1051931), exon 7 (Ile198Thr, Iso195Thr; rs1805018), and exon 4 (Arg92His; rs1805017).13 A prior GWA of Framingham data accessed through dbGaP also identified multiple SNPs clustered in or near PLA2G7 that were associated with Lp-PLA2 mass.6
Lipoprotein-associated phospholipase A2 mass was also influenced by rs247616 (CETP).14 The T allele of rs247616 was associated with higher HDL-C concentration14 and higher mean Lp-PLA2 mass. This finding is in contrast to most epidemiological studies, including ARIC15and Framingham,6 which showed an inverse relation between HDL-C level and Lp-PLA2 mass.3 The mechanism(s) by which reduced CETP activity leading to increased HDL-C would also increase Lp-PLA2 mass is unclear.
Lipoprotein-associated phospholipase A2 activity
All the SNPs associated with Lp-PLA2 activity in our meta-analysis also determine plasma lipoprotein concentrations except for those in PLA2G7. The strongest association for Lp-PLA2 activity was for rs4420638 (APOC1), previously associated with LDL-C concentrations.14,16 rs964184 (ZNF259), near the APOA5–APOA4–APOC3–APOA1 cluster, was associated with increased Lp-PLA2 activity and previously with increased triglycerides;14 elevated triglycerides are associated with increased small dense LDLs, which contain increased Lp-PLA2 mass.17
rs7528419 (CELSR2) located at 1q13.3, which also contains the genes PSRC1 and SORT1, has strong associations with LDL-C levels and CHD.14,16,18 Based on HapMap data, rs7528419 was highly correlated with rs599839 (PSRC1) and rs646776 (CELSR2); minor alleles of rs599839 and rs646776 have been associated with lower LDL-C14,16,18 and lower CHD risk.19 The minor allele of rs7528419 also was associated with lower Lp-PLA2 activity, consistent with the reduction in LDL-C in other studies.14,16,18 rs7528419, or a SNP in LD, might have functional effects due to its location in 3′ UTR of the CELSR2 gene (encoding for cadherin, EGF LAG seven-pass G-type receptor 2), which includes binding sites for transcriptional factors (e.g. Oct-1).
rs6511720 (LDLR) was also associated with lower Lp-PLA2 activity and lower LDL-C.18 It was highly correlated with rs2228671 (LDLR) (r2 = 0.734; D′ = 0.899), which was related to lower LDL-C and reduced CAD risk.20
rs10846744, an intronic SNP within the SCARB1 gene, which encodes for scavenger receptor type B class 1 (SRB1), a major receptor for HDL, was associated with Lp-PLA2 activity. Although this SNP was not associated with lipids in this study, other SNPs in SCARB1 have been shown to be associated with levels of HDL-C.21,22 SCARB1 may also modulate Lp-PLA2 activity through its role as a scavenger receptor for oxidized LDL, which has been shown to increase Lp-PLA2 secretion by human macrophages.23 SCARB1 is expressed in macrophages and has been shown to bind avidly to oxidized LDL.24
Finally, rs7756935 (PLA2G7), associated with increased Lp-PLA2 activity, was in complete LD with rs1051931 (PLA2G7; Ala379Val).24 The V379 allele resulted in two-fold lower Lp-PLA2 activity in in vitro studies25 but increased plasma Lp-PLA2 activity in epidemiological studies.26,27
Association of top single nucleotide polymorphisms with prevalent coronary heart disease/coronary artery disease
Of the top polymorphisms associated with Lp-PLA2 activity, four (in ZNF259, APOC1, CELSR2, SCARB1) were significantly associated with CHD or CAD, as also reported in previous studies.14,16,18,20,28 However, the SNPs in the PLA2G7 gene that were most significantly associated with Lp-PLA2 mass (rs1805017) or activity (rs7756935) were not associated with prevalent CHD or CAD. These findings are consistent with Casas et al.,27 who found a significant association between Lp-PLA2 activity and CHD but did not find any significant association with PLA2G7 genotype (12 tagged SNPs including rs1805017). We had >97% power to detect a 5% increase in risk with these two SNPs, but only 35–40% power to detect a 2.5% increase in risk (Supplementary material online, Table S6); therefore, if there is an increased risk, it is most likely in the range of a few percent or less. However, considering the modest effect of these common alleles of the PLA2G7 locus on Lp-PLA2 mass and activity (Supplementary material online, Table S5) and the modest strength of the association of Lp-PLA2 mass and activity with CHD,3 we may have had insufficient power to detect a genetic association that would still be compatible with a causal role for Lp-PLA2 in CHD, despite our large sample size. In contrast to our findings and those of Casas et al., Sutton et al.29 reported significant associations between SNPs in PLA2G7, including rs1805017, with CAD in case–control and family data sets. This suggests that the nature of the population, or in fact having familial data, may influence the strength and likelihood of observing a significant association.
