Abstract
Background
Generalized anxiety disorder (GAD) is a common, chronic condition that is relatively understudied compared to other psychiatric syndromes. Neuroimaging studies have begun to implicate particular neural structures and circuitry in its pathophysiology; however, no genetically-informative research has examined the potential sources of reported brain differences.
Methods
We acquired spectroscopic, volumetric, and diffusion tensor magnetic resonance imaging data from a pilot study of 34 female subjects selected from monozygotic twin pairs based upon their affection status for GAD and examined brain regions previously implicated in fear and anxiety for their relationship with affection status and genetic risk.
Results
Lifetime GAD associated with increased creatine levels in the amygdala, smaller left hippocampal volume, and lower fractional anisotropy in the uncinate fasciculus which connects amygdala and frontal cortex. In addition, GAD genetic risk predicted increases in myo-inositol in the amygdala and, possibly, glutamate/glutamine/GABA alterations in the hippocampus. The association of lifetime GAD with smaller hippocampal volume was independent of major depression and might represent a common genetic risk marker for internalizing disorders.
Conclusions
These preliminary data suggest that GAD and its genetic risk factors are likely correlated with volumetric and spectroscopic changes in fear-related limbic structures and their connections with the frontal cortex.
Keywords: Anxiety Disorders, Depressive Disorder, Twin Study, Magnetic Resonance Spectroscopy, Diffusion Tensor Imaging
Introduction
Generalized anxiety disorder (GAD) is a common (1), moderately heritable condition (2) that is relatively understudied compared to other psychiatric disorders. It is highly comorbid with other anxiety disorders (3), and it is a potent predictor of the development of major depressive disorder (MDD) (4;5). Twin studies suggest that GAD and MDD share a substantial portion of their genetic susceptibility (6;7).
Neuroimaging studies have implicated various neural structures in the pathophysiology of anxiety, particularly, the amygdala, the insula, the orbitofrontal cortex (OFC), and possibly, the anterior cingulate cortex (ACC) and hippocampus (for reviews, see (8-10)). Several studies have specifically examined GAD, with the majority of findings related to amygdala structure or function. One study of pediatric GAD reported increased amygdala volume in patients compared to healthy controls (11), while another that combined data across subjects with GAD, social phobia, and separation anxiety disorder found an overall reduction in amygdala volume in anxiety patients (12), most likely driven by subjects with GAD. Two studies found larger amygdala volumes in adult patients with GAD (13;14). The Schienle et al. study also found larger right dorsal medial prefrontal cortex (DMPFC) volume in GAD patients and correlations between Penn State Worry Questionnaire (PSWQ) scores and volumes of the ACC and the DMPFC. One group has conducted magnetic resonance spectroscopy (MRS) studies of GAD, reporting several brain metabolite findings, including increased N-acetylaspartate/creatine (NAA/Cr) ratio in right dorsolateral PFC (15) and decreased choline and Cr concentrations in white matter of the centrum semiovale (16).
Using functional imaging, one study of adolescents with GAD found amygdala hyperactivation in response to masked angry faces (17) while a second reported increased activation to fearful faces in a circuit involving the amygdala, ventral prefrontal cortex, and ACC (18). This is in contrast to a study comparing emotionally-invoked responses in adult patients with GAD, social phobia, and healthy controls that reported hyperactivation of the amygdala to fearful faces in social phobia but hypoactivation in GAD (19). Another study of adult GAD found persistent resting state activation of the ACC and prefrontal cortex during a worry task that correlated with PSWQ scores (20) while another reported elevated ACC activation during an anticipatory anxiety-related task (21). Functional connectivity between amygdala and other anxiety-associated structures was found to be altered in adult subjects with GAD (14;22).
Neuroimaging studies have profited from utilizing the twin study design, in which observed similarities or differences between twin pair types is used to infer a genetic or environmental basis for particular findings (reviewed in (23)). Such studies have strongly implicated genetic factors in brain structure, suggesting that combining imaging with genetically-informative samples may provide insight into the etiology of psychopathology. Neuroimaging studies of some psychiatric disorders such as schizophrenia (e.g., (24)), bipolar disorder (25), and MDD (26) have utilized the twin design in this way. One twin study that assessed genetic risk for anxious depression reported hippocampal volume differences that were likely environmental in origin (27). We are aware of no published twin neuroimaging studies of anxiety disorders other than post-traumatic stress disorder (28).
