Abstract
OBJECTIVE
This study was designed to examine the effect of morphine administration on the intestinal mucus barrier and determine its direct effect on the virulence and lethality of Pseudomonas aeruginosa, one of the most frequent pathogens to colonize the gut of critically ill patients.
SUMMARY BACKGROUND DATA
Surgical injury is associated with significant exposure of host tissues to morphine from both endogenous release as well as its use as a potent analgesic agent. Morphine use in surgical patients exposed to extreme physiologic stress is well established to result in increased infection risk. Although morphine is a known immunosuppressant, whether it directly induces virulence expression and lethality in microbes that colonize the human gut remains unknown.
METHODS
Mice were implanted with a slow release morphine or placebo pellet with and without intestinal inoculation of P. aeruginosa created by direct cecal injection. Mucus production and epithelial integrity was assessed in cecal tissue via Alcian Blue staining and histological analysis. In vivo and in vitro P. aeruginosa virulence expression was examined using reporter strains tagged to the epithelial barrier disrupting protein PA-I lectin. P. aeruginosa chemotaxis toward morphine was also assayed in vitro. Finally the direct effect of morphine to induce PA-I lectin expression was determined in the absence and presence of methylnaltrexone, a mu opioid receptor antagonist.
RESULTS
Mice intestinally inoculated with P. aeruginosa and implanted with a morphine pellet demonstrated significant suppression of intestinal mucus, disrupted intestinal epithelium and enhanced mortality whereas exposure of mice to either systemic morphine or intestinal P. aeruginosa alone enhanced intestinal mucus without mortality suggesting a shift in P. aeruginosa during morphine exposure to a mucus suppressing, barrier disrupting, and lethal phenotype. Direct exposure of P. aeruginosa to morphine in vitro confirmed that morphine can transform P. aeruginosa to a more virulent phenotype that is attenuated in part, by methylnaltrexone.
CONCLUSIONS
Morphine administration shifts intestinal P. aeruginosa to express a virulent phenotype and may play a role in its ability to causes lethal gut-derived sepsis in a susceptible host.
INTRODUCTION
Morphine is an analgesic opioid agent used by most practicing surgeons as their preferred drug to treat postoperative pain. Morphine use is especially prevalent in patients undergoing invasive procedures that are associated with long operative times and extended hospitalization. Morphine continues to be used clinically because it is one of the most potent and effective analgesic agents available. Importantly, morphine is also produced endogenously following surgery in direct response to the degree of physiologic stress and injury1–3. Variously tissues and cells produce endogenous morphine including neutrophils that carry morphine to sites of inflammation and infection4. A recent study using mass spectrometry analysis demonstrated that endogenous morphine was present in very high concentrations in the serum of patients with sepsis, especially those with severe sepsis and septic shock compared to patients with systemic inflammatory response syndrome (SIRS) or normal controls4. Endogenous morphine is chemically identical to the poppy (Papaver somniferum) derived morphine used pharmacologically. Therefore in one form or another, exposure of tissues and organs to significant and sustained concentrations of morphine is a virtual certainty for most patients subjected to surgical injury or critical illness. While endogenous morphine exposure may serve some yet-to be identified benefit in terms of tissue healing and organ recovery, the use of pharmacologic doses of morphine to treat pain may, in selected circumstances, result in unintended consequences. Morphine is a known immunosuppressant whose use and activity has been associated with impaired bacterial clearance5–8 and enhanced susceptibility to infectious organisms9–13. Experimental evidence continues to demonstrate that morphine affects various arms of the innate and adaptive immune systems including the IL-23/IL-17 mediated defense system resulting in dysfunction of both dendritic cells and macrophages13. Clinically morphine use has been shown to be an independent risk factor for infection and infectious-related morbidity in burn patients14. Yet the effect of morphine on the gut immune system, one of the main sites adversely affected by the pharmacologic effect of morphine (i.e. ileus), is poorly characterized. While it well known that morphine causes bacterial translocation of the commensal flora that has been postulated to result from its inhibitory action on peristalsis, most animals and humans treated with morphine do not develop spontaneously clinically evident gut-derived sepsis10. However during prolonged exposure, i.e. following severe surgical injury, and when patients become colonized by virulent hospital associated pathogens, the use of high dose morphine may impair local immune function that predisposes to sepsis due to gut derived microbes. Here we created a model in which morphine administration was delivered via a slow release subcutaneously implanted pellet in mice that were simultaneously intestinally inoculated with Pseudomonas aeruginosa via direct cecal puncture. We examined the effect of morphine on innate intestinal immunity by examining intestinal mucus, the first line of barrier function against invading pathogens. We then examined whether morphine could directly affect the virulence state of P. aeruginosa by examining its ability to induce the expression of the PA-I lectin (PA-IL), a quorum sensing dependent virulence factor in P. aeruginosa that we previously demonstrated to play a key role in mucosal adherence, intestinal barrier disruption and lethal gut- derived sepsis in mice. In addition we examined the role of morphine as a chemoattractant for P. aeruginosa. Results indicate that morphine exposure to mice intestinally inoculated with P. aeruginosa leads to lethal gut-derived sepsis characterized by depletion of intestinal mucus, retention of P. aeruginosa in the gut and its adherence to the intestinal mucosa, and in vivo virulence expression of the PA-I lectin/adhesin protein. In vitro experiments demonstrated that morphine can directly induce P. aeruginosa virulence activation and that this effect, in part, can be reversed by a methylnaltrexone (MNTX), an μ opioid receptor antagonist.
MATIERIALS AND METHODS
Bacterial strains
P. aeruginosa strains PAO1 and ATCC 27853 as well their derivatives PA27853/PLL-EGFP, PA27853/EGFP15, PAO1/PLL-EGFP16, PA27853/PLL-RedT1, a red fluorescent PA-I lectin (PA-IL) reporter strain (current work), and P. aeruginosa XEN41, a constitutively luminescent strain that harbors the luxCDABE cassette inserted in a constitutively expressed manner (Caliper, Inc) were used in the experiments.
