SUMMARY
Early childhood is a period of dramatic change in sleep and emotion processing, as well as a time when disturbance in both domains are first detected. Although sleep is recognized as central in emotion processing and psychopathology, the great majority of experimental data have been collected in adults. We examined the effects of acute sleep restriction (nap deprivation) on toddlers’ emotion expression. Ten healthy children (7 females; 30–36 months) followed a strict sleep schedule (≥12.5 hrs time in bed/24 hrs) for 5 days before each of two randomly-assigned afternoon emotion assessments following Nap and No-Nap conditions (resulting in an 11-day protocol). Children viewed emotion-eliciting pictures (5 positive, 3 neutral, 3 negative) and completed puzzles (1 solvable, 1 unsolvable). Children’s faces were video-recorded, and emotion displays were coded. When sleep restricted, children displayed less confusion in response to neutral pictures, more negativity to neutral and negative pictures, and less positivity to positive pictures. Sleep restriction also resulted in a 34% reduction in positive emotion responses (solvable puzzle), as well as a 31% increase in negative emotion responses and a 39% decrease in confused responses (unsolvable puzzle). These findings suggest sleep is a key factor in how young children respond to their world. When sleep restricted, toddlers are neither able to take full advantage of positive experiences nor are they as adaptive in challenging contexts. If insufficient sleep consistently “taxes” young children’s emotion responses, they may not manage emotion regulation challenges effectively, potentially placing them at risk for future emotional/behavioral problems.
Keywords: Sleep Restriction, Napping, Emotion, Facial Coding, Early Childhood, Toddlers
INTRODUCTION
Sleep is increasingly recognized as a central factor in emotion processing and psychopathology (Walker and Harvey, 2010). Sleep deprivation produces decrements in mood, emotion reactivity, and hyper-vigilance, as well as amplified sensitivity to negative stimuli and reduced sensitivity to positive stimuli (Franzen et al., 2009, Gujar et al., 2011, Pilcher and Huffcutt, 1996). The abilities and skills (e.g., executive functioning, working memory, attention, response inhibition) underlying emotion processing are also impaired by prolonged wakefulness (Goel et al., 2009). Furthermore, functional imaging following sleep deprivation suggests a “disconnect” between the amygdala and medial prefrontal cortex – brain regions critical for the expression and regulation of emotion (Yoo et al., 2007). Finally, an established clinical literature indicates sleep disturbance is associated with affective disorder symptoms, which commonly improve after treatment of the sleep disorder (Peterson and Benca, 2006). The present study extends such findings by examining links between sleep and emotion processing in early childhood.
Sleep patterns change substantially during early childhood. Total 24-hour sleep decreases from about 13 hours at age two years to about 11.5 hours by age five (Crosby et al., 2005). The afternoon nap taken by almost all 2-year-olds is commonly dropped between 3 and 4 years of age, either naturally or because caregivers no longer provide children a nap opportunity (Weissbluth, 1995). Against this background of developmental change are reports of sleep problems (e.g., sleep onset delay, bedtime resistance, prolonged nocturnal awakenings) in about 25% of young children (Owens et al., 2000). Whether children obtain adequate sleep is determined by a host of intrinsic and extrinsic factors, such as early developmental changes in biologically-based homeostatic and circadian processes, contemporary family demands, daycare/preschool schedules, and chronic illness (Jenni and LeBourgeois, 2006). Insufficient sleep may result also from one of many common sleep problems, secular changes, or lack of opportunities to nap.
Similar to sleep, developmental shifts in the ability to process (i.e., express, regulate) emotion are also pronounced during early childhood. Maturing language abilities and the development of self allow for more sophisticated emotion processing (Brownell and Kopp, 2007). Increased displays of self-conscious emotions such as pride, shame, and guilt are also observed in toddlers (Lewis et al., 1992), indicating that children this age can reflect on their own behavior. The presence of such emotions can also predict future positive developmental outcomes (Kochanska et al., 2002). Most children begin to manage their emotions more independently by preschool and early school-age (Cole et al., 1994).
Toddlers’ independence, autonomy, and increased involvement in goal-oriented tasks facilitates their emotional investment in the task’s outcome, thus providing more opportunity to practice emotion processing skills (Jennings, 2004). Successfully completing challenging tasks makes it more likely for a young child to experience positive emotions, such as joy, excitement, and pride. On the other hand, task failure may heighten a child’s frustration and experience of sadness, anger, worry/anxiety, or shame (Lewis et al., 1992). Confusion, a “knowledge emotion” associated with periods of active cognitive engagement or disequilibrium (Silvia, 2009), is commonly observed with greater problem-solving efforts and constructive learning (D’Mello et al., 2007). Despite substantial developmental achievements in early childhood, toddlers are far from reliable managers of their emotions. Based on prior work in adolescents and adults, we propose sleep restriction as one important yet relatively unexplored context that may tax children’s developing patterns of emotion processing.
Although a developmental framework for studying functional links between sleep and emotion was proposed over a decade ago (Dahl, 1996), the majority of published studies have been correlational. Such large-scale reports suggest associations between inadequate sleep (i.e., short nighttime sleep, sleep problems, sleep fragmentation) and internalizing and externalizing behavior problems in young children (Coulombe et al., 2010). Furthermore, preschoolers with sleep problems are more likely to have emotional disorders in later childhood and adolescence, even after accounting for stability of such problems and demographic variables (Gregory and O’Connor, 2002). Currently, few well-controlled experimental data on sleep and emotion links in young children exist. One quasi-experimental study found 14-month-old infants fatigued by lack of daytime sleep used fewer mature regulatory strategies and exhibited more distress when separated from their mother (Ross and Karraker, 1999). Another intervention report showed successful treatment of frequent/prolonged nighttime awakenings was associated with less irritability and negative behavior, as well as improved attention and social skills (Minde et al., 1994).
We propose early childhood is an important window for examining relations between sleep and emotion processing because both systems are undergoing rapid developmental change. The current study utilized an experimental design to test the effects of sleep restriction on young children’s emotion responses as assessed objectively with analysis of facial emotion expressions. Specifically, we examine the effects of daytime sleep restriction, or nap deprivation, because napping is prevalent in toddlers. This analysis was one part of a project examining the co-development and co-regulation of sleep, circadian, and emotion processes in young children.
