Abstract
Background
Following a colonoscopy that is negative for cancer, a subset of patients may be diagnosed with colorectal cancer, also termed interval cancer. The frequency and predictors have not been well studied in a population-based U.S. cohort.
Methods
Using the linked SEER-Medicare database, we identified 57,839 patients aged ≥ 69 with colorectal cancer diagnosed between 1994 and 2005 and who underwent colonoscopy within 6 months of cancer diagnosis. Colonoscopy performed between 36 to 6 months prior to cancer diagnosis was a proxy for interval cancer.
Results
Using the case definition, 7.2% of patients developed interval cancers. Factors associated with interval cancers included proximal tumor location (distal colon multivariable OR 0.42, 95% CI 0.390–0.46, rectum OR 0.47, 95% CI 0.42–0.53), increased comorbidity (OR 1.89 95% CI 1.68 2.14 for 3 or more comorbidities), a previous diagnosis of diverticulosis (OR 6.00 95% CI 5.57–6.46), and prior polypectomy (OR 1.74, 95% CI 1.62–1.87). Risk factors at the endoscopist level included a lower polypectomy rate (OR 0.70, 95% CI 0.63–0.78 for the highest quartile), higher colonoscopy volume (OR 1.27, 95% CI 1.13–1.43) and specialty other than gastroenterology (colorectal surgery OR 1.45, 95% CI 1.16–1.83; general surgery OR 1.42, 95% CI 1.24–1.62; internal medicine OR 1.38, 95% CI 1.17–1.63, family practice OR 1.16, 95% CI 1.00–1.35).
Conclusions
A significant proportion of patients develop interval colorectal cancer, particularly in the proximal colon. Contributing factors likely include both procedural and biologic factors, and emphasize the importance of meticulous examination of the mucosa.
Introduction
Colonoscopy is currently considered to be either one of several recommended screening options (1,2) or in some professional guidelines (3), the preferred option for colorectal cancer screening. The evidence about the efficacy of colonoscopy comes primarily from case-control and cohort studies. For example, the National Polyp Study (4), a multicenter study of patients who underwent colonoscopy with removal of one or more adenomas, found a lower observed to expected incidence of cancer in follow-up (5,6).
Whereas few people would dispute the positive attributes of colonoscopy as a screening test, there have been several published studies that have questioned whether this procedure as currently practiced is truly ideal. First, using data derived from audits of newly diagnosed colorectal cancer cases (7) and post-polypectomy patients undergoing surveillance colonoscopy as part of chemoprevention studies (8,9), there is a higher cancer incidence post-colonoscopy than originally reported in the National Polyp Study (4). In addition, population-based studies have questioned the protective effect of colonoscopy on the development of right-sided cancers (10–13) and advanced adenomas (14). Finally, three population-based studies, two from Canada (15,16) and one a Medicare claims study (17) have described the development of colorectal cancer following colonoscopy. Considering patients with a colonoscopy between 6 and 36 months prior to a colorectal cancer diagnosis as having a new or missed cancer, 3.4%–7.9% (15–17) were classified as such. These presumed new or missed lesions are also termed “interval cancers.” However, to date there have been no comparable data from a population-based cohort of US patients that included tumor registry data.
We therefore conducted the present study in a linked tumor registry-health claims database that contained cancer-specific, sociodemographic and procedural data. Our goals were to estimate the frequency of cancers that may have failed colonoscopic detection (interval cancers) and determine factors associated with interval cancers.
Methods
Data Sources
The study used the linked SEER-Medicare database, which consists of Medicare eligible patients who are diagnosed with cancer and reside in one of the geographic areas contained in the SEER registries (18). Through the 1990’s, the SEER Program encompassed about 14% of the US population, but with the addition of several new registries in 2000, approximately 25% of the population is currently captured.
Among the cancer-related variables that were collected, we included demographic characteristics, previous cancer diagnoses, date of cancer diagnosis, and data about the cancer including stage, histology and grade. Medicare claims are contained in three different files, the Carrier file, which includes provider claims, the Outpatient file, which includes claims from institutional outpatient providers, and the Medicare Provider Analysis and Review (MEDPAR) files, which includes all hospitalizations. Each Medicare claim contains diagnoses coded by the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM), and procedures coded according to Common Procedural Terminology, 4th Edition (CPT-4) or ICD-9-CM. The Carrier and Outpatient claims also include physician specialty code and an encrypted version of the physician’s unique personal identifier (UPIN), which was used to categorize practitioners according to specialty.
In addition to patients with a cancer diagnosis, we included the Medicare files from a 5% random sample of beneficiaries who resided in one of the SEER areas but were cancer-free. The Medicare files available for this control group were identical to those of the cancer cases. These files were used to categorize physicians according to two measures of endoscopist performance – the volume of colonoscopies in the database as well as the frequency of polypectomy procedures. The latter measure, which is a representation of the adenoma detection rate (19) was obtained from the ratio of colonoscopy with polypectomy (codes defined below) divided by the total number of colonoscopies by that provider in the database and was adapted from previous studies (20,21).