Morphine model of lethal gut-derived sepsis
Chronic morphine administration via slow release subcutaneously place pellet was used according to a previously described model with minimal modifications17. All experiments were approved by the Animal Care and Use Committee at the University of Chicago (IACUC 71762 and IBC991). Male C57BL/6 mice were ordered from HSD at 7 weeks and allowed one week to acclimate to their new environment prior to surgery. They were randomly assigned to either a morphine or placebo group. Both groups were allowed access to food and water throughout the study period (no fasting). On the day of operation, animals were anesthetized (ketamine 100 mg/kg, xylazine 10 mg/kg) intraperitoneally, and two groups of mice were subcutaneously implanted in the dorsal neck with either a slow release 25 mg morphine pellet obtained from the NIDDK NIH or a placebo pellet according to published methods18. The 25 mg dose of morphine was chosen based on extensive dose response studies (1–40 mg) in preliminary experiments demonstrating respiratory depression with doses higher than 25 mg (data not shown). Immediately following pellet implantation, via a small midline incision, 200 μl of the bacterial suspension containing 107 CFU was injected into the distal ileum while the bowel proximal to the injection site was occluded. This allowed visualized directed filling of the cecum with the Pseudomonas inoculum. The puncture site was swabbed with Acudyne prior to closure of the laparotomy incision. There have been no cases of spillage resulting in local peritonitis in over 500 uses of this model in our lab. Animals were subsequently assessed for mortality, and sacrificed once septic and moribund.
Histology of animal tissues
Animal cecal tissues were collected at 36 hours, fixed in 10% buffered formalin and embedded in paraffin. Paraffin specimens were cut into 4-μm sections and mounted on microslides. Hematoxylin & Eosin and Alcian Blue staining were performed on paraffin embedded sections, and stained slides were reviewed using Zeiss Axioskop. Evaluation of mucus in goblet cells was performed using ACIS software (Automated Cellular Imaging Software) as previously described16.
Cytokine analysis
Cytokines (INFγ, IL1β, IL6, IL9, IL10, IL12(p40), IL12(p70), IL13, IL15, IL17, IP10, and TNFα) were measured using BioPlex (city, state) in both serum and cecal contents in reiterative experiments in mice 12 hrs following morphine or placebo pellet implantation with and without intestinal inoculation of P. aeruginosa via direct cecal puncture (n=6/group × 4 groups).
QRT-PCR Detection of P. aeruginosa in tissues
Real-time qPCR assay was used to amplify and quantify P. aeruginosa rDNA from mouse tissues. The specific qPCR for P. aeruginosa was performed using a mixture of primers Pa23FP, Pa23RPb and fluorescent probe Pa23FAMb as previously described19. Each qPCR assay was performed in 10 μl reaction volume and contained 5μl TaqMan Gene Expression Master mix (Applied Biosystems, CA), 5 ng of extracted DNA, 0.9 μM of each primer and 0.2 μM of the probe. Reactions were carried out in a Prism 7900HT Sequence Detection System (Applied Biosystems, CA) using thermal cycling program consisted of 50°C for 2min, 95°C for 10 min, followed by 40 cycles 95°C for 15 s, 60°C for 1min19. Concentration of DNA was determined using standard curves based on ten-fold serial dilutions of known quantities of P. aeruginosa genomic DNA. Sensitivity of assays was approximately 70 fg of P. aeruginosa genomic DNA per PCR reaction well.
P. aeruginosa chemotaxis assay
PA27853/EGFP cells pre-grown in TSB were used in the chemotaxis assay. Cells were centrifuged at 5,000×g, 5 min, and suspended in 40 mM potassium phosphate buffer, pH 7.0 to get a final density of 0.15 (OD 600nm). Chemotaxis was assayed by creating concentration gradients of the chemoattractant (i.e morphine) that is slowly released from soft agar as previously described20, 21 with following modifications: agarose center cut- outs (i.e agarose “plugs”) containing 1% UltraPure™ Agarose (Invitrogen) in 40 mM potassium phosphate buffer, pH 7.0 were either supplemented or not supplemented with morphine to a final concentrations of 1 μg/μl or 0.1 μg/μl and then a 5 μl “plug” was dropped at the center of 0.15-mm-thick ΔTC3 dishes (Bioptech, Bulter, PA). After 2 minutes, 600 μl of PA27853/EGFP at OD 600 nm = 0.15 was poured into the dish, and bacterial cell movement to and accumulation at the peripheral rim of the agarose droplet was followed in real time using an Olympus Live-cell Digital Imaging Microscope (60× oil objective). Images were captured every 30 sec and bacteria counted at the 5 μm interval at the edge of the agarose droplet. At the end of experiments images of the whole agarose droplets were scanned using a Fluorescence Macroview Stereomicroscope (2.5= objective).
Photon camera imaging of whole intestine and organs using bioluminescent P. aeruginosa
P. aeruginosa XEN41 strain, a constitutively luminescent strain that harbors the luxCDABE cassette inserted in a constitutively expressed manner (Caliper, Inc) was introduced in the cecum of mice implanted with either morphine or placebo pellet. At 6, 9 and 14 hours post-surgery mice were anesthetized with sevofluorane and sacrificed. Organs were immediately harvested, placed in petri dishes, and then placed on ice. Organs were imaged ex-vivo within 30 minutes of harvest using the Xenogen photon camera using a 15 second exposure time. Using the “Living Image” software images were analyzed and the total number of photons was calculated. Mouse organs from mice without bacteria were imaged to confirm the absence of any background luminescence in the absence of bacteria (data not shown).
Construction of red fluorescence PA-IL reporter strain 27853/PLL-RedT1
A pll-redT1 DNA fusion construct was created by overlapping PCR. The pll region comprising the lecA gene (without stop codon) and upstream region was amplified using PA27853 genome DNA as a template, and primers forward, F-2905994, 5'-GTCGGGGTACCTGCCGGTTCGACCCCGGCTCCG-3' and reversed, Red-2905179, 5'-GATGACGTCCTCGGAGGAGGCCATGGACTGATCCTTTCCAATATTG-3'. RedT1 was amplified using the pDsRedT1 as a template, and primers forward, 2905179-Red, 5'-CAATATTGGAAAGGATCAGTCCATGGCCTCCTCCGAGGACGTC-3' and reversed, RedT1-C, 5'-CGCCAAGCTTGCGGCCGCTACAGGAACAGGTGGTG3'. Pll-redT1 was amplified using pll and redT1 PCR products as templates, and primers F-2905994 and RedT1-C. The pll-redT1 DNA construct was cloned in the Escherichia-Pseudomonas shuttle vector pUCP2422 using KpnI and HindIII restriction sites. The pUCP24/-PLL-RedT1 was electroporated in strain P. aeruginosa 27853 to create strain 27853/ PLL-RedT1.
