Abstract
Background and Purpose
Mental practice (MP), which involves cognitive rehearsal of physical movements, is a non-invasive, inexpensive method of enabling repetitive, task specific practice (RTP). Recent, randomized controlled data suggest that MP, when combined with a RTP therapy program, increases affected arm use and function significantly more than RTP only. As a next step, this 10-subject case series examined the possibility that cortical plasticity is a mechanism underlying the treatment effect of MP when combined with RTP.
Method
10 chronic stroke patients (mean = 36.7 months) exhibiting stable, moderate motor deficits received ½ hour therapy sessions for their affected arms, occurring 3 days/week for 10 weeks, and emphasizing valued activities of daily living (ADLs). Directly after therapy, subjects received 30-minute MP sessions, which required MP of the ADLs performed during therapy. Behavioral outcomes were blindly evaluated using the Action Research Arm Test (ARAT) and the Fugl-Meyer Assessment (FM). Functional magnetic resonance imaging (fMRI) was administered before and after intervention to assess cortical changes.
Results
Before intervention, subjects exhibited stable motor deficits. After intervention, subjects exhibited marked ARAT and FM score increases (+ 5.3 and + 4.2, respectively), and clinically significant, new abilities to perform valued ADLs. Post-intervention fMRI revealed significant increases in activation to wrist flexion and extension of the affected hand in the premotor area and primary motor cortex ipsi- and contralaterally to the affected hand, and superior parietal cortex ipsilateral to the affected hand. Decreased activations were noted in parietal cortex of the hemisphere ipsilateral to the affected hand. These changes correlated with anatomical regions in which behavioral changes were observed via the ARAT and FM.
Conclusions
MP is an easy to use, cost effective strategy that was again shown to improve affected arm outcomes after stroke. This is the first study suggesting alteration in the cortical map as a possible MP mechanism for the affected arm.
Declining stroke mortality and stable stroke incidence1 have increased the prevalence of stroke survivors exhibiting motor deficits. Yet, despite billions of dollars spent annually on stroke motor rehabilitation,2 and the widely appreciated impact of hemiparesis, conventional affected arm rehabilitation strategies have a negligible functional impact.3
Newer rehabilitative approaches emphasize repetitive, task-specific practice (RTP) with the affected arm. Some of these RTP programs require participation in high duration therapy sessions.4 Other strategies incorporate mechanized approaches to administer RTP,5,6,7 or use implanted devices to augment or facilitate RTP.8,9 While promising, these approaches are constrained by requiring particular equipment to administer, they can be intensive and/or taxing for the patient and therapist, and some are invasive. Many strategies and devices inherent in the above approaches are also costly and not reimbursed by insurance. Thus, there remains a need for efficacious, easily implemented, inexpensive, non-invasive RTP approaches for arm hemiparesis.
Mental practice (MP) is a non-invasive, inexpensive RTP technique in which physical skills and/or scenarios are cognitively rehearsed. The same musculature and neural structures are activated during MP as during physical practice of the same task.10,11 Consequently, MP has been efficaciously applied as a strategy to enable repetitive practice and skill learning, including for the affected arm after stroke.12,13,14,15,16 Despite its clinical promise, only two small studies have examined MP mechanisms when combined with RTP in stroke; one group17 has suggested that M1 and the orbitofrontal cortex are implicated in motor skill relearning of the affected leg following mental practice. The other case study18 examined possible associated with affected arm RTP. The patient showed increased bilateral cortical activation in both the motor and premotor areas, again including M1. However, the MP was combined with a high-duration affected arm therapy regimen that is impractical in many clinical contexts. Thus, the neural substrates of MP combined with an affected arm RTP program that is analogous to regular clinical care remain unknown.
Given its promise in stroke, our overall goal was to understand possible MP + RTP mechanisms of action in preparation for a larger trial. Using high field, functional magnetic resonance imaging (fMRI), the current study examined the impact of a MP + RTP regimen on cortical organization patterns in stroke. Whereas mere repetitive limb use does not produce cortical changes,19 we hypothesized that a program encouraging skill learning via RTP and MP would elicit changes in cortical output maps corresponding to subjects’ affected hands, arms, and finger regions. We also hypothesized that neural changes would be accompanied by decreases in affected limb functional limitation and impairment, which would conspire to improve movement performance. To our knowledge, this is the first study to examine cortical changes following a clinically-based, MP + RTP program for the affected arm.