METHODS
Recruitment and Screening of Participants
We recruited families of young children through flyers, website advertising, and personal contact at community events. Screening involved parents completing a telephone interview and questionnaires. Study inclusion required children to be 30- to 36-months-old and regularly following a biphasic sleep/wake schedule (nighttime sleep period of a least 10.5 hours and one daytime nap of at least 45 minutes time in bed) in which they fell asleep at least 3 days/week during their nap opportunity. We excluded children for: (a) daily/nightly cosleeping (e.g., with a parent, sibling, pet); (b) a bedtime/rise time sleep schedule varying more than 2 hours between weekdays and weekends; (c) travel beyond 2 time zones within 3 months prior to assessments; (d) medication use possibly affecting sleep, alertness, or the circadian system; (e) diagnosed sleep problems; (f) illness at the time of the assessments; (g) physical handicaps interfering with testing (e.g. blind, deaf), (h) developmental disabilities, (i) chronic medical conditions, infectious illnesses, lead poisoning, and head injury involving loss of consciousness; (j) pre-term or post-term delivery (term= 35–45 weeks); (k) low birth weight (<5.5 lbs); (l) scores in the clinically significant range (T>70) on the Child Behavior Checklist; and (m) a family history (first degree) of diagnosed narcolepsy, psychosis, or bipolar disorder.
For this 11-day protocol, 78 children were screened, 35 met criteria, 16 enrolled, and 11 completed the study. Incomplete assessments were due to children not napping on the day of the assessment, sickness, poor video quality, or study withdrawal. Analysis of data from the emotion eliciting pictures includes 9 children (1 child excluded due to religious practices; 1 child excluded for technical difficulties), and analysis of data from the puzzle task is from 10 children (1 child excluded due to protocol violation). All families signed an IRB-approved consent form. Parents received $25 in cash, and children received a $75 savings bond following study completion.
Participants
Participants were 10 healthy toddlers (7 females; 6 first-born; 8 Caucasian, 1 African-American, 1 mixed-race) aged 30- to 36-months (M=34; SD=1.7). Three attended full-time daycare, three had in-home childcare provided by a non-family member, and four were cared for exclusively by their parents.
Sleep Schedule Protocol and Training
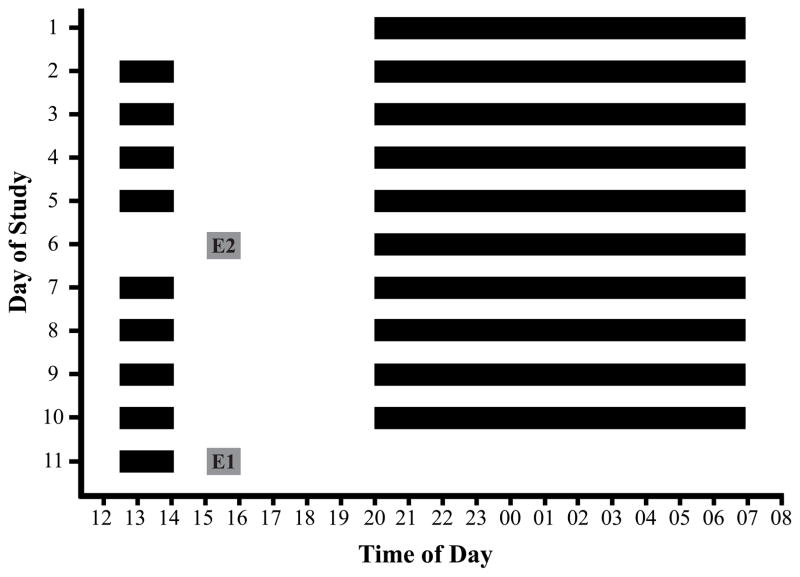
Children followed a strict daytime nap and nighttime sleep schedule for at least five days before each of two emotion assessments to minimize the influence of sleep restriction and to stabilize the circadian system (≥ 12.5 hours time in bed/24 hour day). Thus, the typical 11-day protocol involved 5 days on a strict sleep schedule before the first emotion assessment and then another 5 days on the sleep schedule before the second emotion assessment (Figure 1). Assessments were rescheduled after an additional 5 consecutive days on the sleep schedule if any of the following occurred during the protocol: (a) accidental nap; (b) bedtime or rise time deviating more than 15 minutes from sleep schedule; (c) illness; (d) use of medications affecting sleep and/or alertness; or (e) caffeine consumption. Researchers corresponded daily with parents to ensure compliance with study procedures.
Figure 1.
11-day sample protocol for a child following a strict sleep schedule with a 20:00 bedtime, a 7:00 rise time, and a 12:30–14:00 afternoon nap opportunity (12.5-hours time in bed/24 hour period). Solid black bars represent time in bed; white bars represent periods of wakefulness. Alternate forms of emotion assessments (E1=Nap; E2=No-Nap) occurred after 5 days on this stable sleep schedule. Emotion assessments took place 1 hour after regularly scheduled nap rise time.
Families participated in at least two in-home trainings designed to gradually introduce study procedures. These visits included attaching the actigraph to the child (Tyvek wristband; DuPont, Wilmington, DE, USA), briefing parents on actigraph care/use and in completing the sleep diary, and having children wear headphones while watching a series of “training” pictures on a notebook computer screen. After each visit, children were rewarded with “play time” with researchers and small gifts (e.g. stickers).
Emotion Assessments
Emotion assessments were administered in the child’s home under two different conditions: (a) Nap (baseline – child napped before assessment) and (b) No-Nap (sleep restriction – child did not nap before assessment). To reduce the likelihood of sleep inertia effects on children’s emotion responses, the protocol start time for both conditions was 1 hour past the child’s scheduled nap rise time. The Nap and No-Nap conditions were randomly determined and occurred on non-consecutive study days. Each 30-min emotion assessment used tasks appropriate for 2–3 year-olds. The emotion elicitation protocol was always conducted first, and the challenge protocol conducted second (see below). Because the study was a repeated measures design, assessments included alternate forms of emotion-eliciting tasks not subject to carry over or learning effects.
Upon arrival in the home, we confirmed compliance with the study rules and the sleep schedule by inspecting the child’s wrist actigraphy data, reviewing sleep diary entries, and questioning parents (see below). We then constructed the home-based emotion assessment context, including a child-sized table and chair, a notebook computer placed 10 inches from the front edge of the table, and a video camera positioned to capture the child’s body from the chest up. Children were rewarded with a small non-monetary gift after assessment completion.
Emotion Elicitation Protocol
The first task utilized a computer avatar Bunny to instruct children to remain seated and view a series of pictures on a computer screen. Children listened via headphones to help eliminate noise. Assessments contained alternate forms of 11 emotion-eliciting pictures with comparable valences within and across forms (5 positive, 3 neutral, 3 negative; Table 1) and comparable levels of arousal within valence, as defined by the International Affective Picture System (9=highly positive to 1=highly negative valence; 9=high arousal to 1=low arousal;(Lang et al., 1997). Although the IAPS has not been used extensively with young children, we chose stimuli from this collection because the valence and arousal of most images have been rated and evaluated with other age groups, including school-age children. Also, in previous research with a large sample of 4-year-olds, we found that children responded in predictable ways (positive and negative emotion expressions) to IAPS stimuli that were rated as clearly positive or negative in valence, yet were developmentally appropriate (e.g., shark for negative image, baby for positive image, dustpan for neutral image) and not too distressing. In the current study, the mean valence ratings (across forms) were 8.38 for positive images, 3.17 for negative images, and 5.90 for neutral images. Arousal levels were rated similarly within valence, and as with other studies, arousal ratings of neutral images were lower than for more positive or negatively-valenced images (Table 1). Picture segments were summarized for analyses as follows: Positive, Neutral, and Negative (aggregated across each picture valence). To indicate the range of emotion displays in response to each picture valence, we computed the number of slides of each valence that elicited positive, negative, or confused responses from children.