Patients and Measures
Using the 1994–2005 SEER files, we identified all individuals aged 69 and older with a diagnosis of colorectal adenocarcinoma from 1994–2005. The inclusion criteria were provided to ensure three years of Medicare eligibility (i.e, beginning at age 65) and file availability prior to diagnosis. Patients were excluded if they were enrolled in a Medicare sponsored managed care plan or not enrolled in Medicare Parts A and B from three years prior to diagnosis because of the likely presence of incomplete claims. Patients with a previous diagnosis of cancer at any site according to SEER were also excluded, as were patients with the only colonoscopy procedure coded as incomplete. Due to inconsistent reporting, patients with carcinoma-in-situ at entry were excluded; however, a previous diagnosis of carcinoma-in-situ was considered as a covariate. We also excluded all patients diagnosed with ulcerative colitis or Crohn’s disease during the previous three years, as cancer in this setting is thought to develop through a different biological pathway. In the primary analysis, we excluded all patients without colonoscopy within six months prior to cancer diagnosis. Finally, in order to be able to measure physician performance characteristics associated with the colonoscopy, we excluded patients for whom the colonoscopy could not be linked to an encrypted UPIN.
The Carrier, Outpatient and MEDPAR files from three years through the date of cancer diagnosis were examined for receipt of colonoscopy. Colonoscopies included diagnostic examinations (CPT-4 44388, 44389, 45378, 45380. 45382, G0105, G0121; ICD-9-CM 45.23, 45.41, 45.25, 45.27) and polypectomy (CPT-4 44392, 44393, 44394, 45383, 45384, 45385, ICD-9-CM 45.42, 45.43, 48.36) according to procedure codes, and the dates of all colonoscopies were recorded. Among patients with colonoscopy during both the 36-6 month and < 6 month intervals, the last procedure during the 36-6 month interval was used to derive data about procedure specifics and endoscopist characteristics. Claims data from one year to one month prior to diagnosis were used to derive a previously validated comorbidity score (22).
As characterized in previous studies by our group (23) and others (15–17), colonoscopy procedures between six months and three years before diagnosis were considered to represent interval lesions. The rationale for this distinction assumes that if a malignant lesion is detected at colonoscopy, definitive therapy would be expected to performed within 6 months and that the typical progression from a benign, premalignant lesion to carcinoma occurs on the order of several years (6,24).
In order to determine the robustness of our results, we conducted a series of secondary analyses. First, to account for delays in definitive treatment of a suspicious lesion, we extended the time period for detected lesions to 1 year prior to diagnosis, and interval cancers were accordingly shortened to 36 months to 1 year prior to diagnosis. Second, because patients diagnosed with cancer during hospitalization may differ in clinical presentation and subsequent evaluation, we only considered outpatient colonoscopies. Third, to account for patients who had an index colonoscopy and later presented emergently without undergoing a second preoperative colonoscopy, we also included cases with only a colonoscopy 6–36 months prior to diagnosis in the interval cancer group. The reference group included patients with only a colonoscopy within 6 months of cancer diagnosis.
Analysis
The primary analysis focused on factors associated with the presence of interval colorectal cancers. Differences between these patients and those in the reference or detected group (colonoscopy only within 6 months of diagnosis) were compared using chi-square testing. Variables of interest included demographic factors (age group, gender, race), comorbidity score, previous diagnoses of diverticulosis or colorectal carcinoma-in-situ, and cancer stage, grade and location in the colon. Location was classified as proximal colon (cecum, ascending colon, hepatic flexure, transverse colon), distal colon (splenic flexure, descending colon, sigmoid colon and rectosigmoid junction) and rectum. Procedure characteristics included type of colonoscopy (diagnostic or polypectomy), facility type (inpatient, outpatient, ambulatory surgical center), and year of procedure. Year of diagnosis was also divided into three time intervals based on Medicare colonoscopy reimbursement policies: no coverage for screening (prior to 1998), screening colonoscopy in high risk beneficiaries (January 1998–June 2001) and universal screening colonoscopy (July 2001-). Because a number of SEER registries were added in 2000, we stratified the time period analysis according to membership in the original registries (SEER-9). Physician characteristics included specialty and volume of colonoscopy procedures in the noncancer Outpatient and Carrier files from 1991–2005. Using all colonoscopies from the noncancer sample from 1991–2005, we also included the endoscopist’s polypectomy rate and volume of colonoscopies. For most physicians with missing UPIN data, we were able to obtain specialty through the Medicare specialty code on the claim.
A hierarchical linear model was used to determine the odds of interval cancer with clustering of patients at the physician level. Independent variables included all predictors that were thought to be clinically relevant.
The data were obtained through a Data User Agreement from the National Cancer Institute and the protocol was approved by the Institutional Review Board at the Case Comprehensive Cancer Center.
Results
A total of 299,260 patients were initially identified from the SEER-Medicare database. Patients were excluded for the following reasons: Medicare eligibility based on end stage kidney disease or disability (n=21,268), prior cancer diagnosis (n=49,593), histology other than adenocarcinoma (n=9,170), carcinoma-in-situ at index diagnosis (n=12,117), colorectal cancer diagnosis prior to 1994 (n=34,619), age at diagnosis < 69 (n=42,753), enrollment in Medicare HMO or non-enrollment in Medicare Parts A and B (n=42,386), cancer diagnosis on autopsy or death certificate (n=597), no colonoscopy performed during the study period (n=19,201), only an incomplete colonoscopy performed (n=978), no colonoscopy performed within six months of cancer diagnosis (n=1,119), missing UPIN identifier (n=6,706), and a previous diagnosis of inflammatory bowel disease (n=914). For the primary analysis, our sample consisted of 57,839 patients, including 4,192 with a colonoscopy in the 6 to 36 month period prior to diagnosis and 53,647 with only a colonoscopy within 6 months of diagnosis. The patients with a colonoscopy in the 6–36 month period, which was considered to represent patients with interval cancer, accounted for 7.2%.
