Abstract
Gold nanoparticles (AuNPs) provide attractive vehicles for delivery of drugs, genetic materials, proteins, and small molecules. AuNPs feature low core toxicity coupled with the ability to parametrically control particle size and surface properties. In this review, we focus on engineering of the AuNP surface monolayer, highlighting recent advances in tuning monolayer structures for efficient delivery of drugs and biomolecules. This review covers two broad categories of particle functionalization, organic monolayers and biomolecule coatings, and discusses their applications in drug, DNA/RNA, protein and small molecule delivery.
Keywords: gold, nanoparticles, monolayer, delivery, organic monolayer, biomolecule coated, DNA, drug delivery, protein, small molecule
1. Introduction
Delivery and programmed release of therapeutic materials to specific physiological targets is a key challenge for molecular and macromolecular therapeutics [1]. Several nanocarriers including liposomes [2], polymer micelles and vesicles [3,4], dendrimers [5,6], nanocapsules [7,8], and metal nanoparticles [9] have been used as promising delivery vehicles. Recently, gold nanoparticles (AuNPs) have emerged as a promising delivery system for efficient transport and release of pharmaceuticals into diverse cell types.
AuNPs have a number of desirable properties that make them excellent candidates for use in delivery applications. First, the gold core is essentially inert, non-toxic, and biocompatible, making it an ideal starting point for carrier construction [10]. Secondly, AuNPs with a wide range of core sizes (1 - 150 nm) can be fabricated easily with controlled dispersity [11]; both size and dispersity are key aspects for drug delivery systems. AuNPs can be readily fabricated with sizes commensurate with biomolecules such as proteins and DNA, facilitating their integration into biological systems. Furthermore, the high surface area-to-volume ratio of nanoparticles (NPs) provides dense loading of functionalities incorporating targeting and therapeutic materials [12]. For example, ~100 ligands are covalently conjugated to an AuNP with 2 nm core diameter [13]. Finally, the highly tunable and multivalent surface structures of AuNPs offer the diversity to incorporate multiple therapeutic drugs or biomacromolecules by covalent or non-covalent conjugation on the surface of a NP [14,15].
One important aspect of AuNPs is their ease of functionalization. This ability to tailor the surface has made AuNPs effective in both the active and passive targeting [16]. Similarly, a variety of functional monolayers can be created to provide payload release strategies using internal or external stimuli such as glutathione, pH, heat, and light (vide infra). Therefore, the versatility of the AuNP monolayer platform is central to the appeal of using AuNPs as drug and biomolecule delivery systems.
In this review article, we discuss the recent advances in engineering AuNP surfaces that confer the unique physico-chemical properties required for enhanced delivery of drugs and biomolecules. We have divided this review into two categories based on the type of functionalities (Fig. 1) present on the nanoparticle surface: (1) synthetic monolayers and (2) biomolecule coatings. In both the categories we focus on surface functionalization strategies designed for effective release of payloads into living cells, highlighting a small portion of the numerous reports on AuNP-based delivery systems [17,18,19].
Fig 1.
Schematic presentation of the two AuNP surface structures commonly employed in delivery applications.
2. Organic monolayer coated gold nanoparticles
The initial purpose of introducing synthetic organic ligands on to NPs is to improve their stability by preventing aggregation. A popular approach uses reduction of HAuCl4 by citrate producing AuNPs with ~20 nm diameter, where the citric acid acts as both the reducing agent and stabilizer [20]. The size of the NPs, however, can be controlled by varying the feed ratio of gold salt to sodium [21]. Another elegant method of preparing monolayer-protected clusters (MPCs) of AuNPs was reported by Brust-Schiffrin utilizing biphasic synthetic strategy [22]. In this method, AuCl4- is transferred to organic phase by the surfactant tetraoctylammonium bromide (TOAB) followed by reduction using sodium borohydride (NaBH4) in presence of alkanethiols. The functional diversity of MPCs can be extended through the formation of mixed monolayer protected clusters (MMPCs) either directly or through post-functionalization of MPCs (Fig. 2) using place-exchange reaction with different ligands [23,24].
Fig. 2.
Preparation of monolayer-protected clusters (MPCs) using the Brust-Schiffrin reaction, and MMPCs using the Murray's place-exchange reaction.
2.1. DNA/RNA binding monolayers
Successful transfection of DNA/RNA requires effective complexation and condensation of the genetic materials, cellular uptake through endocytosis coupled with endosomal escape, protection from nuclease in cytoplasm, and finally delivery of the DNA to the nucleus. AuNP provides an attractive platform for DNA/RNA delivery due to its high surface-to-volume ratio maximizing the payload/carrier ratio. Also, the high surface area enables efficient DNA/RNA compaction, an important parameter for gene delivery.
The monolayer coverage of AuNPs allows tuning the charge and hydrophobicity to maximize transfection efficiency while reducing toxicity. NPs functionalized with cationic quaternary ammonium head-groups bind plasmid DNA via electrostatic interaction and can inhibit the transcription of DNA [25]. The efficiency of cellular uptake and/or the subsequent release of DNA from endosomal vesicles is improved by the hydrophobicity of the NPs. Positively charged AuNPs bind to DNA efficiently and protect it from enzymatic digestion [26]. These non-covalent DNA-AuNP conjugates can effectively transfect mammalian 293T cells [27]. A series of NPs (Fig. 3a) showed their transfection efficiencies to be dependent on the number of charged substituents in the monolayer (Fig. 3b), and the hydrophobic packing surrounding the monolayer (Fig. 3c). Among the series of NPs with varying cationic monolayer coverage, the most efficient NP showed ~8-fold more efficiency than 60 kDa polyethylenimine (PEI) (Fig. 3c), a widely used transfecting agent. Simple cationic primary amine-modified monolayers on AuNPs likewise provide enhanced cellular delivery and transfection of plasmid DNA (6.3 fold increase compared to 25 kDa PEI) [28]. Coating of the NPs with ligands having buffering capacity (such as PEI) provides effective release of the nucleic acid payload through the “proton sponge” effect. As demonstrated by Klibanov et al., PEI2 (2 kDa PEI) conjugated to AuNPs (Fig. 4a) enhanced the transfer efficiency of plasmid DNA into cells by ~15-fold compared to unmodified PEI2 [29]. Transfection efficiency of the NP was even higher than PEI25 (25 kDa PEI) or hydrophobically modified PEI25 that forms self-assembled structures (Fig. 4b). Efficient release of bound DNA has also been achieved through place exchange of the cationic sidechains with intracellular concentration of the overall anionic biogenic thiol glutathione (GSH) [30].
Fig. 3.
(a) Structures of cationic NPs varied with amount of cationic ligands (NP 1-5) and alkyl chain length (NP 6-7) used for transfection of DNA. (b) Transfection of β-Galactosidase using NP-DNA complexes (at 2200:1 ratio), measured by β-gal activity. (c) Transfection efficiency of NPs 2, 6, 7 (2200:1 NP/DNA ratio) and commercially available PEI (60 kDa).
Fig. 4.
(a) Structure of AuNPs conjugated to 2 kDa branched polyethylenimine (PEI2) (NP 8), and N-alkylated PEI2 (dodecyl-PEI2). (b) Conjugation of PEI2 on NPs increases the transfection efficiency. Addition of dodecyl-PEI2 along with NP8 synergistically increases the transfection efficiency. The numbers in the parentheses indicate the ratio of PEI nitrogen to DNA phosphate that describes the carrier to DNA ratio.
More sophisticated ligands provide further versatility in particle modification. Liu et al. created a DNA-binding receptor by anchoring cyclodextrin (CD) on oligo(ethylenediamino) modified AuNP (OEA-CD-NP) [31]. The oligo(ethylenediamino) chains attached to the primary side of the CD cavities serve as the electrostatic sites for binding DNA, whereas the CD cavities located at the external surface as a porous hydrophobic environment. Delivery of plasmid DNA using the OEA-CD-NP was moderately efficient (1.2 to 4.8%) as compared with lipofectamine 2000, a widely used transfecting agent. However, the NP-DNA complexes showed less toxicity probably due to the biocompatibility of the extrusive CD cavities on the surface of OEA-CDNP/DNA aggregates. A related study found that DNA bound to AuNPs modified with amine and PEG functionalities possess more stability in the blood circulation compared to naked DNA [32]. Additionally, electroporation assisted restricted gene expression of the circulating AuNP bound DNA in limited areas of liver cells. Another approach was adopted by Lee et al. to enhance the transfection of siRNA [33]. In this strategy, AuNP was modified with poly(ethylene glycol) followed by direct conjugation with siRNA. The particles were then coated with a library of poly(β-amino esters) to provide an overall cationic charge. The modified NPs showed level of in vitro siRNA delivery significantly better than commercially available liposomal delivery agents.
Amino acid functionalized AuNP provides an effective bio-inspired platform for DNA delivery into cells. Binding studies of DNA with AuNPs bearing amino acid sidechains revealed higher affinity with the cationic sidechains compared to the neutral hydrophobic ones [34]. In fact, polycationic lysine-functionalized AuNPs form compact complexes with DNA and provide highly efficient gene delivery without any observed cytotoxicity [35]. Improved compaction of the DNA-AuNP complex through increased density of ammonium groups facilitates in vitro delivery, as demonstrated using first generation lysine dendrons (NP-LysG1) (Fig. 5a). These ligands transfected plasmid DNA with 28-fold higher reporter gene expression compared to polylysine (Fig. 5c) [35]. These amino acid-terminated AuNPs released DNA in response to intracellular glutathione (GSH) levels, as found through GSH depletion and supplementation experiments.
Fig. 5.
(a) Schematic illustration of DNA transfection using nanoplexes containing NP-LysG1 and DNA. The chemical structures of the head groups present on the surface of the NPs are given in the underneath box. (b) Schematic depiction of place-exchange between cationic ligands on NP surface and cellular glutathione (GSH), which is proved to be the mechanism of DNA release in this case. (c) Enhanced transfection using NP-LysG1 and NP-Lys relative to NP-Lys, NP-TMA, polylysin, and naked DNA.
2.2. Protein delivery
Delivery of functional proteins inside living cells has been limited by the poor permeability through the cell membrane [36]. Stability of the protein cargo against digestion by enzymes poses another challenge. Potentially, AuNPs with engineered monolayer are able to overcome these issues. Whereas non-covalent conjugation can retain the structure and activity of the protein cargo, covalent approaches have also been applied without altering the protein's activity.
Non-covalent protein delivery presents a platform where the reversibility of the NP-protein binding plays a key role that can be efficiently tuned by the AuNP monolayer structure. AuNPs capped with cationic monolayer protected clusters (MMPCs) of different chain lengths bind anionic β-galactosidase (β-gal, 465 kDa, pI 4.6) through complementary electrostatic interactions, resulting in complete enzyme activity inhibition [37]. Therefore, these cationic AuNPs can be further tuned for GSH-mediated release of enzymes into cells. Recently, the intracellular delivery of β-gal was demonstrated using cationic AuNPs having a short peptide conjugated to tetra(ethylene glycol) unit (Fig. 6). Significantly, the enzymatic activity of the β-gal was retained, and the β-gal escaped the endosomes and was found in the cytosol [38]. Moreover, the particles showed no apparent cytotoxicity, making this strategy promising for the delivery of protein therapeutics.