Table 1.
International Affective Picture System (IAPS) stimuli (description, number, valence, arousal) used in alternate forms of emotion assessments.
| Form 1 Pictures | Form 2 Pictures | ||||||
|---|---|---|---|---|---|---|---|
| POSITIVE | Number | Valence | Arousal | POSITIVE | Number | Valence | Arousal |
| 3 Puppies | 1710 | 8.69 (0.86) | 5.61 (3.55) | 2 Bunnies | 1750 | 8.67 (0.79) | 5.36 (3.34) |
| Dolphins | 1920 | 8.64 (1.22) | 6.25 (3.21) | Baby | 2070 | 8.51 (1.15) | 5.71 (3.21) |
| Boy/ice-cream | 2650 | 8.44 (1.28) | 6.22 (3.13) | Fireworks | 5910 | 7.47 (2.13) | 6.40 (3.12) |
| Space Shuttle | 5450 | 7.89 (1.94) | 6.64 (3.19) | Candy | 7400 | 8.50 (1.25) | 5.94 (3.31) |
| Ice Cream | 7330 | 8.39 (1.73) | 6.42 (3.02) | Roller Coaster | 8490 | 8.53 (1.06) | 7.50 (2.52) |
| NEUTRAL | NEUTRAL | ||||||
| Dustpan | 7040 | 5.44 (2.05) | 2.51 (2.28) | Flowers | 5020 | 6.43 (2.24) | 3.31 (2.72) |
| Book | 7090 | 5.97 (2.18) | 3.11 (2.94) | Fork | 7080 | 5.64 (2.28) | 2.31 (2.19) |
| Fire Hydrant | 7100 | 6.06 (1.90) | 2.94 (2.54) | Umbrella | 7150 | 5.83 (1.86) | 2.75 (2.70) |
| NEGATIVE | NEGATIVE | ||||||
| Shark | 1930 | 3.91 (2.51) | 7.71 (1.95) | Snake | 1120 | 3.92 (1.93) | 6.58 (2.60) |
| Smoke* | 9280 | 2.80 (1.54) | 4.26 (2.44) | Garbage* | 9290 | 2.88 (1.52) | 4.40 (2.11) |
| Ruins* | 9470 | 3.05 (1.51) | 5.05 (1.98) | Ship* | 9600 | 2.48 (1.62) | 6.46 (2.31) |
Note:
indicates valence and arousal were not available for children; reported values are from adults.
Challenge Protocol
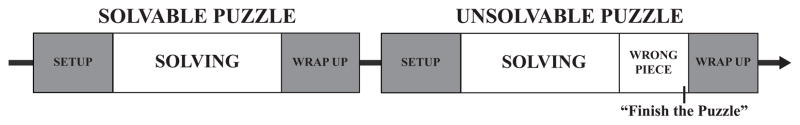
The second set of tasks included two age-appropriate puzzles (1 solvable, 1 unsolvable). The solvable puzzle was designed to elicit positive emotional responses by providing children with an opportunity for task completion. The unsolvable puzzle included one incorrect piece designed to prevent task completion and to elicit negative emotional responses. The order of puzzles was solvable followed by unsolvable; alternate forms were used for Nap and No-Nap conditions.
Figure 2 presents the sequence of puzzles and segments in the challenge protocol. For the Setup segment of both puzzles, children were told, “Here is a picture of a puzzle. Now it’s your turn to do a puzzle just like this one.” Children looked at the picture for four seconds. Then the puzzle was presented, marking the beginning of the Solving segment. After children completed the solvable puzzle they were told “Good job finishing the puzzle. You get a sticker because you finished the puzzle” (solvable puzzle “Wrap Up” segment). The unsolvable puzzle included one incorrect piece. The Solving segment for the unsolvable puzzle denoted the period when children were actively solving the puzzle. When only the incorrect piece remained an electronic marker was inserted to denote the unsolvable puzzle Wrong Piece segment. When children stopped attempting to complete the task they were prompted to “Finish the puzzle.” We analyzed data during the five seconds following this prompt (unsolvable puzzle Finish the Puzzle segment). Finally, children were told they would not receive a reward (sticker) because they did not finish the puzzle (unsolvable puzzle Wrap-up segment). While both puzzles included Setup and Wrap Up segments, the key segments analyzed in this study included the “Solving” segment from the solvable puzzle and the “Solving,” “Wrong Piece,” and “Finish the Puzzle” segments from the unsolvable puzzle (Figure 2). Because the challenge protocol assesses overall emotion reactivity in a lengthy social interaction, we computed the percent time children displayed emotion states (discrete and composite).
Figure 2.
Sequence of puzzles and puzzle segments in the emotion challenge protocol.
Measures
Children’s Sleep Habits Questionnaire
The Children’s Sleep Habits Questionnaire (CSHQ) is a 33-item parent-report instrument. Items are rated on a 3-point scale (usually, sometimes, rarely), with higher scores indicating more problematic sleep. The CSHQ Total Scale adequately discriminates between children with and without sleep problems (Owens et al., 2000).
Child Behavior Checklist
The Child Behavior Checklist (CBCL 1½-5) is a pencil-and-paper 99-item parent-report scale assessing early childhood emotional and behavioral problems, yielding Internalizing and Externalizing scores. Standardized T-scores are categorized as within normal limits (T<60), at-risk (T= 60–69), or clinically significant (T ≥70). The CBCL 1½-5 has adequate reliability (test-retest, multi-informant) and validity for clinical instruments (Achenbach and Rescorla, 2000).
Sleep Diary
Parents completed a 26-item sleep diary on each study day. Evening questions documented children’s daily events, mood, stress level, nap times, caffeine or medicine intake, actigraph off times, bedtime, lights-out time, and activities before bedtime. Morning questions documented children’s night awakenings, reasons for disturbed sleep, sleep quality, and wake time.