The demographic characteristics of the patients in the cohort are shown in Table 1. The mean age of the cohort was 78.9 years, 56.1% were female and 84.6% were Caucasian. Compared to others, those patients with interval cancers were somewhat older, less likely to be Asian and more likely to be African American. Patients with interval cancers had higher comorbidity scores and were more likely to have previous diagnoses of diverticulosis or colorectal carcinoma-in-situ. There was also a higher frequency of interval cancers in the most recent Medicare reimbursement period (after July 2001).
Table 1.
Patient, Geographic, Procedure and Facility Characteristics Comparing Patients with Interval Cancers (Colonoscopy 36-6 months) to Detected Cancers (Colonoscopy within 6 months)
| Characteristic | Overall Population (n=57,839) | Interval Cancers (n=4,192) | Detected Cancers (n=53,647) | P value |
|---|---|---|---|---|
| Patient Measures | ||||
| Age Group | ||||
| 69 – 74 | 16,533 (28.6%) | 1,107 (26.4%) | 15,426 (28.8%) | 0.003 |
| 75 – 79 | 15,744 (27.2%) | 1,214 (29.0%) | 14,530 (27.1%) | |
| 80 – 84 | 13,829 (23.9%) | 1,037 (24.7%) | 12,792 (23.8%) | |
| ≥ 85 | 11,733 (20.3%) | 834 (19.9%) | 10,899 (20.3%) | |
| Gender | ||||
| Male | 25,406 (43.9%) | 1,821 (43.4%) | 23,585 (44.0%) | 0.51 |
| Female | 32,433 (56.1%) | 2,371 (56.6%) | 30,062 (56.0%) | |
| Race | ||||
| Caucasian | 48,920 (84.6%) | 3,584 (85.5%) | 45,336 (84.5%) | < 0.001 |
| African American | 3,957 (6.8%) | 322 (7.7%) | 3,635 (6.8%) | |
| Hispanic | 2,226 (3.9%) | 150 (3.6%) | 2,076 (3.9%) | |
| Asian or Pacific Islander | 2,483 (4.3%) | 117 (2.8%) | 2,366 (4.4%) | |
| Other/Unknown | 253 (0.4%) | 19 (0.4%) | 234 (0.4%) | |
| Comorbidity Score | ||||
| 0 | 35,438 (61.3%) | 2,203 (52.5%) | 33,235 (61.9%) | < 0.001 |
| 1 | 13,623 (23.6%) | 1,087 (25.9%) | 12,536 (23.4%) | |
| 2 | 5,166 (8.9%) | 484 (11.6%) | 4,682 (8.7%) | |
| ≥ 3 | 3,612 (6.2%) | 418 (10.0%) | 3,194 (6.0%) | |
| Diverticulosis | ||||
| No | 40,482 (70.0%) | 1,287 (30.7%) | 39,195 (73.1%) | < 0.001 |
| Yes | 17,357 (30.0%) | 2,905 (69.3%) | 14,452 (26.9%) | |
| Carcinoma in situ | ||||
| No | 56,301 (97.3%) | 4,018 (95.9%) | 52,283 (97.5%) | < 0.001 |
| Yes | 1,538 (2.7%) | 174 (4.1%) | 1,364 (2.5%) | |
| Cancer Characteristics | ||||
| Cancer Stage | ||||
| I | 14,701 (25.4%) | 1,323 (31.6%) | 13,378 (24.9%) | < 0.001 |
| II | 16,915 (29.3%) | 1,121 (26.7%) | 15,794 (29.5%) | |
| III | 13,119 (22.7%) | 929 (22.2%) | 12,190 (22.7%) | |
| IV | 6,950 (12.0%) | 362 (8.6%) | 6,588 (12.3%) | |
| Unknown | 6,154 (10.6%) | 457 (10.9%) | 5,697 (10.6%) | |
| Grade | ||||
| Well or moderately Differentiated | 42,002 (72.6%) | 2,989 (71.3%) | 39,013 (72.7%) | 0.08 |
| Poorly differentiated | 10,412 (18.0%) | 808 (19.3%) | 9,604 (17.9%) | |
| Undifferentiated or Unknown | 5,425 (9.4%) | 395 (9.4%) | 5,030 (9.4%) | |
| Cancer Location | ||||
| Proximal colon | 28,721 (49.7%) | 2,851 (68.0%) | 25,870 (48.2%) | < 0.001 |
| Cecum | 12,286 (21.2%) | 1,270 (30.3%) | 11,016 (20.5%) | |
| Ascending colon | 9,543 (16.2%) | 911 (21.7%) | 8,504 (15.9%) | |
| Hepatic flexure | 3,004 (5.1%) | 280 (6.7%) | 2,677 (5.0%) | |
| Transverse colon | 4,134 (7.0%) | 390 (9.3%) | 3,673 (6.8%) | |
| Distal colon | 18,740 (32.4%) | 819 (19.5%) | 17,921 (33.4%) | |
| Splenic flexure | 1,494 (2.6%) | 92 (2.2%) | 1,402 (2.6%) | |
| Descending colon | 2,145 (3.7%) | 117 (2.8%) | 2,028 (3.8%) | |
| Sigmoid colon | 10,809 (18.7%) | 456 (10.9%) | 10,353 (19.3%) | |
| Rectosigmoid | 4,292 (7.4%) | 154 (3.7%) | 4,138 (7.7%) | |
