Abstract
Objective
The general consensus has been that estrogen is invariably a risk factor for ischemic stroke (IS). We reviewed new observational studies that challenge this simple conclusion.
Methods
This was a review of observational studies of the association of premature or early menopause with stroke or IS published in English from 2006 through 2010.
Results
Three cohort studies showed an increased risk of all stroke in women who underwent bilateral oophorectomy compared with women who conserved their ovaries before age 50 years. The increased risk of stroke was reduced by hormonal therapy (HT) in one of the studies, suggesting that estrogen deprivation is involved in the association. Four additional observational studies showed an association of all stroke or IS with the early onset of menopause or with a shorter lifespan of ovarian activity. In three of the seven studies, the association was restricted to IS. Age at menopause was more important than type of menopause (natural vs induced).
Conclusions
The findings from seven recent observational studies challenge the consensus that estrogen is invariably a risk factor for IS and can be reconciled by a unifying timing hypothesis. We hypothesize that estrogen is protective for IS before age 50 years and may become a risk factor for IS after age 50 years or, possibly, after age 60 years. These findings are relevant to women who experienced premature or early menopause, or to women considering prophylactic bilateral oophorectomy before the onset of natural menopause.
Keywords: Early menopause, Oophorectomy, Estrogen, Ischemic stroke, Risk factors
The general consensus has been that estrogen is invariably a risk factor for ischemic stroke (IS).1–9 For example, the Women’s Health Initiative (WHI) clinical trials reported deleterious effects on IS for estrogen alone or combined with progestin for women older than age 50 years.1,4 These findings were later confirmed by observational data from the Nurses’ Health Study (NHS).6 This review was prompted by the publication of deleterious effects of hormone therapy (HT) on IS from the WHI and the NHS.1,4,6
New data suggest that the effects of estrogen on the progression of atherosclerosis and on the risk of cardiovascular disease (CVD) vary by age at onset of menopause or by age at initiation of exogenous HT, and the effects of estrogen are beneficial rather than harmful at younger ages. This concept has been named the timing hypothesis or window of opportunity hypothesis.10–15 We critically reviewed findings from observational studies of natural or surgically induced premature or early menopause published in the last 5 years to explore the timing hypothesis in relation to estrogen and IS. We also propose a unifying theory for the effects of estrogen on IS across age.
METHODS
Review strategy
PubMed was searched for articles published in English between January 1, 2006, and December 31, 2010, using combinations of the following keywords: “estrogen”, “estrogens”, “oophorectomy”, “ovariectomy”, “premature menopause”, “menopause”, and “stroke”. The search was restricted to articles regarding adult women (aged 19 years or older), indexed in MEDLINE, and written in English. The full abstracts of all of the publications identified were reviewed to identify original reports of observational studies of interest. We also performed a manual search for additional references cited in the articles selected. We focused our review on IS whenever the data were reported separately by type of stroke.
In this review, premature menopause refers to menopause that occurs before age 40 years, and early menopause refers to menopause that occurs between age 40 and 45 years, both ranges being well below the average age of natural menopause (age 51 y).16,17 Premature menopause or early menopause can be spontaneous or induced; if induced, it can be due to medical interventions such as chemotherapy or surgical interventions such as bilateral oophorectomy. The most common cause of premature or early menopause is bilateral oophorectomy.