The Val279Phe substitution is located within the catalytical domain of the encoded enzyme and therefore leads to reduction (heterozygotes) or complete loss (homozygotes) of enzyme activity. In Japan, this mutation occurs in 30% of the population (27% heterozygous, 4% homozygous and therefore completely lacking plasma Lp-PLA2 activity and mass).30 Although Val279Phe-mediated loss of activity was an independent CHD risk factor in Japanese men with hypercholesterolaemia, myocardial infarction, stroke, or non-familial dilated and hypertrophic cardiomyopathies and protective from CAD in South Korean men,31 no association with CHD was observed in a Chinese population.32
Clinical implications
In the present meta-analysis, we identified eight SNPs associated with Lp-PLA2 mass or activity which might contribute to the regulation of plasma levels and give insight as to whether Lp-PLA2 is a risk factor or risk marker for CHD. Most of these genetic loci were in regions that have already been associated with levels of lipoproteins. Of the six top SNPs that could be tested in the CARDIoGRAM consortium, four located in ZNF259, APOC1, CELSR2, and SCARB1 were significantly associated with CHD, whereas the two SNPs in PLA2G7, the gene that encodes for Lp-PLA2, did not have any significant association with CHD. This supports the hypothesis that the genetic regions which are known to affect lipoprotein levels also influence both the level of Lp-PLA2 and the development of CHD and is consistent with the hypothesis that Lp-PLA2 level may be a marker of atherogenic lipoproteins; levels of Lp-PLA2 have been shown to be increased in subpopulations of lipoproteins such as electronegative LDL. Although the lack of association does not provide any evidence in support of the hypothesis that Lp-PLA2 is a risk factor for CHD, our data cannot rule out the possibility that Lp-PLA2 is a risk factor. First, despite the large number of CHD cases in CARDIoGRAM, we lacked sufficient power to rule out a small effect. The SNPs identified in PLA2G7 had modest effects on the levels of Lp-PLA2 mass or activity and would be expected to have even smaller effects on risk for development of CHD. Furthermore, we measured total Lp-PLA2 mass and activity in plasma, not the mass or activity associated with LDL or HDL. It is possible that genes which alter lipoprotein metabolism may also alter the binding of Lp-PLA2 to subspecies of lipoproteins,17 and Lp-PLA2 may only be atherogenic when it is associated with atherogenic lipoproteins such as LDL, not with HDL. Our observation that a SNP in the CETP locus, which is associated with lower CETP activity and higher levels of Lp-PLA2 mass, may have implications for clinical programmes that are developing drugs that inhibit CETP.33
Levels of Lp-PLA2 mass and activity were strongly associated with CHD in a large meta-analysis, supporting the view that circulating Lp-PLA2 indeed represents a biomarker of CHD. Experimental data in the pig model indicate that Lp-PLA2 has important proinflammatory effects in the vessel wall.34 Further, clinical trial data using virtual histology (an intravascular ultrasound–derived modality) as a secondary endpoint suggest that elevated level or increased activity of Lp-PLA2 is related to the progression of atherosclerotic disease.35 With regard to a potential causal role in the pathophysiology of atherosclerosis and its complications, only large randomized clinical trials assessing the effect of Lp-PLA2 inhibition on cardiovascular endpoints (STABILITY36 and SOLID–TIMI 5237) can provide a definitive answer.