Method
Subjects
Study criteria were based on those used by Page and colleagues in their recent, positive MP trial.15 Volunteers were recruited using advertisements placed in rehabilitative clinics in the Midwestern United States. A research team member screened volunteers using the following inclusion criteria from previous MP research: (1) history of no more than one stroke; (2) ability to actively flex at least 10° from neutral at the affected wrist and the metacarpophalangeal and interphalangeal joints of two digits; (3) stroke experienced > 6 months prior to study enrollment; (4) a score ≥ 69 on the Modified Mini Mental Status Examination;20 (5) age > 18 < 80 years. We also applied the following exclusion criteria: (1) excessive spasticity, defined as a score of ≥ 3 on the Modified Ashworth Spasticity Scale;21 (2) excessive pain in the affected arm, as measured by a score of ≥ 4 on a 10-point visual analog scale; (3) still enrolled in any form of physical rehabilitation; (4) participating in any experimental rehabilitation or drug studies.
Using the above inclusion/exclusion criteria, 10 patients were found eligible and agreed to participate (5 males; mean age = 56.5 ± 11.6 years, age range 37 – 69 years; mean time since stroke onset = 36.7 months ± 34.0, range of onset = 10 – 115 months; 8 subjects with right hemiparesis).
Outcome Measures
The current study’s MP and RTP regimens primarily targeted functional limitation. Thus, we administered the Action Research Arm Test, (ARAT)22 which has measured MP-induced changes in functional limitation.14,15 The ARAT is a 19-item test divided into four categories (grasp, grip, pinch, and gross movement), with 16 of the nineteen ARAT items measuring distal regions of the arm, making it an ideal and sensitive measure for this distally based intervention. The ARAT has high intrarater (r = .99) and retest (r = .98) reliability and validity,23,24 all in stroke-induced hemiparesis.
Since perfect or near-perfect performance on the ARAT does not necessarily equate to absence of impairment,25 the upper extremity section of the Fugl-Meyer Scale (FM)26 assessed upper extremity impairment. Data arise from a 3-point ordinal scale (0=cannot perform; 2=can perform fully) applied to each item, and the items are summed to provide a maximum score of 66. The FM has been shown to have impressive test-retest reliability (total=.98–.99; subtests=.87–1.00), interrater reliability, and construct validity.27,28
Testing
A single-blinded, pre- and post-test case series design was applied. This design was consistent with those of other fMRI case series’s examining neural mechanisms of particular interventions.10,29 After screening and signing consent forms approved by the local institutional review board, the FM and ARAT were administered on 2 occasions one week apart (PRE-1; PRE-2).
One week after the second administrations of the FM and ARAT, each subject underwent fMRI. After explaining the procedure, subjects were scanned without sedation using a 4T Varian Unity INOVA whole body MRI scanner (Varian, Inc; Palo Alto, CA). The fMRI evaluators (JPS, JE, HP) were blinded to subject identity and each subject’s therapy outcomes.
