Abstract
Mucosal homeostasis is dependent upon the establishment and maintenance of the cell-cell contacts that comprise the physiological barrier. Breaks in the barrier are linked to multiple diseases such as the inflammatory bowel diseases. While increased cAMP levels limit inflammation by decreasing leukocyte infiltration, the effects of elevated cAMP on intestinal epithelial repair are unknown.
Methods
Restitution in animals administered rolipram was monitored by microscopic examination after laser wounding of the intestinal epithelium or in mice treated with dextran sodium sulfate (DSS). In vitro analysis was conducted using IEC6 and T84 cells to determine the role for elevated cAMP in altering Rho-dependent cellular migration signaling pathways.
Results
We show that treatment with rolipram, forskolin, and cAMP-analogs decrease intestinal epithelial cell migration in vitro. In vivo cell imaging revealed that increased cAMP resulted in a decreased cellular migration rate, with cells at the edge displaying the highest activity. As expected, elevated cAMP elicited increased protein kinase A (PKA) activity, in turn resulting in the inactivation and sequestration of RhoA and decreased actin reorganization. The ablation of restitution by cAMP was not restricted to cell culture as forskolin and rolipram treatment significantly decreased epithelial microwound closure induced by the two photon confocal injury model.
Conclusion
Together, these data suggest that administration of cAMP elevating agents paradoxically decrease infiltration of damage-causing leukocytes while also preventing epithelial repair and barrier maintenance. We propose that treatment with cAMP elevating agents severely limits mucosal re-epithelialization and should be contraindicated for use in chronic inflammatory bowel disorders.
Keywords: cAMP, PKA, restitution, intestinal epithelium, phosphodiesterase inhibitors
Introduction
Intestinal epithelial cells form a barrier and regulate secretion and absorption of nutrients while impeding the entry of noxious substances and microbes. This barrier is unequivocally important in intestinal homeostasis as illustrated by the morbidity encountered in disorders such as the inflammatory bowel diseases (IBD), celiac disease, ischemia/reperfusion and graft-versus-host disease (22;49;61) which are associated with loss of epithelial restitution. The barrier is seriously compromised when either individual cells or sheets of epithelial cells are lost. As intestinal injury is a part of normal function of the intestine, epithelial cells are charged with the tasks of constant regeneration, migration and re-epithelialization over barrier breaks (24;34;37;58). The intestinal epithelium is programmed to rapidly heal such wounds and reseal the barrier within minutes of injury (38;41;48). This healing is dependent on the ability of cells at the edge of the wound to migrate over the denuded area, reform cell contacts and re-establish barrier function (32;42).
Mucosal healing is an important characteristic of the intestinal epithelium in normal physiology as well as recovery and efficacy of treatment in IBD (17). The ability and completeness of mucosal healing is predictive of disease recurrence (1;61). Restitution, defined as the rapid migration of epithelial cells over the site of injury, independent of proliferation, is a key stage in mucosal repair (25;26;33;36;45;57). This stage in wound repair is largely dependent on Rho-mediated modifications in the actin cytoskeleton (32;41;48;50). Monomeric Rho GTP-binding proteins reside in close proximity to the cell membrane and comprise a large superfamily that are activated by the exchange of GDP for GTP and inactivated by the intrinsic GTPase activity. Rho therefore functions as a molecular switch to activate downstream effectors (16;47) in intracellular signaling pathways.
The second messenger cAMP is produced by the activation of adenylyl cyclase which converts ATP into the biologically active signaling mediator, with cAMP subsequently degraded through the action of phosphodiesterase (PDE) enzymes. A growing body of work is focused on elucidating the role for cAMP in regulating inflammatory diseases. In lymphoid cells, increased cytoplasmic concentrations of cAMP can differentially activate integrins, either leading to increased or decreased cellular adhesion through regulation by upstream molecules such as Rho and Rap1 (3;27;28;53). In macrovascular endothelial cells cAMP is linked to increased barrier function while in coronary endothelial monolayers increased endothelial permeability is observed based on the differential activation and disintegration of proteins within the focal adhesion complex (4). These differing responses to this signaling molecule are attributed to subcellular localized production and degradation of cAMP as well as to the primary effector molecules that are activated. The primary downstream signaling effectors of cAMP are protein kinase A (PKA), exchange protein activated by cAMP (EPAC), and cyclic nucleotide-gated channels. Increased cAMP levels can inhibit the ability of inflammatory cells to migrate, modulate endothelial and lung epithelial barrier formation and secretion of cytokines (4;19;30;31).
There is continued interest in cAMP as a therapeutic target to treat inflammatory diseases (7;9;21;51). The aim of this study was to determine the impact of elevated cAMP levels on signaling mechanisms regulating intestinal epithelial restitution. Herein, we utilize cAMP analogs and the phosphodiesterase inhibitor rolipram as a model therapeutic to increase intracellular cAMP. These data demonstrate that cAMP decreases epithelial cell migration through PKA dependent phosphorylation of RhoA. This phosphorylation event allows for sequestration of RhoA away from the cell membrane by Rho-GDI resulting in increased cortical actin formation.
Materials and Methods
Chemicals
Foskolin, rolipram, H89, PKI, prostaglandin-E2 (PGE2), and acriflavine were purchased from Sigma-Aldrich Chemicals (St. Louis, MO, USA). 8-br-cAMP, 6-Bnz-cAMP, Rp-8-CPT-cAMPS were purchased from BioLog Life Science Institute (Axxora LLC, San Diego, CA).
Cell culture
The normal, non- transformed rat small intestinal (IEC-6) cell line (CRL-1592) and the human T84 colonic carcinoma cell line (10) were cultured as described previously (15;54).
Immunoblot analysis
IEC-6 cells were grown to 80% confluence and serum-starved 24 hours before treatment. T84 cells were grown on tissue culture inserts until transepithelial resistance was ≥800Ωcm2. Cells were solublized in modified RIPA buffer [50mM Tris·HCl, pH 7.4, 150mM NaCl, 0.25% (v/v) sodium deoxycholate, 1.0% (v/v) NP-40, 0.1% (v/v) SDS and 1mM EDTA] supplemented with Protease Inhibitor Cocktail Set III (EMD Biosciences) and 10mM sodium orthovanadate, 40mM glycerolphosphate, and 20mM sodium fluoride as phosphatase inhibitors. Lysates were centrifuged at 550 rpm for 5 minutes at 4°C to pellet nuclei. Protein concentration was determined using the Bradford protein assay kit (BCA kit, Pierce Biotechnology, Rockford IL) and 10µg of protein were size-separated using reducing SDS-PAGE. Proteins were electrophoretically transferred to PVDF (Immobilon-P, Millipore) for immunoblot analysis as detailed previously (14).
Live cell imaging
IEC-6 cells were grown to confluence and serum-starved for 24hours prior to treatment with forskolin or the cAMP analogs 6-bnz-cAMP. Cells were wounded by razor and rinsed twice with fresh serum-free DMEM media. Media was then replaced and treatment was applied. Cells were immediately placed in a stage mounted incubation system with flowmeter INU-NI-FI (Tokai Hit) and imaged (Nikon Eclipse Ti). Phase contrast images were taken every 30 minutes for 18 hours using the NIS-Elements AR 3.00 (Nikon) software. Image sequences were converted to .avi format and imported into ImageJ (NIH) software running the MtrackJ macro to track cell migration.
Animal Husbandry
C57BL/6J mice were maintained in a specific-pathogen-free facility. Mice were kept in microisolator cages and provided free access to food and water on an 11–13 hour light-dark cycle. Epithelial restitution and mucosal injury were completed using IACUC approved protocols.