Strengths and limitations
The routine ascertainment of Lp-PLA2 covariates and GWA data in five community-based cohorts based on >13 000 European ancestry subjects are strengths of our study. However, caution should be taken when generalizing these findings to populations with non-European ancestry. In addition, even the most significant SNPs may be in LD with as yet unknown causal variants, and the functional basis of the relation of the identified SNPs with variations in Lp-PLA2 concentrations requires study. Also, it is possible that missense genetic changes may cause a difference in assay immunoreactivity, warranting caution in interpreting genetic effects on levels of Lp-PLA2. Lastly, because many of the cohorts were community based, CHD was largely symptomatic CAD; we acknowledge that asymptomatic CAD may have been misclassified.
Conclusions
We extended the previously reported Framingham findings6 by conducting a meta-analysis with four additional cohorts. Whereas levels of Lp-PLA2 mass were primarily associated with the gene coding for the protein itself, Lp-PLA2 activity was associated with genetic variants related to LDL-C levels, which were also associated with CHD/CAD. Larger association studies and clinical trials will be needed to determine whether Lp-PLA2 is a causal risk factor for CHD.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The Atherosclerosis Risk in Communities (ARIC) Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, N01-HC-55022, R01HL087641, R01HL59367, and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HSN268200625226C. Infrastructure was partly supported by grant number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research. The Cardiovascular Health Study research reported in this article was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, and N01-HC-45133 and grant numbers U01 HL080295 and R01 HL087652 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. DNA handling and genotyping was supported in part by the Cedars-Sinai Board of Governors’ Chair in Medical Genetics (J.L.R.), the National Center for Research Resources grant M01RR00425 to the Cedars-Sinai General Clinical Research Center Genotyping core, and the National Institute of Diabetes and Digestive and Kidney Diseases grant DK063491 to the Southern California Diabetes Endocrinology Research Center. Lp-PLA2 measurements were funded by a grant from GlaxoSmithKline. The Framingham Heart Study was supported by the National Heart, Lung, and Blood Institute's Framingham Heart Study (contract number N01-HC-25195) and its contract with Affymetrix, Inc., for genotyping services (contract number N02-HL-6-4278). A portion of this research utilized the Linux Cluster for Genetic Analysis (LinGA-II) funded by the Robert Dawson Evans Endowment of the Department of Medicine at Boston University School of Medicine and Boston Medical Center. The analyses reflect intellectual input and resource development from the Framingham Heart Study investigators participating in the SNP Health Association Resource (SHARe) project; grants HL64753, HL076784, and AG028321 (E.J.B.); and Deutsche Forschungsgemeinschaft (German Research Foundation) Research Fellowship SCHN 1149/1-1 (R.B.S.). Lp-PLA2 activity measurements were provided by GlaxoSmithKline and mass measurements by diaDexus at no cost to the FHS. The Rotterdam Study is supported by the Erasmus Medical Center and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly; The Netherlands Heart Foundation; the Ministry of Education, Culture and Science; the Ministry of Health Welfare and Sports; the European Commission; and the Municipality of Rotterdam. Support for genotyping was provided by the Netherlands Organization for Scientific Research (NWO) (175.010.2005.011, 911.03.012) and Research Institute for Diseases in the Elderly (RIDE2) (014-93-015). This study was supported by the Netherlands Genomics Initiative (NGI)/NWO project number 050-060-810. Abbas Dehghan is supported by a grant from NWO (Vici, 918-76-619). The MONICA/KORA Augsburg studies were financed by the Helmholtz Zentrum München, German Research Center for Environmental Health, Neuherberg, Germany, and supported by grants from the German Federal Ministry of Education and Research (BMBF). Part of this work was financed by the German National Genome Research Network (NGFNPlus, project number 01GS0834) and through additional funds from the University of Ulm. Furthermore, the research was supported within the Munich Center of Health Sciences (MC Health) as part of LMU innovative.