In this pilot study, we applied an array of neuroimaging techniques to examine the brain-based correlates of GAD and their potential genetic underpinnings using a twin sample. We sought to answer several questions:
Are there differences in the chemical composition, as determined by proton MRS metabolites, for anxiety-relevant brain structures between subjects with GAD and controls?
Do observed spectroscopic metabolites correlate with (a) volumetric changes in the structures themselves, or (b) differences in connectivity between them?
Does genetic risk for (a) GAD in particular, or (b) internalizing disorders in general, explain observed differences?
Materials and Methods
Subjects
The subjects in this study derive from two long-standing twin research projects: the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD) (29;30) and the Young Adult Follow-up of the Adolescent Behavioral Development (ABD) study. Both samples are subsets of the population-based Mid-Atlantic Twin Registry (MATR), all subjects of which are Caucasian and born in Virginia or its neighboring states. This project is the first in MATR to utilize neuroimaging, and, as thus, serves as a pilot study. We chose to study female subjects from monozygotic (MZ) twin pairs selected for their lifetime GAD status (affected or unaffected), as females have higher prevalence of GAD. We sought to recruit subjects who participated in prior studies from affected-affected (AA) pairs, unaffected-unaffected (UU) pairs, and unaffected-affected (UA) pairs. Under the typical assumptions of the classical twin model, those from AA MZ pairs are likely high on genetic risk for GAD while those from UU pairs are likely low, given that (i) twins within MZ pairs are 100% genetically correlated, and (ii) ii) there is evidence for the influence of genetic factors on liability to GAD (2). Discordant UA pairs are hypothesized to differ phenotypically due to unique environmental experiences, since their DNA sequence and family environment are identical. Except for hypertension, we excluded subjects with medical conditions known to affect brain structure or function. Subjects with lifetime substance dependence were also excluded. Approval of the local Institutional Review Board was obtained prior to the study and informed consent was obtained from all subjects prior to data collection.
Phenotypic Measures
In previous assessments, we obtained lifetime psychiatric diagnoses via face-to-face or telephone structured psychiatric interview based on the Structured Clinical Interview for DSM-III-R (SCID) (31). We assigned a lifetime diagnosis of GAD if subjects met criteria at any of the four prior assessments, which took place during the period from 1988-97. Among these, 36% of those positive for GAD at one assessment met criteria at a second. We re-assessed their lifetime GAD status using a telephone-based survey based on DSM-IV symptomatic criteria prior to entry into this study and recruited those whose prior lifetime GAD status we could confirm. In addition, as these subjects were women in mid-life, we also inquired whether they were pre- or post-menopausal and then recorded the date of their last menstrual period or whether they were receiving hormone replacement therapy, respectively.
Imaging Data Acquisition
Each patient underwent one imaging session on a whole-body 3.0 Tesla Signa GE MR scanner: T1- and T2-weighted pulse sequences were used to produce images in the axial and sagittal planes for use as an anatomical reference during proton MR spectroscopy. A high resolution structural scan was obtained using a 3D T1-weighted SGPR sequence (slice thickness = 1.2 mm, FOV = 24 cm, 256 × 256 × 124, TE = 6.0 ms, TR = 20.0 ms, flip angle = 20°, bandwidth = 15.63 kHz). Single-voxel MRS was performed with voxels 4-8 cm3 positioned in regions of interest (ROI) (right and left amygdala, right and left hippocampus, ACC and left OFC) using the PRESS sequence with TR/TE 1500/35 ms and 128 acquisitions. Due to the long scan time of the imaging session, only a subset of the patients (N=20) also underwent diffusion tensor imaging (DTI). Diffusion tensor images were acquired with a spin-echo echo-planar sequence (b=1000, 32 3.5 mm axial slices, 0 gap, 128×128 matrix, 23 cm FOV, 25 directions, 1 b=0 volume, flip angle = 90, TE = 89.5 ms, TR = 5000 ms, NEX=2).
Imaging Data Analysis
Proton MRS
The N-acetyl aspartate (NAA), choline (Cho), creatine (Cr) and glutamate/glutamine/GABA complex (Glx) peak areas for each ROI were computed using a semi-quantitative assessment of the absolute metabolite concentrations by means of a Linear Combination Model of in-vitro spectra (LCModel), an automated frequency-domain fitting routine (32). The model corrects for residual eddy current and rf coil loading effects and allows for an estimate and subtraction of the spectral baseline which is normally present at the short TE spectra used in our study. It does not correct for T1 saturation effects, and hence, the reported concentrations are expressed as institutional units (I.U.).
