Abstract
Background
Guided by the prediction of response-outcome theory of cognitive control (Alexander and Brown, 2010a), the present study examined reward-seeking medial prefrontal cortex (mPFC) activity as a common neuro-functional marker of excessive alcohol consumption, trait disinhibition, and reduced cognitive capacity; all of which have shown consistent patterns of covariation in previous psychometric research (e.g., Bogg and Finn, 2010).
Methods
A sample of 18-to-23-year-old university students with a heterogeneous prevalence of alcohol dependence was assessed with functional magnetic resonance imaging (fMRI) while completing a version of the Balloon Analogue Risk Task (Lejuez et al., 2002). A follow-back typical weekly alcohol consumption interview, self-report measures of trait disinhibition and IQ, and a complex span working memory task also were administered.
Results
Correlational region-of-interest analyses showed greater typical weekly alcohol consumption, greater trait disinhibition, and lower IQ were associated with greater reductions in mPFC activity during reward-seeking behaviors (successive inflation choices). The results also showed greater typical weekly alcohol consumption, greater trait disinhibition, and lower IQ were associated with greater increases in mPFC activity during reward-seeking outcomes (successive successful inflation outcomes). No significant relations with the measure of working memory were found.
Conclusions
The findings suggest mPFC activity during risk/reward appraisal and performance monitoring is a common neuro-functional feature of co-varying expressions of excessive alcohol consumption, trait disinhibition, and lower IQ.
Keywords: Alcohol, disinhibition, IQ, cognitive control, mPFC, fMRI
1. Introduction
Recent statistical modeling research has shown how excessive and illicit alcohol and drug use, a history of anti-social behaviors, and disinhibited dispositions can be organized under a coherent rubric of behavioral disinhibition, or a liability for externalizing tendencies and problems (Bogg and Finn, 2010; Krueger et al., 2007). Behavioral disinhibition is a coherent pattern of intemperate, irresponsible, and undercontrolled tendencies, with concomitant proclivities for various forms of short-term gratification (Iacono et al., 1999; Patrick et al., 2009). Moreover, the common variance among alcohol and co-morbid disinhibited tendencies and problems has been shown to be associated with various forms of reduced cognitive capacity, including intelligence and working memory (Finn et al., 2009).
In spite of the advances in depicting the covariation among alcohol use and co-morbid forms of behavioral disinhibition and its connection with reduced cognitive capacity, far less is known about the neural mechanisms of cognitive control that correspond to these patterns of diminished self-regulatory control. Optimal cognitive control involves an ongoing organization of smaller discrete behaviors in the service of larger goals and related actions (Norman and Shallice, 1986). As such, dysregulated cognitive control likely represents a key feature of the expression of excessive alcohol consumption, as well as co-varying expressions of disinhibition and reduced cognitive capacity (Iacono et al., 2008).
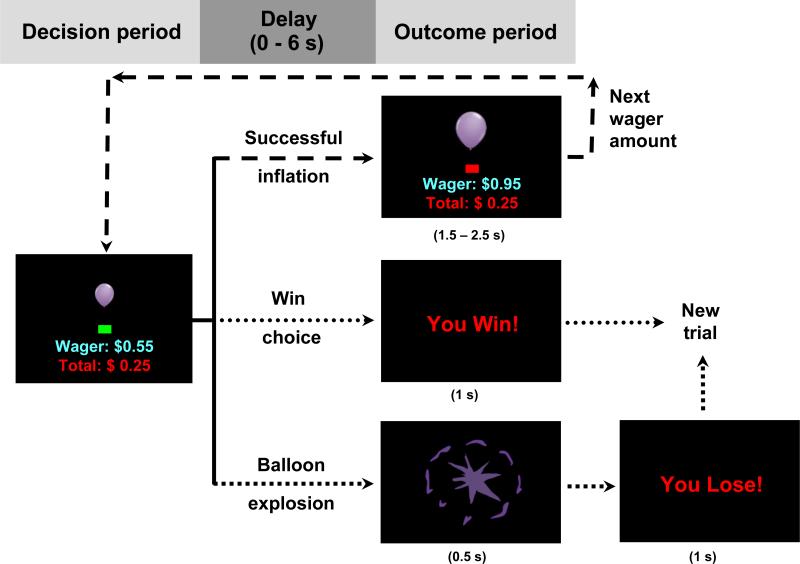
In the current study, the larger goal of the cognitive control system is to maximize winnings on a version of the Balloon Analogue Risk Task (BART; Lejuez et al., 2002; see Figure 1). BART performance has shown associations with substance use, disinhibited personality traits, and neural function during decision-making (Crowley et al., 2009; Lejuez et al., 2002; Skeel et al., 2007). The implementation of the BART in the current study pairs an increasing risk of explosion (whose exact probabilities are unknown to the participant) with an increasing wager amount for each successive inflation response (cf. Rao et al., 2008). The pairing of increasing risk and reward in a single behavioral response is particularly useful for examining cognitive control—the function of which is to negotiate competing signals from current environmental stimuli to facilitate goal-consistent behavior (Miller and Cohen, 2001).