In vivo animal imaging
Mice were anesthetized by i.p. injection of a ketamine (75mg/kg) / xylazine (25mg/kg) mixture. The abdomen was shaved, opened by midline laporatomy and a 3 cm segment of bowel was exteriorized (35). As detailed previously (34) an inflow cannula was placed at the orad end of the exteriorized bowel and an outflow placed distal to exteriorized bowel (I.D. = 0.76mm, O.D.=1.22mm). The cannulas were secured with 6-0 polypropylene suture paying special attention to not restrict the enteric vasculature. Tubing was attached to a perfusion pump (Bio-Rad, Hercules, CA, USA) and Hank’s Balanced Salt Solution (HBSS) was perfused through the intestinal segment at 0.25ml/min and gradually increasing in velocity to 0.75ml/min. After initial flushing of the intestine, experimental animals were administered treatment of 10µM forskolin+10µM rolipram in HBSS+acriflavine (0.05% w/v) and perfused in a recirculating manner for 1 hour while control animals received acriflavine alone. After treatment the cannulas were removed and the abdomen was closed, leaving approximately 1.5cm of bowel exteriorized. The exteriorized gut was opened along the anti-mesenteric border exposing the intestinal epithelium. The mouse was then positioned prone on a 35mm glass bottom dish (MatTek Corporation, Ashland, MA). The epithelium was imaged using a confocal microscope (Ziess LSM510 META-NLO laser scanning microscope, Jena, Germany) equipped with visible wavelength and multiphoton lasers and outfitted with a Zeiss XL-3 incubator to assure stable environmental control. To reproducibly create 12µm wounds, individual cells were exposed to 6µsecond bursts of the Chameleon Ultra II Ti: sapphire laser (Coherent, CA) at 800nm, as modified from Staurodub et al. (56). Each intestine was injured at 3–5 separate sites over a 3-hour period and wound healing was averaged for each mouse.
PKA activation
PKA activity was assessed using the PepTag Assay for Non-Radioactive detection (Promega, Madison, WI, USA) following the manufacturer’s directions. Briefly, cells were grown to confluence, wounded and treated with forskolin or cAMP analogs for 15minutes–2hours. After incubation cells were solubilized, and lysates were collected, clarified and aliquots incubated with the PepTag A1 peptide for 30minutes at room temperature. After incubation phosphorylated and nonphosphorylated PepTag peptides were electrophoretically separated on an agarose gel and levels of phosphorylated peptide were quantified by densitometry.
Animal care and induction of colitis
Acute colitis was induced by allowing mice access to 3.5% (w/v) dextran sodium sulfate (DSS, ~36,000–50,000 daltons; MP Biomedicals, Solon, OH) dissolved in acidified water ad libitum for 5 consecutive days. Animals were allowed to recover for 5 days in the presence or absence of 10µM rolipram administered by oral gavage. Mice were weighed every second day. After the specified recovery time mice were sacrificed, the large intestine removed, formaldehyde fixed, paraffin embedded and sectioned for histological examination by hematoxylin and eosin staining.
Mucosal damage
Tissues were removed and “Swiss-rolled”, fixed in formalin and routinely processed and 4µm sections were stained by hematoxylin and eosin. Damage was assessed by light microscopy, performed in a blinded fashion observing previously defined criteria (60). Briefly, the integrity of the intestinal epithelium was the primary focus for clinical scores; therefore, the histological score was determined for ulceration, inflammation and depth of lesion and combined for a maximal score of 8. The histological score was determined from three sections from each mouse with each section a minimum of 32µm apart into the tissue. On ulcers that lacked an intact epithelium, measurements were taken of the epithelial cells directly adjacent to the wound which had lost their columnar structure. These cells were referred to as the wound associated epithelium (WAE). Unhealed ulcers were those that were not re-epithelialized, whereas healed ulcers successfully re-epithelialized but maintained an inflammatory bed.
Statistical analysis
Differences between unstimulated control and experimental samples were analyzed by unpaired Student’s t-test using SigmaStat (Jandel Scientific Software, San Rafael, CA). Multiple comparisons between groups were analyzed using a two-way ANOVA and a Bonferroni post-hoc analysis used to identify pair wise differences (GraphPad Prism 4, La Jolla, CA). Statistical significance was defined as P≤0.05.
Results
Rolipram reduces basal cell migration and microscopic lesion repair
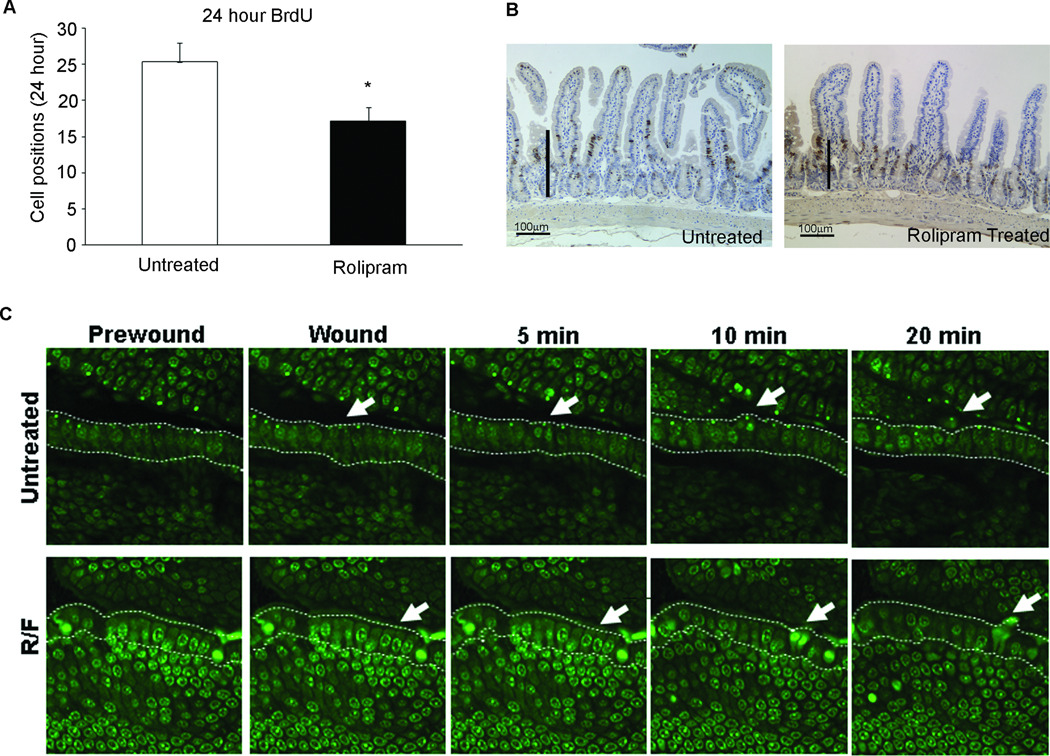
Rolipram increases cAMP concentrations in cells through the inhibition of phosphodiesterase 4 (18;59). Rolipram was administered to mice treated with BrdU and the rate of enterocyte migration was assessed 24 hours later by histological evaluation. Rolipram treatment decreased basal cell migration along the crypt-villus axis (Figure 1A and B). To assess the effects of rolipram on induced cell migration during microscopic lesion repair, consistent with daily injury of the epithelium, we employed the in vivo two-photon laser technique to selectively injure intestinal epithelial cells. Injury progression was qualitatively monitored over time until injured cells lost contact with the basement membrane and were extruded into the intestinal lumen. After initial damage, the nuclei of the injured cells condensed and began to migrate toward the apex of the cell. Underlying tissues appeared to be morphologically unchanged by injury. When the focal plane was favorable, cells adjacent to the damaged cells sent projections under damaged cells as they were shed. Injured intestinal epithelial cells of untreated animals were expelled into the lumen by 18.7±3.5 minutes while expulsion was delayed to 34.5±1.5 minutes in animals treated with both rolipram and forskolin.