Structural
After removal of non-brain tissue, isolated brain images were imported into the imaging software program, Measure (33), and aligned along the anteroposterior commissures and interhemispheric fissure. The amygdala and hippocampus were manually measured in the coronal plane blind to diagnostic status, using reliable protocols that have been described in detail elsewhere (34). Briefly, anterior and lateral boundaries of the amygdala were the anterior commissure and the temporal stem, respectively. The anterior aspect of the hippocampus was identified as the slice in which the temporal horn shifted superiorly, and formed a boundary between the amygdala and hippocampus. The superior border of the hippocampus was identified as the gyrus uncinatus in anterior slices, and the alveus in posterior slices. Inter-rater reliability was excellent, as calculated with the intra-class correlation coefficient (amygdala, 0.95; hippocampus, 0.91).
DTI
Eight a priori ROIs for major white-matter tracts were created from DTI-based white-matter atlases (35;36) using a probability threshold of 40%: bilateral Inferior Fronto-occipital Fasciculus (IFOF), Inferior Longitudinal Fasciculus (ILF), Superior Longitudinal Fasciculus (SLF) and Uncinate Fasciculus (UF). These were chosen based upon their connectivity between our ROI or prior reports of their involvement in anxiety or mood disorders. Mean FA values from GAD and Controls were extracted from these ROIs and tested for significant differences between groups.
Phenotypic Association Analysis
We applied two phenotypic approaches to examine our principle hypotheses regarding the relationship between GAD and the various imaging measures. In the first, we grouped subjects by lifetime GAD status and tested for differences in imaging measures between groups. In the second, we used genetic risk as the main predictor of imaging measures where there was sufficient data to conduct these analyses. Given prior research supporting shared risk between GAD and other internalizing phenotypes, we operationalized genetic risk in two ways. The first assumed GAD genetic risk to be reflected in twin pair status, with subjects from AA pairs at higher genetic risk than those from UU pairs, as described in the subject selection procedure. The second utilized the results of a prior multivariate twin analysis performed in the entire VATSPSUD that identified common genetic factors across GAD and other internalizing disorders (MDD, panic disorder, phobias, neuroticism) (37). The estimated genetic factor scores, designated A1, were available for every subject included in that analysis, which we have utilized as a phenotype in previous genetic association studies (38). Here we use it as a quantitative indicator of genetic risk predicting the imaging measures.
We tested for association between GAD or the genetic risk measures and each of the imaging measures: metabolite levels by ROI, anatomic volumes, and FA. These were done via a series of statistical models designed to account for increasing amounts of variance while controlling for potential confounders. First we performed univariate analyses of all variables (GAD or genetic risk plus potential confounding variables from Table 1) to ascertain their potential association with the imaging measures. Given the small numbers of subjects, we applied screening t-tests with a liberal p-value threshold of 0.1 to identify measures for inclusion in more complex models. For the ROI metabolite levels and volumes, our final analyses consisted of general linear models predicting the imaging measure from the main effects (GAD or genetic risk), covarying for confounding variables passing our initial p-value threshold. These were implemented via Proc Mixed in SAS (39) to account for the non-independence of twins within a pair. Because we did not exclude subjects with MDD, we also performed secondary analyses testing for association between MDD and the imaging measures for comparison to the GAD results. Given the exploratory nature of this study and the small number of subjects, statistical correction for testing of multiple hypotheses was not applied.
Table 1.
Subject characteristics by GAD status
| Lifetime GAD − | Lifetime GAD + | |
|---|---|---|
| N | 17 | 17 |
| Right-handed | 15 | 12 |
| Post-menopausal (on estrogen replacement) | 11 (2) | 9 (2) |
| Median Age (range) | 52 (30-66) | 45 (33-63) |
| Other Lifetime Conditions Major Depression Phobias Panic Disorder Hypertension |
4 5 0 5 |
11 3 1 5 |
| Current Psychotropic Medication Use Antidepressants Benzodiazepines Others (zolpidem, modafinil, buspirone) |
1 0 0 |
10 4 3 (1 each) |
GAD - generalized anxiety disorder
Results
Subjects
We successfully recruited 34 subjects, 50% of whom met criteria for lifetime GAD (Table 1). Among these were 11 complete pairs (AA: 3, UA: 5, UU: 3) and 12 singletons, 6 of whom were from AA pairs, 5 from UU pairs, and 1 unaffected twin from an UA pair. As can be seen from Table 1, the only confounding variables that differed between those with and without GAD were lifetime MDD diagnosis and current psychotropic medications. Nonetheless, we conservatively tested each potential confounder for association with the imaging measures and included those that improved the model fit to the data.