| Rectum | 9,332 (16.1%) | 434 (10.4%) | 8,898 (16.6%) | |
| Unspecified | 1,046 (1.8%) | 88 (2.1%) | 958 (1.8%) | |
| Medicare Reimbursement Policy Change | ||||
| Before January 1998 | 12,358 (21.4%) | 775 (18.5%) | 11,583 (21.6%) | <0.001 |
| January 1998 – July 2001 | 16,699 (28.9%) | 1,107 (26.4%) | 15,592 (29.1%) | |
| After July 2001 | 28,782 (49.8%) | 2,310 (55.1%) | 26,472 (49.3%) | |
| Procedure and Facility Characteristics | ||||
| Type of Colonoscopy | ||||
| Polypectomy | 24,690 (42.7%) | 2,283 (54.5%) | 22,407 (41.8%) | < 0.001 |
| Diagnostic | 33,149 (57.3%) | 1,909 (45.5%) | 31,240 (58.2%) | |
| Facility Type | ||||
| Inpatient | 18,727 (32.4%) | 1,227 (29.3%) | 17,500 (32.6%) | < 0.001 |
| Outpatient | 29,359 (50.7%) | 2,200 (52.5%) | 27,159 (50.6%) | |
| Ambulatory surgical center | 8,141 (14.1%) | 647 (15.4%) | 7,494 (14.0%) | |
| Others | 1,612 (2.8%) | 118 (2.8%) | 1,494 (2.8%) | |
| Physician Specialty | ||||
| Gastroenterology | 34,221 (59.2%) | 2,234 (53.3%) | 31,987 (59.6%) | < 0.001 |
| Colorectal surgery | 2,174 (3.8%) | 170 (4.1%) | 2,004 (3.7%) | |
| General surgery | 6,253 (10.8%) | 514 (12.3%) | 5,739 (10.7%) | |
| Internal medicine | 3,304 (5.7%) | 279 (6.7%) | 3,025 (5.6%) | |
| Family practice | 4,908 (8.5%) | 352 (8.4%) | 4,556 (8.5%) | |
| Other | 3,990 (6.9%) | 342 (8.2%) | 3,648 (6.8%) | |
| Unknown | 2,989 (5.2%) | 301 (7.2%) | 2,688 (5.0%) | |
| Colonoscopy Volume from Noncancer Sample | ||||
| 1 – 48 | 14,806 (25.2%) | 1,055 (23.5%) | 13,751 (25.3%) | < 0.001 |
| 49 – 85 | 14,649 (24.9%) | 1,098 (24.5%) | 13,551 (25.0%) | |
| 86 – 140 | 14,540 (24.8%) | 1,085 (24.2%) | 13,455 (24.8%) | |
| ≥ 141 | 14,758 (25.1%) | 1,247 (27.8%) | 13,511 (24.9%) | |
| Polypectomy Rate (%) from Noncancer Sample | ||||
| 0 – 0.24 | 14,453 (25.0%) | 1,151 (27.5%) | 13,302 (24.8%) | <0.001 |
| 0.24 – 0.33 | 14,499 (25.1%) | 1,066 (25.4%) | 13,433 (25.0%) | |
| 0.33 – 0.43 | 14,415 (24.9%) | 1,014 (24.2%) | 13,401 (25.0%) | |
| ≥ 0.43 | 14,472 (25.05) | 961 (22.9%) | 13,511 (25.2%) | |
Compared to patients in the detected group, patients with interval cancers were more likely to have tumors that were earlier stage. There were significant site differences, with proximal colon tumors much more common in the interval cancer group (Figure 1). Overall, the proportion of interval cancers was 9.9% in the proximal colon, 4.4% in the distal colon and 4.6% in the rectum. Of note, although the prevalence of diverticulosis was similar among interval cancers in each location, the prevalence compared to detected cancers was disproportionately high in the distal colon or rectum (66.5% vs. 20.3% distal, 70.0% vs. 20.3% rectum, 70.0% vs. 51.5% proximal).
Figure 1.
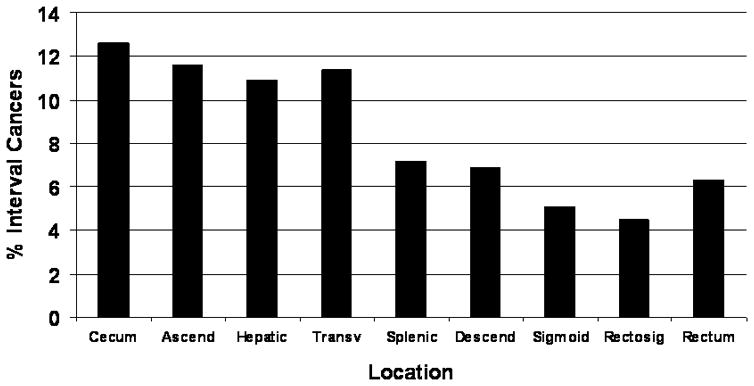
Site Distribution of Interval Colorectal Cancers.
We also examined procedural factors associated with interval cancers. Previous colonoscopies associated with polypectomy were more likely to be associated with interval cancers than diagnostic colonoscopy. Specialty type at initial colonoscopy was associated with interval cancer risk, with gastroenterologists having lower risk than primary care physicians, general surgeons or colorectal surgeons. There was an association of facility type, with procedures performed in hospital outpatient or ambulatory surgical centers more likely to be associated with interval cancers than inpatient procedures. The physician polypectomy rate, which was derived from the noncancer sample, was inversely associated with interval cancer risk, whereas procedure volume was positively correlated with risk.