Expression of PA-IL in the reporter strain 27853/ PLL-RedT1
The reporter strain 27853/ PLL-RedT1 was poured at a concentration of 107 CFU/ml in HDMEM media supplemented with 10% fetal bovine serum with and without morphine (20 μg/ml) into 0.15 mm thick dTC3 dish (Bioptech), and grown under static conditions at 37°C for 5 hours. Dynamic tracking of fluorescence was followed using Olympus Fluoview Confocal microscope (Ix70), 60× NA 1.4 oil objective. Excitation was performed at 543 laser line and data were collected at 605 nm.
GFP fluorescence assay to detect PA-I lectin expression in P. aeruginosa
P. aeruginosa PA27853/PLL-EGFP reporter strain was used to assess the expression of the PA-IL. Overnight culture in tryptic soy broth was diluted 50 fold in HDMEM media, and the culture was allowed to grow for 1 hour followed by the addition of morphine, 20 μg/ml. Fluorescence (excitation 485/20, emission 528/20) was measured dynamically and normalized to cell density evaluated by absorbance at 600 nm. When needed, MNTX (20 μg/ml) was added at the point of inoculation and morphine (20 μg/ml) was added one hour later.
PA-IL expression in vivo in cecal tissues
P. aeruginosa PAO1/PLL-EGFP was introduced in the cecum of mice implanted with either morphine or placebo pellet (3 mice/group). 36 hours later, cecal tissues were collected, fixed in 10% buffered formalin and embedded in paraffin. Paraffin specimens were cut into 4-μm sections and mounted on microslides and evaluated by fluorescence microscopy.
Statistical analysis
All statistical analyses were performed using Student's t-test using Sigma Plot software. Kaplan-Meier Survival analysis was performed using SPSS 17.0 software.
RESULTS
Morphine causes lethal gut-derived sepsis in mice intestinally inoculated with Pseudomonas aeruginosa
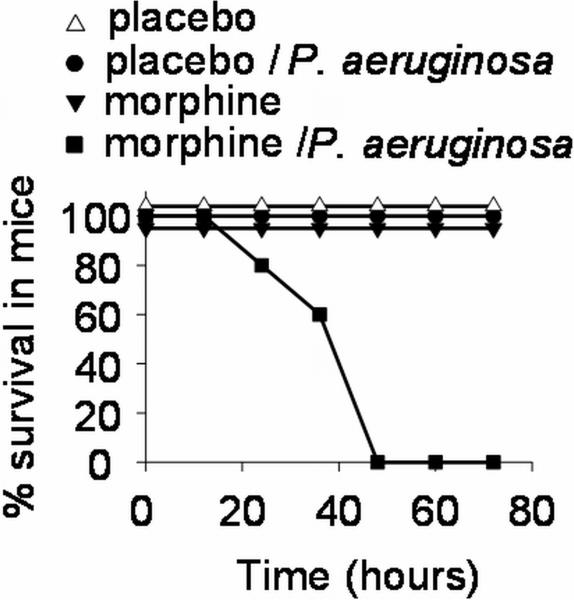
Preliminary dose response experiments were performed varying the doses of the morphine pellet implanted in mice in order to determine the dose of morphine that alone did not cause mortality. Results demonstrated that the implantation of 25 mg pellet alone resulted in no mortality in mice and mice remained completely healthy (data not shown). Four (4) groups of mice were then randomly assigned to receive morphine or a placebo pellet with or without intestinal inoculation with P. aeruginosa via direct cecal puncture- 1. placebo pellet/without P. aeruginosa−; 2. placebo pellet + cecal P. aeruginosa i.e placebo pellet/ P. aeruginosa+; 3. morphine pellet/ P. aeruginosa−; 4. morphine pellet/ P. aeruginosa+. Results demonstrated 100% mortality only in mice in which implantation of 25 mg morphine pellet and injection of P. aeruginosa PAO1 into the distal intestine was carried out. Mice in this group appeared grossly septic prior to death with chromdacctyrrhea, ruffled fur, lethargy, and scant loose stools. Mice in the remaining three groups appeared completely healthy and active for the duration of the observation period (Figure 1).
Figure 1. Synergistic effect of morphine administration and P. aeruginosa intestinal injection on mice mortality.
25 mg of placebo pellet or morphine pellet were implanted in neck of C57BL6 with or without simultaneous direct cecal injection of 200 μl of 107 CFU/ml P. aeruginosa PAO1. Data are presented as Kaplan- Mayer survival curves. n=10/group, p< 0.001.