Figure 1.
Overview of Balloon Analogue Risk Task (BART) procedure and presentation.
The prediction of response-outcome (PRO; Alexander and Brown, 2010a; Alexander and Brown, in press) theory was recently proposed as a synthesis of various accounts of the medial prefrontal cortex (mPFC) in cognitive control, including conflict, discrepancy, and error likelihood models (Brown and Braver, 2005; Scheffers and Coles, 2000; Yeung et al., 2004). Under PRO theory, the first role of mPFC is to appraise the costs and benefits of an action (Kennerley et al., 2009; Alexander and Brown, 2010b), with a preference for maximal rewards and the least effort or risk (Magno et al., 2006). In normal individuals, mPFC activity is weighted toward appraising and avoiding the risks of an action (Brown and Braver, 2007; Magno et al., 2006). The second role of the mPFC according to PRO theory is to identify differences between anticipated and actual action outcomes (Ito et al., 2003), including unexpected outcomes, and then use those differences to update future action responses. As it relates to individual differences in excessive alcohol consumption, trait disinhibition, and reduced cognitive capacity, PRO theory provides for two sets of mPFC-related hypotheses for reward-seeking decisions (inflation choices) and reward-seeking outcomes (explosions and successful inflations) during the BART.
First, most individuals should show a decrease in mPFC activity during choices to inflate as the probability of explosion increases parametrically. This decrease is expected under PRO theory because enacting successive inflation responses should involve a de-escalation of risk appraisal or even non-appraisal of risk. Heavier drinkers with greater disinhibition and reduced cognitive capacity should show an even greater decrease in mPFC activity as the probability of explosion increases parametrically and they, nevertheless, choose to inflate. These individuals are more likely to be biased toward the increasing wager amounts and be less able to gauge the risk of explosion over successive inflations. Lighter drinkers with less disinhibition and increased cognitive capacity should show a smaller decrease in mPFC activity, owing to a comparatively reduced bias for the increasing wager amounts and an increased ability to gauge the risk of explosion.
Second, and consistent with the performance monitoring role of the mPFC, most individuals should show an increase in activity during reward-seeking outcomes (explosions and successful inflations) as the probability of explosion increases parametrically. These increases are expected for two reasons: 1) Although explosions are more likely with successive inflations, they represent an increasingly aversive outcome, as the magnitude of the loss increases with the number of prior successful inflations; 2) Successful inflations, while being wholly consistent with the desired outcome, are less likely as the probability of explosion increases. PRO theory predicts greater activity when the prior probability of an actual event is low or the outcome is somewhat surprising or unexpected (Alexander and Brown, 2010a; Braver et al., 2001). Heavier drinkers with greater disinhibition and reduced cognitive capacity should show greater increases in mPFC activity during successful inflations and explosions because their stronger bias for anticipated reward does not sufficiently compensate for their reduced ability to appraise explosion risk, leading to an experience of greater unexpectedness for both explosions and successful inflations. Conversely, lighter drinkers with less disinhibition and increased cognitive capacity should show smaller increases in mPFC activity during successful inflations and explosions due to their increased ability to appraise explosion risk.
2. Methods
2.1 Participants
The recruitment procedure was designed to yield a sample of 18- to 23-year-old students from Indiana University that varied in its expression of alcohol consumption and disinhibited tendencies. Among college students, one of the most prevalent disinhibitory problems is excessive alcohol use (Hingson et al., 2005). As a result, study advertisements targeted a range of alcohol consumption behaviors, as well as related disinhibited tendencies (e.g., “drink modest amounts of alcohol and who do not take drugs” versus “heavy drinker”; “adventurous” and “impulsive,” versus “introverted” and “reserved”). This approach has been effective in recruiting participants that vary in alcohol use and disinhibited tendencies (Bauer and Hesselbrock, 1993; Finn et al., 2002). Participants were excluded if they [1] were not between 18 and 23 years of age, [2] could not read and/or speak English, [3] had never consumed alcohol, [4] reported any serious head injuries, [5] had a history of psychosis, and [6] were not enrolled in post-secondary coursework (i.e., university classes).