Figure 1. Rolipram treatment slows epithelial cell shedding.
(A) 24 hour migration of BrdU labeled cells was quantified in mice treated with rolipram. *, P≥0.05 compared to untreated control mice. Values are mean±SEM of 3 separate experiments. (B) Micrographs of ileum of adult mice corresponding to panel A. Bars represent BrdU positive cells. (C) Acriflavin stained intestinal epithelium was wounded using a 2-photon laser (wounded area denoted with white arrow). Increased time to shedding was observed in rolipram-treated animals. Images were taken at 400×. Images are representative of 3–4 mice.
Rolipram treatment slows intestinal wound repair
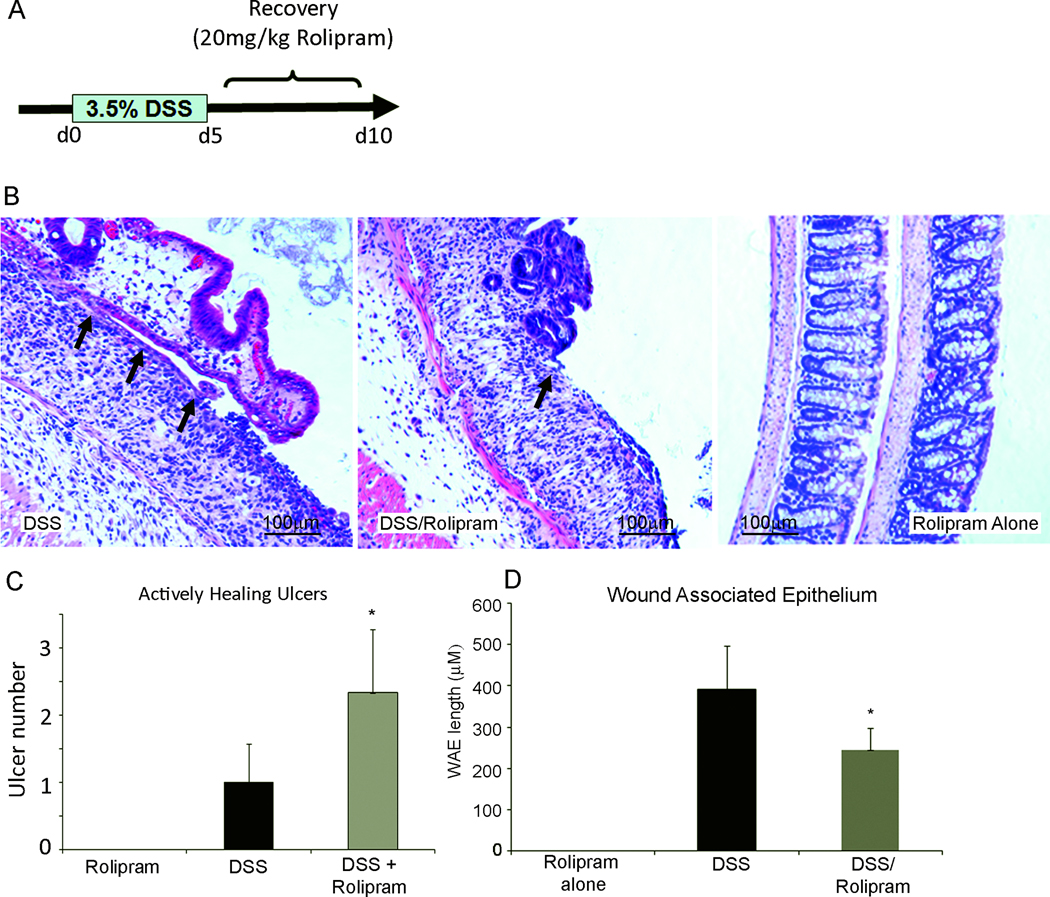
Phosphodiesterase inhibitors are effective in multiple models of inflammation (29;55) including IBD (2;11;20). To delineate the role of increased cAMP levels in epithelial wound repair, DSS was used to induce colonic damage. DSS treatment resulted in marked inflammation and epithelial injury compared to mice treated with rolipram alone (Figure 2B). After 5 days of rolipram administration there was a significant increase in the number of unhealed ulcers (2.3±0.94) compared to animals that received DSS alone (1.0±0.5) (Figure 2C). Rolipram administration to DSS treated (3.6±1.5) mice had little, if any, effect on the total number of ulcers compared to DSS control mice (2.6±1.2) (Supplementary Figure 1). However, as highlighted in Figure 2D, morphological assessment of the wound-associated epithelium (WAE) indicated a marked decrease in re-epithelialization in animals treated with rolipram (243.9±52.6µm) compared to animals receiving DSS alone (391.9±106.4µm). Consistent with previously published reports, animals that received rolipram after DSS treatment began to show an increase in body weight by 5 days of recovery (Supplemental Figure 1C) (11;20). Together, these data indicate that rolipram significantly hinders epithelial cell migration in vivo.
Figure 2. Rolipram decreases enterocyte migration.
(A) Colonic damage was induced by administration of 3.5% Dextran Sodium Sulfate (DSS) for 5 days in drinking water followed by rolipram treatment for 5 days. (B) Wound-associated epithelium (Arrows) was reduced in animals that received rolipram during the recovery phase after DSS damage. Animals received either DSS, DSS followed by rolipram treatment, or rolipram alone. After the tenth day animals were sacrificed and damage assessed histologically. (C) Quantification of actively healing ulcers at day 10 following treatment. *, P≤0.05 compared to DSS alone. While the total number of ulcers was similar between animals that received DSS and those that received DSS and rolipram in combination, DSS/rolipram resulted in the significant increase in ulcers that remained unhealed (actively healing ulcers). (D) Quantification of the length of the wound-associated epithelium (WAE) at day 10. *, P≤0.05 compared to DSS alone. Values are mean±SEM, (n=4).