Morphine administration to mice intestinally inoculated with P. aeruginosa shifts its behavior to express a mucus suppressing phenotype, to disrupt the integrity of the intestinal epithelium, and induce local and systemic cytokine secretion
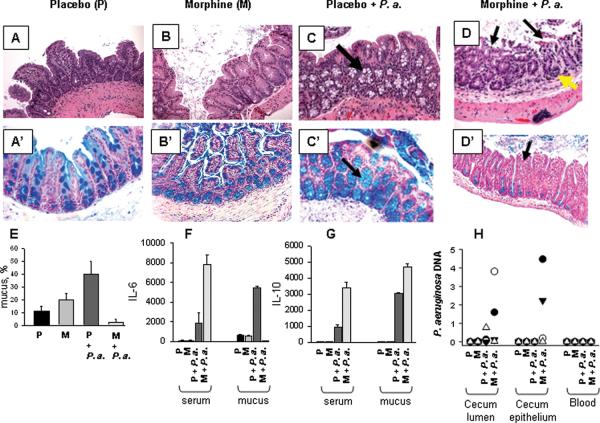
Histological analysis of cecal sections of mice demonstrated an intact intestinal epithelium in mice implanted with the placebo pellet without P. aeruginosa injection (Figure 2A) as did sections in which morphine was implanted without exposure to P. aeruginosa (Figure 2B). However, increased amount of goblet cells appeared on villi and abundant mucus was seen inside the lumen of morphine treated animals. Cecal sections from mice implanted with a placebo pellet and in whom the intestine was injected with P. aeruginosa also displayed an intact epithelium, however goblet cells appeared highly abundant and increased within the crypts compared to mice not receiving intestinal P. aeruginosa (Figure 2C, shown by arrow). In contrast cecal sections from mice implanted with a morphine pellet and intestinally inoculated with P. aeruginosa (Figure 2D), displayed gross disruption in the intestinal epithelium with superficial necrosis, desquamated epithelial cells within the intestinal lumen (shown by black arrows), and islands of lymphocytic infiltration (shown by yellow arrows). To more precisely define the significance of the changes in goblet cells observed between groups by H&E staining, sections were stained with Alcian Blue. Mucus expression was observed to be significantly different between groups. Blue staining goblet cells were significantly increased in morphine treated mice and in placebo treated mice intestinally inoculated with P. aeruginosa with mucus observed to be increased on villi, in crypts and within the intestinal lumen (Figure 2B', C'). However in morphine treated mice intestinally inoculated with P. aeruginosa, significant attenuation in mucus production was observed (Figure 2D'). Figure 2E is a quantitative representation of the percentage of blue stained mucus as assessed by ACIS software with random field counting of images (n=40 spots/image, n=3 per group, *p<0.001). Since either systemic morphine exposure or intestinal P. aeruginosa alone increased mucus production, preserved the intestinal epithelium, and resulted in no mortality whereas systemic morphine together with intestinal P. aeruginosa resulted in suppressed mucus production, epithelial disruption, and lethality, these results raise the possibility that intestinal P. aeruginosa exposed to morphine expresses a mucus suppressing, barrier disrupting and lethal phenotype.
Figure 2. Histology of ileal-cecal intestinal tissue sections.
(A–D), H&E staining; (A'–D'), alcial blue staining. (A, A') mice, implanted with placebo pellet. (B, B') mice, implanted with morphine pellet. (C, C') mice, implanted with placebo pellet and intestinal inoculation with P. aeruginosa PAO1, 200 μl of 107 CFU/ml via direct puncture. Increased amount of goblet cells in crypts is shown by arrow. (D, D') mice, implanted with morphine pellet and injected P. aeruginosa PAO1 similar to C. Focal superficial necrosis (shown by black arrows on panel D, C') and the appearance of lymophocytic infiltration (shown by yellow arrows) are seen. Images were taken with Zeiss Axioskop using a 20 × objective. (E), Quantitative assessment of intestinal mucus abundance represented by % of blue stained mucus/field using ACIS software (Automated Cellular Imaging Software) n=40 fields of evaluation from 4 mice/group, *p<0.05 Placebo vs morphine; **p<0.05 Placebo vs Placebo + P. aeruginosa; ***p<0.001 Placebo vs morphine + P. aeruginosa). (F, G), Production of (F) IL-6 and (G) IL-10 in serum and cecum. (H) QRT-PCR to detect P. aeruginosa PAO1 in cecal lumen, intestinal epithelium, and blood of mice 36 hours following P. aeruginosa inoculation into the cecum. •, mouse 1; ∘, mouse 2; ▾, mouse 3; and Δ, mouse 4.
Cytokine analysis revealed significant differences between IL-6 and IL-10 between groups of mice, whereas all other cytokines tested demonstrated no differences between groups. Serum IL-6 was significantly increased in mice following intestinal inoculation with P. aeruginosa however IL-6 levels in (morphine + intestinal P. aeruginosa) were 4 fold higher compared to (placebo + intestinal P. aeruginosa). In contrast, IL-6 was suppressed in the cecal mucosal of mice exposed to morphine and intestinal P. aeruginosa but enhanced in placebo mice with intestinal P. aeruginosa suggesting that morphine may shift P. aeruginosa to express a phenotype capable of subverting immune clearance. IL-10 levels were significantly increased in both serum and cecal contents in mice treated with morphine and intestinal P. aeruginosa as well as in placebo treated mice intestinally inoculated with P. aeruginosa, although at significantly lower levels. Since bacteria can directly shape the immune repertoire, higher IL-10 levels in morphine treated mice with intestinal P. aeruginosa may viewed as a specific microbial strategy to suppress local and systemic inflammation.
Morphine administration results in the retention of P. aeruginosa within the intestine of mice without any significant difference in bacterial translocation to internal organs
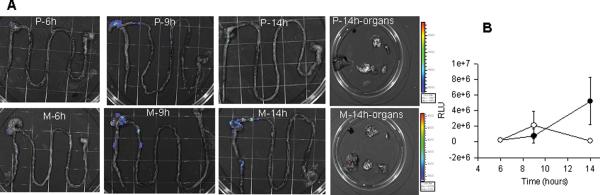
To more precisely determine the effect of morphine administration on the retention of intestinally inoculated P. aeruginosa, a constitutive bioluminescent strain of P. aeruginosa XEN 41 was injected into the cecum of mice with morphine pellets and placebo pellets and tracked for its regional distribution and extra-intestinal migration in whole intestinal segments and whole organs using photon camera imaging (Xenogen) (Fig.3A). Luminescent counts were quantified between groups (Fig.3B). Results demonstrated that bioluminescent strains injected into the cecum remained retained within the ileo-cecal area in morphine treated mice whereas they were mostly absent in placebo treated mice. Translocation of bioluminescent strains to lymph nodes, liver, spleen, and lung was minimal and not statistically different between groups (data not shown). Interestingly morphine did not cause significant translocation to the bloodstream in mice in any treatment groups based on serum DNA specific to P. aeruginosa, although retention in the cecum and adherence to the cecal epithelium, a major determinant of lethal gut-derived sepsis, was observed in 36 hours in mice treated with morphine (Fig.2H).
Figure 3. Effect of morphine administration on the intestinal retention of P. aeruginosa.