fMRI testing began with an initial, fast localizer scan (12 seconds), obtained to assure proper head position. Head padding specifically designed for this scanner was used to restrict head motion. Next, a 3D modified driven equilibrium Fourier transform (MDEFT) high-resolution anatomical T1-weighted scan was acquired using the following protocol: TMD=1.1 s, TR/TE=13 1/6 ms, TE=6 ms, FOV=25.6 × 19.2 × 19.2 cm, matrix 256 × 192 × 96 pixels, resolution 1×1×2 mm, flip angle=22 degrees, zero-filled to 1×1×1 mm during reconstruction. Finally, using the motor task described below, we obtained a T2*-weighted spin-echo Echo Planar Imaging (EPI) pulse sequence (TR/TE = 3000/30 ms, FOV = 25.6 × 25.6 cm, matrix = 64 × 64 pixels, number of slices = 30, slice thickness = 4 mm, flip angle = 75°). The 30 contiguous fMRI slices covered from the apex of the brain to the superior portion of the cerebellum. We recently tested the repeatability and reliability of fMRI BOLD signal changes in patients with chronic stroke and found consistently highly reliable intra-class correlations of R(0.05)~0.8.30
fMRI Task
While in the scanner, subjects performed a self-paced fMRI motor task lasting 4 min 48 sec. This block-design task consisted of an active condition, affected wrist flexion/extension, and a control condition where subjects were asked not to move. The task, which was consistent with other fMRI studies,31,32 was run twice. During each run, subjects received visual prompts every 30 seconds, either asking them to rest or to flex/extend the affected wrist, always starting with rest (with exception of the first control condition, which lasted 18 seconds (6 TRs) and allowed for T2 signal equilibration). These data were not considered in the analyses). During the control condition (“relax”), subjects were asked to hold their affected hand along the body and not to move. Subjects were asked to perform one flexion/extension movement of the wrist at the beginning of each EPI acquisition, which could be audibly detected as a brief pause in the scanning noise of the MRI machine every 3 seconds. Compliance was visually monitored during performance of the task. Movement performance was also visually monitored for the presence of mirror movements. Movement amplitude, frequency, and distance were controlled across subjects using the visual stimulus, and a brace that controlled the distance subjects were able to flex/extend.
Intervention
The PI’s team had identified a battery of familiar activities of daily living (ADLs) that stroke patients commonly wish to relearn. The 5 ADLs shown in Table 1 were chosen from that list for this study because: (a) they had been used in previously efficacious MP work;14–16 (b) individual movements comprising these compound skills transfer positively to performance of other ADLs that stroke patients often desire to relearn; (c) The ADLs could be graded according to subjects’ abilities to be easier or more challenging, and progressed to be increasingly difficult; this was important, since the notion of challenging the patient appears to be a major factor in facilitating cortical plasticity. (d) Some authors have also contended that motor imagery practice should be restricted to tasks in which subjects had previously participated. For instance, Mulder and colleagues33 reported that, following MP, motor performance of a newly learned motor task did not improve as much as motor performance of a previously learned task. It is plausible that the above results may be spurious, and due to differences in terminology used in the above and other studies,34 such as “motor tasks” and “novelty.” Nonetheless, to assure that this phenomenon did not affect patients, all RTP tasks were familiar to subjects, in that all subjects had participated in the tasks prior to their strokes, and the tasks were necessary or desired for subjects’ daily lives. Since the major study goals were to measure MP + RTP impacvt on fMRI, ARAT, and, FM scores, we did not measure ability to perform the tasks.
Table 1.
Sequences on Each Tape, and Where/when Tape Was Used
| Tape Number: |
Functional Task Described: | When Administered: |
|---|---|---|
| 1 | Reaching for and grasping a cup | Weeks 1,2 |
| 2 | Turning a page in a book | Weeks 3,4 |
| 3 | Proper use of a writing utensil | Weeks 5,6 |
| 4 | Proper use of an eating utensil | Weeks 7,8 |
| 5 | Using a hairbrush or comb | Weeks 9,10 |
Subjects practiced the ADLs during ½ hour, one on one therapy sessions, occurring on three days per week for 10 weeks. The ADLs were practiced in the format and order depicted in Table 1, with all therapy administered by a single therapist, in the same order and environment. Each ADL was practiced for 2 weeks. Emphasis for patients was on performing the above ADLs through the entire range of motion. When appropriate to the task (ie, the task was bimanual in nature), subjects were permitted to integrate the unaffected hand into the task (e.g., stabilizing the paper while writing with the affected hand).
Physical practice is believed to create a motor plan or “schema” that MP then augments or emboldens. Thus, directly after their ½ hour RTP sessions, subjects received a 20–30 minute, recorded MP intervention, consisting of: (a) 6–8 opening minutes of relaxation, asking patients to imagine themselves in a warm, relaxing place (e.g., a beach), and asking them to contract and relax their muscles (i.e., progressive relaxation); (b) suggestions for internal, cognitive polysensory (i.e., using both kinesthetic and visual cues) images35 related to using the affected arm in one of the 5 ADLs. For example, subjects were encouraged to envision themselves seated comfortably at their table in a familiar place, to feel the temperature of the cup, and to smell the familiar odors that may be associated with the place where they were seated (e.g., a kitchen). (c) The final minutes allowed patients to refocus into the room.