Fig. 6.
(a) Schematic representation of the complexation between cationic NP and anionic protein (β-Gal), leading to efficient permeation through the cell membrane compared to the uncomplexed protein. (b) Structures of the cationic NPs and the surface ligands.
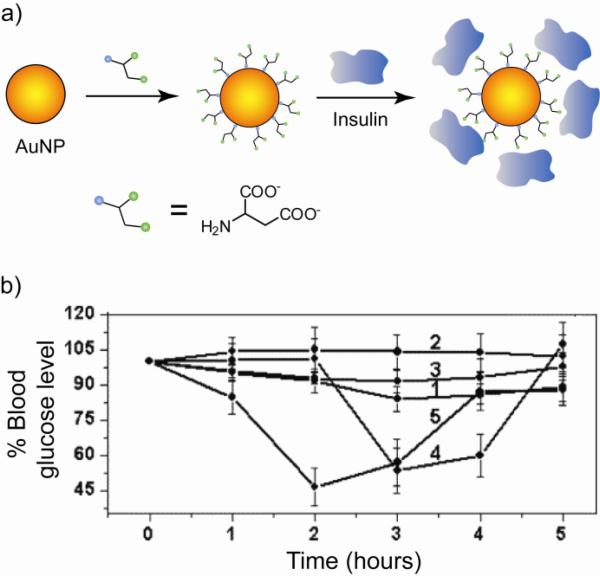
Modified AuNP monolayers have been fabricated for binding insulin and transmucosal delivery of this protein for the therapeutic treatment of diabetes mellitus [39]. Insulin was loaded onto aspartic acid-capped AuNPs (Fig. 7a) and delivered by both oral and transmucosal pathways in diabetic Wistar rats. Significant reduction in blood glucose level was observed compared to the protein only (Fig. 7b). In another example, tumor necrosis factor-alpha (TNF-alpha), a potent cytokine with anticancer efficacy, was loaded on polyethylene glycol coated colloidal AuNPs (PT-cAu-TNF-alpha, 33 nm core) and subsequently delivered into SCK mammary carcinomas grown in mice [40]. Tumor growth was delayed when treated with PT-cAu-TNF-alpha only. In addition, the local heating due to the large core size enhanced the efficacy of these systems. The bio-distribution of AuNPs that target the delivery of TNF-alpha to solid tumor was also studied [41].
Fig. 7.
(a) Functionalization of AuNPs with aspartate ligands followed by loading insulin protein. (b) Plot of time variation of the blood glucose level (expressed in %) in diabetic rats after intranasal administration of insulin-conjugated NP and insulin alone. Control insulin (curve 1), bare AuNPs (curve 2), Au-Asp NPs (curve 3), Au-insulin NPs (curve 4), and Au-Asp-insulin NPs (curve 5). Adapted with permission from [39].
Proteins conjugated to AuNPs through covalent linkage provide enhanced protein stability in complex biological media. Recently, PEGylated AuNPs were conjugated with human transferrin (Tf) by amine-carboxylate reaction and targeted to Neuro2A tumors in mice [42]. In tumor tissue, the number of NPs localized within cancer cells was significantly influenced by the Tf content. However, quantitative biodistributions of the NPs 24 h after i.v. injection showed that the NP accumulations in the tumor and other organs were independent of the Tf content. Nevertheless, the NP localization within a particular organ was influenced by the amount of Tf on a NP, e.g. high Tf contents lead to small amounts of the NPs residing in hepatocytes of liver tissues. A mechanistic study of Tf-mediated cellular uptake has been carried out using mercaptoacetic acid coated AuNP covalently attached with Tf protein [43]. It was shown through AFM imaging that the delivery into cells occurs through transferrin receptor-mediated endocytosis. The structure-function relationship of transferrin-coated AuNPs with different sizes and shapes was studied in mammalian cells [44]. It was observed that transferrin-coated AuNPs enter the cells via clathrin-mediated endocytosis pathway and are exocytosed from the cells with rates that are linearly dependent on particle sizes.
2.3. Drug delivery
The high surface area and tunability of AuNPs provide an excellent platform for attachment of drugs for controlled and sustained release. Various covalent and non-covalent strategies have been employed for loading drugs onto AuNPs. Each approach has its advantages: covalent attachment provides stable delivery vehicles, but generally requires intracellular processing of the prodrug [45]. Non-covalent loading of drugs allows direct release of active drugs; however premature release can be an issue.
Zubarev et al. have covalently conjugated paclitaxel, a chemotherapeutic drug, to AuNPs with 2 nm core [46]. The synthetic strategy involves the attachment of a flexible hexa(ethylene glycol) linker with paclitaxel followed by coupling of the resulting linear analogue to phenol-terminated AuNP. These well-characterized NPs present a well-defined number of paclitaxel molecules with near-uniform composition, and interestingly function as self-therapeutics, i.e. ligand release is not required for drug efficacy. Efficient release of drugs from covalently conjugated prodrugs has also been achieved by various methods including hydrolysis by phosphodiesterase in vitro [47]. Intracellular activation of the prodrug enables delivery of inert forms of the drug, resulting in reduced side effects. This shielding has been demonstrated by covalently conjugating anticancer drug containing Pt(IV) to oligonucleotide AuNPs and delivering the prodrug into tumor cells [48]. Intracellular reduction of the prodrug to the active Pt(II) form results in cytotoxicity. Later, Pt-containing drugs in active form tethered to AuNPs have been shown to have enhanced cytotoxicity relative to the free drugs [49].
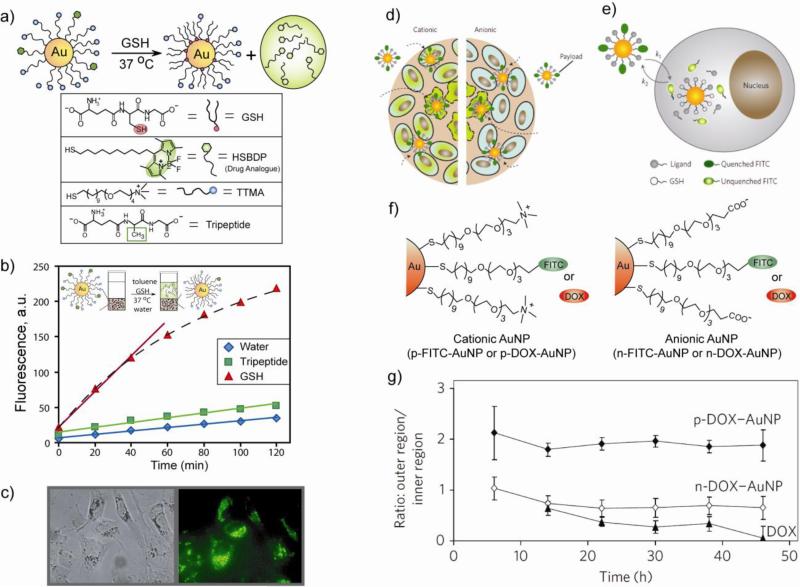
Intracellular activation/release of prodrugs by glutathione [50,51,52] provides a non-enzymatic alternative for drug release. This strategy uses the dramatic difference in the intracellular GSH concentration (1–10 mM) relative to the extracellular thiol levels (2 μM glutathione, 8 μM cysteine) [53]. A number of disulfide-based drug carriers [54,55] relying on this strategy have been generated, however disulfide exchange with cysteines of serum proteins remains a challenge. Monolayer-functionalized AuNPs provide stability against this exchange with plasma proteins due to steric shielding of gold–thiol interface. The effectiveness of cellular delivery and GSH mediated release of a hydrophobic dye (Bodipy) was demonstrated using functionalized AuNPs as shown in Fig. 8a [56]. The cationic surface of AuNPs facilitates in crossing the cell-membrane barrier. Observation of dye release was facilitated by the fluorescence quenching properties of AuNPs [57]. The triggered release of payload by GSH could easily be monitored by fluorescence regeneration upon release of the dye from the AuNP in cuvette (Fig. 8b), or in human liver cells (HepG2) (Fig. 8c). The GSH-controlled release was further confirmed by using glutathione monoester as an external stimulus to release the thiol-terminated dye. It is noteworthy that the release of the dye/drug conjugated ligands by biogenic thiols is determined by the surface charge of the AuNPs, with cationic particles much more labile than anionic analogues [58].
Fig. 8.
(a) Schematic illustration of GSH-mediated surface monolayer exchange reaction/payload release. (b) GSH mediated release of Bodipy ligands in cuvette measured by fluorescence in toluene phase. The slopes were 2.5 (initial period), 0.33, and 0.24 for GSH, tripeptide and water respectively. (c) Bright field and fluorescence images of MCF-7 cells incubated with the above AuNPs displaying GSH-controlled release of the fluorophore. (d) The schematic of delivery of payloads into tumor cylindroids. Viable cells are shown by regular shape with solid boundary and necrotic cells by irregular shape with dashed boundary. Cells containing FITC-SH are shown in green. (e) Cellular uptake and FITC-SH release by intracellular GSH. The rates of particle uptake and FITC release in extracellular regions are represented by k1 and k2, respectively. (f) FITC and doxorubicine (DOX) loaded cationic and anionic AuNPs used in these studies. (g) Ratio of average fluorescence intensities in the outer region to those in the inner region of the cylindroids as a function of time.
A new avenue for the optimization of efficient intratumoral drug delivery was provided by three-dimensional in vitro tumor models that mimic poor vascularization of tumors in vivo [59]. We have shown the timing and location of the NPs in real time using multicellular tumor cylindroids [60]. Detailed studies on intracellular glutathione-mediated release of fluorescein (a dye) and doxorubicin (an anticancer drug) conjugated to AuNPs in tumor cylindroids (Fig. 8d-g) indicated the importance of surface charge on tissue penetration and drug release. Positive NPs, owing to greater cellular uptake, can be more effective in drug delivery into proliferating cells, and the negative NPs can deliver therapeutics deep into tissues due to faster diffusion.