In a multivariable model, we determined sociodemographic, clinical and procedure related factors associated with interval cancers (Table 2). Among sociodemographic characteristics, interval cancers were less frequent in patients ≥ 85 and more frequent in African Americans. Clinical factors associated with interval cancers included increasing comorbidity and previous diagnoses of diverticulosis or carcinoma-in-situ. In addition, interval cancers were more likely Stage I tumors. As in univariate analysis, there was a strong association of proximal cancer site and interval cancers. Interval cancers were more common in the most recent Medicare reimbursement period, but only among SEER registries that were included in the entire study period (SEER-9).
Table 2.
Patient, Geographic, Facility and Procedure Predictors of Interval Colorectal Cancers
| Characteristic | Adjusted Odds Ratio (95% CI) | P Value |
|---|---|---|
| Age Group | ||
| 69 – 74 (reference) | 1 | -- |
| 75 – 79 | 1.02 (0.93, 1.11) | 0.73 |
| 80 – 84 | 0.93 (0.85, 1.03) | 0.16 |
| ≥ 85 | 0.84 (0.76, 0.94) | < 0.001 |
| Gender | ||
| Female (reference) | 1 | -- |
| Male | 1.07 (0.99, 1.16) | 0.07 |
| Race | ||
| Caucasian (reference) | 1 | -- |
| African American | 1.24 (1.09, 1.41) | 0.001 |
| Hispanic | 0.97 (0.81, 1.16) | 0.73 |
| Asians or Pacific Islander | 0.93 (0.76, 1.13) | 0.45 |
| Other/Unknown | 1.03 (0.63, 1.70) | 0.90 |
| Comorbidity score | ||
| 0 | 1 | -- |
| 1 | 1.21 (1.11, 1.31) | < 0.001 |
| 2 | 1.43 (1.28, 1.60) | < 0.001 |
| 3+ | 1.89 (1.68, 2.14) | < 0.001 |
| Diverticulosis | ||
| No (reference) | 1 | -- |
| Yes | 6.00 (5.57, 6.46) | < 0.001 |
| Carcinoma-in-situ | ||
| No (reference) | 1 | -- |
| Yes | 1.61 (1.35, 1.93) | < 0.001 |
| Cancer Stage | ||
| I (reference) | 1 | -- |
| II | 0.76 (0.70, 0.84) | < 0.001 |
| III | 0.85 (0.77, 0.93) | < 0.001 |
| IV | 0.70 (0.62, 0.80) | < 0.001 |
| Unknown | 0.97 (0.86, 1.10) | 0.67 |
| Cancer Location | ||
| Proximal colon (reference) | 1 | -- |
| Distal colon | 0.42 (0.39, 0.46) | < 0.001 |
| Rectum | 0.47 (0.42, 0.53) | < 0.001 |
| Unspecified | 0.93 (0.73, 1.17) | 0.53 |
| Interaction of SEER 9 and Medicare Reimbursement Policy Change | ||
| SEER 9 | ||
| Before January 1998 (reference) | 1 | -- |
| January 1998 – July 2001 | 0.87 (0.77, 0.99) | 0.03 |
| After July 2001 | 1.22 (1.08, 1.36) | < 0.001 |
| Non-SEER 9 | ||
| Before January 1998 (reference) | 1 | -- |
| January 1998 – July 2001 | 0.92 (0.76, 1.13) | 0.43 |
| After July 2001 | 1.05 (0.86, 1.27) | 0.64 |
| Type of Colonoscopy | ||
| Diagnostic (reference) | 1 | -- |
| Polypectomy | 1.74 (1.62, 1.87) | < 0.001 |
| Facility Type | ||
| Inpatient (reference) | 1 | -- |
| Outpatient | 1.43 (1.32, 1.56) | < 0.001 |
| Ambulatory surgical center | 1.58 (1.34, 1.86) | < 0.001 |
| Others | 1.64 (1.33, 2.01) | < 0.001 |
| Physician Specialty | ||
| Gastroenterology (reference) | 1 | -- |
| Colorectal surgery | 1.16 (1.00, 1.35) | 0.05 |
| General surgery | 1.38 (1.17, 1.63) | < 0.001 |
| Family practice | 1.45 (1.16, 1.83) | 0.001 |
| Internal medicine | 1.42 (1.24, 1.62) | < 0.001 |
| Other | 1.22 (0.94, 1.59) | 0.14 |
| Unknown | 1.66 (1.43, 1.94) | < 0.001 |
| Polypectomy rate (%) by physician from non-cancer sample | ||
| 0 – 0.24 (reference) | 1 | -- |
| 0.24 – 0.33 | 0.84 (0.76, 0.93) | 0.001 |
| 0.33 – 0.43 | 0.80 (0.72, 0.89) | < 0.001 |
| ≥ 0.43 | 0.70 (0.63, 0.78) | < 0.001 |
| Colonoscopy volume by physician from non-cancer sample | ||
| 1 – 48 (reference) | 1 | -- |
| 49 – 85 | 1.10 (0.99, 1.22) | 0.07 |
| 86 – 140 | 1.17 (1.04, 1.31) | 0.01 |
| 141 + | 1.27 (1.13, 1.43) | < 0.001 |
Consistent with univariate analyses, interval cancers were more common in patients with previous polypectomy and with colonoscopies performed in locations other than an inpatient setting. There was also a higher likelihood of interval cancers among endoscopists with lower polypectomy rates or highest procedure volume. Finally, compared to gastroenterologists, there was a higher likelihood of interval cancers among other specialties.