Data analyses
Data presented in tables and figures were derived directly from published findings, with some exception. For studies related to premature or early menopause, we also computed measures of association based on a complementary definition of exposure. These inversions were applied to make data about estrogen before menopause (naturally produced) or after menopause (given as HT) directly comparable (associations for estrogen present vs estrogen absent). Thus, for example, instead of using the relative risk for early versus late menopause, we used the relative risk for late versus early menopause. In this review, we have used age 50 years as a simple approximate age cutoff for natural menopause because the average age of natural menopause in the United States is 51.4 years.16,17
RESULTS
Outcome of the literature search
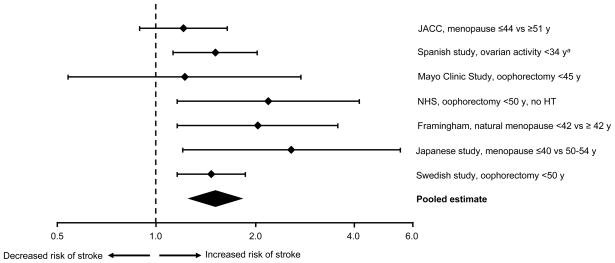
Of 151 articles retrieved from MEDLINE, only 7 met the criteria for inclusion (original reports of observational studies of the association of premature or early menopause with stroke). Comments about particular strengths or weaknesses of the studies and about biases or confounding are described below; however, no studies were excluded because of methodological characteristics. Table 1 and Figure 1 summarize all of the studies of the association of premature or early menopause with the risk of IS identified by our search (in order of publication from 2006 through 2010).
TABLE 1.
Summary of studies on the association of premature or early menopause with risk of stroke (2006 to 2010)
| Authors | Name of study | Study design | Type of stroke | Origin of estrogen | Definition of exposure, stratum | Original OR or HR(95% CI) | Modified OR or HR(95% CI)a |
|---|---|---|---|---|---|---|---|
| Cui et al., 2006 | Japan Collaborative Cohort (JACC) Study | Cohort | All | Endogenous | Menopause ≤ 44 y vs ≥ 51 y | 1.21 (0.89–1.64) | 0.83 (0.61–1.12) |
| de Lecinana et al., 2007 | Spanish study | Case-control | Ischemic | Endogenous | Lifespan of ovarian activity <34 y | 1.51 (1.13–2.03)b | 0.66 (0.49–0.89)b |
| Rivera et al., 2009 | Mayo Clinic study | Cohort | All | Endogenous | Oophorectomy <45 y | 1.22 (0.54–2.74) | 0.82 (0.36–1.85) |
| Parker et al., 2009 | Nurses’ Health Study (NHS) | Cohort | All | Endogenous and exogenous | Oophorectomy <45 y Oophorectomy <50 y, no HT |
1.19 (0.96–1.49) 2.49 (1.16–4.14) |
0.84 (0.67–1.05) 0.46 (0.24–0.86) |
| Lisabeth et al., 2009 | Framingham study | Cohort | Ischemic | Endogenous | Menopause <42 y | 2.03 (1.16–3.56) | 0.49 (0.28–0.86) |
| Baba et al., 2010 | Japanese study | Cohort | Ischemic | Endogenous | Menopause <40 y vs 50–54 y | 2.57 (1.20–5.49) | 0.39 (0.18–0.83) |
| Ingelsson et al., 2010 | Swedish nationwide study | Cohort | All | Endogenous | Hysterectomy + Oophorectomy | 1.47 (1.16–1.87)c | 0.68 (0.54–0.86) |
CI, confidence interval; HT, hormonal therapy; HR, hazard ratio; OR, odds ratio.
The OR or HR was modified by inverting the definition of exposure. This inversion was needed to make the associations directly comparable in studies of estrogen before or after age 50 years (estrogen present vs estrogen absent; Fig. 2).
Odds ratio.
HR for stroke in a cohort of women with hysterectomy considering bilateral oophorectomy as a time-varying covariate. The model was adjusted by age, calendar year, county of residency, and socioeconomic status.
FIG. 1.
Summary of observational studies on the association of premature or early menopause with risk of stroke. The relative risks estimated by OR or HR and 95% confidence interval were plotted using a logarithmic scale. aA length of 34 years of ovarian activity corresponds to the occurrence of estrogen deficiency at approximately age 46 years, assuming a median age at menarche of 12 years. HT = hormone therapy; JACC = Japan Collaborative Cohort Study; NHS = Nurses’ Health Study.