At the beginning of the assessment session, participants were asked about alcohol and drug use in the past 12 hours, the number of hours of sleep during the previous night, the most recent meal, and were given a breath alcohol test using an AlcoSensor IV (Intoximeters, Inc., St. Louis, MO). Participants were rescheduled if their breath alcohol level was greater than 0.000 %, if they consumed any drug within the past 12 hours, if they felt hung-over, or if they reported or appeared to be impaired, high, overly sleepy, or if they were unable to answer questions. All participants (N = 30) met safety requirements for magnetic resonance imaging and were paid $25, in addition to 25 % of banked winnings from the BART. Data for 3 participants were excluded due to an insufficient number of responses or outcomes needed to compute contrast regressors.
The resultant sample (N = 27) was sex-balanced (14 women) and mostly European-American/Caucasian (26), with a mean age of 20.11 years (SD = 1.37 years). Consistent with the recruitment goal of producing a sample with a heterogeneous representation of disinhibited tendencies, 11 participants (5 men, 6 women) met lifetime diagnostic criteria for alcohol dependence using the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; Bucholz et al., 1994) which uses criteria from the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV; American Psychiatric Association, 1994).
2.2 Measures
2.2.1 Balloon Analogue Risk Task (BART)
The implementation of the BART was guided, in part, by prior imaging work using the task (cf. Rao et al., 2008). Participants completed one 8-minute block outside of the scanner using a laptop to become familiar with the response options and task procedure. Participants were instructed to “inflate the balloon as much as possible without popping it” and to “maximize the number of points earned.” Twelve inflation responses were possible for a given trial, with a parametric increase in the probability of explosion over successive inflation responses (i.e., 0 % for $0.0; 2.1 % for $0.05; 4.2 % for $0.15; 6.3 % for $0.25; 14.6 % for $0.55; 23.9 % for $0.95; 31.3% for $1.45; 43.8 % for $2.05; 56.3 % for $2.75; 68.8 % for $3.45; 79.2 % for $4.25; 89.6 % for $5.15).
During fMRI scanning, participants completed two eight-minute blocks of the BART. At the beginning and end of each block, the screen displayed a fixation cross (“+”) for approximately 30 seconds to establish baseline activity. At the start of each trial, an image of a balloon, a square green decision cue, the current wager amount, and the banked winnings across all balloon trials through the most recently completed trial were displayed from the top to the bottom of the screen (see Figure 1). Participants had unlimited time to respond (to inflate the balloon or to take the accumulated wager amount for a given trial). After a response, there was an exponentially distributed delay of 0, 2, 4, or 6 seconds (with a 60% stopping probability after each 2 second interval up to 6 seconds). During the delay, no feedback was given, which allowed separate estimation of the blood-oxygen-level-dependent (BOLD) responses during (inflation) choice and (explosion or successful inflation) outcome periods (Dale, 1999). Following the delay, the outcome was presented. Total winnings were updated after outcome presentation. If the balloon inflated, then the decision cue turned red for an equiprobable 1500, 2000, or 2500 msec, during which further responses were disallowed. After that, the decision cue turned green again to indicate the next choice could be made. Following a win or explosion, the screen was cleared for an equiprobable 2000, 3000, or 4000 msec, after which a new balloon trial was presented.
2.2.2 Typical Weekly Alcohol Consumption Interview
Typical weekly alcohol consumption was determined using an interview during which the participant indicated the typical number and type of alcoholic drinks consumed each day of the week over the past three months. Typical was defined as being “more than half of the [day of week] over the past three months.” Alcohol use was quantified as the total typical number of standard drinks in a week over the past three months. According to skewness and kurtosis diagnostic tests, typical weekly alcohol use deviated from normality by small amounts. Blom transformations—which rank order, then z-transform raw scores—were used to reduce skewness and kurtosis close to zero. These transformed scores are used in all analyses.
2.2.3 Trait Disinhibition
The Sensation Seeking Scale disinhibition subscale (SSS-Dis; Zuckerman, 1979) consists of 10 forced-choice items assessing preferences for exciting, novel, or unrestrained activities or values (e.g., “I am not interested in experience for its own sake” versus “I like to have new and exciting experiences and sensations even if they are a little frightening, unconventional or illegal”). Due to the assessment of alcohol consumption in the present study, three items referencing drinking or drug use were dropped from the scale: “I often like to get high (drinking liquor or smoking marijuana)”; “Keeping drinks full is the key to a good party”; and “I feel best after taking a couple of drinks.”; α = .77.