In order to evaluate the robustness of results, we considered three other samples. First, to account for delays in definitive cancer diagnosis, the time period for interval cancers was changed to 36 months to 12 months prior to diagnosis, with colonoscopies performed within 12 months considered to have detected the cancer. Using this definition, 6.2% of cancers were considered to be interval lesions, and predictors and their magnitude of risk were similar as in the primary analysis. Second, because screening colonoscopy is typically an outpatient procedure, we excluded colonoscopies performed in the inpatient setting. Among 39,112 patients, 2,965 (7.6%) were considered to have interval cancers. Again, the major risk factors for interval cancers were consistent with other analyses. In this analysis, compared with hospital outpatient procedures, colonoscopies performed in ambulatory surgery centers were associated with higher odds of interval cancers (multivariate odds ratio (MOR) 1.26, 95% CI 1.08–1.47). Finally, if patients who only underwent colonoscopy during the 6–36 month period and not within 6 months of diagnosis were included in the interval group, the proportion of interval cancers decreased to 1.8%. Predictors of interval lesions were similar to other case definitions, though for previous polypectomy (MOR 1.16, 95% CI 1.00–1.35) and diverticulosis (MOR 1.97, 95% CI 1.73–2.26), the magnitude of risk was decreased.
Discussion
Although colonoscopy is generally considered to be the most accurate screening modality currently available, a subset of patients may develop colorectal cancer following a colonoscopy that was negative for carcinoma. These lesions, termed interval cancers, have largely been described in studies from Canada (10–13,15,16) and Germany (14), and have a higher prevalence in the proximal colon. Skeptics of findings from these previous studies have questioned the generalizability to U.S. practice. However, the current study, which is the first U.S. population-based analysis that included validation of cancer diagnoses through registry data, documented an interval cancer frequency of 7.2 %. Although risk factors for interval cancers are generally consistent with studies from other countries, the results highlight limitations of colonoscopy as currently practiced in the U.S.
The underlying reason for the consistent findings of a higher frequency of interval cancers in the right colon is unclear and is likely multifactorial. An unknown proportion of interval cancers could be attributed to microsatellite instability (MSI), which even in patients without Lynch syndrome (25), may be more prevalent (26). MSI cancers are associated with more rapid lesion growth and are known to be more common in the right colon. In addition, MSI cancers are more commonly associated with precursor sessile serrated adenomas, lesions that may be more difficult to detect at colonoscopy (27–29). Their presence has been postulated as a mechanism for the failure of colonoscopy to protect against proximal cancers (30). Another potential factor is inability of the endoscopist to reach the cecum. A previous study from Ontario, where billing requirements are to document each segment examined, reported an incomplete colonoscopy frequency of as high as 13% (31). Because in the U.S., reimbursement is potentially the same for advancing to any segment beyond the splenic flexure, comparable data are not available. Although there is a modifier code for an incomplete examination, it is used infrequently (32) and these patients were excluded.
In addition to proximal tumor location, we identified other predictors of interval cancers. Diverticulosis, which has also been found to be a risk factor in other studies (15–17), presumably impedes the endoscopist’s ability to visualize intervening mucosa and/or reach the cecum, and if documented in diagnosis codes, may be associated with more severe disease. In addition, older literature has documented the association between sigmoid diverticulosis and missed cancers on barium enema (33). Our finding of a higher risk for diverticulosis with interval cancers in the distal colon suggests that impaired visualization may be the predominant effect.
As defined in the analysis, prior polypectomy referred to the last procedure performed during the 36-6 month interval or < 6 month interval preceding cancer diagnosis. For patients with an initial colonoscopy shortly prior to diagnosis, it may have been diagnostic of the cancer. This was supported by the lower odds ratio in the secondary analysis that included interval cancers as only within 6–36 months of diagnosis. For patients with an earlier colonoscopy, an incompletely removed polyp may have progressed to cancer. Alternatively, receipt of polypectomy elsewhere in the colon may have served as predictor of subsequent cancer risk. The underlying mechanism for other predictors of interval cancer such as comorbidity is less intuitive. Comorbidity is associated with more frequent contact with the healthcare system and thus could provide more opportunity for detection of subclinical cancer. Alternatively, it could also be an indicator for more difficulty with bowel preparation. We also found lower odds with procedures performed in the inpatient setting, the reason for which is not clear, but may represent differences in the clinical presentation. Importantly, when inpatient cases were excluded, the results remained constant.
Our study documented the association of endoscopist specialty and colonoscopy metrics with risk of interval cancers. A recent study of interval cancers from Ontario (34) documented associations with a lower overall polypectomy rate and colonoscopy completion rate, as well as a non-gastroenterology or surgery specialty. A second recent Ontario based study (35) also found a higher likelihood of interval cancer following a negative colonoscopy if the examination was performed at a hospital by a non-gastroenterologist. Our study also documented an association of interval cancers with the endoscopist’s polypectomy rate, which has been suggested as an indirect measure of the adenoma detection rate (20,21). Given the differences from Canada in billing documentation, we were unable to accurately measure the colonoscopy completion rate.