Effect of increased cAMP concentrations on epithelial migration
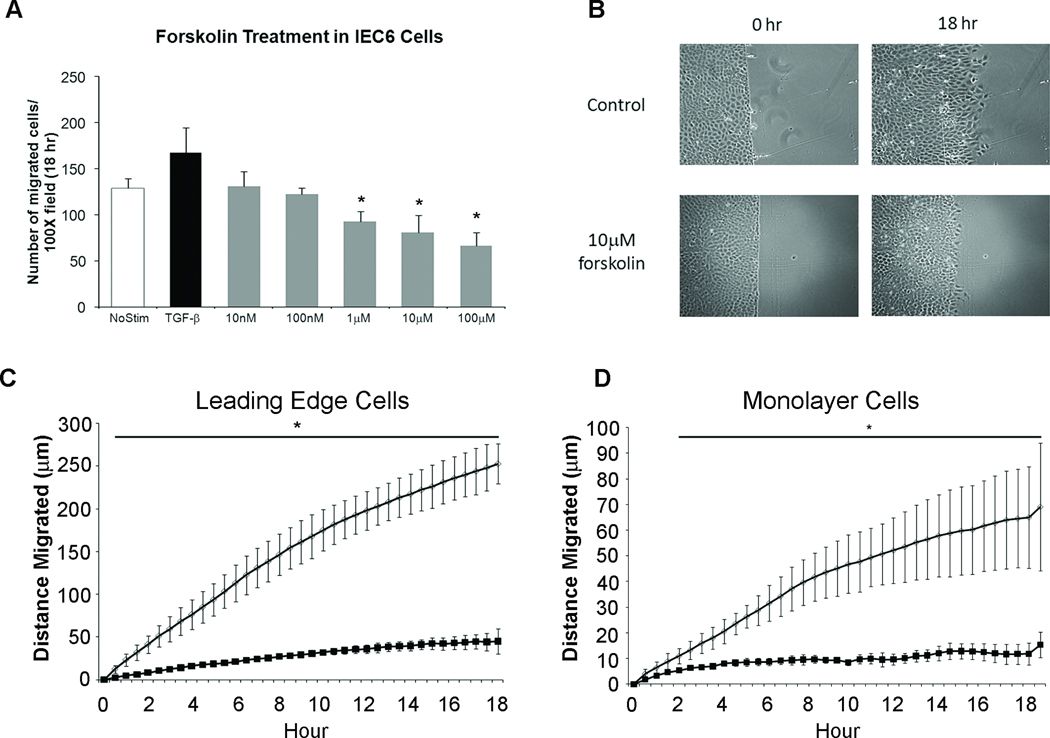
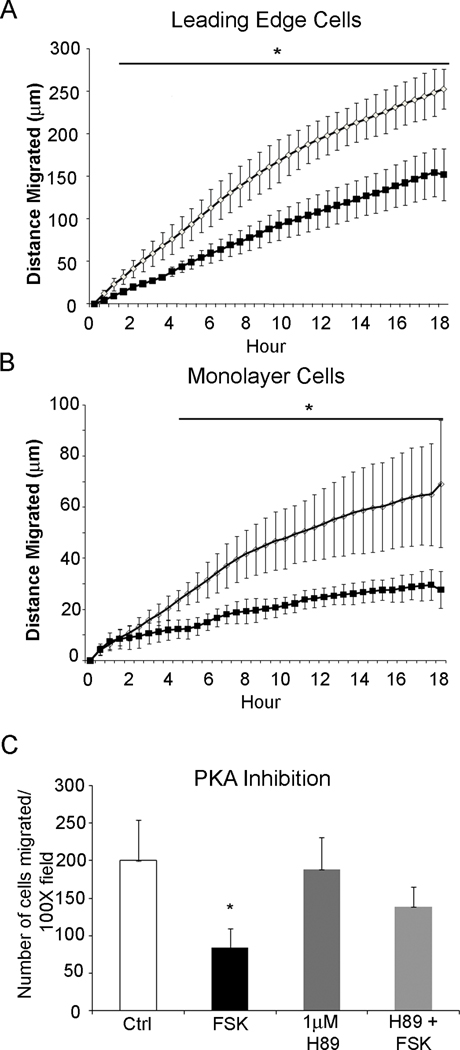
To define the mechanism by which cAMP decreases epithelial cell migration we turned to the IEC-6 cell line model of restitution. Phosphodiesterase inhibitors such as rolipram increase cytoplasmic levels of cAMP. We therefore used the adenylyl cyclase activator forskolin to increase cAMP in cultured epithelial cells. Administration of forskolin (1µM–100µM) dose-dependently reduced cell migration (Figure 3A). Effective inhibition was observed with 10µM forskolin. As expected from our preclinical mouse models, treatment with rolipram dose-dependently inhibited IEC-6 cell migration (Supplemental Figure 2A). Live cell imaging was used to monitor the coordinated restitution response. Using this technique we measured migration of epithelial cells at the migration front (leading edge) as well as cells present 10 rows back from the sheet edge (monolayer cells) (Figure 3). Treatment significantly reduced the distance and velocity of migration in both the leading edge cells and monolayer cells beginning 30 minutes after administration. This decreased migration remained consistent over the 18 hour time frame (Figure 3C). Prostaglandin-E2 (PGE2) binds to a Gαs protein coupled receptor to stimulate cAMP production. IEC-6 cells stimulated with PGE2 demonstrated a significant reduction in migration using this biologically relevant ligand (Supplemental Figure 2B).
Figure 3. Elevated cAMP levels inhibit epithelial cell migration in vitro.
(A) Quantification of IEC-6 migration with increasing doses of forskolin for 18 hours. TGF-β [5ng/ml] served as a positive control for migration. (B) Representative images of wounded monolayers at time 0 and 18 hours after treatment. (C and D) Migration rate (µm/hour) of untreated cells (open circles) and cells treated with forskolin (solid box). Values are mean±SEM from 3–5 experiments. *, P≤0.05; **, P≤0.01 compared to untreated cells.
Elevation in cAMP decreases cell migration in T84 human epithelia
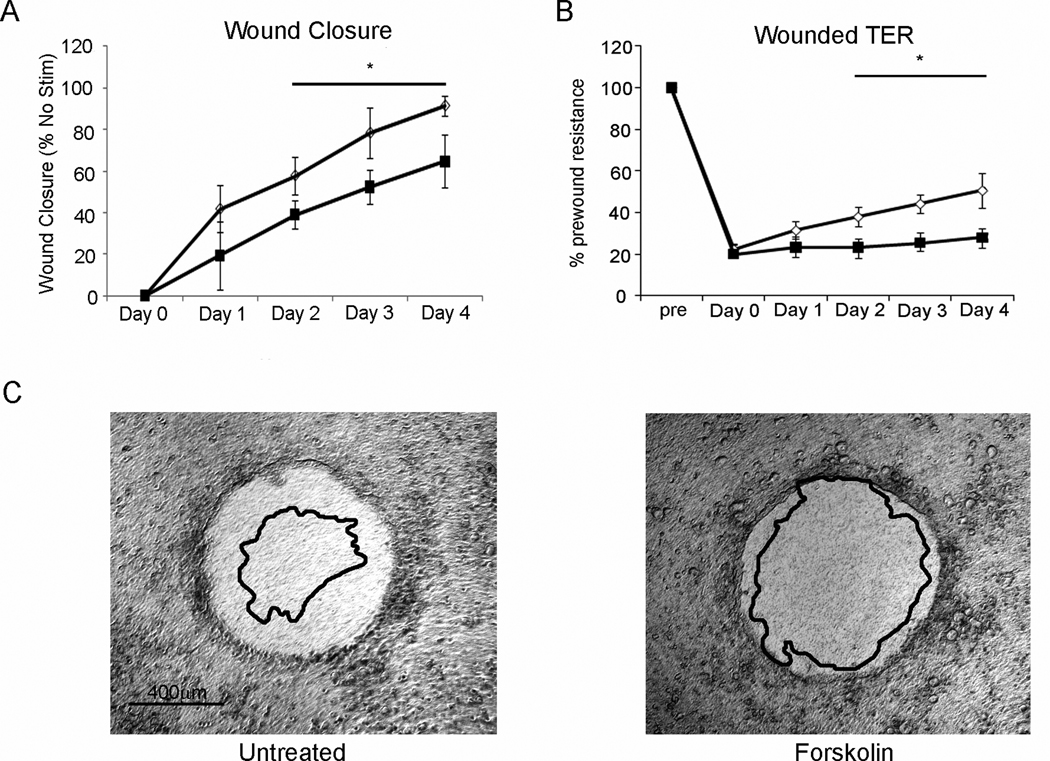
To demonstrate the human relevance of cAMP on restitution, our human T84 polarized epithelial wound model was used (39). Wound closure was diminished in the presence of forskolin, with a significant impairment of restitution observed after day 2 of treatment (Figure 4A). Further, monolayer integrity assessed daily by monitoring the TER (Figure 4B), revealed that forskolin significantly hindered barrier restoration during restitution.