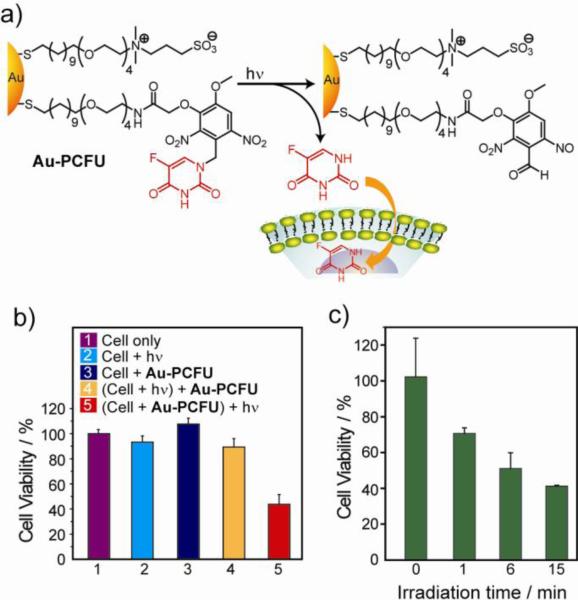
Payload release by external stimuli provides a complementary strategy to endogenous agents for time- and site-specific delivery of drugs [61]. Recently, light-controlled release of caged drugs has been developed where the activity of the drug was suppressed by attaching it to AuNPs through a photocleavable protecting group [62]. In this system, anticancer drug 5-fluorouracil was linked to AuNPs through an o-nitrobenzyl group (Au-PCFU) that could be effectively cleaved using near-UV (365 nm) irradiation (Fig. 9a). The AuNPs also contained a mixed monolayer of zwitterionic ligands that provided solubility and prevented cellular uptake. Alamar blue assay showed an IC50 value of 0.7 μM upon irradiation of Au-PCFU, whereas no significant cell death was observed on cells treated only with NPs or light (Fig. 9b). It was also shown that the cell viability in the presence of the drug decreases with increasing light exposure (Fig. 9c). A related study demonstrated conjugation of histamine, a cell-signaling agent, on AuNPs through carbamate linkage that could be dissociated via the photocleavage reaction of the o-nitrobenzyl group upon near-UV irradiation [63]. Effective caging of histamine enabled its biological activity inhibition while attached to AuNPs and complete recovery after photorelease from the particle surface. Photodynamic therapy (PDT) is another promising non-invasive tumor treatment strategy. A hydrophobic photosensitizing agent phthalocyanine (Pc 4 and Pc 219) has been conjugated to AuNPs both by covalent (AuNP-Pc 4) [64] and non-covalent (AuNP-Pc 219) [65,66] linkages. In vivo release of the PDT drug in tumor-bearing mice indicated highly efficient drug delivery, with preferential accumulation in tumor sites. Recent work showed deep penetration of the drug released from AuNP-Pc 4 into tumors within hours [66]. The biodistribution of these AuNPs over a 7-day period indicated renal clearance from the mice.
Fig. 9.
(a) Schematic of photochemical reaction on Au-PCFU and delivery of payload into cells. (b) Effect of different conditions on the cell viability of MCF-7 cell line. The concentration of Au-PCFU used was 1 μM, and the light exposure time was 20 min. (c) Cell viability with different durations of light exposure.
Non-covalent encapsulation of drugs into AuNP monolayers provides direct release of unmodified drugs. This strategy relies on the use of ligands to generate hydrophobic pockets into the monolayer. Structurally, the radial nature of the ligands [67] creates a hydrophobic interior inside the monolayer of the AuNPs [68], while surface charge can be used to regulate the interactions of the NPs with cell membranes. Recently, biocompatible AuNPs have been developed that encapsulate hydrophobic drugs/dyes into the hydrophobic pockets of zwitterionic ligand-functionalized AuNPs (Fig. 10a) [69]. The zwitterionic headgroup minimizes non-specific binding with biomacromolecules and prevents cell uptake. The entrapped payloads were released into MCF-7 cells by membrane-mediated diffusion, as demonstrated by both fluorescence microscopy (using Bodipy, a fluorophore payload) and through drug efficacy with therapeutic guests (Fig. 10b). Notably, the NPs themselves showed no toxicity even at a concentration of 30 μM, consistent with the lack of cellular uptake of the AuNPs as demonstrated by ICP-MS studies. The biocompatibility of these particles coupled with appropriate sizes (diameter 10-200 nm) make these systems promising candidates for passive targeting using the enhanced permeability and retention (EPR) effect [70,71]. A variety of other strategies have been developed to modify the monolayer of the AuNPs with small molecule payloads. Efficient release of nitric oxide (NO) from AuNPs was achieved at acidic pH [72], providing a potential means of controlling multiple cellular processes including angiogenesis, vasodilation, and the immune response [73]. In this system, NO was efficiently stored on AuNPs by covalent linkage with polyamine ligands via formation of an acid labile N-diazeniumdiolate. These water-soluble NPs exhibited enhanced release profile in acidic condition (pH 3). Controlled release of NO has also been demonstrated in amine derivatized monolayer protected AuNPs [74]. The control over the type and amount of amine in the monolayer allows for a range of NO-release properties.
Fig. 10.
(a) Schematic representation of AuNP containing monolayer entrapped drugs and their delivery into cell through monolayer membrane interaction. (b) Structure of NPs and entrapped guest molecules: Bodipy as a fluorescent probe, and TAF (tamoxifen) and LAP (β-lapachone) as drugs. The number of encapsulated guests per particle is given under the structures. Cytotoxicity of the NPs was measured by alamar blue assay after 24 h incubation with MCF-7 cells. IC50 values of NPs, equivalent drugs, and free drugs are shown in the table.
2.4. Targeted delivery
Successful delivery of therapeutics to disease sites is a major challenge in biomedicine [75]. There are two strategies for targeting therapeutics: ‘passive’ targeting depends upon the leaky vasculature of the diseased tissues (e.g. tumor) [76] to provide EPR effect to the carrier systems; ‘active’ targeting involves the attachment of functionality to the delivery vehicle for interaction with specific cell receptors [77].
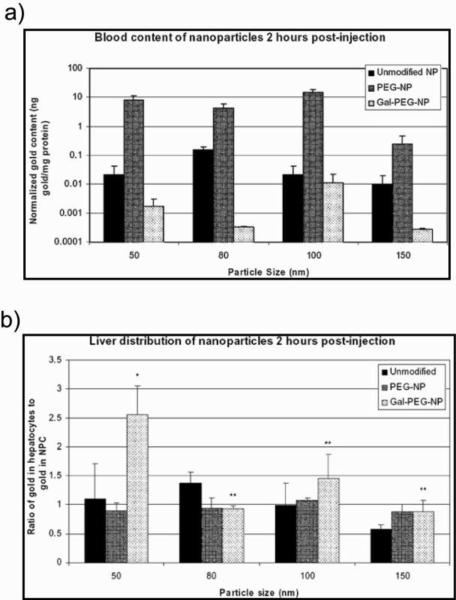
Pun et al. explored the interplay of non-specific versus target-specific uptake as a function of AuNP size, PEGylation, and targeting ligand [78]. They used AuNPs of varying sizes (50, 80, 100, and 150 nm diameter) with or without PEGylation (PEG5000). They targeted asialoglycoprotein receptors on hepatocytes (liver cells) by grafting small sugar molecules such as galactose onto the AuNP surface (Gal-PEG-NP). PEGylation increases blood circulation lifetime of the NPs (Fig. 11a) and galactose facilitates the targeting of Gal-PEG-NPs into the liver (Fig. 11b). In cell uptake studies, Chan et al. reported that gold nanospheres of 50 nm in diameter are internalized by mammalian cells at a higher rate compared to other AuNP sizes (core diameter 14, 30, 74, 100 nm) [79].
Fig. 11.
Distribution of unmodified NP, Pegylated NP (PEG-NP) and galactose modified NP (Gal-PEGNP) have been studied using different particle sizes. Amount of different NPs found in (a) blood and (b) liver 2h after injection of the NPs. The amounts of gold were obtained by instrumental neutron activation analysis. Adapted with permission from [78].
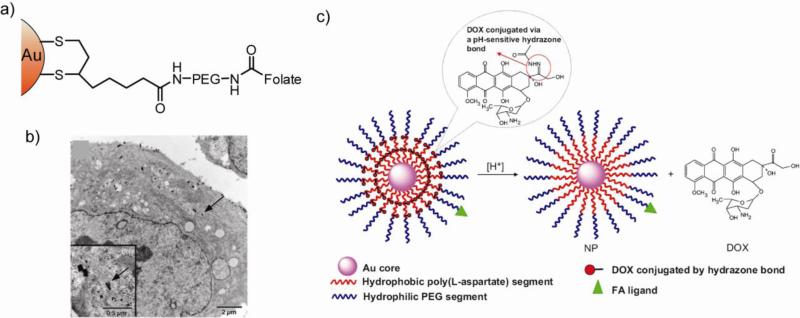
Many tumor cells overexpress folate receptors (FR) on their surfaces that can be targeted using folic acid (FA) and methotrexate (MTX) derivatives [80]. For example, conjugation of folic acid to AuNPs using a PEG spacer provided selective delivery to FR-positive KB cells (Fig. 12a,b) [81]. Recently, folate and doxorubicin (DOX) have simultaneously been conjugated to amphiphillic AuNPs for tumor targeted drug delivery [82]. The AuNPs (viz. Au-P(LA-DOX)-b-PEG-OH/FA) featured a hydrophobic poly(L-aspartate-doxorubicin) (P(LA-DOX)) inner shell, and a hydrophilic poly(ethylene glycol) and folate-conjugated poly(ethylene glycol) outer shell (PEG-OH/FA). DOX was covalently conjugated onto the hydrophobic inner shell by acid-cleavable hydrazone linkage (Fig. 12c). An increased release rate of DOX from the NPs was observed at acidic pH (6.6 and 5.3), which is particularly advantageous for pH-triggered drug release to acidic environment of tumor tissues [83]. Grafting anticancer drugs such as cisplatin, along with FA has been shown to be effective in killing ovarian cancer cells [84]. The targeting moiety methotrexate (MTX) likewise provides effective targeting. It was demonstrated that a MTX–AuNP conjugate inhibits tumor growth in a mouse model of Lewis lung carcinoma [85], where MTX acts as the anticancer agent as well.
Fig. 12.
(a) Structure of folate conjugated AuNP for targeted delivery. (b) TEM Micrographs of thin sections of FR-positive KB cells that were incubated for 2h with the AuNP constructs. The arrows show the internalized NPs inside the cell. Adapted with permission from [81]. (c) Schematic illustration of the Au-P(LA-DOX)-b-PEG-OH/FA NP and its pH-triggered drug release. The acid cleavable hydrazone ligands release Dox at pH 5.3. Adapted with permission from [82].
In addition to the therapeutic applications described above, Paciotti et al. investigated the targeting effect of PEGylated AuNPs (PT-cAu-TNF) with adsorbed protein (tumor necrosis factor, TNF) as targeting agents in vivo [86]. When the NPs were injected intravenously, the large accumulation of NPs was observed in MC-38 colon carcinoma tumors compared to liver, spleen, or other healthy organs. The larger size of the NPs (~33 nm) helped tracking the biodistribution of the PT-cAu-TNF NPs. TNF provided both targeting and therapeutic action via selectively killing the targeted cells. In fact, PT-cAu-TNF was more effective in reducing tumor than native TNF, probably due to the sequestration of TNF to solid tumors. Recent studies have demonstrated enhanced tumor therapy by grafting anticancer drug paclitaxel to cAu-PEG-TNF [87]. Another viable approach for making target-specific AuNPs is attaching aptamer sequence on the particle surface. A novel conjugation approach has been devised using an extended aptamer design where the extension is complementary to an oligonucleotide sequence attached to the surface of the AuNPs [88].
3. Biomolecule-coated gold nanoparticles
Modification of AuNP surfaces with biomolecules has provided efficient transport of biomacromolecules with minimal cytotoxicity. AuNP monolayers generated by biomolecules can address various issues in delivery such as endosomal escape and specific targeting without conjugation of additional moieties. For example, endosomal trapping can be avoided by using a cell penetrating peptide-based coating on AuNP surfaces [89]. We describe in this section oligonucleotide, peptide/protein, carbohydrate, and lipid coated AuNPs for cellular internalization.