Actigraphy
Actigraphy is a noninvasive tool for estimating sleep patterns under non-laboratory conditions (Acebo and LeBourgeois, 2006). The actigraph (model AW2) was worn on the child’s non-dominant wrist and provided continuous recordings of sleep/wake states by measurement of motor activity (MiniMitter Company, Bend, OR, USA). Actiware-Sleep V5.02 software processed 1-minute actigraph epochs for sleep and wake from activity levels produced in the surrounding 2-minute period (medium sensitivity). This algorithm was applied to portions of the record identified as sleep through a combination of diary reports and actigraph event markers at “lights-out” and “lights-on.” In comparison to videosonography in young children, this sleep-wake algorithm shows high overall epoch-by-epoch agreement (94%) and is excellent in detecting sleep (sensitivity=97%); however, it overestimates wake during the sleep period (specificity=24%)(Sitnick et al., 2008). Sleep periods were excluded when the (a) actigraph was off for all/part of the sleep period, (b) concurrent diary report was not available, and (c) sleep period included external motion (e.g., sleeping in a car). For each sleep period, three actigraph variables were derived: (a) time in bed (sleep opportunity)– minutes from lights-out to lights-on; (b) sleep period (duration) – minutes from sleep start (first of three consecutive minutes scored as sleep after lights-out) to sleep end (last of 5 consecutive minutes scored as sleep before lights-on); and (c) sleep efficiency (quality) – % of sleep epochs between sleep start and sleep end time. In this study, we used actigraphy to verify sleep schedule compliance and to assess whether children’s sleep opportunity, duration, and quality differed during the 5 days prior to each emotion assessment.
Observational Coding of Emotion Displays
We videotaped children during emotion assessments for later coding by trained researchers (blind to condition) using The Observer XT software (Noldus Technologies, 2007). We used a modified version of the AFFEX coding system (Izard and Dougherty, 1980), to produce a second-by-second record of the child’s affective state. We coded the following discrete emotion expressions: Joy, Interest/Excitement, Pride, Sadness, Anger, Worry/anxiety, Disgust, Shame, Neutral (default state) and Unscoreable (e.g., child is off-camera). Because the protocol places children in an uncertain situation requiring high cognitive load, we also coded the complex (knowledge) emotion expression “Confused.” Reliability was assessed based on a sample of independently-coded tapes (n=6). Between-coder correlations for emotion codes were uniformly high, ranging from r=.92 to r=1.00. Because certain emotions occurred infrequently (e.g., anger, disgust), we created composite variables representing the percent time in a Positive Emotion state (sum of joy, interest/excitement, pride), percent time in a Confused state (cognitively engaged, yet uncertain), and percent time in a Negative Emotion state (sum of sadness, anger, worry/anxiety, and disgust). We analyzed these composite variables, as well as each specific (discrete) emotion expression that was originally coded (e.g., joy, pride), to determine the effects of sleep restriction on overall emotion valence (Positive/Negative), as well as each individual emotion.
Research Questions and Hypotheses
We examined whether children’s emotion responses to the emotion elicitation and challenge protocols varied as a function of acute sleep restriction. We predicted children would show (a) fewer positive emotional displays, (b) fewer confused displays, and (c) more negative emotional displays in the No-Nap than in the Nap condition.
Analysis Plan
All analyses were performed with the PASW Statistics Package 17.0 (SPSS Inc., Chicago, IL). For the emotion-eliciting protocol, repeated measures analyses (Nap versus No-Nap) of ordinal data (number of pictures eliciting an emotion response) were performed with Wilcoxon matched-pairs signed-ranks tests. For the challenge protocol, repeated measures analyses (Nap versus No-Nap) of continuous data (% time in emotion state during puzzle task segments) were performed with paired t-tests for discrete and composite emotions. Summary measures are presented as means (M) and standard deviations (SD). The significance level for analyses was set at .05 (one-tailed tests). Effect size in SD units was computed for % time in emotion state M comparisons (d =MNo-Nap - MNap/SDpooled).
RESULTS
Behavioral, Emotional, and Sleep Problem Status
All toddlers were below clinical cutoffs on CBCL T-Scores (M=41.45; SD=6.72). With regard to sleep problems, participants’ scores on all CSHQ subscales were well below the mean of published norms for a sleep-clinic-referred sample of children (Owens et al., 2000). Parental reports showed children took about six naps per week (M=4.18 on weekdays; M=1.64 on weekends), confirming they were following a biphasic sleep schedule.
Protocol Sleep Schedule Verification
We found no differences between children’s sleep schedule parameters (i.e., lights-out, rise time, time in bed, sleep period, sleep efficiency) during the first 4 days before each emotion assessment (Nap versus No-Nap conditions; Table 2). Because this study used nap deprivation to restrict children’s sleep, we expected significant differences in sleep parameters during the 24 hours before each emotion assessment. Although average bedtime and rise time on the night prior to assessments was the same, children spent less time in bed (116.8 minutes) and had shorter sleep periods (97.1 minutes) during the 24 hours before No-Nap than Nap emotion assessments. Sleep efficiency between conditions was the same.
Table 2.
Actigraphic sleep variables during study days 1–5 while following a strict sleep schedule and during the 24 hrs prior to each emotion assessment for Nap and No-Nap conditions.
| Study Days 1–4 | Nap | No-Nap | Statistics | ||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | t | d | p | |
| Night Lights-Out Time | 20:00 | 0:29 | 20:04 | 0:28 | .88 | .27 | .40 |
| Morning Rise Time | 6:53 | 0:27 | 6:55 | 0:26 | .32 | .10 | .76 |
| Nap Lights-Out Time | 13:28 | 0:47 | 13:28 | 0:51 | −.03 | .00 | .98 |
| Nap Rise Time | 15:15 | 0:35 | 15:20 | 0:42 | .39 | .12 | .71 |
| Time in Bed (minutes) | 759.84 | 37.00 | 762.73 | 34.81 | .42 | .13 | .69 |
| Sleep Period (minutes) | 687.70 | 43.57 | 692.22 | 34.41 | .54 | .17 | .60 |
| Sleep Efficiency (%) | 86.09 | 3.94 | 85.02 | 4.82 | −1.34 | .42 | .21 |
| 24 Hours Prior to Assessments | M | SD | M | SD | t | d | p |
| Night Lights-Out Time | 19:55 | 0:30 | 19:59 | 0:21 | −.81 | .15 | .44 |
| Morning Rise Time | 7:00 | 0:29 | 7:01 | 0:25 | −.05 | .00 | .96 |
| Nap Lights-Out Time | 13:19 | 0:42 | -- | -- | -- | -- | -- |
| Nap Rise Time | 15:12 | 0:52 | -- | -- | -- | -- | -- |
| Time in Bed (minutes) | 777.50 | 51.64 | 660.70 | 33.17 | −10.25 | 3.24 | .000 |
| Sleep Period (minutes) | 713.14 | 42.27 | 616.00 | 32.44 | −6.84 | 2.58 | .000 |
| Sleep Efficiency (%) | 87.69 | 6.51 | 84.80 | 8.52 | −.99 | .38 | .36 |
Sleep Restriction Effects on Emotion Responses
Emotion Elicitation Results
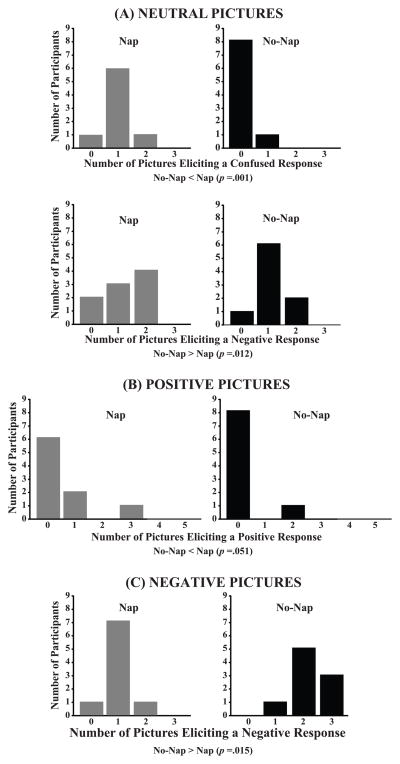
Child emotion responses to the emotion elicitation protocol varied as a function of sleep restriction. When viewing neutral pictures (Figure 3A), children in the No-Nap condition displayed confused responses to fewer slides than they did in the Nap condition (Z=−2.82; p=.001). They also showed negative displays to more neutral slides (Z=2.27; p=.012) in the No-Nap than in the Nap condition. The positive pictures (Figure 3B) did not elicit fewer positive displays from children in the No-Nap than in the Nap condition (Z=−1.63; p=.051). Finally, the negative pictures (Figure 3C), elicited more negative displays from children in the No-Nap than in the Nap condition (Z=−2.17; p=.015).