Figure 4. cAMP inhibits human T84 epithelial cell restitution.
(A) Epithelial restitution was delayed in human T84 wounded by aspiration wound. Open circles represent untreated monolayers, solid boxes represent 10µM forskolin treated monolayers. (B) Transepithelial resistance (TER) was significantly delayed in returning to baseline when treated with forskolin (10µM; solid boxes) compared to untreated cells (Open circles). (C) Representative wounds corresponding to panels A and B, shown at day 0, overlay represents wound edge day 4 post wounding. *, P≤0.05, n=4.
Activation of PKA inhibits intestinal epithelial cell migration
We next evaluated the role for the cAMP effector kinase, PKA in interrupting epithelial restitution. Incubation with the PKA-selective cAMP analog (6-Bnz-cAMP 1–100µM) resulted in a 31% decrease in IEC6 cell migration over 18 hours (Figure 5). Examination of the migration index within the cell monolayer distal to the wound edge indicated an even more robust inhibition, with non-directional migration decreased ~61% over the same 18 hour time span. Moreover, the PKA-induced decrease in migration could be pharmacologically reversed through the addition of the PKA specific inhibitors, H89 (Figure 5C) or PKI (data not shown). Thus, cells stimulated with forskolin but pretreated with H89 maintained migration consistent with untreated cells.
Figure 5. cAMP decreases epithelial cell migration through activation of protein kinase A.
(A and B) Quantification of cell migration rate in untreated cells (open circles) and cells treated with the protein kinase A (PKA) activator, 6-Bnz-cAMP (solid boxes). Cell migration is significantly decreased in cells at the (A) leading edge of the wounded epithelium as well as cells 10 rows back in the (B) monolayer. Mean±SEM, n = 3; *, P≤0.05 from untreated cells. (C) Pharmacological inhibition of PKA with H89 [1µM] reversed the negative effect of 10µM forskolin (FSK) on epithelial migration, with re-establishment of migration to levels consistent with untreated cells. Values are mean±SEM, n=4; *, P≤0.05.
Phosphorylated Rho is sequestered by Rho-GDI in forskolin-treated cells
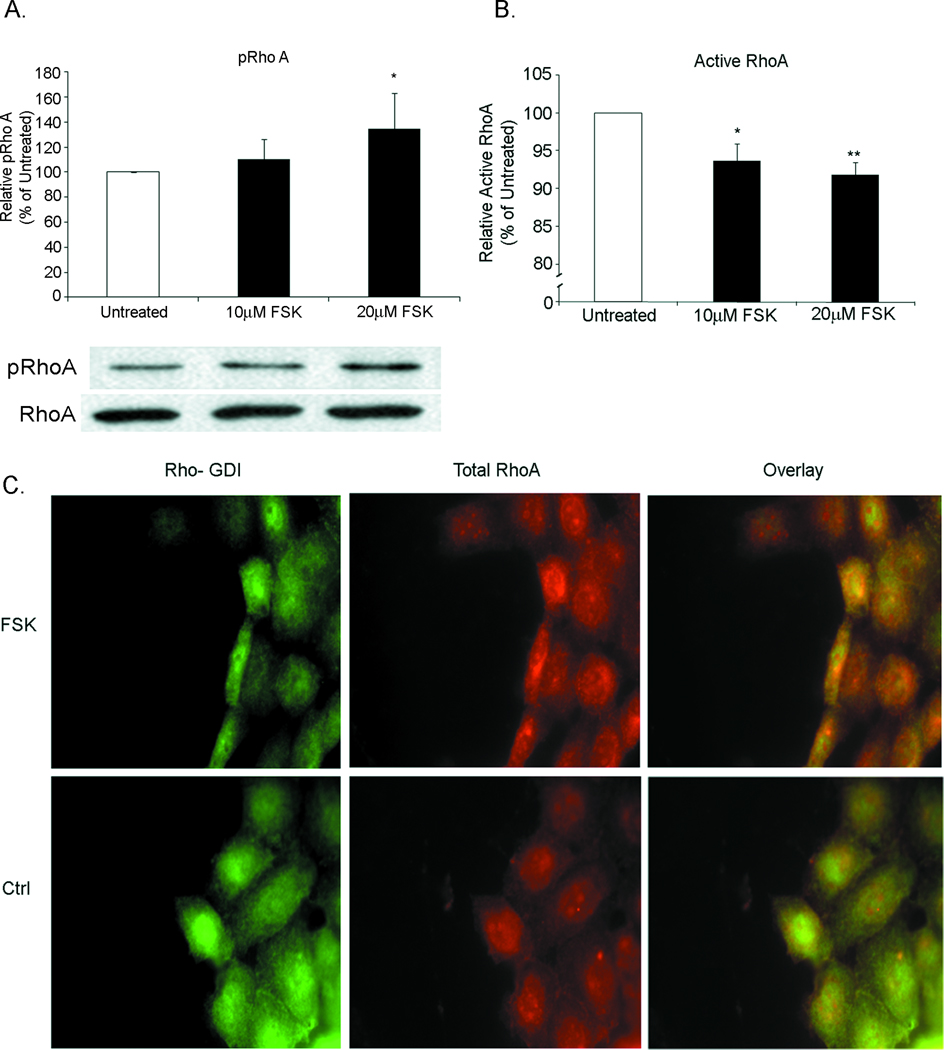
Consistent with the role of RhoA, an integral protein in cell migration and target of PKA phosphorylation (12;27), we assessed phosphorylation of the RhoA negative regulatory serine 188 residue (RhoAser188). Immunoblot analysis of epithelial monolayers treated with forskolin for 30 minutes showed a dose dependent increase in RhoA phosphorylation (Figure 6A). Consistent with those data, pull-down analyses indicated a decreased level of active RhoGTP following forskolin treatment (Figure 6B). Lastly, forskolin resulted in increased co-localization of Rho-GDI and RhoA (Figure 6C), suggesting sequestration of inactive RhoA in response to elevated cAMP. Immunoblot analysis showed no alteration in phosphorylation of the RhoA effector myosin light chain with forskolin treatment compared to untreated controls. Together, these data are consistent with elevated epithelial cAMP inactivating the RhoGTPase signaling cascade.
Figure 6. Elevated cAMP leads to an inhibitory phosphorylation of RhoA by PKA.
(A) Quantification of RhoA phosphorylation in IEC-6 monolayers treated with forskolin showed a dose dependent increase in RhoA phosphorylated at Serine-188, a PKA phosphorylation site. After incubation cells were lysed and immunoblot was performed for total RhoA and ser-188 pRhoA. *, P≤0.05. (B) Quantification of activated RhoA in IEC-6 monolayers demonstrated a decrease in activated RhoA after treatment with forskolin. (C) Fluorescent micrographs of RhoA with Rho-GDI demonstrate colocalization after treatment with forskolin (FSK). Colocalization was not observed in untreated cells (Ctrl). Images are representative of 3 experiments.