2.2.4 Intelligence
Intelligence was measured with the Shipley Institute of Living Scale estimates of IQ (Zachary, 1986), a self-administered measure of intelligence that strongly correlates (median correlation r = .79) with the WAIS Full Scale IQ (Zachary, 1986).
Working Memory Capacity
Working memory functions of dual task ability, divided attention, and maintenance capacity were assessed with the Auditory Consonant Trigram test (ACT: Brown, 1958). The ACT was modified to include four and five nonsensical strings of consonants, in addition to the original three-string stimuli to increase the load on working memory. The dependent variable is the number of correct consonants recalled across all string lengths and delay intervals. This task taps divided attention and the strength of the maintenance/decay of the contents of working memory over time (Brown, 1958; Stuss et al., 1987).
2.3 fMRI Acquisition and Data Processing
Imaging data were collected using a Siemens Magnetom Trio 3.0 Tesla MRI scanner and a 32-channel head coil. Functional BOLD data were collected using a gradient echo T2*-weighted echo-planar imaging sequence with free induction decay for two blocks of 240 whole brain volumes (echo time [TE] = 25 ms, repetition time [TR] = 2000 ms, flip angle = 70°) with 35 axial slices (64 × 64 grid, 3.4 × 3.4 × 3.8 mm voxels, interleaved order, 3.8-mm thickness, 0-mm spacing). A structural scan was collected at the end of each session using three-dimensional MP-RAGE imaging using a high resolution T1-weighted imaging sequence, (TE = 2.67 ms, TR = 1800 ms, flip angle 9°) with nonselective excitation consisting of 192 sagittal slices (512 × 448 grid, 1.0 × 1.0 × 1.0 mm voxels, 1-mm thickness).
Preprocessing was done using SPM5 (Wellcome Trust Centre for Neuroimaging, 2005), except where otherwise specified. The structural scan was skull-stripped using FSL's BET2 with default parameters (Péchaud et al., 2006). Functional data were spike-corrected on a voxel-byvoxel basis to reduce the impact of artifacts using AFNI's 3dDespike. The functional images were slice-timing corrected using sinc-interpolation (Oppenheim et al., 1999), motion corrected by means of a least-squares 6-parameter rigid-body, and coregistered with the structural scan. Once the structural scan was normalized to the SPM MNI template and the warps applied to the functional data, the normalized images were smoothed with an 8 mm3 FWHM isotropic Gaussian kernel.
2.4 fMRI Analysis
A general linear model (GLM) with random effects was computed for each participant with a canonical hemodynamic response function with no derivatives, a microtime resolution of 16 time bins per scan, a high-pass filter cutoff at 128 seconds using a residual forming matrix, autoregressive AR(1) to account for serial correlations, and restricted maximum likelihood (ReML) for model estimation. The model included a constant term, 6 motion regressors using the parameters from the preprocessing motion correction, and event-related regressors to model activation during the decision and outcome periods. The decision periods for each trial included regressors for inflation choices. Inflation choice events were aligned to the time of response. The outcome events for each trial were modeled with separate regressors for successful inflations or explosions. Win decision and win outcome event regressors also were included to account for variance from these events, although these regressors are not analyzed further here. The estimated activation for each of these regressors is relative to a baseline of any remaining unmodeled event-related activation. Because regressors were included for all events, the remaining unmodeled activity can be expected to be negligible. Parametrically modulated (by probability of balloon explosion) inflation choice, successful inflation, and explosion event contrasts were included and represent the focus of the group-level analyses.
The second-level analyses used linear regression on the per-participant measures with ReML estimation in SPM5. The statistical threshold for cluster-level significance was determined using p < .01, with false-discovery rate (FDR) correction, with a minimum cluster size of 20 voxels. Peak Montreal Neurological Institute (MNI) coordinates with family-wise error (FWE) voxel-level correction of p < .05 and voxels contiguous with those passing this corrected threshold are reported and presented. The WFU PickAtlas (Maldjian et al. 2003) and Talairach Daemon (Lancaster et al., 2000) were used for anatomical labeling. MRIcron software was used to display the regions of interest.
Region-of-interest (ROI) analyses were performed using SPM5 and MarsBaR (Brett et al., 2002) within significant regions from the group analyses. Mean parameter estimates within ROIs are reported as percent magnetic resonance signal change within the region. The ROI analyses were used to examine relationships between the measures of trait disinhibition, IQ, ACT working memory, and alcohol consumption and the neural correlates of BART-related decision and outcome signals found using the whole-brain voxel-by-voxel tests. In order to avoid selection bias, these correlation analyses were used only in ROIs identified by other contrasts.