Findings from individual studies
The Japan Collaborative Cohort (JACC) Study showed a 32% increased risk of all stroke mortality in women who experienced later menarche (age ≥ 17 y) compared with women with earlier menarche (age ≤ 13 y). In addition, the study showed a 21% increased risk of all stroke mortality for women with younger age at menopause (≤44 y vs ≥ 51 y), regardless of the type of menopause (natural vs induced). However, neither of these hazard ratios was statistically significant.18 A Spanish case-control study of non-cardioembolic IS showed a 51% increased odds ratio for a shorter lifespan of ovarian activity (<34 y between menopause and menarche).19
The Mayo Clinic Cohort Study of Oophorectomy and Aging showed a 44% increased long-term risk of CVD including stroke in women who underwent oophorectomy before menopause.15 However, women who underwent bilateral oophorectomy before age 45 years but received HT through age 45 years did not experience an increased risk of CVD including stroke.15 These analyses stratified by treatment suggest that the association of oophorectomy with increased CVD mortality may be mediated by the premature estrogen deficiency caused by oophorectomy. The risk for all stroke was increased 22%; however, the association did not reach statistical significance. The Mayo Clinic study examined mortality rather than morbidity and did not separate IS from hemorrhagic stroke. Therefore, the association of oophorectomy with stroke risk may have been underestimated.15 Finally, information about possible cardiovascular risk factors at baseline or genetic confounding factors was not available.
The NHS showed a 19% increased risk of stroke in women who underwent hysterectomy with bilateral oophorectomy before age 45 years compared with women who underwent hysterectomy but conserved their ovaries. The finding was not statistically significant after multivariable adjustment. However, the study showed a statistically significant 149% increased risk of all stroke in women who underwent hysterectomy with bilateral oophorectomy before age 50 years and did not receive HT (Table 1; Fig. 1).20 Therefore, the NHS showed that age at the time of bilateral oophorectomy is important and that the use of HT may reduce the effect of oophorectomy on the risk of stroke. The NHS provided observational evidence that the association of oophorectomy with increased risk of stroke may be mediated by the premature estrogen deficiency caused by oophorectomy. Unfortunately, IS was not separated from hemorrhagic stroke, and the association of oophorectomy and stroke may have been underestimated.20
To address the concern that the increased risk of stroke after oophorectomy could be due to confounding by some other cardiovascular risk factor, the analyses in the NHS were adjusted by history of diabetes, high blood pressure, hypercholesterolemia, smoking, alcohol consumption, physical exercise, body mass index, and use of aspirin. In addition, to control for possible genetic confounding factors, the investigators included in the regression models family history of myocardial infarction before age 60 years and family history of breast cancer. Finally, they included in the regression models parity, tubal ligation, use of oral contraceptives, and use of HT (when applicable).21 These adjustments did not eliminate the association.
The Framingham study showed a 103% increased risk of IS in women who experienced natural menopause before age 42 years compared to women with later menopause, and a trend of increasing risk of IS with decreasing age at the time of natural menopause.22 A second, more recent Japanese study showed a 56% increased risk of all stroke and a 157% increased risk of IS in women who experienced either natural or surgical menopause before age 40 years (vs 50 to 54 y). Results did not vary after adjustment for age, hypertension, cholesterol level, body mass index, smoking, and alcohol consumption.23 Finally, a recent nationwide Swedish cohort study showed a 47% increased risk of stroke in women who underwent hysterectomy plus bilateral oophorectomy before age 50 years compared to women who did not have either of the surgeries.24