A recent study from Manitoba also found a higher likelihood of colorectal cancer following a negative colonoscopy if the procedure was performed by a non-gastroenterologist (12). The similar findings are noteworthy despite differences in demographics of endoscopists in the U.S. versus Canada, where a much lower proportion are gastroenterologists (10–13,15,31). Also of note is that two recently published Medicare-based studies have found the prevalence of other potential quality measures such as polyp detection and removal rates (36) and need for repeat colonoscopy (37) to be inferior among non-gastroenterologists.
One potential method to increase lesion detection at colonoscopy is the use of newer imaging modalities (38). Unfortunately, the most commonly used technology, narrow band imaging, has not been shown to increase adenoma detection (39). However, other less commonly used techniques such as indigo carmine spraying, may increase the detection of nonpolypoid neoplasia, which is more common in the right colon and may be more likely to harbor carcinoma (40).
We recognize several limitations of the current study. First, the study was conducted in a cohort of older Medicare beneficiaries receiving care in fee-for-service arrangements. Thus, the generalizability of findings to other patient groups is unknown. Second, procedure related details such as size and morphology of polyps detected, quality of bowel preparation and ability to complete the examination to the cecum were not available. Third, we could not ascertain whether the follow up colonoscopy that diagnosed the cancer was a scheduled or unscheduled examination. In the former case, the endoscopist may have recognized the risk for subsequent cancer based on procedural factors or limited visualization and arranged for repeat colonoscopy. Fourth, specifics about colonoscopy, including use of polypectomy and physician specialty were obtained from administrative data. Although data are collected for billing purposes and not research, the completeness of Medicare claims for measuring colonoscopy use is thought to be relatively high (41). A recent study that compared Medicare claims to colonoscopy reports found a high sensitivity and specificity for a diagnosis of polyps as well as interventions that were performed (32). Fifth, our measures of endoscopist performance characteristics such as frequency of polypectomy were derived from Medicare beneficiaries alone and do not reflect colonoscopy in other patients. However, for other procedures such as cancer resection, there is a strong correlation between provider-specific volume in Medicare and non-Medicare patients (42). Sixth, given the large sample size, certain statistically significant differences may not have been clinically relevant. Seventh, the study was limited to patients who underwent colonoscopy prior to cancer diagnosis. In related work, we examined the nearly 20% of patients who did not undergo colonoscopy within six months of diagnosis (43). These patients were more likely to be elderly with multiple comorbidities, nursing home residents, presenting emergently and with late stage disease, and thus their exclusion may have biased the sample toward healthier patients. Finally, a subset of patients may have received part of their care, including colonoscopies, at a VA facility, and data on these procedures would not be available.
In conclusion, we have used population-based U.S. data to demonstrate a frequency of interval cancers following colonoscopy of 7.2%. The frequencies were particularly high in the proximal colon, which may be attributed to procedural and biologic factors. The findings emphasize the importance of meticulous inspection at the time of colonoscopy to detect precursor lesions. Moreover, if the impact of colonoscopy on cancer prevention is to be maximized, quality metrics proposed by professional societies should be targeted (19).
Supplementary Material
Acknowledgments
Supported by the National Cancer Institute at the National Institutes of Health, R01 CA132862
References
- 1.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 2.U S. Preventive Services Task Force. Screening for colorectal cancer: recommendations and rationale. Ann Intern Med. 2002;137:129–31. doi: 10.7326/0003-4819-137-2-200207160-00003. [DOI] [PubMed] [Google Scholar]
- 3.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol. 2009;104:739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 4.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 5.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med. 1992;326:658–62. doi: 10.1056/NEJM199203053261002. [DOI] [PubMed] [Google Scholar]
- 6.Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology. 1987;93:1009–13. doi: 10.1016/0016-5085(87)90563-4. [DOI] [PubMed] [Google Scholar]
- 7.Haseman JH, Lemmel GT, Rahmani EY, Rex DK. Failure of colonoscopy to detect colorectal cancer. Gastrointest Endosc. 1997;45:451–5. doi: 10.1016/s0016-5107(97)70172-x. [DOI] [PubMed] [Google Scholar]
- 8.Pabby A, Schoen RE, Weissfeld JL, et al. Analysis of colorectal cancer occurrence during surveillance colonoscopy in the dietary Polyp Prevention Trial. Gastrointest Endosc. 2005;61:385–91. doi: 10.1016/s0016-5107(04)02765-8. [DOI] [PubMed] [Google Scholar]
- 9.Robertson DJ, Greenberg ER, Beach M, et al. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology. 2005;129:34–41. doi: 10.1053/j.gastro.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 11.Singh H, Turner D, Xue L, Targownik LE, Bernstein CN. Risk of developing colorectal cancer following a negative colonoscopy examination. JAMA. 2006;295:2366–73. doi: 10.1001/jama.295.20.2366. [DOI] [PubMed] [Google Scholar]
- 12.Singh H, Nugent Z, Mahmud SM, Demers AA, Bernstein CN. Predictors of colorectal cancer after negative colonoscopy: a population-based study. Am J Gastroenterol. 2010;105:663–73. doi: 10.1038/ajg.2009.650. [DOI] [PubMed] [Google Scholar]