Figure 3.
Frequency histograms of the number of pictures eliciting emotion responses by picture valence (neutral, positive, and negative) in Nap and No-Nap conditions. Wilcoxon matched-pairs signed-ranks tests (p<.05).
Challenge Results
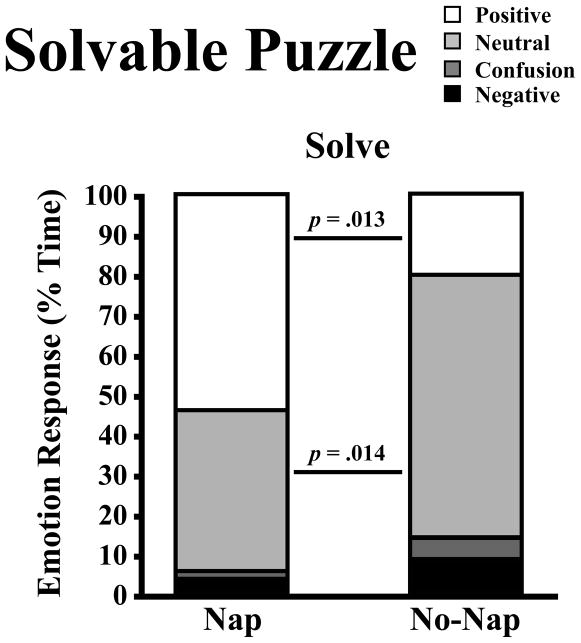
Emotion displays during the solvable and unsolvable puzzle tasks also differed in response to sleep restriction. During the Solving segment of the solvable puzzle, children spent 34% less time on average displaying positive emotion responses in the No-Nap than in the Nap condition (Table 3& Figure 4). Specifically, when children were given the opportunity to complete an age-appropriate puzzle, they showed less joy and pride when sleep restricted than when optimally rested (Table 3). The observed decrease in positive displays with nap deprivation was associated with a reciprocal increase in the percent time children displayed neutral or no emotion (e.g., as opposed to an increase in Negative [Nap: 40.2±38.3%; No-Nap: 65.7±28.0%; t(9)=3.09, d=.77, p=.006].
Table 3.
Percent time in discrete and composite emotion states during Solvable Puzzle and Unsolvable Puzzle Task segments (n=10).
| Solvable Puzzle Task (Solving Segment) | Nap | No-Nap | Statistics | ||||
|---|---|---|---|---|---|---|---|
| M | sd | M | sd | t | d | p | |
| Positive (Composite) | 54.6 | 40.0 | 20.5 | 28.9 | −2.66 | .98 | .013 |
| Joy | 41.8 | 37.8 | 17.6 | 28.8 | −2.26 | .72 | .025 |
| Pride | 5.9 | 7.4 | 1.3 | 2.6 | −2.25 | .45 | .025 |
| Interest/Excitement | 6.8 | 1.6 | 1.3 | 2.3 | −1.31 | .31 | .111 |
| Confused | 1.0 | 2.6 | 5.3 | 8.9 | −1.65 | .74 | .067 |
| Negative (Composite) | 4.1 | 7.4 | 8.5 | 13.9 | 0.84 | .41 | .212 |
| Anger | 0 | 0 | 0 | 0 | |||
| Sad | 0 | 0 | 0 | 0 | |||
| Worry/Anxiety | 4.1 | 7.4 | 8.5 | 13.9 | 0.84 | .41 | .212 |
| Shame | 0 | 0 | 0 | 0 | |||
| Disgust | 0 | 0 | 0 | 0 | |||
| Unsolvable Puzzle Task (Solving Segment) | Nap | No-Nap | Statistics | ||||
| M | sd | M | sd | t | d | p | |
| Positive (Composite) | 26.8 | 26.5 | 23.8 | 30.4 | −0.34 | .11 | .373 |
| Joy | 19.9 | 21.7 | 21.7 | 31.1 | 0.23 | .03 | .411 |
| Pride | 1.2 | 2.4 | 0 | 0 | −1.53 | .97 | .08 |
| Interest/Excitement | 5.8 | 14.0 | 2.1 | 6.2 | −1.38 | .36 | .101 |
| Confused | 22.6 | 22.0 | 7.5 | 9.0 | −1.94 | .97 | .042 |
| Negative (Composite) | 6.9 | 11.4 | 12.2 | 17.6 | 1.02 | .36 | .167 |
| Anger | 0.2 | 0.7 | 0 | 0 | −1.00 | .30 | .172 |
| Sad | 0 | 0 | 0 | 0 | |||
| Worry/Anxiety | 6.7 | 11.5 | 11.9 | 17.7 | 1.02 | .36 | .168 |
| Shame | 0 | 0 | 0 | 0 | |||
| Disgust | 0 | 0 | 0.2 | 0.8 | 1.00 | .63 | .172 |
| (Wrong Piece Segment) | Nap | No-Nap | Statistics | ||||
| M | sd | M | sd | t | d | p | |
| Positive (Composite) | 2.8 | 3.8 | 4.2 | 9.6 | 0.63 | .21 | .272 |
| Joy | 1.6 | 3.7 | 3.1 | 6.6 | 1.15 | .29 | .141 |
| Pride | 1.1 | 2.1 | 1.0 | 3.2 | −0.01 | .03 | .499 |
| Interest/Excitement | 0.1 | 0.2 | 0 | 0 | −1.00 | .06 | .172 |
| Confused | 34.3 | 23.9 | 6.0 | 7.0 | −3.66 | 1.83 | .003 |
| Negative (Composite) | 41.4 | 34.2 | 63.7 | 25.1 | 3.73 | .79 | .003 |
| Anger | 3.9 | 6.2 | 1.9 | 6.0 | −0.84 | .33 | .212 |
| Sad | .42 | .89 | 7.9 | 18.5 | 1.33 | .78 | .108 |
| Worry/Anxiety | 31.8 | 34.2 | 50.0 | 26.5 | 2.21 | .60 | .027 |
| Shame | 4.9 | 5.0 | 3.2 | 6.3 | −0.91 | .30 | .181 |
| Disgust | .35 | .73 | .69 | 2.2 | 0.45 | .24 | .330 |
| (Finish Puzzle Segment) | Nap | No-Nap | Statistics | ||||
| M | sd | M | sd | t | d | p | |
| Positive (Composite) | 3.0 | 6.3 | .13 | .41 | −1.40 | 0.85 | .097 |
| Joy | 1.3 | 4.2 | .13 | .41 | −0.89 | 0.52 | .199 |
| Pride | 1.6 | 5.1 | 0 | 0 | −1.00 | 0.64 | .172 |
| Interest/Excitement | 0 | 0 | 0 | 0 | |||