3. Results
3.1 Behavioral Data
Table 1 displays descriptive statistics and intercorrelations for BART responses and outcomes, typical weekly alcohol use, trait disinhibition, Shipley IQ, and ACT working memory. These results show individuals with more balloon trials tended to have fewer inflations per trial, more explosions, more win choices, and less winnings per trial than individuals who had fewer total trials. In addition, individuals with more inflations per trial tended to have more explosions, fewer win choices, and more winnings per trial than individuals with fewer inflations per trial. Moreover, individuals with more inflations per trial tended to score lower on trait disinhibition and higher on IQ than those who had fewer inflations per trial. Individuals scoring lower on trait disinhibition also tended to have more explosions and fewer win choices than individuals scoring higher on trait disinhibition. Consistent with research showing relations between forms of behavioral disinhibition and cognitive capacity, the correlational results showed individuals scoring higher on trait disinhibition reported greater typical weekly alcohol use and tended to score lower on IQ and than individuals scoring lower on trait disinhibition. ACT working memory capacity did not show significant relations with typical weekly alcohol use, trait disinhibition, IQ, or BART responses or outcomes.
Table 1.
BART, Typical Weekly Alcohol Use, Trait Disinhibition, IQ, and Working Memory and Descriptive Statistics and Intercorrelations
| BART Responses and Outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Trials | Inflations Per Trial | Total Explosions | Total Win Choices | Winnings Per Trial | Typical Weekly Alcohol Use | SSS-Dis | Shipley IQ | M (SD) | |
| Total Trials | -- | -- | -- | -- | -- | -- | -- | -- | 36.63 (4.79) |
| Inflations Per Trial | -.83* | -- | -- | -- | -- | -- | -- | -- | 5.04 (.86) |
| Total Explosions | .38* | .55* | -- | -- | -- | -- | -- | -- | 12.56 (4.70) |
| Total Win Choices | .84* | -.83* | -.83* | -- | -- | -- | -- | -- | 24.07 (7.89) |
| Winnings Per Trial | -.75* | .79* | .16 | -.55* | -- | -- | -- | -- | $0.64 ($0.13) |
| Typical weekly alcohol use | .14 | -.22 | -.31 | .27 | -.13 | -- | -- | -- | 10.65 (11.70) |
| SSS-Dis | .36 | -.41* | -.41* | .46* | -.25 | .72* | -- | -- | 3.04 (2.18) |
| Shipley IQ | -.32 | .40* | .22 | -.33 | .29 | -.30 | -.40* | -- | 110.26 (7.81) |
| ACT working memory | -.18 | 32 | .30 | -.29 | .20 | .01 | .05 | .30 | 35.62 (7.76) |
Note.
p < .05.
BART = Balloon Analogue Risk Task; SSS-Dis = Sensation Seeking Scale – Disinhibition; ACT = Auditory consonant trigram.
Mean (M) and standard deviation (SD) of typical weekly alcohol use is based on raw scores. All correlations with typical weekly alcohol use are based on Blom-transformed scores.
3.2 mPFC Signal Change During Inflation Choices, Successful Inflations, and Explosions
Table 2 presents the cluster size, peak voxel coordinates, Brodmann area, and z scores for BOLD signal changes during inflation choices and successful inflation outcomes when these regressors are parametrically modulated by the probability of explosion (See Figure 2 for displays of clusters). As was expected under PRO theory, there was a significant decrease in BOLD signal in the mPFC during inflation choices and a significant increase in signal during successful inflation outcomes as a function of the increasing probability of explosion across successive inflation responses. Specifically, the clusters peaked in the right anterior cingulate cortex (ACC). No mPFC clusters or peak voxels passed significance thresholds for explosion outcomes. The results show ACC down-regulation during inflation choices and, conversely, up-regulation for successful inflation outcomes, both as a function of the probability of explosion. Supplementary material presents additional regions of signal change during inflation choices, successful inflations outcomes, and explosion outcomes that were not considered in the mPFC-specific hypotheses derived from PRO theory1.
Table 2.
Medial Prefrontal Cortical Regions with BOLD Signal Change during Inflation Choices and Successful Inflations
| Peak MNI Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| BART Contrast | Region | Cluster size | x | y | z | Brodmann Area | Z Score |
| Decision period decreased signal: InflationChoice * P(explode) | R. Anterior Cingulate | 1,322 | 8 | 26 | 24 | 32 | 5.36 |
| Outcome period increased signal: SuccessfulInflation * P(explode) | R. Anterior Cingulate | 232 | 8 | 28 | 24 | 32 | 5.13 |
Note. Peak Montreal Neurological Institute (MNI) coordinates with family-wise error (FWE) voxel-level correction of p < .05 are presented. BART contrast effects are computed as increasing or decreasing blood-oxygen-level-dependent (BOLD) signal as a parametric function of the increasing probability of explosion across successive inflation responses, i.e., P(explode).