3.1. Oligonucleotide coated nanoparticles
Cellular internalization of negatively charged oligonucleotide-functionalized AuNPs has emerged as a new approach to gene therapeutics [90]. A new approach has been reported to conjugate thiolated double-stranded (ds) DNA fragments (EGFP) directly to AuNPs using ligase-dependent strategy [91]. The Au-EGFP nanoconjugates could be digested by restriction enzymes and expressed as functional proteins inside mammalian cells, demonstrating retained activity of the dsDNA conjugated to AuNPs. Similar strategies were used to inhibit transcription using double-stranded promoter DNA on the surface of AuNPs [92]. DNA-functionalized AuNPs have proved to be effective contrast agents that could be functionalized with a variety of biomolecules ranging from small molecules to peptides and antibodies [93].
A novel class of DNA-AuNPs, attached through mono- or tetrathiol terminals, were prepared by Mirkin and co-workers (Fig. 13a). Whereas the latter particles exhibited binding constant to its complementary sequence that was approximately similar to the unmodified nucleotide, the former particles showed ~35 times higher binding affinity [94]. This observation was consistent with the cooperative binding properties of the monothiol AuNPs arising from denser packing efficiency. These “antisense nanoparticle” target the mRNA sequence encoding proteins, serving as both delivery agent and antisense entity. Effective suppression of EGFP signal was observed in C166 cells using these NPs in low concentrations. Later on, densely functionalized locked nucleic acid-NP (LNP) conjugates were synthesized that form remarkably stable duplexes with complementary nucleic acids [95,96]. LNPs incorporate bridged sugars in their backbones, and feature increased binding affinity and duplex stability [97]. As demonstrated in lung carcinoma cell model, LNPs readily enter cells where they are more effective at controlling gene expression than their DNA functionalized NP analogues [95]. Investigation on the mechanism of cellular uptake of these negatively charged DNA coated NPs revealed the endocytosis pathway initiated by adsorption of numerous serum proteins onto the particle surfaces (Fig. 13b) [98].
Fig. 13.
(a) Preparation of antisense AuNPs (ASNP). Citrate-stabilized AuNPs (~13 nm) were functionalized with oligonucleotides that were premodified with A10 tether and two cyclic disulfides (DTPA) or one alkyl-thiol anchoring group. (b) Proposed mechanism for cellular uptake of ASNP. Adapted with permission from [94].
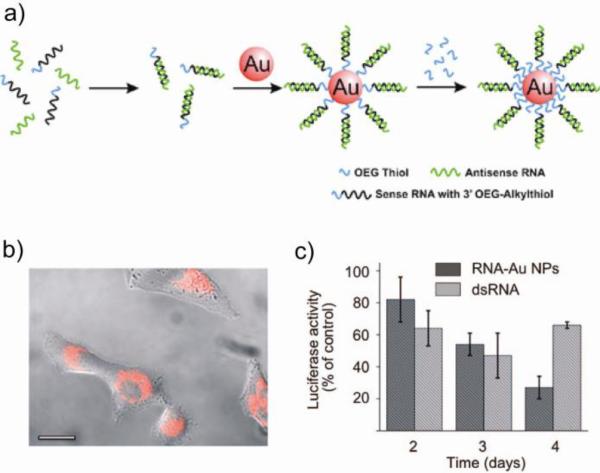
Recently, siRNA coated AuNPs have been synthesized in RNase free conditions (Fig. 14a) and delivered into cells [99]. The RNA interference pathway uses molecular RNAs that have short half-lives as a result of the instability due to RNase degradation, thus limiting their efficacy [100]. In the case of siRNA-AuNPs, the dense covalently attached RNA monolayer increases the protection from nonspecific degradation. These nanoconjugates are serum stable and provide excellent gene regulation [99]. Efficient protein knockdown without any cytotoxicity was demonstrated in HeLa cells using luciferase plasmid (Fig. 14b,c). Similarly, thiolated siRNA was grafted onto AuNPs coated with poly(ethyleneglycol)-block-poly(2-(N,N-dimethylamino)ethylmethacrylate) copolymer (PEG-PMMA) to enhance cellular internalization for gene silencing in HuH-7 cells [101].
Fig. 14.
(a) Synthesis of polyvalent RNA AuNP conjugates. (b) Fluorescence microscopy image of HeLa cells after 6 h incubation with RNA AuNPs. (c) Knockdown of luciferase expression over 4 days. Adapted with permission from [99].
Hairpin DNA-coated AuNPs (hAuNPs) add useful functionality to delivery systems. An efficient method for live cell imaging of messenger RNA was demonstrated using hAuNPs [102]. The hairpin-DNA was conjugated to AuNP through the 5' thiol groups, consisting of a stem-loop-stem sequence that is designed to bind specific target mRNA. A recent study showed that the AuNPs stabilized by weakly bound surface ligands caused significant cellular responses such as changes in gene expression, cell-cycle progression, or apoptosis induction [103]. On the other hand, AuNPs stably functionalized with nucleic acids do not show measurable change in biological responses. The enhanced cellular responses have been attributed to the relative instability of these particles and the interaction of the aggregated particles with the cells.
Overall, oligonucleotide coated AuNPs possess multivalency arising from the densely packed monolayer. The multivalent properties lead to their higher binding affinity for the complementary sequence and hence higher genetic payload carrying capacity. Dense packing provides greater stability of the carrier in cell culture media as well.
3.2. Peptide/Protein coated nanoparticles
NPs directly attached with peptides or proteins present another paradigm for delivering cargo into cells. Most of the delivery studies using peptide/protein coated AuNPs focus on attaching targeting peptide sequences or receptor specific antibodies. Significantly, these coatings enhance internalization of the particles into different organelles or targeting specific cancer cells. In an elegant approach, AuNPs have been modified with bovine serum albumin (BSA) (Fig. 15a,b) conjugated to various cellular targeting peptides [104,105]. These NPs enter the nucleus of cultured HepG2 cells. Interestingly, these studies showed that NPs functionalized with targeting peptides containing both receptor-mediated endocytosis (RME) and nuclear localization signal (NLS) sequences are able to enter the nucleus of cells. Conjugation with the sequences as separate pieces rather than a sequence containing both, imparts more efficient nuclear targeting (Fig. 15c,d). The intracellular distributions of different NLS sequences in different cells were also investigated using AuNPs conjugated to a series of NLS peptides. Studies in HeLa, 3T3 and HepG2 cells showed that the transport of NPs into the cytoplasm and nucleus is dependent on peptide sequence and cell line [105]. AuNPs capped with protein transduction domains (PTD) from HIV Tat protein get internalized into the cytoplasm, but not the nucleus of 3T3 and HepG2 cells. Cellular uptake of these NP conjugates was temperature dependent, suggesting an endosomal pathway of uptake. On the other hand, adenovirus nuclear localization signal and the integrin binding domain capped AuNP enters cells via an energy dependent mechanism and achieves the nuclear localization in cells [104]. These observations suggest that the most efficient nuclear targeting may result from a combination of targeting peptides attached to a single carrier.
Fig. 15.
(a) Synthesis of peptide modified AuNP mediated by BSA. A desired peptide containing terminal cysteine was conjugated to MBS (3-maleimidobenzoic acid N-hydroxysuccinimide ester) on surface lysine residues of BSA. Adapted with permission from [104]. (b) Peptide sequences used in NP-BSA-Peptide complexes. (c-d) NP-peptide complexes incubated with HepG2 cells for 2 h: peptide 2 (c), peptide 3 (d). Adapted with permission from [105].
Direct conjugation of functional peptides onto AuNPs provides a number of tunable properties that can modulate their functions inside living cells [106]. For example, intracellular component targeting could be achieved using AuNPs stabilized by a transmembrane sequence-containing peptide. The intracellular distributions of the particles changed from the nucleus to the endoplasmic reticulum by increasing the density of the peptide at a constant NP diameter [106]. Additionally, increasing the particle diameter at a constant density of peptide leads to less cellular uptake. Another effort directed towards enhancing macrophage proliferation using peptide conjugated AuNPs suggested that the activation is independent of peptide length and polarity, and is instead dependent on the peptide pattern at the NP surface [107]. Likewise, the effect of the peptide packing sequence in stabilizing NPs and entering cells have been investigated using AuNPs modified with both peptides and polyethylene glycol moieties [108]. The stability of the assemblies of AuNPs with PEG and mixed peptide/PEG monolayers was enhanced with smaller particle size, higher PEG length, and higher PEG mole fraction. Receptor mediated endocytosis of these AuNPs was observed in HeLa cells, providing a promising lead for AuNP-based delivery vehicles.
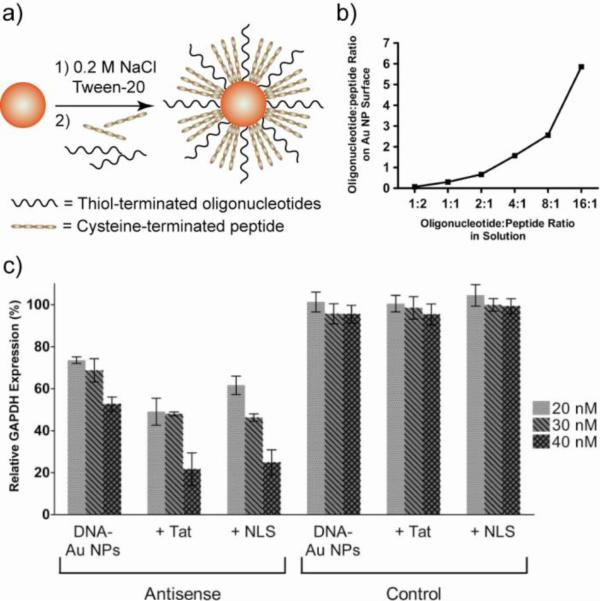
Effective gene silencing vectors can be generated through co-functionalization of AuNPs with antisense oligonucleotides and NLS or HIV Tat peptides. Hetero-functionalized AuNPs were synthesized by mixing thiolated oligonucleotides and cysteine-terminated peptides with 13 nm AuNPs [109]. Addition of salt was required to screen the electrostatic attraction between the oppositely charged oligopeptides and oligonucleotides, leading to densely functionalized stable NPs (Fig. 16a,b). The AuNPs were efficiently internalized into cells and localized in the prenuclear region, featuring high gene silencing ability (>75% decrease in target protein expression) (Fig. 16c). A study with AuNPs conjugated with both nuclear targeting peptide and gene splicing oligonucleotide revealed that the intrinsic targeting ability of the oligonucleotide was not improved by the conjugation of targeting peptide that facilitates endocytosis or uptake [110]. However, sequestration of the oligonucleotides or other therapeutic cargo from the harsh conditions of the endosomes enhances their efficacy in cell targeting applications.
Fig. 16.
(a) Preparation of AuNPs co-functionalized with antisense oligonucleotide (to GAPDH: glyceraldehyde phosphate dehydrogenase) and peptide (Tat or NLS sequences). Pre-addition of salt helps in stabilizing the NPs. (b) Oligonucleotide and peptide loading as a function of solution stoichiometry determined by fluorescent labeling. (c) Relative decrease in GAPDH expression in HeLa cells. β-Tubulin was used as a loading control and for subsequent normalization of GAPDH knockdown. Adapted with permission from [109].