Association between Phenotypes and ROI Metabolite Levels
Several metabolites from the ROIs were predicted from our phenotypic measures. Table 2 provides the best-fitting multivariate models for the ROI metabolite concentrations, including main effects with other significant covariates. As denoted, lifetime GAD and GAD genetic risk (as operationalized by contrasts between AA and UU pairs) predicted increased concentrations of Cr and mI, respectively, in the amygdala. GAD genetic risk also predicted right hippocampal Glx concentrations with marginal significance. MDD predicted increased concentrations of both Cr and NAA in the OFC. Controlling for other covariates tended to increase the significance level of the respective main phenotypic predictor. In several of the models, age predicted increasing metabolite concentrations. Interestingly, including handedness improved the fit of the models of GAD genetic risk predicting increase in both right and left amygdalar mI, allowing us to combine these into a single model.
Table 2.
Best-fit multivariate models (MRS)
| Metabolite | Predictor Variable / Covariates (direction) |
Significance of Predictor (type 3 tests of fixed effects) |
|---|---|---|
| L-amygdala Cr | GAD ↑ Handedness |
F=8.01, P=0.009 F=4.62, P=0.052 |
| Amygdala mI (L+R) | GAD genetic risk ↑ Handedness |
F=12.5, P=0.0064 F=5.94, P=0.033 |
| R-hippocampus Glx | GAD genetic risk ↑ Estrogen ↓ |
F=6.19, P=0.025 F=6.14, P=0.026 |
| OFC Cr | MDD ↑ Age ↑ |
F=24.1, P=0.0004 F=10.8, P=0.0065 |
| OFC NAA (total) | MDD ↑ Age ↑ BNZ ↑ AntiDep ↓ |
F=23.3, P=0.0004 F=24,2, P=0.0004 F=28.6, P=0.0002 F=14.2, P=0.0027 |
MRS = magnetic resonance spectroscopy; L=left; R=right; GAD = lifetime generalized anxiety disorder; GAD genetic risk = contrasts between GAD concordant affected (AA) vs. unaffected (UU) pairs; MDD = lifetime major depressive disorder; OFC = orbital frontal cortex; Cr = creatine; mI = myo-inositol; NAA (total) = total N-acetylaspartate; Cho = choline; Glx = glutamate/glutamine/GABA; BNZ = current benzodiazepine use; AntiDep = current antidepressant use; estrogen = post-menopausal estrogen replacement therapy
Association between Phenotypes and ROI Volumes
Smaller left hippocampal volume was predicted by lifetime GAD diagnosis (p=0.026) and internalizing genetic factor A1 (p=0.034), controlling for hypertension and whole brain volume. Trend level results in the same direction were obtained for GAD genetic risk as indexed by twin pair concordance (AA vs. UU). MDD was not associated with any ROI volumetric differences, although it marginally predicted larger whole brain volume (p=0.046). Age predicted lower left amygdalar, left hippocampal, and whole brain volumes. Hypertension, benzodiazepine use, and postmenopausal status each predicted lower left hippocampal volume, with hypertension having the strongest association and the only one whose inclusion in the regression models improved their fit.
Association between Phenotypes and FA
We note that each of the following sections present results for FA as predicted by GAD, A1, and MDD. Because we had too few complete twin pairs with DTI data, we were unable to test for genetic differences using pair-wise comparisons.
GAD vs. Control
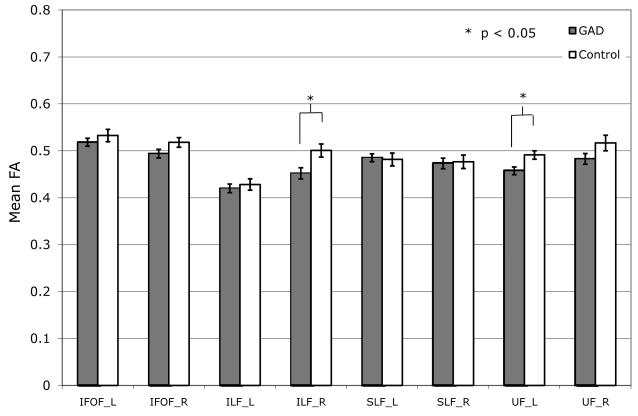
Mean FA in nearly all ROIs was found to be lower in GAD as compared to the Control group, with the difference reaching significance in the right ILF (mean FA±SEM; GAD: 0.452±0.012, Controls: 0.501±0.013, P<0.02) and left UF (GAD: 0.457±0.008, Controls: 0.491±0.009, P<0.02) (Fig.1a).