A new cohort study conducted as part of the WHI Observational Study was published in 2011 and is outside of the time frame of this formal review (2006 to 2010).25 The results are summarized here for completeness. This study showed a 13% increased risk for all stroke in women who underwent hysterectomy plus bilateral oophorectomy before age 40 years compared to hysterectomy alone, and a 44% increased risk for women who had the oophorectomy before age 40 years but did not receive HT. In addition, the hazard ratio for stroke declined with increasing age at the time of oophorectomy among women who did not receive subsequent HT (44% for age <40 y; 35% for ages 40 to 49 y; 37% for age ≥ 50 y; data not shown in Table 1, Fig. 1, and Fig. 2).25 Because none of these hazard ratios reached statistical significance, the authors concluded that oophorectomy is not associated with an increased risk of stroke. Unfortunately, the study did not have adequate power to test for the associations reported and suffered from additional limitations partly acknowledged by the authors.25
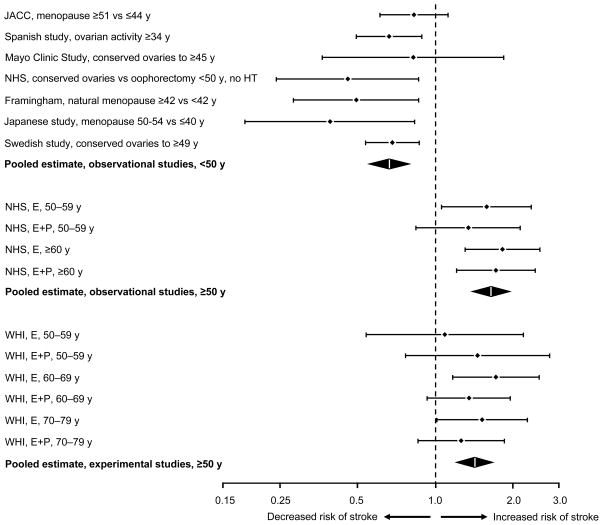
FIG. 2.
Illustration of a unifying theory for the effects of estrogen on ischemic stroke across age (timing hypothesis). The relative risks estimated by OR or HR and 95% confidence interval for estrogen present versus estrogen absent were plotted using a logarithmic scale. Studies or strata were grouped into three blocks by age and study design: observational studies before age 50 years (from this review, with inversion of the definition of exposure to compare later menopause vs earlier menopause), observational studies of HT after age 50 years (using the NHS as an example), and experimental studies of HT after age 50 years (using the WHI clinical trials as an example). Most studies focused on ischemic stroke but some considered all stroke (see Table 1 for details of studies before age 50 y). E = estrogen; E + P = estrogen plus progestin therapy; HT = hormone therapy; JACC = Japan Collaborative Cohort Study; NHS = Nurses’ Health Study; WHI = Women’s Health Initiative.
Possible biases or confounding
Because these seven studies showing a consistently increased risk of all stroke or IS in women who experienced early or premature menopause (either naturally or surgically induced) were observational, we cannot exclude the possibility that findings were caused by bias or confounding. The major concern about confounding by other risk factors for IS that were present at baseline is adequately addressed by the multivariable analyses in both the NHS and one of the Japanese studies that included most conventional cardiovascular risk factors.23,26 The multivariable analyses in the NHS also partly addressed the concern about confounding by genetic factors (inclusion of family history variables).26 Finally, analyses in the nationwide Swedish study were adjusted for socioeconomic status.24
It remains uncertain whether premature or early menopause causes an increased risk of IS because of the reduction in circulating estrogen or through some other hormonal effect such as a decrease in circulating progesterone or testosterone, or an increased production of gonadotropins. However, the relatively lower risk of CVD for women who received HT in the Mayo Clinic study, and the reduced risk of stroke for women who received HT in the NHS, suggests that estrogen is a key mediator of the effects of bilateral oophorectomy.15,20,27
Ischemic versus hemorrhagic stroke
Stroke is generally classified as ischemic, including embolic and thrombotic mechanisms, or hemorrhagic, including subarachnoid or intracerebral locations.28,29 Because the pathogenetic mechanisms of ischemic and hemorrhagic stroke are different, we might expect different effects of estrogen on different types of stroke. The increased risk associated with early natural menopause was limited to IS in the Framingham cohort study.22 The association with early natural or surgical menopause was stronger for IS than for all types of stroke combined in one of the Japanese cohort studies (157% increase vs 56% increase).23 The association with a shorter lifespan duration of ovarian activity was also limited to IS in the Spanish case-control study.19 In summary, the association appears to be restricted to IS in studies that considered IS separately from hemorrhagic stroke.