Figure 2.
Clusters of medial prefrontal cortex (mPFC) and anterior cingulate cortex (ACC) showing decreased or increased activity as a function of the probability of balloon explosion for choices to inflate and successful inflation outcomes. The cluster on the left in blue shows a region with significant negative loading on the parametrically modulated regressor of InflationChoice * P(explode). The cluster on the right in green shows a region with significant positive loading on the parametrically modulated regressor of SuccessfulInflation * P(explode).
3.3 Alcohol Consumption, Trait Disinhibition, Cognitive Capacity, and mPFC Signal Change During Inflation Choices and Successful Inflations
Pearson product-moment correlations were used to examine relations between mPFC/ACC signal change and typical weekly alcohol use, trait disinhibition, IQ, and working memory capacity. Figure 3, panels A-C, displays the scatter plots for the correlations between the ROI clusters of the mPFC/ACC identified above during inflation choices and successful inflation outcomes and the scores for typical weekly alcohol use, trait disinhibition, and IQ. Over successive inflation responses, individuals who showed greater down-regulation (i.e., greater signal decrease with increasing probability of explosion) in the mPFC/ACC during inflation choices tended to have greater typical weekly alcohol use (r = -.55, p < .05), score higher on trait disinhibition (r = -.40, p < .05), and score lower on IQ (r = .50, p < .05). Moreover, individuals who showed greater up-regulation (i.e., greater signal increase with increasing probability of explosion) in the mPFC/ACC during successful inflation outcomes tended to have greater typical weekly alcohol use (r = .54, p < .05), score higher on trait disinhibition (r = .39, p < .05), and score lower on IQ (r = -.69, p < .05). There were no significant relations between ACT working memory capacity and signal change during inflation choices (r = -.03, p > .05) or successful inflation outcomes (r = -.23, p > .05). The results indicate that heavier-drinking, disinhibited, and less intelligent individuals tend to show greater functional fluctuations in the mPFC/ACC during reward-seeking choices and successful reward-seeking outcomes compared to lighter-drinking, less disinhibited, and more intelligent individuals.
Figure 3.
Scatter plots for the correlations between the ROI clusters of the mPFC/ACC identified during choices to inflate and successful inflation outcomes and the scores for typical weekly alcohol use (Panel A), trait disinhibition (Panel B), and IQ (Panel C).
4. Discussion
The goal of the present study was to examine cognitive control via reward-seeking medial prefrontal cortex (mPFC) activity as a common neuro-functional marker of excessive alcohol consumption, trait disinhibition, and reduced cognitive capacity. Using the prediction of response-outcome (PRO) theory as a model for the role of the medial prefrontal cortex (mPFC) in cognitive control and using the Balloon Analogue Risk Task (BART) to elicit reward-seeking behavior and outcomes, two sets of hypotheses related to the postulated mPFC functions of risk/reward appraisal and performance monitoring were formulated (Alexander and Brown, 2010a). The results, which were generally consistent with these predictions, and their implications are discussed below.
Consistent with the risk/reward appraisal role of mPFC function in cognitive control, there was a decrease in mPFC/ACC signal during inflation choices as the probability of balloon explosion increased. That is, across successive inflation choices (ongoing reward-seeking behavior), there was down-regulation (signal decrease) in the mPFC/ACC, indicating a failure of the mPFC/ACC to drive risk avoidance (Magno et al., 2006). In line with PRO theory's posited performance-monitoring role of the mPFC in cognitive control, there was an increase in mPFC signal during successful inflation outcomes as the probability of balloon explosion increased. That is, across successive inflation responses, there was up-regulation (signal increase) in the mPFC (specifically, the ACC) during successful inflation outcomes. This is consistent with a role for the mPFC in detecting surprising outcomes (Jessup et al., 2010), as accounted for by PRO theory. It should be noted that the parametrically modulated effect for explosion outcomes (reward-seeking failures) did not pass the relatively conservative thresholds and corrections used in the present study. Moreover, as is shown in the supplementary Table 1, a number of brain regions outside the mPFC were associated with BART responses and outcomes. Currently, PRO theory focuses on the mPFC, but other regions play a complementary role in BART performance and are likely involved in the larger expression of cognitive control.