Figure 1.
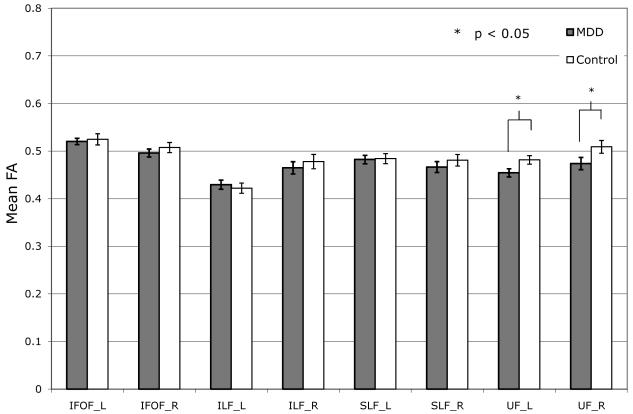
Group differences in fractional anisotropy (FA) by region of interest for (a) GAD vs. Controls and (b) MDD vs. Controls.
GAD = generalized anxiety disorder; MDD = major depressive disorder; IFOF = Inferior Fronto-occipital Fasciculus; ILF = Inferior Longitudinal Fasciculus; SLF = Superior Longitudinal Fasciculus; UF = Uncinate Fasciculus.
MDD vs. Control
Similar to the trend seen in GAD vs. Control comparison, mean FA overall tended to be lower in MDD as compared to Controls. However, significant differences between groups were only found in the left UF (MDD: 0.454±0.009, Controls: 0.482±0.009, P<0.03) and right UF (MDD: 0.474±0.013, Controls: 0.51±0.013, P<0.04) (Fig.1b).
A1 Predicting FA
Internalizing genetic risk score, A1, was negatively correlated with FA in right ILF (r = −0.51, p = 0.030) and at a trend level in left UF (r = −0.46, p = 0.056).
Discordant Twin Pairs
Given the small number of twin pairs discordant for GAD, there was limited power to detect differences in brain measures. The only significant difference obtained was for mean whole brain volume, which was about 25 cc (~2%) smaller in twins with GAD than in their discordant cotwin without GAD (p=0.040).
Discussion
In this study, we collected pilot neuroimaging data from subjects selected from female-female MZ twin pairs from the MATR. We examined lifetime GAD diagnosis and genetic risk as predictors of differences in neuronal metabolites, volumes, and structural connectivity associated with several anxiety-related regions. Controlling for potential confounders, GAD predicted increased left amygdalar Cr levels, smaller left hippocampal volume, and reduced fractional anisotropy in the left uncinate fasciculus (UF) and right inferior fronto-occipital fasciculus. GAD genetic risk, as operationalized by contrasts between affected-affected and unaffected-affected twin pairs, predicted increased right hippocampal Glx and bilateral amygdalar mI levels. Higher internalizing disorder genetic risk score A1 predicted smaller left hippocampal volume.
We compare our findings with those from previous GAD studies. The only comparable prior study examining the relationship between GAD diagnosis and brain metabolite levels reported increased right DLPFC NAA/Cr ratio (15). Two prior adult studies reported larger amygdala volumes in patients with GAD (13;14), which we did not replicate. The Schienle et al. study differed from ours by examining pure GAD without comorbid depression. We are aware of no published findings regarding structural connectivity in GAD. Prior fMRI studies reported altered “functional connectivity” between amygdala and other anxiety-relevant brain regions in GAD patients (14;22). For comparison, DTI studies have been conducted in other anxiety phenotypes, with the following findings: lower FA in UF tracts in generalized social phobia (40) as well as in trait anxiety (41). Similar abnormalities have been reported in mood disorders (reviewed in (42)). The UF is a major white matter tract connecting the amygdala and the OFC (43). Taken together, these data suggest potential abnormalities in connectivity between limbic and frontal structures that may represent a source of common pathology among anxiety and mood disorders.