Posttesting
One week after the final therapy session, each subject returned to the laboratory at which pretesting occurred, and the ARAT and FM were again administered by the same examiner who administered pretests. The examiner was blinded in that he was unaware of the intervention in which subjects had participated, or even if they had participated in any intervention. fMRI was also re-administered at this time, and at the same location as pre-intervention scanning.
fMRI Data Processing and Analysis
Images were processed, analyzed, and visualized with AFNI (Analysis of Functional NeuroImages).36,37 The functional runs were motion corrected to the third image of the first run using a six-parameter rigid-body transformation with Fourier interpolation.38 Next, following motion correction (3dvolreg in AFNI), the fMRI data were assessed by AFNI function 3dToutcount for timeseries outliers. Then, each image corresponding to a likely outlier was visually inspected for uncorrectable head movements or other signal artifact and censored from further analysis if significant signal abnormalities affected approximately 30% or more of voxels in the image. The MDEFT image was normalized to Talairach space using tools in AFNI. The EPI datasets were then spatially smoothed with a 6 mm full-width, half-maximum Gaussian filter, normalized by adopting the transformation for MDEFT normalization, and re-sampled to 3×3×3mm. For participants with an affected left-hand, fMRI involved left-hand movements and all brain images were reversed to allow group analysis of pre- post-interventional changes. All changes were described in terms of right or left hemisphere. Only affected-hand movements were scanned.
The two runs for the affected hand were concatenated and a box-car reference function was fitted to the EPI time series to assess the magnitude of the fMRI response. Additionally, the signal mean and low frequency linear, quadratic and cubic drifts for each run were also fitted. We also included the motion correction parameters as regressors of no interest to account for signal intensity variations that might occur in images due to head motion within the scanner. After deconvolution, the activation magnitude estimates were entered as dependent measures in t-tests and linear regression analyses to identify brain activation effects of interest across the group of 10 subjects and regions of pre- to post-intervention change in activation. The activation maps generated by these t-tests and regressions were then thresholded at a voxel-level p ≤ 0.05 and a cluster threshold of 10 voxels or more. The Talairach daemon39 was used to identify the locations of clusters exceeding these thresholds.
Results
During the pretesting phase, all subjects exhibited stable motor deficits. This was evidenced by ARAT scores that did not differ from each other between pretesting interventions (PRE-1 = 29.8, PRE-2 = 29.7; T = 0.019 {p = 0.98}), and by FM scores that did not differ from each other between pretesting interventions (PRE-1 = 35.2, PRE-2 = 34.5; T=0.13 {p = 0.89}). Comparison of each subject’s motor deficits at pretesting with those reported at therapy discharge in medical records further confirmed motor deficit stability.
After intervention, the mean ARAT score was 35.1 (mean change = +5.3), with most changes occurring on the ARAT pinch and grasp scales. Likewise, the mean FM score increased after intervention to 38.7 (mean change = +4.2), with most changes observed in distal impairment items (e.g., wrist extension; pincer grasp). Clinically, subjects reported new ability to perform components of the ADLs listed in Table 1 (e.g., hold and use a fork independently). These new abilities transferred to new ability to perform other valued ADLs (e.g., holding and dialing a push button telephone). However, as stated previously, the study purpose was to measure fMRI, ARAT, and FM changes in response to intervention. As such, the above data are anecdotal, as transfer to ADL was not formally measured. Future researchers are encouraged to examine this construct more fully.
Changes were also noted in the BOLD signal volume between the pre-MP and post-MP fMRI scans (Figures 1a; 1b). Analysis of the differences between the pre- and post-intervention fMRI images revealed significant increases in BOLD signal to wrist flexion and extension of the affected hand in the premotor area and primary motor cortex ipsi- and contralaterally to the affected hand and superior parietal cortex ipsilateral to the affected hand (Figure 1c; Table 2). Decreases in BOLD signal were noted in parietal cortex of the hemisphere ipsilateral to the affected hand. Individual time points from each person’s fMRI data were edited out of the statistical deconvolution analysis (see methods), so residual, uncorrected head movements did not contribute to activation effects
Figure 1.