- 13.Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128–37. doi: 10.1053/j.gastro.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 14.Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Hang U. Protection from right- and left-sided colorectal neoplasms after colonoscopy: a population-based study. J Natl Cancer Inst. 2010;102:89–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 15.Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96–102. doi: 10.1053/j.gastro.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Singh H, Nugent Z, Demers A, Bernstein CN. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol. 2010;105:2588–96. doi: 10.1038/ajg.2010.390. [DOI] [PubMed] [Google Scholar]
- 17.Singh A, Kuo YF, Freeman JL, et al. Colon cancer miss rates in Medicare population (abstract) Gastrointest Endosc. 2009;69:AB134–5. [Google Scholar]
- 18.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER- Medicare data. Med Care. 2002;40:IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 19.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2006;63:S16–S28. doi: 10.1016/j.gie.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Francis DL, Rodriguez-Correa DT, Buchner A, Harewood GC, Wallace M. Application of a conversion factor to estimate the adenoma detection rate from the polyp detection rate. Gastrointest Endosc. 2011;73:493–7. doi: 10.1016/j.gie.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Williams JE, Le TD, Faigel DO. Polypectomy rate as a quality measure for colonoscopy. Gastrointest Endosc. 2011;73:498–506. doi: 10.1016/j.gie.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data. Med Care. 2002;40 (Suppl):26–35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 23.Cooper GS, Payes JD. Receipt of colorectal testing prior to colorectal cancer diagnosis: a population-based study. Cancer. 2005;103:696–701. doi: 10.1002/cncr.20839. [DOI] [PubMed] [Google Scholar]
- 24.Brenner H, Hoffmeister M, Stegmaier C, Altenhofer L, Haug U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 810,149 screening colonoscopies. Gut. 2007;56:1585–9. doi: 10.1136/gut.2007.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindblom A. Different mechanisms of tumorigenesis of proximal and distal colon cancers. Curr Opin Oncol. 2001;13:63–9. doi: 10.1097/00001622-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology. 2006;131:1700–5. doi: 10.1053/j.gastro.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 27.Harvey NT, Ruszkiewicz A. Serrated neoplasia of the colorectum. World J Gastroenterol. 2007;13:3792–8. doi: 10.3748/wjg.v13.i28.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groff RJ, Nash R, Ahnen DJ. Significance of serrated polyps of the colon. Current Gastroenterology Reports. 2008;10:490–8. doi: 10.1007/s11894-008-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawkins N, Ward R. Sporadic colorectal cancers with microsatellite instability and their possible origin in hyperplastic polyps and serrated adenomas. J Natl Cancer Inst. 2001;93:1307–13. doi: 10.1093/jnci/93.17.1307. [DOI] [PubMed] [Google Scholar]
- 30.Markowitz SD, Itzkowitz SH, Berger BM. The effectiveness of colonoscopy in reducing mortality from colorectal cancer (letter) Ann Intern Med. 2009;150:816–7. doi: 10.7326/0003-4819-150-11-200906020-00013. [DOI] [PubMed] [Google Scholar]
- 31.Shah HA, Paszat LF, Saskin R, Stukel TA, Rabeneck L. Factors associated with incomplete colonoscopy: a population-based study. Gastroenterology. 2007;132:2297–2303. doi: 10.1053/j.gastro.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 32.Ko CW, Dominitz JA, Green P, Kreuter W, Baldwin LM. Accuracy of Medicare claims for identifying findings and procedures performed during colonoscopy. Gastrointest Endosc. 2011;73:447–53. doi: 10.1016/j.gie.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker SR, Alterman DD. False-negative barium enema in patients with sigmoid cancer and diverticulosis. Gastrointest Radiol. 1985;10:171–3. doi: 10.1007/BF01893095. [DOI] [PubMed] [Google Scholar]
- 34.Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011;140:65–72. doi: 10.1053/j.gastro.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Rabeneck L, Paszat LF, Saskin R. Endocopist specialty is associated with incident colorectal cancer after a negative colonoscopy. Clin Gastroenterol Hepatol. 2010;8:275–9. doi: 10.1016/j.cgh.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Ko CW, Dominitz JA, Green P, Kreuter W, Baldwin LM. Specialty differences in polyp detection, removal, and biopsy during colonoscopy. Am J Med. 2010;123:528–35. doi: 10.1016/j.amjmed.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 37.Ko CW, Dominitz JA, Green P, Kreuter W, Baldwin LM. Utilization and predictors of early repeat colonoscopy in Medicare beneficiaries. Am J Gastroenterol. 2010;105:2670–9. doi: 10.1038/ajg.2010.344. [DOI] [PubMed] [Google Scholar]
- 38.Rex DK. Update on colonoscopic imaging and projections for the future. Clin Gastroenterol Hepatol. 2010;8:318–21. doi: 10.1016/j.cgh.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Paggi S, Radaelli F, Amato A, et al. The impact of narrow band imaging in screening colonoscopy: a randomized controlled trial. Clin Gastroenterol Hepatol. 2009;7:1049–54. doi: 10.1016/j.cgh.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 40.Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027–35. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 41.Schenck AP, Klabunde CN, Warren JL, et al. Data sources for measuring colorectal endoscopy use among Medicare enrollees. Cancer Epidemiol Biomarkers Prev. 2007;16:2118–27. doi: 10.1158/1055-9965.EPI-07-0123. [DOI] [PubMed] [Google Scholar]
- 42.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–51. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 43.Cooper GS, Xu F, Koroukian S, Barnholtz-Sloan J, Schluchter M. Colorectal cancer diagnosis without colonoscopy: frequency and predictive factors (abstract). Presented at Digestive Disease Week; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