(A) Distribution of constitutive bioluminescent P. aeruginosa XEN41 following direct ileal inoculation by intestinal region at varying time points in mice implanted with placebo (P) or morphine (M) pellets. (B) Photon counts of P. aeruginosa XEN41 following 6, 9 and 14 hours after direct ileal inoculation by intestinal region in mice implanted with placebo (∘) or morphine (•) pellets. Data are expressed as mean ±SEM.
P. aeruginosa recognizes the presence of morphine and responds by expressing clumping behavior and PA-IL expression
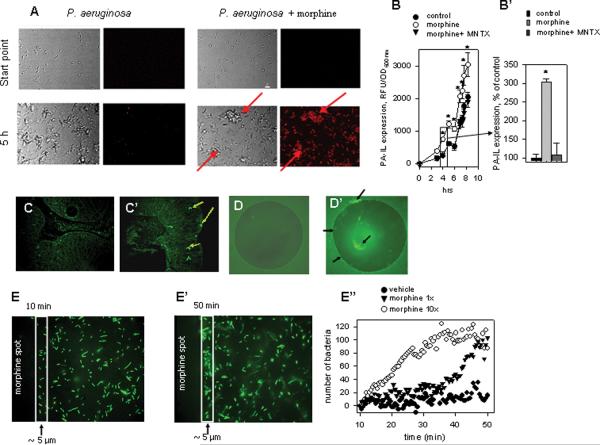
Next, to determine whether morphine is directly recognized and responded to by P. aeruginosa, we exposed a red fluorescent protein reporter construct of P. aeruginosa tagged to the promoter of the virulence related protein PA-IL, to morphine. Reporter strains were exposed to morphine or placebo and time lapsed video analysis demonstrated activation of the PA-IL in response to morphine as judged by the time dependent activation of red fluorescence and the appearance of clumping behavior, a known virulence related behavior that develops prior to biofilm formation23 (see supplemental video https://wiki.uchicago.edu/pages/viewpageattachments.action?pageId=40894480&sortBy=date&highlight=Real-time+tracking+of+PA-I+expression+in+P.ppt&). Start point and 5 hrs time point are represented in Fig.4A. PA-IL expression was also directly quantified using a PA-IL reporter strain PA27853/PLL-EGFP. Strains were tracked over time and demonstrated a statistically significant increase in PA-IL expression following exposure to morphine that was attenuated in the presence of methylnaltrexone, a known μ opioid receptor antagonist (Fig 4B,B'). We also used the PA-IL reporter strain PAO1/PLL-EGFP in vivo by inoculating it into the cecum of mice implanted with either a placebo or morphine pellet. Mice were sacrificed at 36 hrs and cecal tissues visualized by fluorescence microscopy to determine if PA-IL was in vivo expressed in morphine treated mice. Cecal tissue images demonstrated P. aeruginosa localized within the epithelium of mice implanted with morphine pellet which were not observed in placebo treated mice (Fig.4C,C') consistent with the DNA detection data (see Figure 2F).
Figure 4. P. aeruginosa recognizes morphine.
(A) PA-IL reporter strain demonstrating enhanced PA-IL expression detected by red fluorescence, and clumping formation (precursor to biofilm development) shown by red arrows. (B,B') Exposure to mu opioid receptor antagonists MNTX attenuates the PA-I lectin inducing effect of morphine. (B), dynamic tracking of PA-IL expression, (B'), PA-IL expression following 4 hrs of culture represented as % of control. (C,C') Enhanced expression of PA-IL is seen in P. aeruginosa adherent to the intestinal epithelium in mice implanted with (C') morphine containing pellet but not (C) placebo pellet. (D,D') P. aeruginosa accumulating at the peripheral rim and on the agarose droplet containing (D') morphine but not (D) blank agarose. (E–E") P. aeruginosa migrates to the morphine agarose droplet as seen in images captured at (E) 10 min and (E') 50 min after P. aeruginosa inoculation on the blank non- morphine containing agarose droplet (E") Bacterial counts inside a rectangle of 5 μm width.
P. aeruginosa strains display chemotaxis toward morphine
In order to determine whether P. aeruginosa could migrate along a concentration gradient of morphine (i.e. chemotaxis), experiments were designed in which morphine was included in the melted agarose and dropped in the center of 0.15-mm-thick ΔTC3 culture dishes (Bioptech, Bulter, PA). P. aeruginosa PA27853/EGFP was placed in the dish and fluorescence measured over several hours. As seen on Figure 4D, there was essentially no accumulation of P. aeruginosa around a blank agarose drop, however morphine-containing agarose was surrounded by fluorescent bacteria (Fig. 4D'). Dynamic image capture in time lapse frames allowed detection of accumulated P. aeruginosa at the site of the morphine-containing agarose drop (Fig. 4E, E'). Fluorescent bacteria were quantified on images captured every 30 sec with a geometric area of 5 μm and demonstrated a time- and morphine dose-dependent attraction of P. aeruginosa to the morphine-containing agarose drop (Fig.4E"). In control dishes containing blank agarose, the number of bacteria at the edge of the agarose fluctuated whereas the number of bacteria at the edge of morphine-containing agarose increased. This effect was enhanced at higher concentrations of morphine (1 μg/μl (10×) versus 0.1 μg/μl (1×)). Reiterative experiments were performed with methylnaltrexone to determine if migration could be attenuated however no differences were observed (data not shown).
DISCUSSION
Morphine is becoming increasingly recognized as a major contributing factor in infection related outcome during critical illness, burn injury, following trauma, and in narcotic abusing patients. Although many mechanisms underlying these observations have been proposed, much of the focus of this line of inquiry has identified morphine as a potent immunosuppressant. The current study is focused on yet an unexplored mechanism by which morphine might increase the susceptibility to infection by examining its ability to directly affect the expression of virulence in colonizing microbes. We have previously shown that endogenously released opioids such as dynorphin can directly activate the quorum sensing virulence machinery of P. aeruginosa24, one of the most common bacteria to cause lethal gut-derived sepsis following a variety of severe catabolic stresses including burn injury, prolonged critical illness, radiation injury, and bone marrow transplantation25–27. The finding in the present study that P. aeruginosa senses the presence of morphine and can respond by expressing proteins that are known to cause lethal gut-derived sepsis (i.e. PA-I lectin)28, may be clinically important given the difficulty of eliminating this highly immunoelusive pathogen which resists immune clearance by forming an antibiotic impenetrable biofilm.