While MDD was not the target phenotype, it is frequently comorbid with GAD, and prior analyses in this sample suggest this occurs due to shared genetic risk (6). Per Table 1, 11 of the 17 GAD subjects met criteria for lifetime MDD and 4 subjects had MDD without GAD, so we performed secondary analyses of MDD in relation to our brain-based measures. MDD predicted increases of Cr and total NAA levels in OFC. Interestingly, these results for MDD did not overlap those for GAD. On the other hand, the FA findings regarding MDD were similar to those from GAD; given the overlap in diagnoses for these subjects, it is difficult to differentiate whether GAD or MDD drives these differences.
Our finding of smaller hippocampal volume in association with GAD and internalizing genetic risk is intriguing in light of its association with MDD in other studies. In order to attempt to unravel the potential confounding effects of GAD-MDD comorbidity in our study, we re-ran this analysis after removing all fifteen subjects with lifetime MDD. In this highly reduced sample of six subjects with lifetime GAD and thirteen controls, our best-fit model still found a trend for smaller left hippocampus in subjects with GAD (p=0.067). (We note that there were too few subjects with DTI data meeting criteria for GAD without MDD to attempt the same phenotypic dissection for the UF FA findings.) Given the findings of shared genetic risk factors between GAD and MDD, and the fact that onset of GAD usually precedes that of MDD, one hypothesis is that it is this common genetic risk, rather than either phenotype directly, that is associated with smaller hippocampal volume. There is only one other published study of hippocampal volumes in an anxiety disorder using twins, and that study potentially informs this hypothesis. That study of PTSD in the male Vietnam Era Twin Registry sample reported smaller hippocampal volumes in both the exposed and unexposed members of MZ twin pairs in which the combat-exposed brother developed PTSD (28). The authors’ interpretation of that finding is that a smaller hippocampus, rather than being an outcome of severe trauma or PTSD, likely represents a marker for increased PTSD genetic risk. Adding support from our study, we hypothesize that smaller hippocampal volume is a marker for common genetic risk to internalizing disorders more broadly. We note that this is in contrast to the conclusion reached by another study of twins classified by risk for anxious depression, in which hippocampal volume differences observed in discordant MZ pairs suggested differential environmental risk (27).
The strengths of the current study include (1) selection of well characterized subjects from a population-based twin registry, (2) analyses of both lifetime GAD diagnosis and genetic risk measures, (3) use of multi-modal imaging, and (4) analytic control for a wide range of potential confounders. The limitations included (1) small sample size, (2) few discordant pairs, (3) lack of information on current GAD status, (4) current medication use by some subjects. The analyses are limited to women, so the findings might not generalize to men. Also, because of the small sample size and novel character of the data, many of the analyses were exploratory and not corrected for multiple testing. For example, the MRS analyses included four metabolites in six ROI, requiring a corrected p-value of 0.05/(24)=.002 to declare significance. Only two of the phenotypic measures in Table 2 meet this criterion. The volumetric and FA results also do not reach statistical significance if corrected for the number of regions analyzed. Thus, our findings are tentative until adequately replicated.
Conclusions
Our study found preliminary evidence that lifetime GAD, or its genetic risk, is associated with metabolite changes in the amygdala and hippocampus, two structures previously reported to be involved with fear and anxiety. GAD diagnosis also predicted smaller hippocampal volume independent of MDD, and this finding might be due to genetic risk for (a) GAD specifically, or (b) internalizing disorders in general. We also found that MDD is associated with metabolite changes in the OFC independent of GAD. GAD and MDD both correlated with lower fractional anisotropy in the uncinate fasciculus, the primary tract connecting amygdala and OFC. Such findings, together with the extant literature on genetic epidemiology of internalizing disorders, tentatively suggest that future imaging studies might benefit from selecting subjects based upon shared or specific genetic risk in addition to their diagnostic phenotypes. Findings from such studies have the potential to better inform psychiatric nosology by grouping disorders according to underlying neurobiologic and genetic factors.
Acknowledgements
This work was supported by NIH grant K08 MH-66277 (JMH). We wish to thank Lisa Halberstadt, M.S., and Karen Hough, study coordinators, and Barbara Brooke and Rebecca Ortiz for their subject recruitment efforts. Carol Prescott, PhD, provided critical help in the collection of the VATSPSUD sample. We acknowledge the contribution of the Virginia Twin Registry, now part of the Mid-Atlantic Twin Registry (MATR), for the ascertainment of subjects for this study. The MATR is currently supported by UL1RR031990 from the National Center for Research Resources.