| Confused | 43.2 | 33.5 | 4.17 | 5.7 | −3.72 | 1.99 | .002 |
| Negative (Composite) | 39.7 | 28.9 | 70.8 | 20.5 | 3.58 | 1.26 | .003 |
| Anger | 8.3 | 14.7 | 3.6 | 11.3 | −1.11 | 0.37 | .148 |
| Sad | .23 | .71 | 4.9 | 10.6 | 1.48 | 0.82 | .086 |
| Worry/Anxiety | 29.5 | 33.9 | 58.9 | 28.1 | 2.82 | 0.95 | .010 |
| Shame | 1.7 | 5.2 | 3.5 | 7.7 | 0.58 | 0.28 | .288 |
| Disgust | 0 | 0 | 0 | 0 | |||
Figure 4.
Percent time children displayed neutral, confusion, and composite emotion responses (positive, negative) during the solving segment of the solvable puzzle in Nap and No-Nap conditions. One-tailed paired t-tests (p<.05).
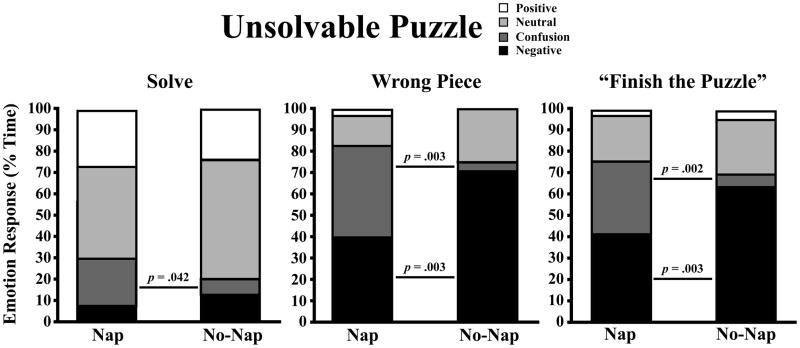
During all segments of the unsolvable puzzle, children spent less time displaying confusion (i.e., cognitive engagement) in the No-Nap than in the Nap condition (Table 3& Figure 5). That is, in the No-Nap condition, children spent 15% less time displaying confusion when solving the puzzle, 28% less time displaying confusion when only the wrong piece remained, and 39% less time displaying confusion when prompted to “finish the puzzle.” Children also spent more time displaying negative emotion in the No-Nap than in the Nap condition (22% increase in Wrong Piece segment; 31% increase in Finish the Puzzle segment). Specifically, when children were faced with a puzzle with no solution, they showed significantly more worry/anxiety when sleep restricted than when well rested (Table 3).
Figure 5.
Percent time children displayed neutral, confusion, and composite emotion states (positive, negative) during the solving, wrong piece, and “finish the puzzle” segments of the unsolvable puzzle in Nap and No-Nap conditions. One-tailed paired t-tests (p<.05).
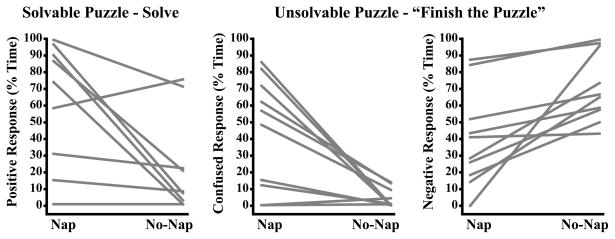
Finally, we observed wide variability (0%–100%) in the percent time children displayed positive emotion responses during the Solving segment of solvable puzzle (Figure 6A) in the Nap condition. In the No-Nap condition, over half the sample showed a decrease in positivity (four were dramatic), while the remaining children showed little-to-no change (one even showed a minor increase). Individual variation in confused displays during the Finish the Puzzle segment of the unsolvable puzzle in the Nap condition (Figure 6B) was also considerable (0%–86%), and the effect of sleep restriction was dramatic in the majority of participants. Finally, we observed substantial variability (0%–89%) in negative displays during the Finish the Puzzle segment of the unsolvable puzzle (Figure 6C). In the No-Nap condition, one participant had a dramatic increase in negative displays, one showed no change, and the majority showed small-to-moderate increases.
Figure 6.
Individual differences in children’s emotion responses in the Nap and No-Nap conditions.
DISCUSSION
To our knowledge, this is the first experimental study to examine acute sleep restriction effects on young children’s emotion responses. In our healthy, normally-developing, good-sleeping 30- to 36-month-olds, we found that merely eliminating a nap dampened children’s positive emotions, amplified their negative emotions, and decreased their confusion displays. Such responses were observed in a non-social context where children were presented with emotion-eliciting stimuli and in a more social context where children attempted to complete solvable and unsolvable puzzles with a familiar examiner. Sleep restriction effects on emotion displays were strongest in the social context. Results are discussed with regard to the role of sleep in optimal emotion processing and the importance of extending rigorous investigations of sleep-emotion links into early childhood.