Conflict of interest: Lp-PLA2 activity measurements were provided by GlaxoSmithKline and mass measurements by diaDexus at no cost to the FHS. R.R. has been consultant to Cumberland Pharmaceuticals and Celera. R.C.H. has received research/grant support from diaDexus for Lp-PLA2 work in ARIC. J.J.N. is an employee of GlaxoSmithKline. H.S. has received research grants from the EU, project Cardiogenics; NGFN, project Atherogenomics; and CADnet BMBF. H.A.S.-F. is an employee of GlaxoSmithKline. B.M.P. serves on a data and safety monitoring board for a device clinical trial funded by Zoll Lifecor. M.C. has been consultant for GlaxoSmithKline in relation to Lp-PLA2 and had grant funding from them for same. R.P.T.'s laboratory has received support from GlaxoSmithKline in the form of free assay kits for Lp-PLA2 measurements. W.K. has received honoraria for lectures from diaDexus and GlaxoSmithKline, and is a member of the Steering Committee of the GlaxoSmithKline-sponsored STABILITY and SOLID trials. C.M.B. has received research/grant support from GlaxoSmithKline and diaDexus, and consulting honoraria from GlaxoSmithKline. All other authors have no conflict of interest to declare.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the individual studies for their important contributions, and acknowledge the essential role of the Cohorts for Heart and Aging Research in Genome Epidemiology (CHARGE) Consortium in the development and support of this manuscript. CHARGE members include NHLBI's ARIC Study, Cardiovascular Health Study, and Framingham Heart Study; NIA's Iceland Age, Gene/Environment Susceptibility (AGES) Study; and the Netherland's Rotterdam Study.
References
- 1.Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol. 2005;25:923–931. doi: 10.1161/01.ATV.0000160551.21962.a7. doi:10.1161/01.ATV.0000160551.21962.a7. [DOI] [PubMed] [Google Scholar]
- 2.Kolodgie FD, Burke AP, Skorija KS, Ladich E, Kutys R, Makuria AT, Virmani R. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2523–2529. doi: 10.1161/01.ATV.0000244681.72738.bc. doi:10.1161/01.ATV.0000244681.72738.bc. [DOI] [PubMed] [Google Scholar]
- 3.Lp-PLA2 Studies Collaboration. Lipoprotein-associated phospholipase A2 and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–1544. doi: 10.1016/S0140-6736(10)60319-4. doi:10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerra R, Zhao B, Mooser V, Stafforini D, Johnston JM, Cohen JC. Determinants of plasma platelet-activating factor acetylhydrolase: heritability and relationship to plasma lipoproteins. J Lipid Res. 1997;38:2281–2288. [PubMed] [Google Scholar]
- 5.Schnabel R, Dupuis J, Larson MG, Lunetta KL, Robins SJ, Zhu Y, Rong J, Yin X, Stirnadel HA, Nelson JJ, Wilson PW, Keaney JF, Vasan RS, Benjamin EJ. Clinical and genetic factors associated with lipoprotein-associated phospholipase A2 in the Framingham Heart Study. Atherosclerosis. 2009;204:601–607. doi: 10.1016/j.atherosclerosis.2008.10.030. doi:10.1016/j.atherosclerosis.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suchindran S, Rivedal D, Guyton JR, Milledge T, Gao X, Benjamin A, Rowell J, Ginsburg GS, McCarthy JJ. Genome-wide association study of Lp-PLA2 activity and mass in the Framingham Heart Study. PLoS Genet. 