The findings suggest cognitive control vis-à-vis the mPFC shows increased down-regulation during reward-seeking choices as the probability of an aversive outcome increases. Moreover, this decrease as a function of greater explosion probability is larger in those with greater typical alcohol use and trait disinhibition, and lower IQ. The findings also suggest the presentation of a successful reward-seeking outcome tends to result in an up-regulation of the mPFC as the probability of a reward-seeking failure increases, and this effect is larger in those with greater typical alcohol use and trait disinhibition, and lower IQ. The dearth of relations between working memory and mPFC/ACC function suggests BART-related mPFC/ACC activity is more closely tied to general cognitive and analytic ability than it is to dual task ability and maintenance capacity.
The greater reward-seeking down-regulation of the mPFC for heavier-drinking, disinhibited, and less intelligent individuals suggests a more precipitous decrease in cognitive control, which, under PRO theory, is consistent with a more pronounced attenuation of the risk appraisal role of the mPFC during reward-seeking behavior. The greater up-regulation of the mPFC during successful reward-seeking outcomes aligns with the PRO theory prediction of greater activity when the prior probability of an actual event is low. As the probability of a balloon explosion increases, a successful inflation outcome becomes less likely and therefore, more unexpected, especially when an individual does not recognize or understand the nature of the probabilities.
Over many trials, BART winnings are maximized when inflations are made until the balloons are approximately half-inflated. Too few or too many inflations results in less winnings. For heavier-drinking, disinhibited, and less intelligent individuals, the greater up-regulation in the mPFC during successful reward-seeking outcomes might reflect a poorer comprehension of the probabilities for success or failure, and hence, greater unexpectedness. The behavioral results for the BART support this contention, showing disinhibited and less intelligent individuals being more likely to make fewer inflation choices per trial (which predicts less winnings per trial). The behavioral results suggest these individuals could be characterized as loss-averse reward takers, in that they tend to bank winnings too soon. Conversely, lighter drinking, less disinhibited, and smarter individuals could be characterized as loss-tolerant reward optimizers, requiring less cognitive control via the mPFC/ACC to maximize winnings. At first glance, the findings related to these two patterns are slightly counterintuitive. One might expect disinhibited individuals to seek out the largest reward possible (i.e., more liberal inflation style), whereas the less disinhibited individuals might attempt to avoid explosions (i.e., more conservative inflation style). While the results showed the opposite trend, the findings are consistent with studies using delay discounting tasks, which have shown that disinhibited and impulsive individuals tend to choose short-term (more immediate) payoffs and more intelligent individuals tend to choose larger long-term payoffs (Bobova et al., 2009; Shamosh and Gray, 2008).
In recent years, a great deal of research has focused on the role of the mPFC in cognitive control. The current study shows how mPFC/ACC function during risk/reward appraisal and performance monitoring can be understood as a common neuro-functional factor of co-varying expressions of excessive alcohol consumption, trait disinhibition, and lower IQ.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
References
- Alexander WH, Brown JW. Computational models of performance monitoring and cognitive control. Top. Cogn. Sci. 2010a;2:658–677. doi: 10.1111/j.1756-8765.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WH, Brown JW. Competition between learned reward and error outcome predictions in anterior cingulate cortex. Neuroimage. 2010b;49:3210–3218. doi: 10.1016/j.neuroimage.2009.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nature Neurosci. doi: 10.1038/nn.2921. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Bauer LO, Hesselbrock VM. EEG, autonomic and subjective correlates of the risk for alcoholism. J. Stud. Alcohol Drugs. 1993;54:577–589. doi: 10.15288/jsa.1993.54.577. [DOI] [PubMed] [Google Scholar]
- Bogg T, Finn PR. A self-regulatory model of behavioral disinhibition in late adolescence: integrating personality traits, externalizing psychopathology, and cognitive capacity. J. Pers. 2010;78:441–470. doi: 10.1111/j.1467-6494.2010.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb. Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Marseille Boîte à Région d’Intérêt (MarsBaR) 2002 From http://marsbar.sourceforge.net/
- Bobova L, Finn PR, Rickert ME, Lucas J. Disinhibitory psychopathology and delay discounting in alcohol dependence: personality and cognitive correlates. Exp. Clin. Psychopharmacol. 2009;17:51–61. doi: 10.1037/a0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. Some tests of the decay of immediate memory. Q. J. Exp. Psychol. 1958;10:12–21. [Google Scholar]