Elevated cAMP stabilizes cortical actin
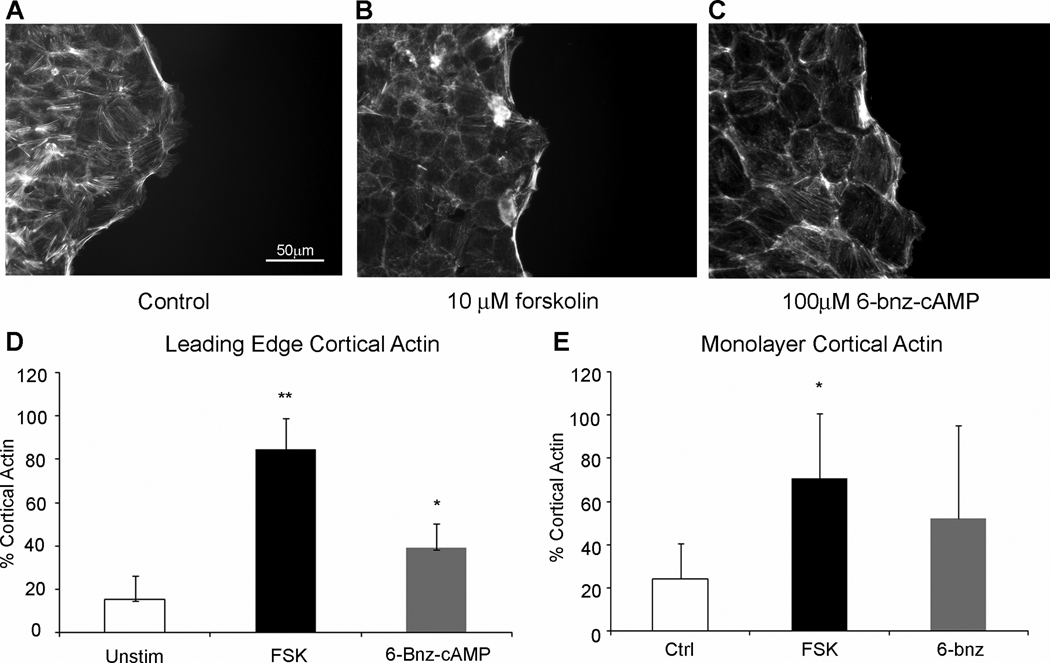
Active RhoA increases filamentous actin formation during restitution (39;44;48). Using Alexafluor-488-labeled phalloidin we assessed the impact of elevated cAMP concentration on the actin cytoskeleton. While the overall levels of filamentous actin in wounded monolayers were unchanged, the cellular localization of F-actin was altered by addition of forskolin or 6-bnz-cAMP (Figure 7A–C). Consistent with actively migrating cells, long bundles of stress fiber actin, running in the direction of cell migration, were present in leading edge cells of untreated wounded monolayers (Figure 7A). However, cells treated with forskolin (Figure 7B) or 6-bnz-cAMP (Figure 7C) demonstrated increased levels of actin along the perimeter of the cell. Thus, as shown in Figure 7D, cells at the wound edge displayed increased regions of cortical actin after treatment with forskolin (84.8±12.0%) or 6-bnz-cAMP (39.3±8.9%) compared to untreated cells (14.2±9.4%). Moreover, consistent with the observed decrease in migration velocity, cells 10 rows back from the leading edge demonstrated a similar increase in cortical actin (Figure 7E). In agreement with the lack of migration, we also noted that stress fiber orientation was altered in cells with elevated cAMP. Taken together these findings agree with the inactivation of RhoA and suggest cAMP and PKA decrease formation of properly oriented stress fiber actin needed for cell motility.
Figure 7. PKA activation decreases cell migration through altered actin regulation.
Fluorescence micrographs of wounded monolayers (A) untreated, (B) forskolin, or (C) 6-bnz-cAMP. (D) Quantification of cortical and stress fiber actin at the leading edge of the wounded epithelium. *, P≤0.05; **, P≤0.01; mean±SEM of 4 experiments. (E) Quantification of cortical and stress fiber actin at 5–10 rows back into the monolayer from the leading edge. *, P≥0.05. Images are representative of 5–7 fields of view from 4 experiments.
Discussion
Numerous reports suggest an underlying defect in the strength and integrity of the intestinal epithelial barrier in chronic human intestinal inflammatory diseases (6;13;23;43;61). Previous reports suggest the importance of cAMP elevating agents in controlling and treating inflammatory disorders of the intestine (11;20;60). A limitation of those studies was their broad evaluation of the overall disease state through clinical scores and the disease progression, without isolating individual aspects of disease recovery or tissue repair. The goal of these studies was to investigate the impact of cAMP elevating agents on the cells of the intestinal epithelial barrier. Herein, we indicate a key inhibitory effect in the migratory potential of intestinal epithelial cells with elevated cAMP levels and increased activation of PKA. We establish that treatment with rolipram decreases basal epithelial migration in vivo. Further, we demonstrate that cAMP elevating agents reduced the ability of colonocytes to migrate and re-epithelialize ulcers induced by DSS treatment and hindered injured cell shedding from microlesions. In vitro analyses demonstrated a link between increased PKA activity and decreased actin polymerization necessary for cell motility, through the sequestration of monomeric RhoA GTPase. Therefore, phosphodiesterase inhibitors fail to address the underlying barrier defects intrinsic to human inflammatory diseases.
The lessened clinical scores observed after treatment with rolipram in prior studies (11;20;60) are likely indicative of the decrease in inflammatory cells, with a perhaps indirect effect on tissue damage within the intestine. The current report focuses specifically on the response of the intestinal epithelium and barrier function of the intestine. We demonstrate a decrease in epithelial restitution after rolipram treatment and DSS colitis, suggesting that the clinical score may not accurately predict the enduring health of the tissue. These findings provide clinical relevance to the pharmacological treatment of inflammation as well as the state of the epithelial barrier in regard to disease remission and recurrence. This report suggests that while inflammation and subsequent intestinal damage may be decreased by phosphodiesterase inhibition (11;20;60), the damage done to the intestinal epithelium may be prolonged thereby extending the time to recover barrier function and increasing susceptibility to disease recurrence. These findings, in conjunction with previous studies, suggest that the decrease in the clinical manifestations of disease as a result of treatments with cAMP regulating drugs likely occurs before the barrier function of the intestine has been adequately restored.
Several lines of evidence in our work indicate that cAMP is acting through PKA in decreased epithelial cell migration. First, cells treated with forskolin demonstrated an increase in PKA activity compared to untreated monolayers. This initial observation led us to further expand the inquiry into the mechanism used by PKA to inhibit cell migration. Subsequently, when IEC-6 cells were stimulated with the PKA-specific analog of cAMP, 6-Bnz-cAMP, migration was decreased. Similarly, the inhibition of PKA activation by pharmacological means demonstrated a release of the cAMP induced brake in cell migration in forskolin treated cells. These data together further substantiated the involvement of PKA in epithelial arrest.