Age versus type of menopause
It remains unknown whether age at menopause (and corresponding onset of estrogen deficiency) is the only predictor of the risk of IS or whether the type of menopause is also important.30 On the one hand, women who undergo premature or early natural menopause may have some underlying processes that caused the early ovarian insufficiency and that may also increase the risk of IS above and beyond the effect of estrogen deficiency. On the other hand, women who undergo bilateral oophorectomy experience a more abrupt drop in circulating ovarian hormones and a more severe disruption of the hypothalamic-pituitary-ovarian axis.16,27
The studies summarized in Table 1 and Figure 1 suggest that the increased risk of stroke or IS is observed both for women who underwent premature or early natural menopause18,19,22,23 and for women who underwent bilateral oophorectomy.15,21,23,24 This pattern suggests that age at estrogen deficiency is a major determinant of risk; however, we cannot exclude that type of menopause may further modify the magnitude of the association.
DISCUSSION
Our review of observational studies of women experiencing premature or early menopause suggested that lack of endogenous estrogen is associated with an increased risk of IS and that HT before age 50 years may partly offset the increased risk. These findings are in clear contrast with the general consensus that estrogen is invariably a risk factor for IS.1–9 For example, the experimental data from the WHI clinical trials and the observational data from the NHS are remarkably consistent and indicate that estrogen (primarily conjugated equine estrogens) used alone or in combination with a progestin at the dosage of 0.625 mg/day and administered orally after age 50 years is a risk factor for IS.1,4,6 The same conclusion was reached by three meta-analyses of clinical trials of HT and risk of stroke.2,5,7
The apparent contradiction in the results of studies of endogenous estrogen or HT before age 50 years with the results of studies of HT after age 50 years may be explained by the timing hypothesis. In Figure 2, we combined the observational findings from this review with the findings from the NHS cohort and the WHI clinical trials to illustrate our proposal for a unifying theory. The data presented in Figure 2 are compatible with the theory that estrogen has protective effects before age 50 years and deleterious effects after age 50 years (particularly if used orally at 0.625 mg/day of conjugated estrogens). Observational data and experimental data are shown separately.
The timing hypothesis for estrogen and IS may be shifted by approximately 10 years compared with the timing hypothesis for estrogen and coronary heart disease. For women aged 50 to 59 years, oral estrogen at a dose equivalent to 0.625 mg/day of conjugated estrogens is possibly beneficial for coronary heart disease but is a risk factor for IS.12–14 By contrast, for women who underwent premature or early menopause, oral estrogen taken before age 50 years may be safe and beneficial for both coronary heart disease and IS.15,26
The reasons for this 10-year shift in risk remain unexplained and may be interpreted as evidence against the timing hypothesis. By contrast, the shift may be caused by the heterogeneity of IS mechanisms. Approximately 15% to 30% of IS is due to large vessel disease, 15% to small vessel disease, 30% to cardiac causes (cardioembolism), 5% to other defined causes, and the remaining IS has undetermined (or cryptogenic) etiology.31,32 Because estrogen induces several beneficial anti-atherogenetic and vasoprotective effects as well as detrimental pro-thrombotic and pro-inflammatory effects, estrogen might modify the risk of IS differentially, based on IS subtype.13,33,34 In addition, the balance of these differential effects might vary with age. Thus, the pathogenetic mechanisms leading to IS are more heterogeneous than those leading to coronary heart disease.29,35 For example, it is possible that HT between the ages of 50 and 59 years increases the short-term risk of IS due to cardiac sources of emboli, despite offering a long-term protective effect against atherosclerotic disease progression in large vessels and small vessels of the brain and heart. Under this hypothesis, the different risk for stroke and coronary heart disease associated with HT at ages 50 to 59 years may be in part due to the differential mechanisms of cardioembolic IS.31,32
On the other hand, a 2011 report from the WHI clinical trials considering a post-intervention phase of follow-up showed that the use of unopposed estrogen for a median of 5.9 years in women with hysterectomy was not associated with an increased risk of stroke or any other adverse outcomes after 10.7 years of follow-up.36,37 In addition, women experienced a significantly decreased risk of breast cancer. Approximately 40% of the women in this WHI trial had undergone bilateral oophorectomy along with hysterectomy.36 These new findings may suggest that estrogen is not a long-term risk factor for IS in women treated with estrogen alone at ages 50 to 59 years, and that the timing hypothesis for estrogen and IS may be synchronous with the timing hypothesis for estrogen and coronary heart disease (rather than shifted 10 y earlier as discussed above).