Footnotes
Preliminary results from this study were presented at the 30th Annual Anxiety Disorders Association of America Conference, March 4-7, 2010, Baltimore, MD.
Disclosure / Conflicts of Interest
None.
Reference List
- (1).Wittchen HU, Zhao S, Kessler RC, Eaton WW. DSM-III-R generalized anxiety disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1994 May;51(5):355–64. doi: 10.1001/archpsyc.1994.03950050015002. [DOI] [PubMed] [Google Scholar]
- (2).Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001 Oct;158(10):1568–78. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- (3).Judd LL, Kessler RC, Paulus MP, Zeller PV, Wittchen HU, Kunovac JL. Comorbidity as a fundamental feature of generalized anxiety disorders: results from the National Comorbidity Study (NCS) Acta Psychiatr Scand Suppl. 1998;393:6–11. doi: 10.1111/j.1600-0447.1998.tb05960.x. [DOI] [PubMed] [Google Scholar]
- (4).Kessler RC, Nelson CB, McGonagle KA, Liu J, Swartz M, Blazer DG. Comorbidity of DSM-III-R major depressive disorder in the general population: results from the US National Comorbidity Survey. Br J Psychiatry Suppl. 1996 Jun;(30):17–30. [PubMed] [Google Scholar]
- (5).Hettema JM, Prescott CA, Kendler KS. The effects of anxiety, substance use and conduct disorders on risk of major depressive disorder. Psychol Med. 2003 Nov;33(8):1423–32. doi: 10.1017/s0033291703008365. [DOI] [PubMed] [Google Scholar]
- (6).Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Major depression and generalized anxiety disorder. Same genes, (partly) different environments? Arch Gen Psychiatry. 1992 Sep;49(9):716–22. doi: 10.1001/archpsyc.1992.01820090044008. [DOI] [PubMed] [Google Scholar]
- (7).Kendler KS, Gardner CO, Gatz M, Pedersen NL. The sources of co-morbidity between major depression and generalized anxiety disorder in a Swedish national twin sample. Psychol Med. 2006 Nov 23;37(3):453–62. doi: 10.1017/S0033291706009135. [DOI] [PubMed] [Google Scholar]
- (8).Damsa C, Kosel M, Moussally J. Current status of brain imaging in anxiety disorders. Curr Opin Psychiatry. 2009 Jan;22(1):96–110. doi: 10.1097/YCO.0b013e328319bd10. [DOI] [PubMed] [Google Scholar]
- (9).Ferrari MC, Busatto GF, McGuire PK, Crippa JA. Structural magnetic resonance imaging in anxiety disorders: an update of research findings. Rev Bras Psiquiatr. 2008 Sep;30(3):251–64. doi: 10.1590/s1516-44462008000300013. [DOI] [PubMed] [Google Scholar]
- (10).Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007 Oct;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, et al. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000 Jul 1;48(1):51–7. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- (12).Milham MP, Nugent AC, Drevets WC, Dickstein DP, Leibenluft E, Ernst M, et al. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biol Psychiatry. 2005 May 1;57(9):961–6. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- (13).Schienle A, Ebner F, Schafer A. Localized gray matter volume abnormalities in generalized anxiety disorder. Eur Arch Psychiatry Clin Neurosci. 2010 Sep 5; doi: 10.1007/s00406-010-0147-5. [DOI] [PubMed] [Google Scholar]
- (14).Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009 Dec;66(12):1361–72. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- (15).Mathew SJ, Mao X, Coplan JD, Smith EL, Sackeim HA, Gorman JM, et al. Dorsolateral prefrontal cortical pathology in generalized anxiety disorder: a proton magnetic resonance spectroscopic imaging study. Am J Psychiatry. 2004 Jun;161(6):1119–21. doi: 10.1176/appi.ajp.161.6.1119. [DOI] [PubMed] [Google Scholar]
- (16).Coplan JD, Mathew SJ, Mao X, Smith EL, Hof PR, Coplan PM, et al. Decreased choline and creatine concentrations in centrum semiovale in patients with generalized anxiety disorder: relationship to IQ and early trauma. Psychiatry Res. 2006 Jun 30;147(1):27–39. doi: 10.1016/j.pscychresns.2005.12.011. [DOI] [PubMed] [Google Scholar]
- (17).Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008 May;65(5):568–76. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007 Jan;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- (19).Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, et al. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry. 2008 Sep;165(9):1193–202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Paulesu E, Sambugaro E, Torti T, Danelli L, Ferri F, Scialfa G, et al. Neural correlates of worry in generalized anxiety disorder and in normal controls: a functional MRI study. Psychol Med. 2010 Jan;40(1):117–24. doi: 10.1017/S0033291709005649. [DOI] [PubMed] [Google Scholar]