Conjugation of proteins to AuNPs, provides excellent biocompatibility and reduces the nonspecific toxicity towards normal cells [111]. A number of different approaches have been adopted for direct conjugation of proteins to AuNPs through thiols or amines on the proteins [112], but retaining the native structure of the proteins remains a challenge [113]. Antibody conjugated AuNPs provide a useful tool for cellular application including imaging and photothermal therapy. Various synthetic methods such as adsorption [114], N-hydroxysuccinimidyl ester coupling [115], and oligonucleotide-directed immobilization [116] have been employed to modify NP surfaces with antibodies. In one example, AuNPs conjugated with monoclonal anti-epidermal growth factor receptor (anti-EGFR) were incubated with one non-malignant and two malignant oral epithelial cells [117]. The nanoconjugates bound specifically to the cancerous cells with six time greater affinity than the non-cancerous cells, making this method a potential cancer detection and targeting technique. The anti-EGFR conjugated NPs (~40 nm size) were incubated with normal and carcinoma cells and subsequently irradiated with laser (514 nm) to induce heating [118]. The results indicated that malignant cells require less than half the laser energy to be killed than the benign cells [119]. In another example, monoclonal anti-HER2 antibodies were conjugated with AuNPs to target breast cancer cells overexpressing HER2 receptors on the cell surfaces [120]. Irradiation of the targeted cells with near IR laser destroyed the tumors effectively.
3.3. Carbohydrate coated nanoparticles
Mammalian cell surfaces are covered with a dense coating of carbohydrates (the glycocalyx) that is critically involved in cellular processes including cell-cell recognition, pathogenesis, inflammation, cancer, and immune surveillance of tumors [121,122]. Cell surface carbohydrates play an important role in various physiological and pathological processes including metastasis, inflammation, and infection [123]. In addition to protein-carbohydrate interactions, carbohydrate-carbohydrate interactions are also involved in a wide variety of processes [124,125]. Utilizing these interactions, carbohydrate coated AuNPs have proved to be effective therapeutic agents. Conjugating carbohydrates on AuNP surfaces improves the biocompatibility, targeting ability, and provides higher delivery efficiency.
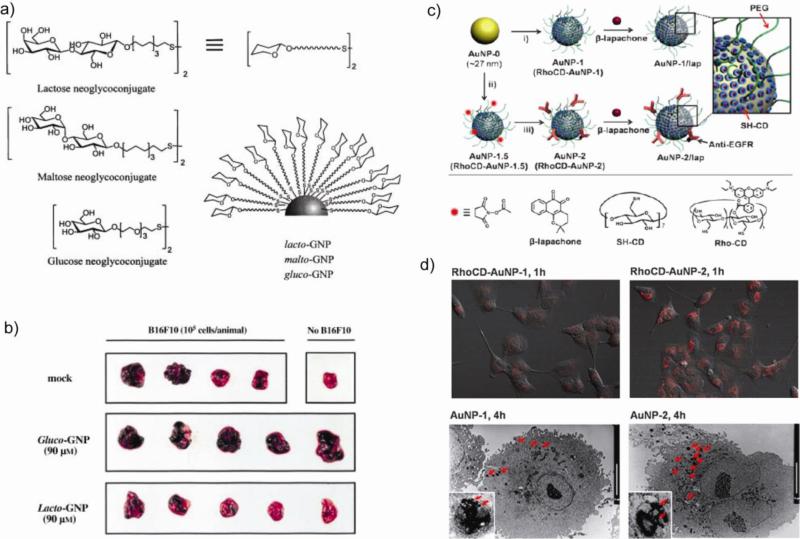
Penades et al. have developed AuNPs functionalized with specific carbohydrate antigens (glycoNPs) as a scaffold for providing antiadhesion therapy and consequently preventing tumor metastasis [126]. These glycoNPs display multivalent carbohydrates that possess the advantages of high polyvalency, water solubility, and resistance to enzymatic hydrolysis. Lactose conjugated AuNPs (lacto-GNPs) were tested as a potential inhibitor of the binding of melanoma cells to endothelium cells (Fig. 17a) based on the involvement of tumor associated antigens in cell adhesion [127]. Disaccharide maltose (malto-GNPs) and monosaccharide glucose (gluco-GNPs) were used as negative controls. A strong protective effect against lung metastasis was observed by direct visual inspection of lungs obtained from animals inoculated with murine melanoma cells (B16F10) pretreated with lacto-GNPs. However, the protective effect, as demonstrated by the absence of metastases, was not observed in the lungs obtained from animals primed with B16F10 cells or B16F10 cells pretreated with gluco-GNPs (Fig. 17b). A diversity of glycoNPs presenting varying carbohydrate antigens can be prepared, providing a controlled model for studying the role of carbohydrate presentation and density on recognition events [128]. Multifunctional glycoNPs incorporating T-cell helper peptides (TT) and glucose in well defined proportions and with differing density provide polyvalent anticancer vaccine candidates and drug delivery carriers with defined average chemical composition [129].
Fig. 17.
(a) Structure of AuNPs coated with lactose, maltose and glucose. (b) A representative picture of lungs corresponding to animals included in each group in comparison with the lungs obtained from a control animal not injected with B16F10 cells. Lacto-GNP protects against development of tumoral foci (black spots). Adapted with permission from [126]. (c) Schematic illustration of the functionalization of AuNPs with β-lapachone. (d) Enhanced intracellular release shown by CLSM images of A549 cells incubated with Rho-AuNP-1 or Rho-AuNP-2 for 1 h and TEM images of A549 cells incubated with AuNP-1 or AuNP-2 for 4 h (scale bars: 10 μm). Adapted with permission from [130].
EGFR targeted cyclodextrin-covered AuNP carriers (Fig. 17c) have been developed for non-covalent encapsulation of an anti-cancer drug β-lapachone [130]. Glutathione-mediated release of drugs from the surface of these AuNPs was also demonstrated in MCF-7 (low glutathione concentration) and A549 (high glutathione concentration) cell lines. Images of A549 cells incubated with Rhodamine-labeled RhoCD-AuNP-1 and RhoCD-AuNP-2 for 1 h are shown in Figure 17d. The cells incubated with targeted NPs showed high RhoCD fluorescence and high intracellular uptake as observed by transmission electron microscopy study. The introduction of an anti-EGFR antibody onto the AuNPs was shown to both enhance the uptake of AuNPs and increase the degree of apoptosis.
3.4. Lipid coated nanoparticles
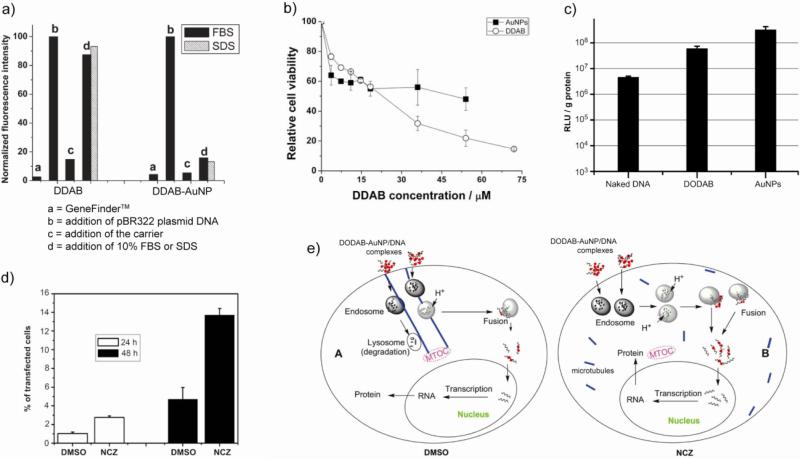
Lipids have been used extensively to modify AuNPs for cellular delivery. Despite the promising use of cationic lipids as non-viral carriers, their practical application is limited due to lower transfection efficiency arising from lower stability of lipid-DNA complexes [131,132], especially in serum [133,134]. The stability of the cationic lipids-DNA complexes increases dramatically after coating the lipids on AuNPs (Fig. 18a), as shown in case of didodecyldimethyl ammonium bromide (DDAB), a cationic lipid that also featured low toxicity (Fig. 18b) [135]. Similar lipid coated AuNPs with enhanced stability have been prepared using dimethyldioctadecylammonium bromide (DODAB), and proved to be five times more efficient than DODAB alone (Fig. 18c) [136]. However, most of the DODAB-AuNPs/DNA complexes were trapped in the endosomes/lysosomes even after 48 h of transfection. To avoid trapping and to further improve the transfection efficient of DODAB-AuNPs, the influence of nocodazole (NCZ), a microtubule depolymerizing agent [137], has been studied [138]. The experimental results showed that NCZ increased the transfection efficiency about 3-fold on cultured HEK 293T cells (Fig. 18d). The cellular trafficking of these AuNPs-DNA complexes was studied using TEM to investigate the mechanism of the transfection. In DMSO pretreated cells the complexes were transported along the microtubules and most of them were translocated to the lysosomes near the MTOC (microtubule organizing center) region. In contrast, in the NCZ pre-treated cells the complexes were dispersed in the cytoplasm and subsequently entered the nucleus. The mechanism of transport of DODAB-AuNP/DNA complexes is schematically shown in Figure 18e.
Fig. 18.
(a) Change in relative fluorescence intensity of GeneFinderTM (a highly sensitive DNA detection dye) at 523 nm as a measure of stability of lipid/DNA and lipid-AuNP/DNA complexes. While bound to DNA, the dye showed higher fluorescence revealing that DDAB-AuNP was stable in FBS (fetal bovine serum) or SDS. (b) Cell viability of DDAB and DDAB-AuNP measured by MTT assay after incubation for 26 hrs. (c) Transfection of PGL3 plasmid DNA inside 293 cells using naked DNA, DNA/DODAB, and DNA/DODAB-AuNP complexes. (d) Transfection efficiency quantified by the percentage of fluorescent cells by flow cytometry. (e) Schematic of transport process of DODAB-AuNPs/DNA complexes in the DMSO and NCZ pre-treated cells. Adapted with permission from [135,136,138].
The incorporation of AuNPs with liposomes provides carriers with enhanced properties. Different ways of preparing liposome-AuNP conjugates include adsorption of AuNPs onto the liposome, loading of AuNPs into the liposome bilayer, and encapsulation of AuNPs inside the liposome. Physical adsorption of AuNPs on liposome membranes promotes the formation of stable dispersions of AuNPs under isotonic conditions without membrane disruption [139]. Loading AuNPs inside the dipalmitoylphosphatidylcholine (DPPC) bilayer increases the membrane fluidity, providing potential in temperature sensitive delivery systems. Similarly, photo-induced hyperthermia on liposome-encapsulated gold nanoshells has been applied in human mammary carcinoma cells [140].
Biomimetic high density lipoprotein (HDL) nanostructures prepared from lipid modified AuNPs have emerged as promising candidates for in vivo imaging and therapeutics. Typically, the synthesis involves thiolated lipids or alkanethiols along with apolipoprotein A1 (an HDL protein) adsorbed onto the AuNP surface and a second layer of lipid adsorbed through hydrophobic interactions between lipid tails and thiolated species [141]. Transportation of cholesterol by natural HDL is physiologically very critical [142,143] and determining the binding constant is essential to provide new synthetic therapeutic agents. It has been shown that the HDL AuNPs are capable of binding fluorescent cholesterol analogues with nanomolar binding affinity (Kd = 4 nM). The HDL AuNPs have also been applied in in vivo imaging of macrophages, whose high density is indicative of high-risk atherosclerosis plaque and other inflammatory diseases [144,145]. The HDL AuNPs were injected in mice on a high cholesterol diet for 10 months, an established model for atherosclerosis [146]. The co-localization studies on macrophages in the sections of aortas excised from the mice 24h post-injection showed that each HDL-coated particle was associated with macrophages. In addition, tomography images of the mice aorta showed deposition of the NPs on the aortic wall. Taken together, nanocrystal HDL can be very successfully applied for molecular imaging of atherosclerosis.