Mechanistically, PKA is known to regulate the actin cytoskeleton through its effects on the RhoGTPase RhoA. We found that phosphorylation of RhoA was increased in intestinal epithelial cells with elevated cAMP concentrations. Thus, the inhibitory phosphorylation at ser188 resulted in decreased activation of Rho A and colocalization of RhoA by Rho-GDI. Presumably this colocalization resulted in the subsequent sequestration of RhoA away from the cell membrane by Rho-GDI resulting in less interaction with the actin cytoskeleton. While cytosolic RhoA is protected from PKA phosphorylation by GDI (27), only membrane bound RhoA is available to be phosphorylated by PKA. Membrane localized RhoA accounts for only 2–3% of total cellular RhoA (27). Therefore, the low levels of Rho phosphorylation and inactivation observed in the colocalization and GLISA pull-down analyses are not surprising. Interestingly, the induction of Rho-GDI expression has been observed specifically in the intestinal epithelial cells of patients with IBD (52). Our data suggest that the sequestration of RhoA away from the cell membrane can lead to a catastrophic decrease in RhoA activity upon PKA activation in IBD patients and an inability for the epithelial cells to migrate across a wound. This mechanism is consistent with that observed in other cell types such as lymphocytes (27), endothelial cells (5;40;46) and gastric cancer cells (8). As we detected no alteration in myosin light chain phosphorylation we hypothesize that RhoA inhibition is the primary mechanism of PKA induced intestinal epithelial arrest. However, while treatment with forskolin provided nearly complete abrogation of epithelial migration, 6-bnz-cAMP had an intermediate effect, suggesting the involvement of additional pathways.
In summary, we show that increased levels of cAMP in intestinal epithelial cells inhibit the migratory and restitutive potential in the intestine. This decrease in migration occurs through the PKA-dependent phosphorylation of RhoA and the subsequent decrease in actin polymerization. These data suggest a caveat to treatment of inflammatory diseases with phosphodiesterase inhibitors or elevating agents of cAMP in patients who have or are predisposed to barrier dysfunction in the intestine.
Supplementary Material
Acknowledgements
We thank Sheena Faherty for technical assistance with the animal maintenance and Soonyean Hwang for assistance with 2-photon imaging studies. We appreciate the free use of MtrackJ software which was developed and made available by Dr. Erik Meijering. This work was supported by grants from the National Institutes of Health including F32DK083209, R01DK06827, R01DK062066 and UL1RR031973.
References
- 1.Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology. 2010;138:463–468. doi: 10.1053/j.gastro.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 2.Banner KH, Trevethick MA. PDE4 inhibition: a novel approach for the treatment of inflammatory bowel disease. Trends Pharmacol Sci. 2004;25:430–436. doi: 10.1016/j.tips.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Berends C, Dijkhuizen B, de Monchy JG, et al. Inhibition of PAF-induced expression of CD11b and shedding of L-selectin on human neutrophils and eosinophils by the type IV selective PDE inhibitor, rolipram. Eur Respir J. 1997;10:1000–1007. doi: 10.1183/09031936.97.10051000. [DOI] [PubMed] [Google Scholar]
- 4.Bindewald K, Gunduz D, Hartel F, et al. Opposite effect of cAMP signaling in endothelial barriers of different origin. Am J Physiol Cell Physiol. 2004;287:C1246–C1255. doi: 10.1152/ajpcell.00132.2004. [DOI] [PubMed] [Google Scholar]
- 5.Birukova AA, Liu F, Garcia JG, et al. Protein kinase A attenuates endothelial cell barrier dysfunction induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol. 2004;287:L86–L93. doi: 10.1152/ajplung.00441.2003. [DOI] [PubMed] [Google Scholar]
- 6.Buhner S, Buning C, Genschel J, et al. Genetic basis for increased intestinal permeability in families with Crohn's disease: role of CARD15 3020insC mutation? Gut. 2006;55:342–347. doi: 10.1136/gut.2005.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cazzola M, Picciolo S, Matera MG. Roflumilast in chronic obstructive pulmonary disease: evidence from large trials. Expert Opin Pharmacother. 2010;11:441–449. doi: 10.1517/14656560903555201. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Wang Y, Yu H, et al. The cross talk between protein kinase A- and RhoA-mediated signaling in cancer cells. Exp Biol Med (Maywood) 2005;230:731–741. doi: 10.1177/153537020523001006. [DOI] [PubMed] [Google Scholar]
- 9.Dastidar SG, Rajagopal D, Ray A. Therapeutic benefit of PDE4 inhibitors in inflammatory diseases. Curr Opin Investig Drugs. 2007;8:364–372. [PubMed] [Google Scholar]
- 10.Dharmsathaphorn K, McRoberts JA, Mandel KG, et al. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984;246:G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Granados N, Howe K, Lu J, et al. Dextran sulfate sodium-induced colonic histopathology, but not altered epithelial ion transport, is reduced by inhibition of phosphodiesterase activity. Am J Pathol. 2000;156:2169–2177. doi: 10.1016/S0002-9440(10)65087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong JM, Leung T, Manser E, et al. cAMP-induced morphological changes are counteracted by the activated RhoA small GTPase and the Rho kinase ROKalpha. J Biol Chem. 1998;273:22554–22562. doi: 10.1074/jbc.273.35.22554. [DOI] [PubMed] [Google Scholar]
- 13.Duerksen DR, Wilhelm-Boyles C, Parry DM. Intestinal permeability in long-term follow-up of patients with celiac disease on a gluten-free diet. Dig Dis Sci. 2005;50:785–790. doi: 10.1007/s10620-005-2574-0. [DOI] [PubMed] [Google Scholar]
- 14.Dwinell MB, Johanesen PA, Smith JM. Immunobiology of epithelial chemokines in the intestinal mucosa. Surgery. 2003;133:601–607. doi: 10.1067/msy.2003.143. [DOI] [PubMed] [Google Scholar]
- 15.Dwinell MB, Ogawa H, Barrett KE, et al. SDF-1/CXCL12 regulates cAMP production and ion transport in intestinal epithelial cells via CXCR4. Am J Physiol Gastrointest Liver Physiol. 2004;286:G844–G850. doi: 10.1152/ajpgi.00112.2003. [DOI] [PubMed] [Google Scholar]
- 16.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 17.Froslie KF, Jahnsen J, Moum BA, et al. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–422. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 18.Frossard N, Landry Y, Pauli G, et al. Effects of cyclic AMP- and cyclic GMP- phosphodiesterase inhibitors on immunological release of histamine and on lung contraction. Br J Pharmacol. 1981;73:933–938. doi: 10.1111/j.1476-5381.1981.tb08748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giordano D, Magaletti DM, Clark EA, et al. Cyclic nucleotides promote monocyte differentiation toward a DC-SIGN+ (CD209) intermediate cell and impair differentiation into dendritic cells. J Immunol. 2003;171:6421–6430. doi: 10.4049/jimmunol.171.12.6421. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann G, Bidlingmaier C, Siegmund B, et al. Specific type IV phosphodiesterase inhibitor rolipram mitigates experimental colitis in mice. J Pharmacol Exp Ther. 2000;292:22–30. [PubMed] [Google Scholar]
- 21.Hatzelmann A, Morcillo EJ, Lungarella G, et al. The preclinical pharmacology of roflumilast--a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2010;23:235–256. doi: 10.1016/j.pupt.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Hollander D. The intestinal permeability barrier. A hypothesis as to its regulation and involvement in Crohn's disease. Scand J Gastroenterol. 1992;27:721–726. doi: 10.3109/00365529209011172. [DOI] [PubMed] [Google Scholar]