The beneficial effects of estrogen on blood vessels and on the overall cardiocirculatory system shown in studies of tissue cultures, small mammals, and primates have been reviewed in detail elsewhere.10,13,33,34,38–44 Additional biological mechanisms specific for the protection of neuronal or glial cells against brain ischemia have also been reviewed elsewhere.45,46 These beneficial effects of estrogen are probably acting over the full lifespan of women, so that an adequate amount of endogenous or exogenous estrogen early in life may change the overall trajectory of atherosclerosis and vascular aging, and may reduce the risk of IS 30 or 40 years later (long-term protective effects). By contrast, the deleterious effects of HT after age 50 years on the risk of IS appear to be related to pro-thrombotic and pro-inflammatory effects and tend to be more proximate or contemporary to the ischemic event (short-term deleterious effects).13,33,34
CONCLUSIONS
Our review of observational studies of the association of premature or early menopause with risk of stroke suggests that estrogen is protective for stroke in women younger than age 50 years. These findings have two clinical implications. First, women considering bilateral oophorectomy for the prevention of cancer should include the increased risk of IS in the assessment of risks and benefits of the surgery.15,20,47 Second, women who experienced an early estrogen deficiency, before approximately age 45 years, either naturally or because of medical or surgical interventions, should consider taking HT unless there is a clear contra-indication.16,48,49 Unfortunately, the results from the WHI clinical trials have been inappropriately extrapolated from women who were older than age 50 years at the time of initiation of treatment to younger women, and many women have discontinued HT or avoided starting HT at all ages, including women younger than age 50 years.50–55
Acknowledgments
Funding/Support: The Mayo Clinic Cohort Study of Oophorectomy and Aging was funded by the National Institutes of Health (grant NS 033978) and was made possible by the Rochester Epidemiology Project (AG 034676).
The authors thank Barbara J. Balgaard for typing the manuscript.
Footnotes
Financial disclosures/Conflicts of interest: None reported.
Author contributions: Walter A. Rocca reviewed the literature and drafted the manuscript. Brandon R. Grossardt conducted the analyses required for Figures 1 and 2, and reviewed the manuscript. Virginia M. Miller reviewed the manuscript and made specific contributions related to vascular physiology and laboratory studies. Lynne T. Shuster reviewed the manuscript and made specific contributions related to menopause and hormone therapy. Robert D. Brown, Jr. reviewed the manuscript and made specific contributions related to stroke and neurological issues.
References
- 1.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 2.Bath PM, Gray LJ. Association between hormone replacement therapy and subsequent stroke: a meta-analysis. BMJ. 2005;330:342. doi: 10.1136/bmj.38331.655347.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bushnell CD. Oestrogen and stroke in women: assessment of risk. Lancet Neurol. 2005;4:743–751. doi: 10.1016/S1474-4422(05)70220-9. [DOI] [PubMed] [Google Scholar]
- 4.Hendrix SL, Wassertheil-Smoller S, Johnson KC, et al. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 5.Magliano DJ, Rogers SL, Abramson MJ, Tonkin AM. Hormone therapy and cardiovascular disease: a systematic review and meta-analysis. BJOG. 2006;113:5–14. doi: 10.1111/j.1471-0528.2005.00797.x. [DOI] [PubMed] [Google Scholar]