4. Summary and outlook
AuNPs present versatile synthetic scaffolds for efficient delivery of drugs and biomolecules. The combination of their low inherent toxicity, high surface area and tunable surface chemistry contributes to their growing applications in clinical practice. Here, we have described the diversity of surface monolayers on the particles, organic ligands or biomolecules, employed to improve the transfection efficiency of the payloads. These studies hint at the potential for AuNPs in both in vitro and in vivo applications.
There are, however, a number of issues to be resolved before AuNPs mediated delivery can be applied in clinical trials. First, accurate mechanisms of uptake for AuNPs should be known to facilitate optimization of AuNP platforms for efficient internalization. The mechanism of cellular uptake of AuNPs is likely to differ for different classes of AuNPs based on their surface monolayer structure, size, and charge. For example, little is known about the uptake mechanism of negatively charged AuNPs that might involve a different set of proteins, identification of which still remains a challenge. Also, the continued effort for further development of the AuNPs has been directed to address the important challenge of targeting specific cells and eventually tissues and organs. Integration of targeting moieties such as antibodies, aptamers, peptides, and small molecules can be potentially useful (vide supra). However, attaching the targeting moiety without compromising functionality of the monolayer is not trivial. Taken together, there remains a need for continued improvement in the design and synthesis of AuNPs to achieve the ultimate goals of serving as effective delivery vehicles.
Acknowledgements
Research support from the NIH (GM077173) is acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 2.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 3.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 4.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004;56:1649–1659. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Lee CC, MacKay JA, Frechet JMJ, Szoka FC. Designing dendrimers for biological applications. Nat. Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 6.Crampton HL, Simanek EE. Dendrimers as drug delivery vehicles: Non-covalent interactions of bioactive compounds with dendrimers. Polym. Int. 2007;56:489–496. doi: 10.1002/pi.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huynh NT, Passirani C, Saulnier P, Benoit JP. Lipid nanocapsules: A new platform for nanomedicine. Int. J. Pharm. 2009;379:201–209. doi: 10.1016/j.ijpharm.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 8.van Dongen SFM, de Hoog HPM, Peters R, Nallani M, Nolte RJM, van Hest JCM. Biohybrid polymer capsules. Chem. Rev. 2009;109:6212–6274. doi: 10.1021/cr900072y. [DOI] [PubMed] [Google Scholar]
- 9.Peer D, Karp JM, Hong S, FaroKhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 10.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 11.Daniel MC, Astruc D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 12.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005;105:1103–1169. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 13.Hostetler MJ, Wingate JE, Zhong CJ, Harris JE, Vachet RW, Clark MR, Londono JD, Green SJ, Stokes JJ, Wignall GD, Glish GL, Porter MD, Evans ND, Murray RW. Alkanethiolate gold cluster molecules with core diameters from 1.5 to 5.2 nm: core and monolayer properties as a function of core size. Langmuir. 1998;14:17–30. [Google Scholar]
- 14.Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Kim CK, Ghosh P, Rotello VM. Multimodal drug delivery using gold nanoparticles. Nanoscale. 2009;1:61–67. doi: 10.1039/b9nr00112c. [DOI] [PubMed] [Google Scholar]
- 16.Gao XH, Cui YY, Levenson RM, Chung LWK, Nie SM. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 17.Gindy ME, Prud'homme RK. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin. Drug Deliv. 2009;6:865–878. doi: 10.1517/17425240902932908. [DOI] [PubMed] [Google Scholar]
- 18.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. 2010;49:3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie J, Lee S, Chen XY. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010;62:1064–1079. doi: 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951:55–75. [Google Scholar]
- 21.Frens G. Controlled nucleation for regulation of particle-size in monodisperse gold suspensions. Nat.-Phys. Sci. 1973;241:20–22. [Google Scholar]
- 22.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol-derivatized gold nanoparticles in a 2-phase liquid-liquid system. J. Chem. Soc.-Chem. Commun. 1994:801–802. [Google Scholar]
- 23.Templeton AC, Wuelfing MP, Murray RW. Monolayer protected cluster molecules. Acc. Chem. Res. 2000;33:27–36. doi: 10.1021/ar9602664. [DOI] [PubMed] [Google Scholar]
- 24.Fan J, Chen SW, Gao Y. Coating gold nanoparticles with peptide molecules via a peptide elongation approach. Colloids Surf., B. 2003;28:199–207. [Google Scholar]
- 25.McIntosh CM, Esposito EA, Boal AK, Simard JM, Martin CT, Rotello VM. Inhibition of DNA transcription using cationic mixed monolayer protected gold clusters. J. Am. Chem. Soc. 2001;123:7626–7629. doi: 10.1021/ja015556g. [DOI] [PubMed] [Google Scholar]
- 26.Han G, Martin CT, Rotello VM. Stability of gold nanoparticle-bound DNA toward biological, physical, and chemical agents. Chem. Biol. Drug Des. 2006;67:78–82. doi: 10.1111/j.1747-0285.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 27.Sandhu KK, McIntosh CM, Simard JM, Smith SW, Rotello VM. Gold nanoparticle-mediated transfection of mammalian cells. Bioconjugate Chem. 2002;13:3–6. doi: 10.1021/bc015545c. [DOI] [PubMed] [Google Scholar]
- 28.Noh SM, Kim WK, Kim SJ, Kim JM, Baek KH, Oh YK. Enhanced cellular delivery and transfection efficiency of plasmid DNA using positively charged biocompatible colloidal gold nanoparticles. Biochim. Biophys. Acta-Gen. Subj. 2007;1770:747–752. doi: 10.1016/j.bbagen.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Thomas M, Klibanov AM. Conjugation to gold nanoparticles enhances polyethylenimine's transfer of plasmid DNA into mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 2003;100:9138–9143. doi: 10.1073/pnas.1233634100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han G, Chari NS, Verma A, Hong R, Martin CT, Rotello VM. Controlled recovery of the transcription of nanoparticle-bound DNA by intracellular concentrations of glutathione. Bioconjugate Chem. 2005;16:1356–1359. doi: 10.1021/bc050173j. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Chen Y, Li XY, Liu Y. Synthesis of oligo(ethylenediamino)-beta- cyclodextrin modified gold nanoparticle as a DNA concentrator. Mol. Pharm. 2007;4:189–198. doi: 10.1021/mp060045s. [DOI] [PubMed] [Google Scholar]
- 32.Kawano T, Yamagata M, Takahashi H, Niidome Y, Yamada S, Katayama Y, Niidome T. Stabilizing of plasmid DNA in vivo by PEG-modified cationic gold nanoparticles and the gene expression assisted with electrical pulses. J. Control. Release. 2006;111:382–389. doi: 10.1016/j.jconrel.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 33.Lee JS, Green JJ, Love KT, Sunshine J, Langer R, Anderson DG. Gold, poly(beta-amino ester) nanoparticles for small interfering RNA delivery. Nano Lett. 2009;9:2402–2406. doi: 10.1021/nl9009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh PS, Han G, Erdogan B, Rosado O, Krovi SA, Rotello VM. Nanoparticles featuring amino acid-functionalized side chains as DNA receptors. Chem. Biol. Drug Des. 2007;70:13–18. doi: 10.1111/j.1747-0285.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh PS, Kim CK, Han G, Forbes NS, Rotello VM. Efficient gene delivery vectors by tuning the surface charge density of amino acid-functionalized gold nanoparticles. ACS Nano. 2008;2:2213–2218. doi: 10.1021/nn800507t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murthy N, Xu MC, Schuck S, Kunisawa J, Shastri N, Frechet JMJ. J. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4995–5000. doi: 10.1073/pnas.0930644100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verma A, Simard JM, Worrall JWE, Rotello VM. Tunable reactivation of nanoparticle-inhibited beta-galactosidase by glutathione at intracellular concentrations. J. Am. Chem. Soc. 2004;126:13987–13991. doi: 10.1021/ja046572r. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh P, Yang XC, Arvizo R, Zhu ZJ, Agasti SS, Mo ZH, Rotello VM. Intracellular delivery of a membrane-impermeable enzyme in active form using functionalized gold nanoparticles. J. Am. Chem. Soc. 2010;132:2642–2645. doi: 10.1021/ja907887z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joshi HM, Bhumkar DR, Joshi K, Pokharkar V, Sastry M. Gold nanopartncles as carriers for efficient transmucosal insulin delivery. Langmuir. 2006;22:300–305. doi: 10.1021/la051982u. [DOI] [PubMed] [Google Scholar]
- 40.Visaria RK, Griffin RJ, Williams BW, Ebbini ES, Paciotti GF, Song CW, Bischof JC. Enhancement of tumor thermal therapy using gold nanoparticle-assisted tumor necrosis factor-alpha delivery. Mol. Cancer Ther. 2006;5:1014–1020. doi: 10.1158/1535-7163.MCT-05-0381. [DOI] [PubMed] [Google Scholar]
- 41.Goel R, Shah N, Visaria R, Paciotti GF, Bischof JC. Biodistribution of tnf-alpha-coated gold nanoparticles in an in vivo model system. Nanomedicine. 2009;4:401–410. doi: 10.2217/nnm.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi CHJ, Alabi CA, Webster P, Davis ME. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc. Natl. Acad. Sci. U.S.A. 2010;107:1235–1240. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang PH, Sun XS, Chiu JF, Sun HZ, He QY. Transferrin-mediated gold nanoparticle cellular uptake. Bioconjugate Chem. 2005;16:494–496. doi: 10.1021/bc049775d. [DOI] [PubMed] [Google Scholar]
- 44.Chithrani BD, Chan WCW. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7:1542–1550. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 45.Morgan MT, Nakanishi Y, Kroll DJ, Griset AP, Carnahan MA, Wathier M, Oberlies NH, Manikumar G, Wani MC, Grinstaff MW. Dendrimer-encapsulated camptothecins: increased solubility, cellular uptake, and cellular retention affords enhanced anticancer activity in vitro. Cancer Res. 2006;66:11913–11921. doi: 10.1158/0008-5472.CAN-06-2066. [DOI] [PubMed] [Google Scholar]
- 46.Gibson JD, Khanal BP, Zubarev ER. Paclitaxel-functionalized gold nanoparticles. J. Am. Chem. Soc. 2007;129:11653–11661. doi: 10.1021/ja075181k. [DOI] [PubMed] [Google Scholar]