2010;6:e1000928. doi: 10.1371/journal.pgen.1000928. doi:10.1371/journal.pgen.1000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Psaty BM, O'Donnell CJ, Gudnason V, Lunetta KL, Folsom AR, Rotter JI, Uitterlinden AG, Harris TB, Witteman JC, Boerwinkle E on behalf of the CHARGE Consortium. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet. 2009;2:73–80. doi: 10.1161/CIRCGENETICS.108.829747. doi:10.1161/CIRCGENETICS.108.829747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Preuss M, Konig IR, Thompson JR, Erdmann J, Absher D, Assimes TL, Blankenberg S, Boerwinkle E, Chen L, Cupples LA, Hall AS, Halperin E, Hengstenberg C, Holm H, Laaksonen R, Li M, Marz W, McPherson R, Musunuru K, Nelson CP, Burnett MS, Epstein SE, O'Donnell CJ, Quertermous T, Rader DJ, Roberts R, Schillert A, Stefansson K, Stewart AF, Thorleifsson G, Voight BF, Wells GA, Ziegler A, Kathiresan S, Reilly MP, Samani NJ, Schunkert H on behalf of the CARDIoGRAM Consortium. Design of the Coronary ARtery DIsease Genome-Wide Replication And Meta-Analysis (CARDIoGRAM) Study: a genome-wide association meta-analysis involving more than 22 000 cases and 60 000 controls. Circ Cardiovasc Genet. 2010;3:475–483. doi: 10.1161/CIRCGENETICS.109.899443. doi:10.1161/CIRCGENETICS.109.899443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. doi:10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeggini E, Ioannidis JP. Meta-analysis in genome-wide association studies. Pharmacogenomics. 2009;10:191–201. doi: 10.2217/14622416.10.2.191. doi:10.2217/14622416.10.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devlin B, Roeder K, Wasserman L. Genomic control, a new approach to genetic-based association studies. Theor Popul Biol. 2001;60:155–166. doi: 10.1006/tpbi.2001.1542. doi:10.1006/tpbi.2001.1542. [DOI] [PubMed] [Google Scholar]
- 12.Pe'er I, Yelensky R, Altshuler D, Daly MJ. Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol. 2008;32:381–385. doi: 10.1002/gepi.20303. doi:10.1002/gepi.20303. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann MM, Winkler K, Renner W, Winkelmann BR, Seelhorst U, Wellnitz B, Boehm BO, Marz W. Genetic variants and haplotypes of lipoprotein associated phospholipase A2 and their influence on cardiovascular disease (The Ludwigshafen Risk and Cardiovascular Health Study) J Thromb Haemost. 2009;7:41–48. doi: 10.1111/j.1538-7836.2008.03216.x. doi:10.1111/j.1538-7836.2008.03216.x. [DOI] [PubMed] [Google Scholar]
- 14.Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS, Stringham HM, Strait J, Duren WL, Maschio A, Busonero F, Mulas A, Albai G, Swift AJ, Morken MA, Narisu N, Bennett D, Parish S, Shen H, Galan P, Meneton P, Hercberg S, Zelenika D, Chen WM, Li Y, Scott LJ, Scheet PA, Sundvall J, Watanabe RM, Nagaraja R, Ebrahim S, Lawlor DA, Ben-Shlomo Y, Davey-Smith G, Shuldiner AR, Collins R, Bergman RN, Uda M, Tuomilehto J, Cao A, Collins FS, Lakatta E, Lathrop GM, Boehnke M, Schlessinger D, Mohlke KL, Abecasis GR. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–169. doi: 10.1038/ng.76. doi:10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2004;109:837–842. doi: 10.1161/01.CIR.0000116763.91992.F1. doi:10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 16.Sandhu MS, Waterworth DM, Debenham SL, Wheeler E, Papadakis K, Zhao JH, Song K, Yuan X, Johnson T, Ashford S, Inouye M, Luben R, Sims M, Hadley D, McArdle W, Barter P, Kesaniemi YA, Mahley RW, McPherson R, Grundy SM, Bingham SA, Khaw KT, Loos RJ, Waeber G, Barroso I, Strachan DP, Deloukas P, Vollenweider P, Wareham NJ, Mooser V. LDL-cholesterol concentrations: a genome-wide association study. Lancet. 