That we are aware, data from the present study are the first to demonstrate that an opportunistic pathogen such as P. aeruginosa possesses the ability to switch from a mucus enhancing to a mucus suppressing phenotype depending on the presence or absence of a local environmental “cue” such as morphine. One of the first lines of innate immune defense in the gastrointestinal tract is the presence and secretion of mucus. Virtually all disorders of the intestinal mucosa have alterations in mucus as a characteristic feature29, 30. An intact mucus layer is critical not only to maintain intestinal epithelial barrier function by stabilizing the cell cytoskeleton, but also acts as a phospholipid rich physical barrier that repels bacteria through both electrostatic repulsion and the presence of varying concentrations of antimicrobials31, 32.
Yet, in order for bacteria to successfully colonize the gut, they must adhere to and persist within the mucus33. Recent in depth analysis of mucus dynamics in health and stress indicate that this occurs in the outer layer of mucus whereas the inner adherent layer remains devoid of bacteria34. During health, epithelial cells in the gut remain sterile and therefore are not affected by bacterial mediated actions. When bacteria are cued to invade, they must first traverse this line of defense. It is presumed this occurs via phospholipases that degrade the phospholipid rich mucus35. Yet most commensal and pathogenic organisms elicits enhanced mucus production as intestinal cells sense their presence and respond by secreting more antimicrobial rich mucus29. In fact, in germ free mice, as might be predicted, mucus production is dramatically decreased36. In the present study when P. aeruginosa was intestinally inoculated into mice receiving a placebo pellet, as would be expected, mucus production was increased. Yet when P. aeruginosa was intestinally inoculated into mice implanted with a morphine pellet, mucus production was dramatically suppressed. One plausible explanation for this combined effect, given the response of the individual agents alone, is that morphine shifted the behavior of P. aeruginosa to express a mucus suppressing phenotype. This conclusion is reinforced by the high probability that intestinal P. aeruginosa was exposed to morphine given the previous observation that parenteral morphine enters the intestinal lumen in rodents37. Second, the rodent pathogen C. rodentium, known to play a causative role in experimental inflammatory bowel disease, has recently been demonstrated to inhibit muc2 and trefoil factor gene expression in mice via modulation of the immune system38. We are currently exploring methods to stabilize the transformed phenotype of P. aeruginosa that develops in morphine treated mice to determine if it can independently suppress muc2 gene expression in cultured intestinal epithelium as well as in vivo.
It is becoming increasingly clear that in vivo phenotypic switching can explain the observation that the mere presence of a pathogen alone appears to be less important than how its virulence machinery is dynamically activated by local cues within a physiologically stressed host This has been recently confirmed in clinical reports demonstrating that quorum sensing signaling molecules (i.e. those specific to P. aeruginosa), that indicate activation of virulence in a given pathogen, are present in the serum of patients with sepsis and septic shock that correlate with the degree of clinical sepsis and mortality39. These studies and others are beginning to shed light on the both experimental and clinical findings that indicate that the presence of a pathogen alone, even when disseminated from the primary site of colonization or infection, does not necessarily indicate a pathologic state of inflammation or predict mortality. Converging lines of evidence suggest that mortality is likely to be predicted by the state of virulence of a given pathogen at its primary site of colonization37. It may be for this reason that neither blood cultures nor the presence of microbial DNA in the serum accurately or consistently predict mortality from sepsis40. Finally, whether a given intestinal pathogen when exposed to a given local environmental cue (i.e morphine) can subvert local immunity to prevent its clearance, such as was observed here with both mucus production and local IL-6, may play a role in its success to cause lethality from within the local site. For these reasons, the precise pathogen or community of pathogens that cause lethal gut-derived sepsis often remain unknown.
We have previously shown that host derived factors released in response to surgical injury can bind to and transduce the virulence machinery of P. aeruginosa via highly specific outer membrane bound receptors41. In the case of endogenous opioid signals, we previously identified dynorphin to activate the quorum sensory MvfR-PQS system in P. aeruginosa leading to enhanced virulence expression and altered intestinal epithelial barrier function24. This effect appeared to be mediated principally by kappa (k) receptors although not exclusively. In the present study we demonstrated a similar role for exogenous morphine. In the present study, expression of the virulence related protein PA-IL in response to morphine, appeared to be mediated by mu (μ) receptor since its expression was attenuated in the presence of a specific mu inhibitor, methylnaltrexone. However chemotaxis was not inhibited by methylnaltrexone, suggesting that additional morphine agonist properties such as delta or kappa agonist activity may play a role in activation of this particular virulence related behavior given that morphine is not a pure μ agonist. We did not use methylnaltrexone in our mouse model as rodents possess a demethylating enzyme for MNTX4. This is in contrast to humans where MNTX has been studied in clinical trials to reduce postoperative ileus and restore bowel function in critically ill patients. Another problem with mouse studies using systemic competitive opioid antagonists is that many of these compounds have agonist like activity depending on the dose, timing, and tissue compartment in which they are active. Finally, while parenteral use of opioid antagonists may reverse the host adverse effects of morphine and other endogenous opioids released during surgical injury, differentiating this effect from its direct effect on colonizing microbes is problematic. For these reasons application of these opioid antagonists to our mouse model has presented various methodological challenges. Nonetheless, we did observe an attenuation of virulence in response to morphine when methylnaltrexone was directly applied to bacteria cells. This suggests that μ-like receptors may be present on or within P. aeruginosa and play a role in the mechanism by which P. aeruginosa recognizes and responds to morphine. Confirming this notion is the observation over 15 years ago that a high affinity opioid binding site with mixed mu and delta properties is present in E. coli and involves the EnvY protein42. We are in the process of localizing and identifying the receptors and their precise mechanism of transduction of the quorum sensing signaling system in P. aeruginosa in response to morphine similar to our previous studies with analogous compounds released during surgical injury.