- (21).Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, et al. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009 Mar;166(3):302–10. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010 May;167(5):545–54. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Schmitt JE, Eyler LT, Giedd JN, Kremen WS, Kendler KS, Neale MC. Review of twin and family studies on neuroanatomic phenotypes and typical neurodevelopment. Twin Res Hum Genet. 2007 Oct;10(5):683–94. doi: 10.1375/twin.10.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).van Erp TG, Saleh PA, Huttunen M, Lonnqvist J, Kaprio J, Salonen O, et al. Hippocampal volumes in schizophrenic twins. Arch Gen Psychiatry. 2004 Apr;61(4):346–53. doi: 10.1001/archpsyc.61.4.346. [DOI] [PubMed] [Google Scholar]
- (25).van der Schot AC, Vonk R, Brans RG, van Haren NE, Koolschijn PC, Nuboer V, et al. Influence of genes and environment on brain volumes in twin pairs concordant and discordant for bipolar disorder. Arch Gen Psychiatry. 2009 Feb;66(2):142–51. doi: 10.1001/archgenpsychiatry.2008.541. [DOI] [PubMed] [Google Scholar]
- (26).Munn MA, Alexopoulos J, Nishino T, Babb CM, Flake LA, Singer T, et al. Amygdala volume analysis in female twins with major depression. Biol Psychiatry. 2007 Sep 1;62(5):415–22. doi: 10.1016/j.biopsych.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).de Geus EJ, van’t Ent D, Wolfensberger SP, Heutink P, Hoogendijk WJ, Boomsma DI, et al. Intrapair differences in hippocampal volume in monozygotic twins discordant for the risk for anxiety and depression. Biol Psychiatry. 2007 May 1;61(9):1062–71. doi: 10.1016/j.biopsych.2006.07.026. [DOI] [PubMed] [Google Scholar]
- (28).Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002 Nov;5(11):1242–7. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Kendler KS, Prescott CA. A population-based twin study of lifetime major depression in men and women. Arch Gen Psychiatry. 1999 Jan;56(1):39–44. doi: 10.1001/archpsyc.56.1.39. [DOI] [PubMed] [Google Scholar]
- (30).Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. Guilford Press; New York: 2006. [Google Scholar]
- (31).Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-III-R (SCID) Biometrics Research Department, New York State Psychiatric Institute; New York: 1985. [Google Scholar]
- (32).Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993 Dec;30(6):672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- (33).Barta PE, Dhingra L, Royall R, Schwartz E. Improving stereological estimates for the volume of structures identified in three-dimensional arrays of spatial data. J Neurosci Methods. 1997 Aug 22;75(2):111–8. doi: 10.1016/s0165-0270(97)00049-6. [DOI] [PubMed] [Google Scholar]
- (34).Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res. 1997 Aug 8;75(1):31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- (35).Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007 Jul 1;36(3):630–44. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Mori S, Wakana S, van Zijl PC, Nagae-Poetscher LM. MRI Atlas of Human White Matter. Elsevier; Amsterdam: 2005. [Google Scholar]
- (37).Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS. A population-based twin study of the relationship between neuroticism and internalizing disorders. Am J Psychiatry. 2006 May;163(5):857–64. doi: 10.1176/ajp.2006.163.5.857. [DOI] [PubMed] [Google Scholar]
- (38).Hettema JM, An SS, Bukszar J, van den Oord EJ, Neale MC, Kendler KS, et al. Catechol-O-methyltransferase contributes to genetic susceptibility shared among anxiety spectrum phenotypes. Biol Psychiatry. 2008 Aug 15;64(4):302–10. doi: 10.1016/j.biopsych.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).SAS. SAS Institute . SAS/STAT Software: Version 8. SAS Institute Inc.; Cary, NC: 1999. [Google Scholar]
- (40).Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, et al. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry. 2009 Oct 1;66(7):691–4. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009 Sep 16;29(37):11614–8. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Sexton CE, Mackay CE, Ebmeier KP. A systematic review of diffusion tensor imaging studies in affective disorders. Biol Psychiatry. 2009 Nov 1;66(9):814–23. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- (43).Ebeling U, von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir (Wien) 1992;115(3-4):143–8. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]