2008;371:483–491. doi: 10.1016/S0140-6736(08)60208-1. doi:10.1016/S0140-6736(08)60208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaubatz JW, Gillard BK, Massey JB, Hoogeveen RC, Huang M, Lloyd EE, Raya JL, Yang CY, Pownall HJ. Dynamics of dense electronegative low density lipoproteins and their preferential association with lipoprotein phospholipase A2. J Lipid Res. 2007;48:348–357. doi: 10.1194/jlr.M600249-JLR200. doi:10.1194/jlr.M600249-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, Cooper GM, Roos C, Voight BF, Havulinna AS, Wahlstrand B, Hedner T, Corella D, Tai ES, Ordovas JM, Berglund G, Vartiainen E, Jousilahti P, Hedblad B, Taskinen MR, Newton-Cheh C, Salomaa V, Peltonen L, Groop L, Altshuler DM, Orho-Melander M. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–197. doi: 10.1038/ng.75. doi:10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muendlein A, Geller-Rhomberg S, Saely CH, Winder T, Sonderegger G, Rein P, Beer S, Vonbank A, Drexel H. Significant impact of chromosomal locus 1p13.3 on serum LDL cholesterol and on angiographically characterized coronary atherosclerosis. Atherosclerosis. 2009;206:494–499. doi: 10.1016/j.atherosclerosis.2009.02.040. doi:10.1016/j.atherosclerosis.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 20.Linsel-Nitschke P, Gotz A, Erdmann J, Braenne I, Braund P, Hengstenberg C, Stark K, Fischer M, Schreiber S, El Mokhtari NE, Schaefer A, Schrezenmeir J, Rubin D, Hinney A, Reinehr T, Roth C, Ortlepp J, Hanrath P, Hall AS, Mangino M, Lieb W, Lamina C, Heid IM, Doering A, Gieger C, Peters A, Meitinger T, Wichmann HE, Konig IR, Ziegler A, Kronenberg F, Samani NJ, Schunkert H. Lifelong reduction of LDL-cholesterol related to a common variant in the LDL-receptor gene decreases the risk of coronary artery disease—a Mendelian Randomisation study. PLoS One. 2008;3:e2986. doi: 10.1371/journal.pone.0002986. doi:10.1371/journal.pone.0002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin Cho Y, Jin Go M, Jin Kim Y, Lee JY, Park T, Kim K, Sim X, Twee-Hee Ong R, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Hua Zhao J, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RY, Wright AF, Witteman JC, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJ, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BW, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O'Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, McArdle W, Masson D, Martin NG, Marroni F, Mangino M, Magnusson PK, Lucas G, Luben R, Loos RJ, Lokki ML, Lettre G, Langenberg C, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Kronenberg F, Konig IR, Khaw KT, Kaprio J, Kaplan LM, Johansson A, Jarvelin MR, Janssens AC, Ingelsson E, Igl W, Kees Hovingh G, Hottenga JJ, Hofman A, Hicks AA, Hengstenberg C, Heid IM, Hayward C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Gyllensten U, Guiducci C, Groop LC, Gonzalez E, Gieger C, Freimer NB, Ferrucci L, Erdmann J, Elliott P, Ejebe KG, Doring A, Dominiczak AF, Demissie S, Deloukas P, de Geus EJ, de Faire U, Crawford G, Collins FS, Chen YD, Caulfield MJ, Campbell H, Burtt NP, Bonnycastle LL, Boomsma