- 6.Grodstein F, Manson JE, Stampfer MJ, Rexrode K. Postmenopausal hormone therapy and stroke: role of time since menopause and age at initiation of hormone therapy. Arch Intern Med. 2008;168:861–866. doi: 10.1001/archinte.168.8.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sare GM, Gray LJ, Bath PM. Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. Eur Heart J. 2008;29:2031–2041. doi: 10.1093/eurheartj/ehn299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JS, Yaffe K, Lui LY, et al. Prospective study of endogenous circulating estradiol and risk of stroke in older women. Arch Neurol. 2010;67:195–201. doi: 10.1001/archneurol.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17:242–255. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- 10.Mikkola TS, Clarkson TB. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc Res. 2002;53:605–619. doi: 10.1016/s0008-6363(01)00466-7. [DOI] [PubMed] [Google Scholar]
- 11.Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357–365. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 12.Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 13.Mendelsohn ME, Karas RH. HRT and the young at heart. N Engl J Med. 2007;356:2639–2641. doi: 10.1056/NEJMe078072. [DOI] [PubMed] [Google Scholar]
- 14.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 15.Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15–23. doi: 10.1097/gme.0b013e31818888f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.North American Menopause Society. Menopause Practice: A Clinician’s Guide. 4. Cleveland, OH: North American Menopause Society; 2010. [Google Scholar]
- 18.Cui R, Iso H, Toyoshima H, et al. Relationships of age at menarche and menopause, and reproductive year with mortality from cardiovascular disease in Japanese postmenopausal women: the JACC study. Journal of epidemiology/Japan Epidemiological Association. 2006;16:177–184. doi: 10.2188/jea.16.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Leciñana MA, Egido JA, Fernández C, et al. Risk of ischemic stroke and lifetime estrogen exposure. Neurology. 2007;68:33–38. doi: 10.1212/01.wnl.0000250238.69938.f5. [DOI] [PubMed] [Google Scholar]
- 20.Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:1027–1037. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker WH, Jacoby V, Shoupe D, Rocca W. Effect of bilateral oophorectomy on women’s long-term health. Womens Health (Lond Engl) 2009;5:565–576. doi: 10.2217/whe.09.42. [DOI] [PubMed] [Google Scholar]
- 22.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: The Framingham Heart Study. Stroke. 2009;40:1044–1049. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baba Y, Ishikawa S, Amagi Y, Kayaba K, Gotoh T, Kajii E. Premature menopause is associated with increased risk of cerebral infarction in Japanese women. Menopause. 2010;17:506–510. doi: 10.1097/gme.0b013e3181c7dd41. [DOI] [PubMed] [Google Scholar]
- 24.Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32:745–750. doi: 10.1093/eurheartj/ehq477. [DOI] [PubMed] [Google Scholar]
- 25.Jacoby VL, Grady D, Wactawski-Wende J, et al. Oophorectomy vs ovarian conservation with hysterectomy: cardiovascular disease, hip fracture, and cancer in the Women’s Health Initiative Observational Study. Arch Intern Med. 2011;171:760–768. doi: 10.1001/archinternmed.2011.121. [DOI] [PubMed] [Google Scholar]
- 26.Parker WH, Manson JE. Oophorectomy and cardiovascular mortality: is there a link? Menopause. 2009;16:1–2. doi: 10.1097/gme.0b013e31818d64d6. [DOI] [PubMed] [Google Scholar]
- 27.Rocca WA, Shuster LT, Grossardt BR, et al. Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health (Lond Engl) 2009;5:39–48. doi: 10.2217/17455057.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson CS, Jamrozik KD, Burvill PW, Chakera TM, Johnson GA, Stewart-Wynne EG. Determining the incidence of different subtypes of stroke: results from the Perth Community Stroke Study, 1989–1990. Med J Aust. 1993;158:85–89. doi: 10.5694/j.1326-5377.1993.tb137529.x. [DOI] [PubMed] [Google Scholar]
- 29.Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58:688–697. doi: 10.1002/ana.20617. [DOI] [PubMed] [Google Scholar]
- 30.Mack WJ, Slater CC, Xiang M, Shoupe D, Lobo RA, Hodis HN. Elevated subclinical atherosclerosis associated with oophorectomy is related to time since menopause rather than type of menopause. Fertil Steril. 2004;82:391–397. doi: 10.1016/j.fertnstert.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 31.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. 1999;30:2513–2516. doi: 10.1161/01.str.30.12.2513. [DOI] [PubMed] [Google Scholar]