- 47.Hwu JR, Lin YS, Josephrajan T, Hsu MH, Cheng FY, Yeh CS, Su WC, Shieh DB. Targeted paclitaxel by conjugation to iron oxide and gold nanoparticles. J. Am. Chem. Soc. 2009;131:66–68. doi: 10.1021/ja804947u. [DOI] [PubMed] [Google Scholar]
- 48.Dhar S, Daniel WL, Giljohann DA, Mirkin CA, Lippard SJ. Polyvalent oligonucleotide gold nanoparticle conjugates as delivery vehicles for platinum(IV) warheads. J. Am. Chem. Soc. 2009;131:14652–14653. doi: 10.1021/ja9071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown SD, Nativo P, Smith JA, Stirling D, Edwards PR, Venugopal B, Flint DJ, Plumb JA, Graham D, Wheate NJ. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J. Am. Chem. Soc. 2010;132:4678–4684. doi: 10.1021/ja908117a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem.-Biol. Interact. 1998;112:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 51.Sies H. Glutathione and its role in cellular functions. Free Radical Biol. Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 52.Jones DP, Carlson JL, Mody VC, Cai JY, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radical Biol. Med. 2000;28:625–635. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 53.Jones DP, Carlson JL, Samiec PS, Sternberg P, Mody VC, Reed RL, Brown LAS. Glutathione measurement in human plasma, evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin. Chim. Acta. 1998;275:175–184. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 54.Bajaj A, Kondaiah P, Bhattacharya S. Effect of the nature of the spacer on gene transfer efficacies of novel thiocholesterol derived gemini lipids in different cell lines: a structure-activity investigation. J. Med. Chem. 2008;51:2533–2540. doi: 10.1021/jm7010436. [DOI] [PubMed] [Google Scholar]
- 55.Khemtong C, Kessinger CW, Gao JM. Polymeric nanomedicine for cancer MR imaging and drug delivery. Chem. Commun. 2009;24:3497–3510. doi: 10.1039/b821865j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hong R, Han G, Fernandez JM, Kim BJ, Forbes NS, Rotello VM. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J. Am. Chem. Soc. 2006;128:1078–1079. doi: 10.1021/ja056726i. [DOI] [PubMed] [Google Scholar]
- 57.Sapsford KE, Berti L, Medintz IL. Materials for fluorescence resonance energy transfer analysis: beyond traditional donor-acceptor combinations. Angew. Chem. Int. Ed. 2006;45:4562–4588. doi: 10.1002/anie.200503873. [DOI] [PubMed] [Google Scholar]
- 58.Chompoosor A, Han G, Rotello VM. Charge dependence of ligand release and monolayer stability of gold nanoparticles by biogenic thiols. Bioconjugate Chem. 2008;19:1342–1345. doi: 10.1021/bc8000694. [DOI] [PubMed] [Google Scholar]
- 59.Kim BJ, Forbes NS. Single-cell analysis demonstrates how nutrient deprivation creates apoptotic and quiescent cell populations in tumor cylindroids. Biotechnol. Bioeng. 2008;101:797–810. doi: 10.1002/bit.21985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim B, Han G, Toley BJ, Kim CK, Rotello VM, Forbes NS. Tuning payload delivery in tumour cylindroids using gold nanoparticles. Nat. Nanotechnol. 2010;5:465–472. doi: 10.1038/nnano.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giri S, Trewyn BG, Stellmaker MP, Lin VSY. Stimuli-responsive controlled-release delivery system based on mesoporous silica nanorods capped with magnetic nanoparticles. Angew. Chem. Int. Ed. 2005;44:5038–5044. doi: 10.1002/anie.200501819. [DOI] [PubMed] [Google Scholar]
- 62.Agasti SS, Chompoosor A, You CC, Ghosh P, Kim CK, Rotello VM. Photoregulated release of caged anticancer drugs from gold nanoparticles. J. Am. Chem. Soc. 2009;131:5728–5729. doi: 10.1021/ja900591t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakanishi J, Nakayama H, Shimizu T, Ishida H, Kikuchi Y, Yamaguchi K, Horiike Y. Light-regulated activation of cellular signaling by gold nanoparticles that capture and release amines. J. Am. Chem. Soc. 2009;131:3822–3823. doi: 10.1021/ja809236a. [DOI] [PubMed] [Google Scholar]
- 64.Cheng Y, Samia AC, Meyers JD, Panagopoulos I, Fei BW, Burda C. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J. Am. Chem. Soc. 2008;130:10643–10647. doi: 10.1021/ja801631c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng Y, Samia AC, Li J, Kenney ME, Resnick A, Burda C. Delivery and efficacy of a cancer drug as a function of the bond to the gold nanoparticle surface. Langmuir. 2010;26:2248–2255. doi: 10.1021/la902390d. [DOI] [PubMed] [Google Scholar]
- 66.Cheng Y, Meyers JD, Broome AM, Kenney ME, Basilion JP, Burda C. Deep penetration of a pdt drug into tumors by noncovalent drug-gold nanoparticle conjugates. J. Am. Chem. Soc. 2011;133:2583–2591. doi: 10.1021/ja108846h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hostetler MJ, Stokes JJ, Murray RW. Infrared spectroscopy of three-dimensional self-assembled monolayers: N-alkanethiolate monolayers on gold cluster compounds. Langmuir. 1996;12:3604–3612. [Google Scholar]
- 68.Lucarini M, Franchi P, Pedulli GF, Pengo P, Scrimin P, Pasquato L. Epr study of dialkyl nitroxides as probes to investigate the exchange of solutes between the ligand shell of monolayers of protected gold nanoparticles and aqueous solutions. J. Am. Chem. Soc. 2004;126:9326–9329. doi: 10.1021/ja048554f. [DOI] [PubMed] [Google Scholar]
- 69.Kim CK, Ghosh P, Pagliuca C, Zhu ZJ, Menichetti S, Rotello VM. Entrapment of hydrophobic drugs in nanoparticle monolayers with efficient release into cancer cells. J. Am. Chem. Soc. 2009;131:1360–1361. doi: 10.1021/ja808137c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 71.Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011;63:131–135. doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 72.Polizzi MA, Stasko NA, Schoenfisch MH. Water-soluble nitric oxide-releasing gold nanoparticles. Langmuir. 2007;23:4938–4943. doi: 10.1021/la0633841. [DOI] [PubMed] [Google Scholar]
- 73.Mocellin S, Bronte V, Nitti D. Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities. Med. Res. Rev. 2007;27:317–352. doi: 10.1002/med.20092. [DOI] [PubMed] [Google Scholar]
- 74.Rothrock AR, Donkers RL, Schoenfisch MH. Synthesis of nitric oxide-releasing gold nanoparticles. J. Am. Chem. Soc. 2005;127:9362–9363. doi: 10.1021/ja052027u. [DOI] [PubMed] [Google Scholar]
- 75.Kim DK, Dobson J. Nanomedicine for targeted drug delivery. J. Mater. Chem. 2009;19:6294–6307. [Google Scholar]
- 76.Baban DF, Seymour LW. Control of tumour vascular permeability. Adv. Drug Deliv. Rev. 1998;34:109–119. doi: 10.1016/s0169-409x(98)00003-9. [DOI] [PubMed] [Google Scholar]
- 77.Phillips MA, Gran ML, Peppas NA. Targeted nanodelivery of drugs and diagnostics. Nano Today. 2010;5:143–159. doi: 10.1016/j.nantod.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bergen JM, Von Recum HA, Goodman TT, Massey AP, Pun SH. Gold nanoparticles as a versatile platform for optimizing physicochemical parameters for targeted drug delivery. Macromol. Biosci. 2006;6:506–516. doi: 10.1002/mabi.200600075. [DOI] [PubMed] [Google Scholar]
- 79.Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 80.Low PS, Antony AC. Folate receptor-targeted drugs for cancer and inflammatory diseases - preface. Adv. Drug Deliv. Rev. 2004;56:1055–1058. doi: 10.1016/j.addr.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 81.Dixit V, Van den Bossche J, Sherman DM, Thompson DH, Andres RP. Synthesis and grafting of thioctic acid-PEG-folate conjugates onto Au nanoparticles for selective targeting of folate receptor-positive tumor cells. Bioconjugate Chem. 2006;17:603–609. doi: 10.1021/bc050335b. [DOI] [PubMed] [Google Scholar]
- 82.Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong SQ. Gold nanoparticles with a monolayer of doxorubicin-conjugated amphiphilic block copolymer for tumor-targeted drug delivery. Biomaterials. 2009;30:6065–6075. doi: 10.1016/j.biomaterials.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 83.Lee ES, Oh KT, Kim D, Youn YS, Bae YH. Tumor pH-responsive flower-like micelles of poly(L-lactic acid)-b-poly(ethylene glycol)-b-poly(L-histidine) J. Control. Release. 2007;123:19–26. doi: 10.1016/j.jconrel.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patra CR, Bhattacharya R, Mukherjee P. Fabrication and functional characterization of gold nanoconjugates for potential application in ovarian cancer. J. Mater. Chem. 2010;20:547–554. doi: 10.1039/b913224d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen YH, Tsai CY, Huang PY, Chang MY, Cheng PC, Chou CH, Chen DH, Wang CR, Shiau AL, Wu CL. Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol. Pharm. 2007;4:713–722. doi: 10.1021/mp060132k. [DOI] [PubMed] [Google Scholar]
- 86.Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11:169–183. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 87.Paciotti GF, Kingston DGI, Tamarkin L. Colloidal gold nanoparticles: a novel nanoparticle platform for developing multifunctional tumor-targeted drug delivery vectors. Drug Dev. Res. 2006;67:47–54. [Google Scholar]
- 88.Javier DJ, Nitin N, Levy M, Ellington A, Richards-Kortum R. Aptamer-targeted gold nanoparticles as molecular-specific contrast agents for reflectance imaging. Bioconjugate Chem. 2008;19:1309–1312. doi: 10.1021/bc8001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nativo P, Prior IA, Brust M. Uptake and intracellular fate of surface-modified gold nanoparticles. ACS Nano. 2008;2:1639–1644. doi: 10.1021/nn800330a. [DOI] [PubMed] [Google Scholar]
- 90.Uhlmann E, Peyman A. Antisense oligonucleotides - a new therapeutic principle. Chem. Rev. 1990;90:543–584. [Google Scholar]
- 91.Tsai CY, Shiau AL, Cheng PC, Shieh DB, Chen DH, Chou CH, Yeh CS, Wu CL. A biological strategy for fabrication of Au/EGFP nanoparticle conjugates retaining bioactivity. Nano Lett. 2004;4:1209–1212. [Google Scholar]
- 92.Agbasi-Porter C, Ryman-Rasmussen J, Franzen S, Feldheim D. Transcription inhibition using oligonucleotide-modified gold nanoparticles. Bioconjugate Chem. 2006;17:1178–1183. doi: 10.1021/bc060100f. [DOI] [PubMed] [Google Scholar]
- 93.Nitin N, Javier DJ, Richards-Kortum R. Oligonucleotide-coated metallic nanoparticles as a flexible platform for molecular imaging agents. Bioconjugate Chem. 2007;18:2090–2096. doi: 10.1021/bc0701242. [DOI] [PubMed] [Google Scholar]
- 94.Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312:1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 95.Seferos DS, Giljohann DA, Rosi NL, Mirkin CA. Locked nucleic acid-nanoparticle conjugates. ChemBioChem. 2007;8:1230–1232. doi: 10.1002/cbic.200700262. [DOI] [PubMed] [Google Scholar]