DI, Boekholdt SM, Bergman RN, Barroso I, Bandinelli S, Ballantyne CM, Assimes TL, Quertermous T, Altshuler D, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Adair LS, Taylor HA, Jr, Borecki IB, Gabriel SB, Wilson JG, Holm H, Thorsteinsdottir U, Gudnason V, Krauss RM, Mohlke KL, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJ, Schadt EE, Rotter JI, Boerwinkle E, Strachan DP, Mooser V, Stefansson K, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, van Duijn CM, Peltonen L, Abecasis GR, Boehnke M, Kathiresan S. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. doi:10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edmondson AC, Braund PS, Stylianou IM, Khera AV, Nelson CP, Wolfe ML, Derohannessian SL, Keating BJ, Qu L, He J, Tobin MD, Tomaszewski M, Baumert J, Klopp N, Doring A, Thorand B, Li M, Reilly MP, Koenig W, Samani NJ, Rader DJ. Dense genotyping of candidate gene loci identifies variants associated with high-density lipoprotein cholesterol. Circ Cardiovasc Genet. 2011;4:145–155. doi: 10.1161/CIRCGENETICS.110.957563. doi:10.1161/CIRCGENETICS.110.957563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Keyzer D, Karabina SA, Wei W, Geeraert B, Stengel D, Marsillach J, Camps J, Holvoet P, Ninio E. Increased PAFAH and oxidized lipids are associated with inflammation and atherosclerosis in hypercholesterolemic pigs. Arterioscler Thromb Vasc Biol. 2009;29:2041–2046. doi: 10.1161/ATVBAHA.109.196592. doi:10.1161/ATVBAHA.109.196592. [DOI] [PubMed] [Google Scholar]
- 24.Gillotte-Taylor K, Boullier A, Witztum JL, Steinberg D, Quehenberger O. Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J Lipid Res. 2001;42:1474–1482. [PubMed] [Google Scholar]
- 25.Kruse S, Mao XQ, Heinzmann A, Blattmann S, Roberts MH, Braun S, Gao PS, Forster J, Kuehr J, Hopkin JM, Shirakawa T, Deichmann KA. The Ile198Thr and Ala379Val variants of plasmatic PAF-acetylhydrolase impair catalytical activities and are associated with atopy and asthma. Am J Hum Genet. 2000;66:1522–1530. doi: 10.1086/302901. doi:10.1086/302901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ninio E, Tregouet D, Carrier JL, Stengel D, Bickel C, Perret C, Rupprecht HJ, Cambien F, Blankenberg S, Tiret L. Platelet-activating factor-acetylhydrolase and PAF-receptor gene haplotypes in relation to future cardiovascular event in patients with coronary artery disease. Hum Mol Genet. 2004;13:1341–1351. doi: 10.1093/hmg/ddh145. doi:10.1093/hmg/ddh145. [DOI] [PubMed] [Google Scholar]
- 27.Casas JP, Ninio E, Panayiotou A, Palmen J, Cooper JA, Ricketts SL, Sofat R, Nicolaides AN, Corsetti JP, Fowkes FG, Tzoulaki I, Kumari M, Brunner EJ, Kivimaki M, Marmot MG, Hoffmann MM, Winkler K, Marz W, Ye S, Stirnadel HA, Khaw KT, Humphries SE, Sandhu MS, Hingorani AD, Talmud PJ. PLA2G7 genotype, lipoprotein-associated phospholipase A2 activity, and coronary heart disease risk in 10 494 cases and 15 624 controls of European ancestry. Circulation. 2010;121:2284–2293. doi: 10.1161/CIRCULATIONAHA.109.923383. doi:10.1161/CIRCULATIONAHA.109.923383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, Barrett JH, Konig IR, Stevens SE, Szymczak S, Tregouet DA, Iles MM, Pahlke F, Pollard H, Lieb W, Cambien F, Fischer M, Ouwehand W, Blankenberg S, Balmforth AJ, Baessler A, Ball SG, Strom TM, Braenne I, Gieger C, Deloukas P, Tobin MD, Ziegler A, Thompson JR, Schunkert H. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–453. doi: 10.1056/NEJMoa072366. doi:10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutton BS, Crosslin DR, Shah SH, Nelson SC, Bassil A, Hale AB, Haynes C, Goldschmidt-Clermont PJ, Vance JM, Seo D, Kraus WE, Gregory SG, Hauser ER. Comprehensive genetic analysis of the platelet activating factor acetylhydrolase (PLA2G7) gene and cardiovascular disease in case-control and family datasets. Hum Mol Genet. 2008;17:1318–1328. doi: 10.1093/hmg/ddn020. doi:10.1093/hmg/ddn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stafforini DM, Satoh K, Atkinson DL, Tjoelker LW, Eberhardt C, Yoshida H, Imaizumi T, Takamatsu S, Zimmerman GA, McIntyre TM, Gray PW, Prescott SM. Platelet-activating factor acetylhydrolase deficiency: a missense mutation near the active site of an anti-inflammatory phospholipase. J Clin Invest. 1996;97:2784–2791. doi: 10.1172/JCI118733. doi:10.1172/JCI118733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang Y, Waterworth D, Lee JE, Song K, Kim S, Kim HS, Park KW, Cho HJ, Oh IY, Park JE, Lee BS, Ku HJ, Shin DJ, Lee JH, Jee SH, Han BG, Jang HY, Cho EY, Vallance P, Whittaker J, Cardon L, Mooser V. Carriage of the V279F null allele within the gene encoding Lp-PLA is protective from coronary artery disease in South Korean males. PLoS One. 2011;6:e18208. doi: 10.1371/journal.pone.0018208. doi:10.1371/journal.pone.0018208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stafforini DM. Functional consequences of mutations and polymorphisms in the coding region of the PAF acetylhydrolase (PAF-AH) gene. Pharmaceuticals. 2009;2:94–117. doi: 10.3390/ph2030094. doi:10.3390/ph2030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Register TC, Burdon KP, Lenchik L, Bowden DW, Hawkins GA, Nicklas BJ, Lohman K, Hsu FC, Langefeld CD, Carr JJ. Variability of serum soluble intercellular adhesion molecule-1 measurements attributable to a common polymorphism. Clin Chem. 2004;50:2185–2187. doi: 10.1373/clinchem.2004.036806. doi:10.1373/clinchem.2004.036806. [DOI] [PubMed] [Google Scholar]
- 34.Wilensky RL, Shi Y, Mohler ER, III, Hamamdzic D, Burgert ME, Li J, Postle A, Fenning RS, Bollinger JG, Hoffman BE, Pelchovitz DJ, Yang J, Mirabile RC, Webb CL, Zhang L, Zhang P, Gelb MH, Walker MC, Zalewski A, Macphee CH. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat Med. 2008;14:1059–1066. doi: 10.1038/nm.1870. doi:10.1038/nm.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serruys PW, Garcia-Garcia HM, Buszman P, Erne P, Verheye S, Aschermann M, Duckers H, Bleie O, Dudek D, Botker HE, von Birgelen C, D'Amico D, Hutchinson T, Zambanini A, Mastik F, van Es GA, van der Steen AF, Vince DG, Ganz P, Hamm CW, Wijns W, Zalewski A. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118:1172–1182. doi: 10.1161/CIRCULATIONAHA.108.771899. doi:10.1161/CIRCULATIONAHA.108.771899. [DOI] [PubMed] [Google Scholar]
- 36. The Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy Trial (STABILITY) http://clinicaltrials.gov/ct2/show/NCT00799903. 8 Jun 2011. [Google Scholar]
- 37. The Stabilization Of pLaques usIng Darapladib-Thrombolysis In Myocardial Infarction 52 Trial (SOLID-TIMI 52) http://clinicaltrials.gov/ct2/show/NCT01000727. 8 Jun 2011.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.