- 23.Hollander D, Vadheim CM, Brettholz E, et al. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 24.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177:512–524. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson LR, McCormack SA. Healing of Gastrointestinal Mucosa: Involvement of Polyamines. News Physiol Sci. 1999;14:12–17. doi: 10.1152/physiologyonline.1999.14.1.12. [DOI] [PubMed] [Google Scholar]
- 26.Lacy ER, Ito S. Rapid epithelial restitution of the rat gastric mucosa after ethanol injury. Lab Invest. 1984;51:573–583. [PubMed] [Google Scholar]
- 27.Lang P, Gesbert F, Delespine-Carmagnat M, et al. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J. 1996;15:510–519. [PMC free article] [PubMed] [Google Scholar]
- 28.Laudanna C, Campbell JJ, Butcher EC. Elevation of intracellular cAMP inhibits RhoA activation and integrin-dependent leukocyte adhesion induced by chemoattractants. J Biol Chem. 1997;272:24141–24144. doi: 10.1074/jbc.272.39.24141. [DOI] [PubMed] [Google Scholar]
- 29.Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet. 2005;365:167–175. doi: 10.1016/S0140-6736(05)17708-3. [DOI] [PubMed] [Google Scholar]
- 30.Lorenowicz MJ, Fernandez-Borja M, Hordijk PL. cAMP signaling in leukocyte transendothelial migration. Arterioscler Thromb Vasc Biol. 2007;27:1014–1022. doi: 10.1161/ATVBAHA.106.132282. [DOI] [PubMed] [Google Scholar]
- 31.Lorenowicz MJ, Fernandez-Borja M, van Stalborch AM, et al. Microtubule dynamics and Rac-1 signaling independently regulate barrier function in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1321–L1331. doi: 10.1152/ajplung.00443.2006. [DOI] [PubMed] [Google Scholar]
- 32.Lotz MM, Rabinovitz I, Mercurio AM. Intestinal restitution: progression of actin cytoskeleton rearrangements and integrin function in a model of epithelial wound healing. Am J Pathol. 2000;156:985–996. doi: 10.1016/S0002-9440(10)64966-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mammen JM, Matthews JB. Mucosal repair in the gastrointestinal tract. Crit Care Med. 2003;31:S532–S537. doi: 10.1097/01.CCM.0000081429.89277.AF. [DOI] [PubMed] [Google Scholar]
- 34.Marchiando AM, Shen L, Graham WV, et al. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology. 2011;140:1208–1218. doi: 10.1053/j.gastro.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchiando AM, Shen L, Graham WV, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCormack SA, Viar MJ, Johnson LR. Migration of IEC-6 cells: a model for mucosal healing. Am J Physiol. 1992;263:G426–G435. doi: 10.1152/ajpgi.1992.263.3.G426. [DOI] [PubMed] [Google Scholar]
- 37.McNeil PL, Ito S. Gastrointestinal cell plasma membrane wounding and resealing in vivo. Gastroenterology. 1989;96:1238–1248. doi: 10.1016/s0016-5085(89)80010-1. [DOI] [PubMed] [Google Scholar]
- 38.Moore R, Carlson S, Madara JL. Rapid barrier restitution in an in vitro model of intestinal epithelial injury. Lab Invest. 1989;60:237–244. [PubMed] [Google Scholar]
- 39.Moyer RA, Wendt MK, Johanesen PA, et al. Rho activation regulates CXCL12 chemokine stimulated actin rearrangement and restitution in model intestinal epithelia. Lab Invest. 2007;87:807–817. doi: 10.1038/labinvest.3700595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Netherton SJ, Sutton JA, Wilson LS, et al. Both protein kinase A and exchange protein activated by cAMP coordinate adhesion of human vascular endothelial cells. Circ Res. 2007;101:768–776. doi: 10.1161/CIRCRESAHA.106.146159. [DOI] [PubMed] [Google Scholar]
- 41.Nusrat A, Delp C, Madara JL. Intestinal epithelial restitution. Characterization of a cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Invest. 1992;89:1501–1511. doi: 10.1172/JCI115741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nusrat A, Delp C, Madara JL. Intestinal epithelial restitution. Characterization of a cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Invest. 1992;89:1501–1511. doi: 10.1172/JCI115741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearson AD, Eastham EJ, Laker MF, et al. Intestinal permeability in children with Crohn's disease and coeliac disease. Br Med J (Clin Res Ed) 1982;285:20–21. doi: 10.1136/bmj.285.6334.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pertz O, Hodgson L, Klemke RL, et al. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 45.Podolsky DK. Review article: healing after inflammatory injury--coordination of a regulatory peptide network. Aliment Pharmacol Ther. 2000;14(Suppl 1):87–93. doi: 10.1046/j.1365-2036.2000.014s1087.x. [DOI] [PubMed] [Google Scholar]
- 46.Qiao J, Huang F, Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2003;284:L972–L980. doi: 10.1152/ajplung.00429.2002. [DOI] [PubMed] [Google Scholar]
- 47.Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713–2722. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 48.Russo JM, Florian P, Shen L, et al. Distinct temporal-spatial roles for rho kinase and myosin light chain kinase in epithelial purse-string wound closure. Gastroenterology. 2005;128:987–1001. doi: 10.1053/j.gastro.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 50.Santos MF, McCormack SA, Guo Z, et al. Rho proteins play a critical role in cell migration during the early phase of mucosal restitution. J Clin Invest. 1997;100:216–225. doi: 10.1172/JCI119515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842–855. doi: 10.1111/j.1476-5381.2009.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shkoda A, Ruiz PA, Daniel H, et al. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology. 2007;132:190–207. doi: 10.1053/j.gastro.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 53.Smalley DM, Ley K. L-selectin: mechanisms and physiological significance of ectodomain cleavage. J Cell Mol Med. 2005;9:255–266. doi: 10.1111/j.1582-4934.2005.tb00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith JM, Johanesen PA, Wendt MK, et al. CXCL12 activation of CXCR4 regulates mucosal host defense through stimulation of epithelial cell migration and promotion of intestinal barrier integrity. Am J Physiol Gastrointest Liver Physiol. 2005;288:G316–G326. doi: 10.1152/ajpgi.00208.2004. [DOI] [PubMed] [Google Scholar]
- 55.Sousa LP, Lopes F, Silva DM, et al. PDE4 inhibition drives resolution of neutrophilic inflammation by inducing apoptosis in a PKA-PI3K/Akt-dependent and NF-kappaB-independent manner. J Leukoc Biol. 2010;87:895–904. doi: 10.1189/jlb.0809540. [DOI] [PubMed] [Google Scholar]
- 56.Starodub OT, Demitrack ES, Baumgartner HK, et al. Disruption of the Cox-1 gene slows repair of microscopic lesions in the mouse gastric epithelium. Am J Physiol Cell Physiol. 2008;294:C223–C232. doi: 10.1152/ajpcell.00395.2006. [DOI] [PubMed] [Google Scholar]
- 57.Svanes K, Ito S, Takeuchi K, et al. Restitution of the surface epithelium of the in vitro frog gastric mucosa after damage with hyperosmolar sodium chloride. Morphologic and physiologic characteristics. Gastroenterology. 1982;82:1409–1426. [PubMed] [Google Scholar]
- 58.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verghese MW, McConnell RT, Strickland AB, et al. Differential regulation of human monocyte-derived TNF alpha and IL-1 beta by type IV cAMP-phosphodiesterase (cAMP-PDE) inhibitors. J Pharmacol Exp Ther. 1995;272:1313–1320. [PubMed] [Google Scholar]
- 60.Videla S, Vilaseca J, Medina C, et al. Selective inhibition of phosphodiesterase-4 ameliorates chronic colitis and prevents intestinal fibrosis. J Pharmacol Exp Ther. 2006;316:940–945. doi: 10.1124/jpet.105.090837. [DOI] [PubMed] [Google Scholar]
- 61.Wyatt J, Vogelsang H, Hubl W, et al. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.