Acute Sleep Restriction Affects Toddlers’ Emotion Expression
Our results support and extend previous findings regarding sleep deprivation effects on emotion. Adult studies identify increased negative mood and anxiety under sleep deprivation conditions (Pilcher and Huffcutt, 1996, Franzen et al., 2009, Gujar et al., 2011). When toddlers skipped just one regular afternoon nap of about 120 minutes and were faced with instructions to finish the unsolvable puzzle, we observed a 31% increase in expression of negative emotion, specifically worry/anxiety. Our puzzle task approximates situations similar to what children may encounter at school; it is worth considering whether children who obtain inadequate (daytime and/or nighttime) sleep are experiencing negative emotions, perhaps specifically worry/anxiety, in school settings. The fact that we see this so early in development is also notable, given that worry/anxiety is associated with poor school performance in later years, yet is a behavior easily missed in young children, particularly in busy classroom settings (Winsler and Wallace, 2002).
Missing a nap was also associated with a 34% decrease in positive emotion displays, specifically less joy and pride in the solvable puzzle task. In adults, napping has been associated with enhanced ratings of happiness (Gujar et al., 2011). Positive emotions are critical for many aspects of healthy psychological development, such as initiating and extending social interactions, as well as helping individuals to broaden their outlook and promote openness to new ideas (Fredrickson, 2001). In sum, sleepy children may view and respond to the world differently than children who are well-rested; they may not be able to take full advantage of positive experiences and may not be as able to manage challenges.
Children who were nap-deprived also spent 39% less time displaying confusion when prompted to finish the unsolvable puzzle task. Confusion has been described as a “knowledge emotion,” resulting when a stimulus is of high novelty and low comprehensibility (Silvia, 2009). Confusion may represent an opportunity for cognitive engagement and a need to retrieve information from the environment (D’Mello et al., 2007, Silvia, 2009). Young children likely experience many stimuli in their daily interactions with the environment that are not yet comprehensible, and confusion may help motivate them to find a solution. Here, children’s lack of sleep may have deprived them of the attentional and cognitive sharpness needed to register the challenge or motivation to address the situation. Considering broader implications, a lack of sleep in contexts that rely on young children’s mastery of new information (e.g., preschool) may have significant and potentially dire longer-term consequences.
Zohar’s (Zohar et al., 2005) cognitive energy theory states that when sleep-deprived, facing a challenge should result in increased negative affect because the energy required for managing that event is drained from lack of sleep. In contrast, in “goal-enhancing” contexts like our solvable puzzle task, sleep restriction would result in decreased positive emotion displays, because there are limited energy reserves available for positive engagement. Zohar (Zohar et al., 2005) showed that medical residents with disrupted sleep experienced increased negative affect in response to challenge and diminished positive affect in response to [objectively positive] events. Our findings that acute sleep restriction causes dampened positive emotion displays when positive responses are expected (solvable puzzle), as well as increased negative emotion and decreased confusion displays under challenging conditions (unsolvable puzzle) support this and extend this theory to a much younger age range.
Developmental Implications
Dahl (Dahl, 1996) proposed a developmental framework in which sleep, affect, and arousal are interrelated across behavioral, clinical, and neurological domains. Sleep problems in young children are often associated with poor emotional and behavioral adjustment, including internalizing and externalizing problems (Coulombe et al., 2010). Although the mechanisms of association are not yet entirely clear, the observed differences in emotion may be the result of one or more changes, for example in 1) the brain’s ability in registering emotion; 2) the brain’s ability to process and integrate that registered emotion; or in 3) the final outward behavioral expression of these registered, integrated emotions. The current study provides unique experimental evidence that inadequate sleep due to missing a daytime nap in toddlerhood leads to non-adaptive emotion processing. Over time, young children who are chronically sleep restricted may develop longer-lasting dysregulated emotion patterns. The emotion expressions we observed (i.e., increased negativity; decreased positivity) in response to simple nap deprivation mirror the symptoms of major depressive disorder; and indeed, difficulties with emotion regulation during early childhood can signal behavioral problems later in development (Cole et al., 1994).
Identifying factors underlying individual differences in the development of emotion processing in early childhood is a critical step in elucidating pathways to psychopathology and identifying avenues for early intervention. Early childhood represents a window during which the neural substrates influencing expression and regulation of emotion are in a state of rapid change, and obtaining adequate sleep may be essential to facilitate these plastic processes (Shonkoff and Philips, 2000). Our descriptive data lend credence to this general proposition. Although beyond the scope of the data we collected, others (e.g., Walker and Harvey, 2010) have speculated that sleep-emotion associations are driven by neurological mechanisms involved in both emotion processing and sleep regulation, such as those involving the amygdala and the prefrontal cortex regions.
Finally, another important developmental feature of this study is that we examined the impact of restricting daytime sleep, or napping. Even in adults, napping is associated with adaptive emotion processing (Gujar et al., 2011). The majority of toddlers do not sleep only at night (Weissbluth, 1995), thus, daytime sleep is likely essential for meeting their individual physiologic sleep need. Our results suggest that merely removing one nap from otherwise well-rested children produces substantial changes in young children’s emotion expression. Whether such responses would be observed as a consequence of other common types of acute sleep restriction (e.g., early morning awakenings, bedtime delay) or amplified when children experience chronic restriction of sleep, remain important unanswered questions. Furthermore, the preschool years represent a developmental window when children begin to give up their afternoon naps (Crosby et al., 2005, Weissbluth, 1995). For many young children, this transition is not smooth – they experience inconsistent nap schedules or do not nap enough, which may result in the increase of sleep debt across the day/week, delayed sleep onset, and/or disrupted nighttime sleep. Clearly, further longitudinal research is needed to identify sleep-related vulnerabilities in early childhood, as they may influence not only emerging behavioral styles and emotion processing but also affective neural development.
Limitations and Future Directions
Although this study used a well-controlled experimental design, it is not without limitations. First, although the IAPS is the best available set of validated emotion stimuli, the images we used to elicit emotion responses had not been previously used in children under the age of 4 years. The stimuli elicited mostly neutral expressions, perhaps in part be due to the passive nature of the task (i.e., watching a computer). Thus, it may be more useful in future work to examine the effects of sleep restriction on emotions in more naturalistic interactional contexts. Indeed, our puzzle tasks, which were designed to be more social and interactive in nature, elicited a greater range of emotion responses. Second, although observational coding is often the “gold standard” of emotion studies, particularly for young children who cannot report reliably on their emotional experience, internal sensations are also critical elements of emotion experience (Mauss et al., 2005). Reliance on differential emotions theory, which underlies coding and interpretation of facial expressions, limits our ability to access the inner experience of our participants. Third, while a recent report in adults suggests REM-rich naps are associated with increased daytime adaptive emotion functioning (Gujar et al., 2011), we did not obtain such data in our sample. Fourth, ideally we would have liked to balance the Nap and No-Nap conditions; however, this was not feasible in the context of the parent project. Seven of the 10 children in the final analysis received the Nap condition first. We found no order effect on any of the reported outcome measures, although we are likely underpowered to detect such effects. Finally, due to the relatively demanding nature of our experimental protocol, our sample was relatively small and consisted of very good sleepers, which is not typically the norm for children this age (Owens et al., 2000). This limits the full generalizability of our results. Future research should aim to include young children with sleep difficulties or emotional/behavioral problems to assess whether the present results generalize to a wider group of toddlers.