The recent introduction of the peripheral mu opiate antagonists methylnaltrexone (MNTX) and alvimopan43 into clinical practice could potentially permit human testing of our findings. MNTX, given subcutaneously, is approved to treat opioid induced constipation in advanced illness in palliative care patients when response to conventional laxatives has not been sufficient. Alvimopan, an orally administered peripheral mu opiate antagonist has been used to facilitate gut recovery following large or small bowel resection with primary anastomosis43. However, alvimopan is very insoluble and would be difficult to employ is our model system. In a recent retrospective observational case control study of more than 7000 patients, use of alvimopan resulted in a statistically significant reduction in postoperative infection rate http://clinicaltrials.gov/ct2/show/results/NCT01150760?term=alvimopan&rank=1§=Xb015#outcome6. These human findings are consistent with our laboratory observations and may suggest that colonizing microbes can be shielded, in part, from the effects of host activating signals released during surgical injury by using opioid antagonists. However more expanded human trials of these agents will be needed to confirm the preventative effects of peripheral opioid antagonism on clinical infection following surgery.
In summary, here we present evidence that P. aeruginosa is directly activated by morphine to express a virulent phenotype against the intestinal epithelium leading to lethal sepsis. Further work is needed to more completely understand the mechanism of this response and determine its role in clinical infection.
Here we demonstrate that direct exposure of Pseudomonas aeruginosa, an organism that commonly colonizes the gut of critically ill patients, to morphine activates its virulence as judged by enhanced chemotaxis and virulence factor production. In mice intestinally colonized by P. aeruginosa and implanted with a slow release morphine pellet, lethal gut derived sepsis develops in association with altered intestinal mucus production.
Acknowledgments
This study was funded by NIH RO1 GM062344-11 and by the University of Chicago BSD Imagine Research Institute for Pilot Research Projects Using Animal Imaging.
Footnotes
Disclosure: Dr. Jonathan Moss serves as a paid consultant to Progenics Pharmaceuticals, Inc., has a financial interest in MNTX as a patent holder through the University of Chicago, and receives stock options from Progenics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Muller A, Glattard E, Taleb O, et al. Endogenous morphine in SH-SY5Y cells and the mouse cerebellum. PLoS ONE. 2008;3(2):e1641. doi: 10.1371/journal.pone.0001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshida S, Ohta J, Yamasaki K, et al. Effect of surgical stress on endogenous morphine and cytokine levels in the plasma after laparoscopoic or open cholecystectomy. Surg Endosc. 2000;14(2):137–40. doi: 10.1007/s004649900085. [DOI] [PubMed] [Google Scholar]
- 3.Pryor SC, Zhu W, Cadet P, et al. Endogenous morphine: opening new doors for the treatment of pain and addiction. Expert Opin Biol Ther. 2005;5(7):893–906. doi: 10.1517/14712598.5.7.893. [DOI] [PubMed] [Google Scholar]
- 4.Glattard E, Welters ID, Lavaux T, et al. Endogenous morphine levels are increased in sepsis: a partial implication of neutrophils. PLoS ONE. 5(1):e8791. doi: 10.1371/journal.pone.0008791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacerdote P. Opioids and the immune system. Palliat Med. 2006;20(Suppl 1):s9–15. [PubMed] [Google Scholar]
- 6.Rahim RT, Meissler JJ, Jr., Adler MW, Eisenstein TK. Splenic macrophages and B cells mediate immunosuppression following abrupt withdrawal from morphine. J Leukoc Biol. 2005;78(6):1185–91. doi: 10.1189/jlb.0304123. [DOI] [PubMed] [Google Scholar]
- 7.Ohara T, Itoh T, Takahashi M. Immunosuppression by morphine-induced lymphocyte apoptosis: is it a real issue? Anesth Analg. 2005;101(4):1117–22. doi: 10.1213/01.ane.0000167772.16584.0f. table of contents. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Barke RA, Charboneau R, et al. Morphine induces defects in early response of alveolar macrophages to Streptococcus pneumoniae by modulating TLR9-NF-kappa B signaling. J Immunol. 2008;180(5):3594–600. doi: 10.4049/jimmunol.180.5.3594. [DOI] [PubMed] [Google Scholar]
- 9.Sax HC. Effective total parenteral nutrition plus morphine on bacterial translocation in rats. JPEN J Parenter Enteral Nutr. 1994;18(4):380–1. doi: 10.1177/014860719401800422. [DOI] [PubMed] [Google Scholar]
- 10.Runkel NS, Moody FG, Smith GS, et al. Alterations in rat intestinal transit by morphine promote bacterial translocation. Dig Dis Sci. 1993;38(8):1530–6. doi: 10.1007/BF01308616. [DOI] [PubMed] [Google Scholar]
- 11.Li PF, Hao YS, Huang DA, et al. Morphine-promoted survival of CEMx174 cells in early stages of SIV infection in vitro: involvement of the multiple molecular mechanisms. Toxicol In Vitro. 2004;18(4):449–56. doi: 10.1016/j.tiv.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Feng P, Wilson QM, Meissler JJ, Jr., et al. Increased sensitivity to Salmonella enterica serovar Typhimurium infection in mice undergoing withdrawal from morphine is associated with suppression of interleukin-12. Infect Immun. 2005;73(12):7953–9. doi: 10.1128/IAI.73.12.7953-7959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma J, Wang J, Wan J, et al. Morphine disrupts interleukin-23 (IL-23)/IL-17-mediated pulmonary mucosal host defense against Streptococcus pneumoniae infection. Infect Immun. 78(2):830–7. doi: 10.1128/IAI.00914-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwacha MG, McGwin G, Jr., Hutchinson CB, et al. The contribution of opiate analgesics to the development of infectious complications in burn patients. Am J Surg. 2006;192(1):82–6. doi: 10.1016/j.amjsurg.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Wu L, Zaborina O, Zaborin A, et al. High-molecular-weight polyethylene glycol prevents lethal sepsis due to intestinal Pseudomonas aeruginosa. Gastroenterology. 2004;126(2):488–98. doi: 10.1053/j.gastro.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Valuckaite V, Zaborina O, Long J, et al. Oral PEG 15–20 protects the intestine against radiation: role of lipid rafts. Am J Physiol Gastrointest Liver Physiol. 2009;297(6):G1041–52. doi: 10.1152/ajpgi.00328.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilburger ME, Adler MW, Truant AL, et al. Morphine induces sepsis in mice. J Infect Dis. 1997;176(1):183–8. doi: 10.1086/514021. [DOI] [PubMed] [Google Scholar]