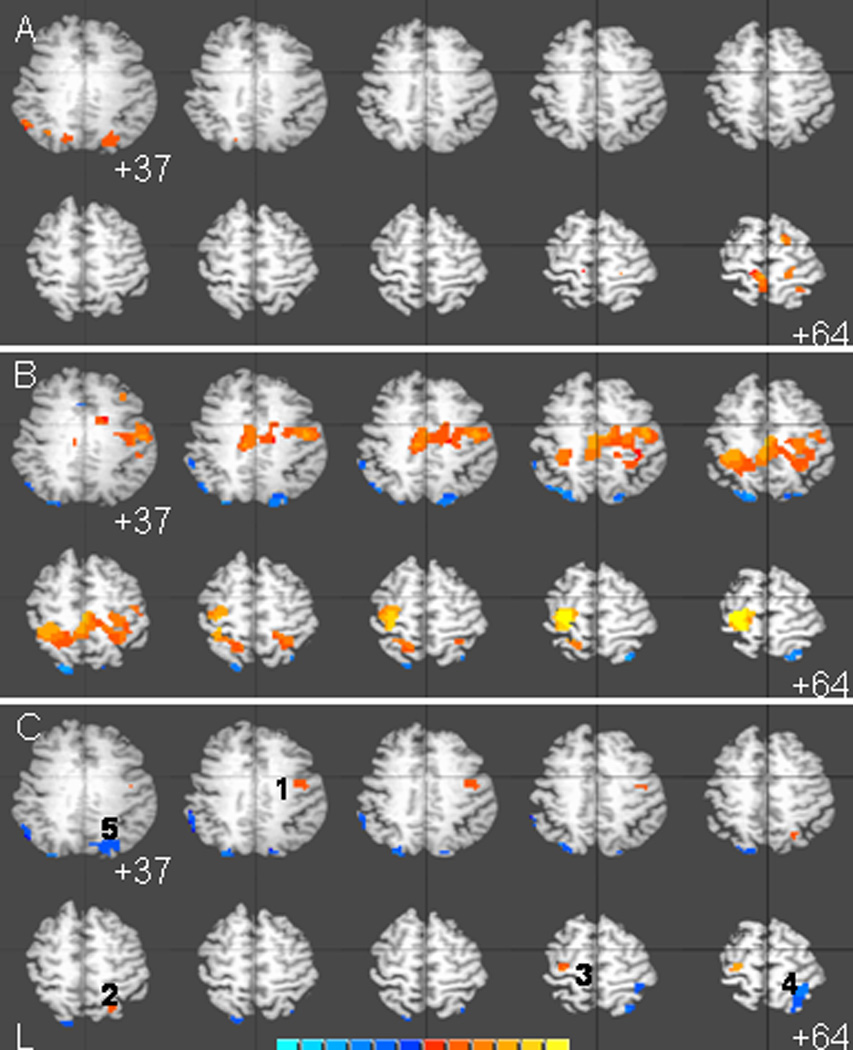
Average brain activation maps for 10 stroke subjects at pre-intervention (A), post-intervention (B), and the statistical difference between them (C). Activation maps are thresholded at p < 0.05 with a cluster of 10 or more voxels (270uL). Brain activation maps are overlaid on a single subject's anatomical MRI. Image left is subject's left. Color panel indicate strength of activation (A and B) or activation difference (C) in raw mr units.
Table 2.
Talairach coordinates of active clusters shown in Figure 1
| Region of Interest | X | Y | Z | Max. t | Active Vol. (uL) |
|---|---|---|---|---|---|
| R. Precentral gyrus (BA4) | 42.9 | −8.6 | 43.5 | 3.271 | 918 |
| R. Superior Parietal Lobule | 25 | −63.8 | 52.3 | 2.7326 | 378 |
| L. Precentral gyrus (BA4,6) | −30 | −19.4 | 65.3 | 2.4179 | 648 |
| R. Superior Parietal Lobule (BA7) | 31.5 | −49.6 | 65.8 | −3.081 | 2295 |
| R. Intraparietal sulcus (BA7) | 20.3 | −75 | 37.1 | −3.4815 | 2430 |
Discussion
Mental practice has emerged as a non-invasive RTP strategy that increases affected arm use and function, even years after stroke. The current study examined MP effects on movement in a cohort of patients exhibiting stable hemiparesis, and whether neural changes may underlie movement changes.