- 32.Woo D, Gebel J, Miller R, et al. Incidence rates of first-ever ischemic stroke subtypes among blacks: a population-based study. Stroke. 1999;30:2517–2522. doi: 10.1161/01.str.30.12.2517. [DOI] [PubMed] [Google Scholar]
- 33.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 34.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 36.LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jungheim ES, Colditz GA. Short-term use of unopposed estrogen: a balance of inferred risks and benefits. JAMA: the journal of the American Medical Association. 2011;305:1354–1355. doi: 10.1001/jama.2011.405. [DOI] [PubMed] [Google Scholar]
- 38.Duckles SP, Krause DN, Stirone C, Procaccio V. Estrogen and mitochondria: a new paradigm for vascular protection? Mol Interv. 2006;6:26–35. doi: 10.1124/mi.6.1.6. [DOI] [PubMed] [Google Scholar]
- 39.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30:201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chambliss KL, Wu Q, Oltmann S, et al. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120:2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendelsohn ME, Karas RH. Rapid progress for non-nuclear estrogen receptor signaling. J Clin Invest. 2010;120:2277–2279. doi: 10.1172/JCI43756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Register T, Sophonsritsuk A, Appt SE. Estrogen inhibits carotid artery inflammation in early but not late menopause in cynomolguis monkeys. The North American Menopause Society 21st Annual Meeting: Transition and Change - The Future is Upon Us; Chicago, IL. 2010. p. Abstract S-2. [Google Scholar]
- 44.Zhang B, Subramanian S, Dziennis S, et al. Estradiol and G1 reduce infarct size and improve immunosuppression after experimental stroke. J Immunol. 2010;184:4087–4094. doi: 10.4049/jimmunol.0902339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bushnell CD, Hurn P, Colton C, et al. Advancing the study of stroke in women: summary and recommendations for future research from an NINDS-Sponsored Multidisciplinary Working Group. Stroke. 2006;37:2387–2399. doi: 10.1161/01.STR.0000236053.37695.15. [DOI] [PubMed] [Google Scholar]
- 46.Raval AP, Bhatt A, Saul I. Chronic nicotine exposure inhibits 17beta-estradiol-mediated protection of the hippocampal CA1 region against cerebral ischemia in female rats. Neurosci Lett. 2009;458:65–69. doi: 10.1016/j.neulet.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 47.Shuster LT, Grossardt BR, Gostout BS, Rocca WA. Prophylactic bilateral oophorectomy jeopardizes long-term health. Sexuality Reprod Menopause. 2010;8:S1, S3–S5. [Google Scholar]
- 48.De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376:911–921. doi: 10.1016/S0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- 49.Vujovic S, Brincat M, Erel T, et al. EMAS position statement: Managing women with premature ovarian failure. Maturitas. 2010;67:91–93. doi: 10.1016/j.maturitas.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Buist DS, Newton KM, Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104:1042–1050. doi: 10.1097/01.AOG.0000143826.38439.af. [DOI] [PubMed] [Google Scholar]
- 51.Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140:184–188. doi: 10.7326/0003-4819-140-3-200402030-00009. [DOI] [PubMed] [Google Scholar]
- 52.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 53.Chubaty A, Shandro MT, Schuurmans N, Yuksel N. Practice patterns with hormone therapy after surgical menopause. Maturitas. 2011;69:69–73. doi: 10.1016/j.maturitas.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Parente L, Uyehara C, Larsen W, Whitcomb B, Farley J. Long-term impact of the women’s health initiative on HRT. Arch Gynecol Obstet. 2008;277:219–224. doi: 10.1007/s00404-007-0442-1. [DOI] [PubMed] [Google Scholar]
- 55.Silverman BG, Kokia ES. Use of hormone replacement therapy, 1998–2007: sustained impact of the Women’s Health Initiative findings. Ann Pharmacother. 2009;43:251–258. doi: 10.1345/aph.1L438. [DOI] [PubMed] [Google Scholar]