- 18.Cerletti C, Keinath SH, Reidenbery MM, Alder MW. Chronic morphine administration: plasma levels and withdrawal syndrome in rats. Pharmacol Biochem Behav. 1976;4(3):323–7. doi: 10.1016/0091-3057(76)90249-5. [DOI] [PubMed] [Google Scholar]
- 19.Volkmann H, Schwartz T, Kirchen S, et al. Evaluation of inhibition and cross-reaction effects on real-time PCR applied to the total DNA of wastewater samples for the quantification of bacterial antibiotic resistance genes and taxon-specific targets. Mol Cell Probes. 2007;21(2):125–33. doi: 10.1016/j.mcp.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Langer R, Fefferman M, Gryska P, Bergman K. A simple method for studying chemotaxis using sustained release of attractants from inert polymers. Can J Microbiol. 1980;26(2):274–8. doi: 10.1139/m80-045. [DOI] [PubMed] [Google Scholar]
- 21.Doyle TB, Hawkins AC, McCarter LL. The complex flagellar torque generator of Pseudomonas aeruginosa. J Bacteriol. 2004;186(19):6341–50. doi: 10.1128/JB.186.19.6341-6350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West SE, Schweizer HP, Dall C, et al. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene. 1994;148(1):81–6. doi: 10.1016/0378-1119(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 23.Diggle SP, Stacey RE, Dodd C, et al. The galactophilic lectin, LecA, contributes to biofilm development in Pseudomonas aeruginosa. Environ Microbiol. 2006;8(6):1095–104. doi: 10.1111/j.1462-2920.2006.001001.x. [DOI] [PubMed] [Google Scholar]
- 24.Zaborina O, Lepine F, Xiao G, et al. Dynorphin activates quorum sensing quinolone signaling in Pseudomonas aeruginosa. PLoS Pathog. 2007;3(3):e35. doi: 10.1371/journal.ppat.0030035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alverdy J, Holbrook C, Rocha F, et al. Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: evidence for in vivo virulence expression in Pseudomonas aeruginosa. Ann Surg. 2000;232(4):480–9. doi: 10.1097/00000658-200010000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alverdy JC, Chang EB. The re-emerging role of the intestinal microflora in critical illness and inflammation: why the gut hypothesis of sepsis syndrome will not go away. J Leukoc Biol. 2008;83(3):461–6. doi: 10.1189/jlb.0607372. [DOI] [PubMed] [Google Scholar]
- 27.Alverdy JC, Laughlin RS, Wu L. Influence of the critically ill state on host-pathogen interactions within the intestine: gut-derived sepsis redefined. Crit Care Med. 2003;31(2):598–607. doi: 10.1097/01.CCM.0000045576.55937.67. [DOI] [PubMed] [Google Scholar]
- 28.Laughlin RS, Musch MW, Hollbrook CJ, et al. The key role of Pseudomonas aeruginosa PA-I lectin on experimental gut-derived sepsis. Ann Surg. 2000;232(1):133–42. doi: 10.1097/00000658-200007000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson ME, Holmen Larsson JM, Hansson GC. Microbes and Health Sackler Colloquium: The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001;73(6):1131S–1141S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- 31.Bergstrom KS, Kissoon-Singh V, Gibson DL, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 6(5):e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheth SU, Lu Q, Twelker K, et al. Intestinal mucus layer preservation in female rats attenuates gut injury after trauma-hemorrhagic shock. J Trauma. 68(2):279–88. doi: 10.1097/TA.0b013e3181caa6bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linden SK, Florin TH, McGuckin MA. Mucin dynamics in intestinal bacterial infection. PLoS ONE. 2008;3(12):e3952. doi: 10.1371/journal.pone.0003952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansson ME, Phillipson M, Petersson J, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105(39):15064–9. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu R, Goldberg MB. Bacterial exploitation of host cell signaling. Sci Transl Med. 2(51):51ps48. doi: 10.1126/scitranslmed.3001612. [DOI] [PubMed] [Google Scholar]
- 36.Petersson J, Schreiber O, Hansson GC, et al. Importance and Regulation of the Colonic Mucus Barrier in a Mouse Model of Colitis. Am J Physiol Gastrointest Liver Physiol. doi: 10.1152/ajpgi.00422.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doherty MM, Pang KS. Route-dependent metabolism of morphine in the vascularly perfused rat small intestine preparation. Pharm Res. 2000;17(3):291–8. doi: 10.1023/a:1007548905772. [DOI] [PubMed] [Google Scholar]
- 38.Bergstrom KS, Guttman JA, Rumi M, et al. Modulation of intestinal goblet cell function during infection by an attaching and effacing bacterial pathogen. Infect Immun. 2008;76(2):796–811. doi: 10.1128/IAI.00093-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boontham P, Robins A, Chandran P, et al. Significant immunomodulatory effects of Pseudomonas aeruginosa quorum-sensing signal molecules: possible link in human sepsis. Clin Sci (Lond) 2008;115(11):343–51. doi: 10.1042/CS20080018. [DOI] [PubMed] [Google Scholar]
- 40.Struelens MJ. Detection of microbial DNAemia: does it matter for sepsis management? Intensive Care Med. 36(2):193–5. doi: 10.1007/s00134-009-1710-2. [DOI] [PubMed] [Google Scholar]
- 41.Wu L, Estrada O, Zaborina O, et al. Recognition of host immune activation by Pseudomonas aeruginosa. Science. 2005;309(5735):774–7. doi: 10.1126/science.1112422. [DOI] [PubMed] [Google Scholar]
- 42.Cabon F, Morser J, Parmantier E, et al. The E. coli envY gene encodes a high affinity opioid binding site. Neurochem Res. 1993;18(7):795–800. doi: 10.1007/BF00966775. [DOI] [PubMed] [Google Scholar]
- 43.Moss J, Rosow CE. Development of peripheral opioid antagonists' new insights into opioid effects. Mayo Clin Proc. 2008;83(10):1116–30. doi: 10.4065/83.10.1116. [DOI] [PubMed] [Google Scholar]