Consistent with two of our hypotheses, ARAT and FM scores increased after intervention, indicative of decreased affected limb functional limitation and impairment, respectively. These changes conveyed improved ability to perform ADLs in Table 1, as well as valued ADLs in subjects’ homes. The valence and magnitude of these motor changes were highly similar to those reported in recent MP studies using the same outcome measures.14–16 These findings replicate the outcome of previous studies using the same MP program, and strengthen the argument for MP efficacy. Importantly, subjects were exhibiting stable motor deficits before intervention. All subjects and their caregivers also reported not engaging in any additional MP or therapies outside of study participation. Given our motor outcomes, the stroke chronicity in our sample, and the size of the motor changes in a relatively short timeframe, it is likely that motor changes were attributable to the MP + RTP intervention.
Previous MP authors14–16 have hypothesized that repetitive affected arm use, and repeated activation of the neural networks involved in this use,10,11 may induce cortical changes that underlie the MP effect. However, relatively few studies have examined the neural substrates of motor recovery as facilitated by therapeutic interventions, and none had examined neural substrates of the MP treatment effect in the affected arm. In order to elucidate the mechanisms that underlie stroke recovery we combined subjects into a single group. Despite the difference in lesion size, location, and degree of functional deficit, this approach has allowed us to identify sites associated with improved recovery at a group rather than individual level. As hypothesized, we found significant BOLD fMRI signal changes that coincided with above-noted motor changes. Specific regions of post-intervention activation were the premotor area and primary motor cortex ipsi- and contralaterally to the affected hand, and superior parietal cortex ipsilateral to the affected hand. These patterns were consistent with other fMRI intervention studies in minimally impaired subjects,40,41 which reported treatment-induced activations within the ipsilesional primary motor cortex, dorsal premotor cortex, and supplementary motor areas. The activation areas were also consistent with our previous MP neuroimaging work,18 which constitutes the only other MP neuroimaging study in the affected arm. However, our previous study utilized a much higher duration protocol than the one herein described. The notable neural and functional changes herein observed is of potential clinical significance, as it suggests that such changes can be observed without intensive practice that may be impractical in some clinics. Collectively, the fact that one can attain similar neural and functional changes as other RTP interventions, but with substantially lower treatment duration and no equipment, should be exciting news for clinicians wanting to apply RTP strategies in their environments.
Although data are encouraging, some study limitations should be noted. First, no control group was used. However, previous studies have already shown that MP + RTP conveys larger motor changes than RTP alone,15 and, thus, the goal of this trial was to “take the next step,” and understand the MP + RTP neural mechanisms. Given that this was our goal, a RTP-only group may have been less necessary, given the scope of this study. Moreover, several, valid fMRI case series’s with the same goal as the present study have also not used a control group,28,29,42,43,42 yet their data have been useful in discerning neural mechanisms. Using the same intervention as efficacious, controlled, MP trials,15 this study offers value by explaining the mechanisms of the MP treatment effect. While this is one of the larger fMRI-intervention studies reported, more data with a larger number of subjects would further validate findings herein reported. This is a second limitation, but will be overcome in future work. Matching subjects in lesion location, impairment and other relevant variables will also be important in this future work.
Conclusion
An affected arm rehabilitative program incorporating MP appears to improve movement via significant cortical reorganization. Moreover, the patterns and magnitude of both cortical reorganization and motor change observed herein were comparable to those observed after other interventions. However, unlike other strategies, MP can be administered with an amount of therapist contact conforming to current care, no special devices, and minimal cost.
Footnotes
This study was supported by grants from the National Institutes of Health (R21 AT002110-01A1; K01 AT002637-01).