Acknowledgments
This research was supported by a grant from the National Institute of Mental Health (K01MH74643; LeBourgeois). Mary A. Carskadon, Ph.D. and Oskar Jenni, M.D. gave valuable advice in designing the study. We are very grateful to the children and families for their time and effort in making this study possible.
Footnotes
Financial Disclosure: The authors have nothing to declare.
References
- Acebo C, Lebourgeois MK. Actigraphy. Respir Care Clin N Am. 2006;12:23–30. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for ASEBA Preschool Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2000. [Google Scholar]
- Brownell CA, Kopp CB. Socioemotional Development in the Toddler Years: Transitions and Transformations. Guilford Press; New York: 2007. [Google Scholar]
- Cole PM, Michel MK, Teti LO. The development of emotion regulation and dysregulation: A clinical perspective. In: Fox NA, editor. The Development of Emotion Regulation: Biological and Behavioral Considerations, Monographs of the Society for Research in Child Development. 2–3. Vol. 59. University of Chicago Press; Chicago: 1994. pp. 73–100. Series 240. [PubMed] [Google Scholar]
- Coulombe JA, Reid GJ, Boyle MH, Racine Y. Concurrent associations among sleep problems, indicators of inadequate sleep, psychopathology, and shared risk factors in a population-based sample of healthy Ontario children. J Pediatr Psychol. 2010;35:790–9. doi: 10.1093/jpepsy/jsp097. [DOI] [PubMed] [Google Scholar]
- Crosby B, Lebourgeois MK, Harsh J. Racial differences in reported napping and nocturnal sleep in 2- to 8-year-old children. Pediatrics. 2005;115:225–32. doi: 10.1542/peds.2004-0815D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’mello SK, Taylor R, Graesser AC. Monitoring Affective Trajectories during Complex Learning. In: McNamara DS, Trafton JG, editors. Proceedings of the 29th Annual Cognitive Science Society. Cognitive Science Society; Austin, TX: 2007. pp. 203–08. [Google Scholar]
- Dahl RE. The regulation of sleep and arousal: development and psychopathology. Development and Psychopathology. 1996;8:3–27. [Google Scholar]
- Franzen PL, Buysse DJ, Dahl RE, Thompson W, Siegle GJ. Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biol Psychol. 2009;80:300–5. doi: 10.1016/j.biopsycho.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL. The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am Psychol. 2001;56:218–26. doi: 10.1037//0003-066x.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–39. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, O’connor TG. Sleep problems in childhood: a longitudinal study of developmental change and association with behavioral problems. J Am Acad Child Adolesc Psychiatry. 2002;41:964–71. doi: 10.1097/00004583-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Gujar N, Mcdonald SA, Nishida M, Walker MP. A role for rem sleep in recalibrating the sensitivity of the human brain to specific emotions. Cereb Cortex. 2011;21:115–23. doi: 10.1093/cercor/bhq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard CE, Dougherty LM. A system for identifying affect expression by holistic judgements (AFFEX) Instructional Resources Center; Newark, DE: 1980. [Google Scholar]
- Jenni OG, Lebourgeois MK. Understanding sleep-wake behavior and sleep disorders in children: the value of a model. Curr Opin Psychiatry. 2006;19:282–7. doi: 10.1097/01.yco.0000218599.32969.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings KD. Development of goal-directed behaviour and related self-processes in toddlers. International Journal of Behavioral Development. 2004;28:319–27. [Google Scholar]
- Kochanska G, Gross JN, Lin MH, Nichols KE. Guilt in young children: development, determinants, and relations with a broader system of standards. Child Dev. 2002;73:461–82. doi: 10.1111/1467-8624.00418. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Unpublished manual. NIMH Center for the Study of Emotion and Attention; 1997. International Affect Picture System (IAPS): Technical Manual and affective ratings. [Google Scholar]
- Lewis M, Alessandri SM, Sullivan MW. Differences in shame and pride as a function of children’s gender and task difficulty. Child Dev. 1992;63:630–8. [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, Mccarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5:175–90. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Minde K, Faucon A, Falkner S. Sleep problems in toddlers: effects of treatment on their daytime behavior. J Am Acad Child Adolesc Psychiatry. 1994;33:1114–21. doi: 10.1097/00004583-199410000-00007. [DOI] [PubMed] [Google Scholar]
- Owens JA, Spirito A, Mcguinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–51. [PubMed] [Google Scholar]
- Peterson MJ, Benca RM. Sleep in mood disorders. Psychiatr Clin North Am. 2006;29:1009–32. doi: 10.1016/j.psc.2006.09.003. abstract ix. [DOI] [PubMed] [Google Scholar]
- Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19:318–26. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- Ross CN, Karraker KH. Effects of fatigue on infant emotional reactivity and regulation. Infant Mental Health Journal. 1999;20:410–28. [Google Scholar]
- Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17:201–7. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Philips D. From Neurons to Neighborhoods: The Science of Early Child Development. National Academies Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- Silvia PJ. Looking Past Pleasure: Anger, Confusion, Disgust, Pride, Surprise, and Other Unusual Aesthetic Emotions. Psychology of Aesthetics, Creativity, and the Arts. 2009;3:48–51. [Google Scholar]
- Sitnick SL, Goodlin-Jones BL, Anders TF. The use of actigraphy to study sleep disorders in preschoolers: some concerns about detection of nighttime awakenings. Sleep. 2008;31:395–401. doi: 10.1093/sleep/31.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Harvey AG. Obligate symbiosis: sleep and affect. Sleep Med Rev. 2010;14:215–7. doi: 10.1016/j.smrv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Weissbluth M. Naps in children: 6 months-7 years. Sleep. 1995;18:82–7. doi: 10.1093/sleep/18.2.82. [DOI] [PubMed] [Google Scholar]
- Winsler A, Wallace GL. Behavior problems and social skills in preschool children: Parent-teacher agreement and relations with classroom observations. Early Education and Development. 2002;13:41–58. [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep--a prefrontal amygdala disconnect. Curr Biol. 2007;17:R877–8. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Zohar D, Tzischinsky O, Epstein R, Lavie P. The effects of sleep loss on medical residents’ emotional reactions to work events: a cognitive-energy model. Sleep. 2005;28:47–54. doi: 10.1093/sleep/28.1.47. [DOI] [PubMed] [Google Scholar]