- Brown JW, Braver TS. Risk prediction and aversion by anterior cingulate cortex. Cogn. Affect. Behav. Neurosci. 2007;7:266–277. doi: 10.3758/cabn.7.4.266. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Bucholz K, Cadoret R, Cloninger CR, Dinwiddie S, Hesselbrock V, Nurnberger J, Reich T, Schmit I, Schuckit M. A new semistructured psychiatric interview for use in genetic linkage studies: a report of the reliability of the SSAGA. J. Stud. Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, Crutcher C, Bailey CA, Lejuez CW, Mayes LC. Risk-taking and the feedback negativity response to loss among at-risk adolescents. Dev. Neurosci. 2009;31:137–148. doi: 10.1159/000207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum. Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Mazas C, Justus A, Steinmetz JE. Early-onset alcoholism with conduct disorder: go/nogo learning deficits, working memory capacity, and personality. Alcohol. Clin. Exp. Res. 2002;26:186–206. [PubMed] [Google Scholar]
- Finn PR, Rickert ME, Miller MA, Lucas J, Bogg T, Bobova L, Cantrell H. Reduced cognitive ability in alcohol dependence: examining the role of externalizing psychopathology. J. Abnorm. Psychol. 2009;118:100–116. doi: 10.1037/a0014656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R, Heeren T, Winter M, Wechsler H. Magnitude of alcohol-related mortality and morbidity among U.S. college students ages 18–24: changes from 1998 to 2001. Annu. Rev. Public Health. 2005;26:259–279. doi: 10.1146/annurev.publhealth.26.021304.144652. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin Family Study. Dev. Psychopathol. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addition: common and specific influences. Annu. Rev. Clin. Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown J, Schall JD. Performance monitoring by anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Jessup RK, Busemeyer JR, Brown JW. Error effects in anterior cingulate cortex reverse when error likelihood is high. J. Neurosci. 2010;30:3467–3472. doi: 10.1523/JNEUROSCI.4130-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Dahmubed AF, Lara AH, Wallis JD. Neurons in the frontal lobe encode the value of multiple decision variables. J. Cogn. Neurosci. 2009;21:1162–1178. doi: 10.1162/jocn.2009.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick PJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. J. Abnorm. Psychol. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task BART. J. Exp. Psychol. Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Magno E, Foxe JJ, Molholm S, Robertson IH, Garavan H. The anterior cingulate and error avoidance. J. Neurosci. 2006;26:4769–4773. doi: 10.1523/JNEUROSCI.0369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;21:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- MRIcron software (downloaded from http://www.cabiatl.com/mricro/mricron/index.html)
- Norman D, Shallice T. Attention to action: willed and automatic control of behavior. In: Davidson R, Schwartz G, Shapiro D, editors. Consciousness and Self Regulation: Advances in Research and Theory. Vol. 4. Plenum; New York: 1986. [Google Scholar]
- Oppenheim AV, Schafer RW, Buck JR. Discrete-time signal processing. 2nd Edition Prentice Hall; Upper Saddle River, NJ: 1999. [Google Scholar]
- Patrick CJ, Fowles DC, Krueger RF. Triarchic conceptualization of psychopathy: developmental origins of disinhibition, boldness, and meanness. Dev. Psychopathol. 2009;21:913–938. doi: 10.1017/S0954579409000492. [DOI] [PubMed] [Google Scholar]
- Péchaud M, Jenkinson M, Smith S. Brain Extraction Tool (BET) [Software] Oxford University Centre for Functional MRI of the Brain; Oxford: 2006. [Google Scholar]
- Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA. Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI study of the Balloon Analog Risk Task (BART). Neuroimage. 2008;42:902–910. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamosh NA, Gray JR. Delay discounting and intelligence: a meta-analysis. Intelligence. 2008;38:289–305. [Google Scholar]
- Skeel RL, Neudecker J, Pilarski C, Pytlak K. The utility of personality variables and behaviorally-based measures in the prediction of risk-taking behavior. Pers. Individ. Dif. 2007;43:203–214. [Google Scholar]
- Scheffers MK, Coles MG. Performance monitoring in a confusing world: error-related brain activity, judgments of response accuracy, and types of errors. J. Exp. Psychol. Hum. Percept. Perform. 2000;26:141–151. doi: 10.1037//0096-1523.26.1.141. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Seethem LL, Poirier CA. Comparison of three tests of attention and rapid information processing across six age groups. Clin. Neuropsychol. 1987;1:139–152. [Google Scholar]
- Wellcome Trust Centre for Neuroimaging . Statistical parametric mapping (SPM) [Software] Wellcome Trust Centre for Neuroimaging; London: 2005. [Google Scholar]
- Yeung N, Cohen JD, Botvinick MM. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol. Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale: Revised Manual. Western Psychological Services; Los Angeles, CA: 1986. [Google Scholar]
- Zuckerman M. Sensation Seeking: Beyond the Optimal Level of Arousal. Erlbaum; Hillsdale, NJ: 1979. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.