References
- 1.Kleindorfer D, Broderick J, Khoury J, et al. The unchanging incidence and case-fatality of stroke in the 1990s: A population-based study. Stroke. 2006;37:2473–2478. doi: 10.1161/01.STR.0000242766.65550.92. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 Jan 29;117(4):e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 3.Duncan PW. Synthesis of intervention trials to improve motor recovery following stroke. Top Stroke Rehabil. 1997;3:1–20. doi: 10.1080/10749357.1997.11754126. [DOI] [PubMed] [Google Scholar]
- 4.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D EXCITE Investigators. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006 Nov 1;296(17):2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 5.Volpe BT, Ferraro M, Lynch D, Christos P, Krol J, Trudell C, Krebs HI, Hogan N. Robotics and other devices in the treatment of patients recovering from stroke. Curr Neurol Neurosci Rep. 2005 Nov;5(6):465–470. doi: 10.1007/s11910-005-0035-y. [DOI] [PubMed] [Google Scholar]
- 6.Whitall J, McCombe Waller S, Silver KH, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke. 2000 Oct;31(10):2390–2395. doi: 10.1161/01.str.31.10.2390. [DOI] [PubMed] [Google Scholar]
- 7.Taub E, Lum PS, Hardin P, Mark VW, Uswatte G. AutoCITE: automated delivery of CI therapy with reduced effort by therapists. Stroke. 2005 Jun;36(6):1301–1304. doi: 10.1161/01.STR.0000166043.27545.e8. Epub 2005 May 5. [DOI] [PubMed] [Google Scholar]
- 8.Northstar Neuroscience, Inc. Safety and effectiveness of cortical stimulation in hemiparetic stroke patients. Northstar study code “Everest”; study # VO267 [Google Scholar]
- 9.Chae J, Hart R. Intramuscular hand neuroprosthesis for chronic stroke survivors. Neurorehabil Neural Repair. 2003 Jun;17(2):109–117. doi: 10.1177/0888439003017002005. [DOI] [PubMed] [Google Scholar]
- 10.Lafleur MF, Jackson PL, Richards C, Malouin F, Doyon J. Motor learning produces parallel dynamic functional changes during the execution and the imagination of sequential foot movements. NeuroImage. 2002;16:142–157. doi: 10.1006/nimg.2001.1048. [DOI] [PubMed] [Google Scholar]
- 11.Lacourse MG, Turner JA, Randolph-Orr E, Schandler SL, Cohen MJ. Cerebral and cerebellar sensorimotor plasticity following motor imagery-based mental practice of a sequential movement. J Rehabil Res Dev. 2004 Jul;41(4):505–524. doi: 10.1682/jrrd.2004.04.0505. [DOI] [PubMed] [Google Scholar]
- 12.Crosbie JH, McDonough SM, Gilmore DH, Wiggam MI. The adjunctive role of mental practice in the rehabilitation of the upper limb after hemiplegic stroke: a pilot study. Clin Rehabil. 2004 Feb;18(1):60–68. doi: 10.1191/0269215504cr702oa. [DOI] [PubMed] [Google Scholar]
- 13.Dijkerman HC, Letswaart M, Johnston M, MacWalter RS. Does motor imagery training improve hand function in chronic stroke patients? A pilot study. Clin Rehabil. 2004 Aug;18(5):538–549. doi: 10.1191/0269215504cr769oa. [DOI] [PubMed] [Google Scholar]
- 14.Page SJ, Levine P, Leonard AC. Effects of mental practice on affected limb use and function in chronic stroke. Arch Phys Med Rehabil. 2005 Mar;86(3):399–402. doi: 10.1016/j.apmr.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Page SJ, Levine P, Leonard A. Mental practice in chronic stroke: Results of a randomized, placebo controlled trial. Stroke. 2007 Apr;38(4):1293–1297. doi: 10.1161/01.STR.0000260205.67348.2b. [DOI] [PubMed] [Google Scholar]
- 16.Hewitt TE, Ford K, Levine P, Page SJ. Reaching Kinematics to Measure Motor Changes After Mental Practice in Stroke. Top Stroke Rehabil. 2007 Jul–Aug;14(4):23–29. doi: 10.1310/tsr1404-23. [DOI] [PubMed] [Google Scholar]
- 17.Jackson PL, Lafleur MF, Malouin F, Richards CL, Doyon J. Functional cerebral reorganization following motor sequence learning through mental practice with motor imagery. Neuroimage. 2003 Oct;20(2):1171–1180. doi: 10.1016/S1053-8119(03)00369-0. [DOI] [PubMed] [Google Scholar]
- 18.Butler AJ, Page SJ. Mental practice with motor imagery: evidence for motor recovery and cortical reorganization after stroke. Arch Phys Med Rehabil. 2006 Dec;87(12 Suppl 2):S2–S11. doi: 10.1016/j.apmr.2006.08.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 2000 Jul;74(1):27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- 20.Teng EL, Chui HC. The Modified Mini-Mental State Exam. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 21.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 22.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4:483–492. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Van der Lee JH, De Groot V, Beckerman H, Wagenaar RC, Lankhorst GJ, Bouter LM. The intra- and interrater reliability of the action research arm test: a practical test of upper extremity function in patients with stroke. Arch Phys Med Rehabil. 2001 Jan;82(1):14–19. doi: 10.1053/apmr.2001.18668. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh CL, Hsueh IP, Chiang FM, Lin PH. Inter-rater reliability and validity of the action research arm test in stroke patients. Age Ageing. 1998;27:107–113. doi: 10.1093/ageing/27.2.107. [DOI] [PubMed] [Google Scholar]
- 25.Dromerick AW, Lang CE, Birkenmeier R, Hahn MG, Sahrmann SA, Edwards DF. Relationships between upper-limb functional limitation and self-reported disability three months after stroke. J Rehabil Res Dev. 2006;43:401–408. doi: 10.1682/jrrd.2005.04.0075. [DOI] [PubMed] [Google Scholar]
- 26.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. I. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 27.Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606–1610. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- 28.DiFabio RP, Badke RB. Relationship of sensory organization to balance function in patients with hemiplegia. Phys Ther. 1990;70(9):542–548. doi: 10.1093/ptj/70.9.542. [DOI] [PubMed] [Google Scholar]
- 29.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000 Jun;31(6):1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 30.Eaton KP, Szaflarski JP, Altaye M, Ball AL, Kissela BM, Banks C, Holland SK. Reliability of fMRI for studies of language in post-stroke aphasia subjects. Neuroimage. 2008 Jun 1;41(2):311–322. doi: 10.1016/j.neuroimage.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cramer SC, Benson RR, Himes DM, et al. Use of functional MRI to guide decisions in a clinical stroke trial. Stroke. 2005;36:e50–e52. doi: 10.1161/01.STR.0000163109.67851.a0. [DOI] [PubMed] [Google Scholar]
- 32.Szaflarski JP, Page SJ, Kissela B, Levine P, Lee J, Strakowski S. Cortical reorganization following modified constraint-induced therapy: A study of four patients with chronic stroke. Arch Phys Med Rehabil. 2006 Aug;87(8):1052–1058. doi: 10.1016/j.apmr.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Mulder T, Zijlstra S, Zijlstra W, Hochstenbach J. The role of motor imagery in learning a totally novel movement. Exp Brain Res. 2004 Jan;154(2):211, 217. doi: 10.1007/s00221-003-1647-6. Epub 2003 Sep 24. [DOI] [PubMed] [Google Scholar]
- 34.Imbiriba LA, Rodrigues EC, Magalhaes J, Vargas CD. Motor imagery in blind subjects: the influence of the previous visual experience. Neurosci Lett. 2006 May 29;400(1–2):181–185. doi: 10.1016/j.neulet.2006.02.042. Epub 2006 Mar 10. [DOI] [PubMed] [Google Scholar]
- 35.Paivio A. Cognitive and motivational functions of imagery in human performance. J Applied Sport Sci. 1985;10(4):22–28. [PubMed] [Google Scholar]
- 36.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 37.Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10:171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 38.Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 39.Lancaster JL, Woldorff MG, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YH, Park JW, Ko MH, Jang SH, Lee PK. Plastic changes of motor network after constraint-induced movement therapy. Yonsei Med J. 2004;45:241–246. doi: 10.3349/ymj.2004.45.2.241. [DOI] [PubMed] [Google Scholar]
- 41.Johansen-Berg J, Dawes C, Guy SM, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125(Pt 12):2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- 42.Liepert J, Hamzei F, Weiller C. Lesion-induced and training-induced brain reorganization. Restor Neurol Neurosci. 2004;22:269–277. [PubMed] [Google Scholar]