- 96.McKenzie F, Faulds K, Graham D. Sequence-specific DNA detection using high-affinity LNA-functionalized gold nanoparticles. Small. 2007;3:1866–1868. doi: 10.1002/smll.200700225. [DOI] [PubMed] [Google Scholar]
- 97.Koshkin AA, Nielsen P, Meldgaard M, Rajwanshi VK, Singh SK, Wengel J. LNA (locked nucleic acid): an RNA mimic forming exceedingly stable LNA : LNA duplexes. J. Am. Chem. Soc. 1998;120:13252–13253. [Google Scholar]
- 98.Giljohann DA, Seferos DS, Patel PC, Millstone JE, Rosi NL, Mirkin CA. Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles. Nano Lett. 2007;7:3818–3821. doi: 10.1021/nl072471q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Giljohann DA, Seferos DS, Prigodich AE, Patel PC, Mirkin CA. Gene regulation with polyvalent siRNA-nanoparticle conjugates. J. Am. Chem. Soc. 2009;131:2072–2073. doi: 10.1021/ja808719p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chiu YL, Rana TM. siRNA function in RNAi: a chemical modification analysis. RNA. 2003;9:1034–1048. doi: 10.1261/rna.5103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oishi M, Nakaogami J, Ishii T, Nagasaki Y. Smart PEGylated gold nanoparticles for the cytoplasmic delivery of siRNA to induce enhanced gene silencing. Chem. Lett. 2006;35:1046–1047. [Google Scholar]
- 102.Jayagopal A, Halfpenny KC, Perez JW, Wright DW. Hairpin DNA-functionalized gold colloids for the imaging of mRNA in live cells. J. Am. Chem. Soc. 2010;132:9789–9796. doi: 10.1021/ja102585v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Massich MD, Giljohann DA, Schmucker AL, Patel PC, Mirkin CA. Cellular response of polyvalent oligonucleotide-gold nanoparticle conjugates. ACS Nano. 2010;4:5641–5646. doi: 10.1021/nn102228s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tkachenko AG, Xie H, Liu YL, Coleman D, Ryan J, Glomm WR, Shipton MK, Franzen S, Feldheim DL. Cellular trajectories of peptide-modified gold particle complexes: comparison of nuclear localization signals and peptide transduction domains. Bioconjugate Chem. 2004;15:482–490. doi: 10.1021/bc034189q. [DOI] [PubMed] [Google Scholar]
- 105.Tkachenko AG, Xie H, Coleman D, Glomm W, Ryan J, Anderson MF, Franzen S, Feldheim DL. Multifunctional gold nanoparticle-peptide complexes for nuclear targeting. J. Am. Chem. Soc. 2003;125:4700–4701. doi: 10.1021/ja0296935. [DOI] [PubMed] [Google Scholar]
- 106.Sun LL, Liu DJ, Wang ZX. Functional gold nanoparticle-peptide complexes as cell-targeting agents. Langmuir. 2008;24:10293–10297. doi: 10.1021/la8015063. [DOI] [PubMed] [Google Scholar]
- 107.Bastus NG, Sanchez-Tillo E, Pujals S, Farrera C, Lopez C, Giralt E, Celada A, Lloberas J, Puntes V. Homogeneous conjugation of peptides onto gold nanoparticles enhances macrophage response. ACS Nano. 2009;3:1335–1344. doi: 10.1021/nn8008273. [DOI] [PubMed] [Google Scholar]
- 108.Liu YL, Shipton MK, Ryan J, Kaufman ED, Franzen S, Feldheim DL. Synthesis, stability, and cellular internalization of gold nanoparticles containing mixed peptide-poly(ethylene glycol) monolayers. Anal. Chem. 2007;79:2221–2229. doi: 10.1021/ac061578f. [DOI] [PubMed] [Google Scholar]
- 109.Patel PC, Giljohann DA, Seferos DS, Mirkin CA. Peptide antisense nanoparticles. Proc. Natl. Acad. Sci. U.S.A. 2008;105:17222–17226. doi: 10.1073/pnas.0801609105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu YL, Franzen S. Factors determining the efficacy of nuclear delivery of antisense oligonucleotides by gold nanoparticles. Bioconjugate Chem. 2008;19:1009–1016. doi: 10.1021/bc700421u. [DOI] [PubMed] [Google Scholar]
- 111.Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde RR, Sastry M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir. 2005;21:10644–10654. doi: 10.1021/la0513712. [DOI] [PubMed] [Google Scholar]
- 112.Aubin-Tam ME, Hwang W, Hamad-Schifferli K. Site-directed nanoparticle labeling of cytochrome c. Proc. Natl. Acad. Sci. U.S.A. 2009;106:4095–4100. doi: 10.1073/pnas.0807299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rana S, Yeh YC, Rotello VM. Engineering the nanoparticle-protein interface: applications and possibilities. Curr. Opin. Chem. Biol. 2010;14:828–834. doi: 10.1016/j.cbpa.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Faulk WP, Taylor GM. Immunocolloid method for electron microscope. Immunochemistry. 1971;8:1081–1083. doi: 10.1016/0019-2791(71)90496-4. [DOI] [PubMed] [Google Scholar]
- 115.Qian XM, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie SM. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 116.Niemeyer CM, Ceyhan B. DNA-directed functionalization of colloidal gold with proteins. Angew. Chem. Int. Ed. 2001;40:3685–3688. doi: 10.1002/1521-3773(20011001)40:19<3685::aid-anie3685>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 117.El-Sayed IH, Huang XH, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005;5:829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 118.Link S, Ei-Sayed MA. Optical properties and ultrafast dynamics of metallic nanocrystals. Annu. Rev. Phys. Chem. 2003;54:331–366. doi: 10.1146/annurev.physchem.54.011002.103759. [DOI] [PubMed] [Google Scholar]
- 119.El-Sayed IH, Huang XH, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006;239:129–135. doi: 10.1016/j.canlet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 120.Au L, Zheng DS, Zhou F, Li ZY, Li XD, Xia YN. A quantitative study on the photothermal effect of immuno gold nanocages targeted to breast cancer cells. ACS Nano. 2008;2:1645–1652. doi: 10.1021/nn800370j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dube DH, Bertozzi CR. Glycans in cancer and inflammation – potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 122.Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim YJ, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconjugate J. 1997;14:569–576. doi: 10.1023/a:1018580324971. [DOI] [PubMed] [Google Scholar]
- 124.Hakomori S, Zhang YM. Glycosphingolipid antigens and cancer therapy. Chem. Biol. 1997;4:97–104. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- 125.Du JA, Yarema KJ. Carbohydrate engineered cells for regenerative medicine. Adv. Drug Deliv. Rev. 2010;62:671–682. doi: 10.1016/j.addr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rojo J, Diaz V, de la Fuente JM, Segura I, Barrientos AG, Riese HH, Bernade A, Penades S. Gold glyconanoparticles as new tools in antiadhesive therapy. ChemBioChem. 2004;5:291–297. doi: 10.1002/cbic.200300726. [DOI] [PubMed] [Google Scholar]
- 127.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco) lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 128.Barrientos AG, de la Fuente JM, Rojas TC, Fernandez A, Penades S. Gold glyconanoparticles: synthetic polyvalent ligands mimicking glycocalyx-like surfaces as tools for glycobiological studies. Chem. Eur. J. 2003;9:1909–1921. doi: 10.1002/chem.200204544. [DOI] [PubMed] [Google Scholar]
- 129.Ojeda R, de Paz JL, Barrientos AG, Martin-Lomas M, Penades S. Preparation of multifunctional glyconanoparticles as a platform for potential carbohydrate-based anticancer vaccines. Carbohydr. Res. 2007;342:448–459. doi: 10.1016/j.carres.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 130.Park C, Youn H, Kim H, Noh T, Kook YH, Oh ET, Park HJ, Kim C. Cyclodextrin-covered gold nanoparticles for targeted delivery of an anti-cancer drug. J. Mater. Chem. 2009;19:2310–2315. [Google Scholar]
- 131.Takahashi T, Harada A, Emi N, Kono K. Preparation of efficient gene carriers using a polyamidoamine dendron-bearing lipid: improvement of serum resistance. Bioconjugate Chem. 2005;16:1160–1165. doi: 10.1021/bc050012f. [DOI] [PubMed] [Google Scholar]
- 132.Tarahovsky YS, Koynova R, MacDonald RC. DNA release from lipoplexes by anionic lipids: correlation with lipid mesomorphism, interfacial curvature, and membrane fusion. Biophys. J. 2004;87:1054–1064. doi: 10.1529/biophysj.104.042895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bajaj A, Kondaiah P, Bhattacharya S. Gene transfection efficacies of novel cationic gemini lipids possessing aromatic backbone and oxyethylene spacers. Biomacromolecules. 2008;9:991–999. doi: 10.1021/bm700930y. [DOI] [PubMed] [Google Scholar]
- 134.Bajaj A, Paul B, Kondaiah P, Bhattacharya S. Structure-activity investigation on the gene transfection properties of cardiolipin mimicking gemini lipid analogues. Bioconjugate Chem. 2008;19:1283–1300. doi: 10.1021/bc700474r. [DOI] [PubMed] [Google Scholar]
- 135.Li PC, Zhang LX, Ai KL, Li D, Liu XH, Wang EK. Coating didodecyldimethyl-ammonium bromide onto au nanoparticles increases the stability of its complex with DNA. J. Control. Release. 2008;129:128–134. doi: 10.1016/j.jconrel.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 136.Li PC, Li D, Zhang LX, Li GP, Wang EK. Cationic lipid bilayer coated gold nanoparticles-mediated transfection of mammalian cells. Biomaterials. 2008;29:3617–3624. doi: 10.1016/j.biomaterials.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 137.Hasegawa S, Hirashima N, Nakanishi M. Microtubule involvement in the intracellular dynamics for gene transfection mediated by cationic liposomes. Gene Ther. 2001;8:1669–1673. doi: 10.1038/sj.gt.3301573. [DOI] [PubMed] [Google Scholar]
- 138.Li D, Li PC, Li GP, Wang J, Wang EK. The effect of nocodazole on the transfection efficiency of lipid-bilayer coated gold nanoparticles. Biomaterials. 2009;30:1382–1388. doi: 10.1016/j.biomaterials.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 139.Kojima C, Hirano Y, Yuba E, Harada A, Kono K. Preparation and characterization of complexes of liposomes with gold nanoparticles. Colloids Surf.-B. 2008;66:246–252. doi: 10.1016/j.colsurfb.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 140.Kasili PM, Vo-Dinh T. Liposome encapsulated gold nanoshells for nanophototherapy induced hyperthermia. Int. J. Nanotechnol. 2005;2:397–410. [Google Scholar]
- 141.Thaxton CS, Daniel WL, Giljohann DA, Thomas AD, Mirkin CA. Templated spherical high density lipoprotein nanoparticles. J. Am. Chem. Soc. 2009;131:1384–1385. doi: 10.1021/ja808856z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Linsel-Nitschke P, Tall AR. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug Discov. 2005;4:648–648. doi: 10.1038/nrd1658. [DOI] [PubMed] [Google Scholar]
- 144.Morand EF, Leech M, Jurgen B. Mif: A new cytokine link between rheumatoid arthritis and atherosclerosis. Nat. Rev. Drug Discov. 2006;5:399–410. doi: 10.1038/nrd2029. [DOI] [PubMed] [Google Scholar]
- 145.Shi L, Kishore R, McMullen MR, Nagy LE. Chronic ethanol increases lipopolysaccharide-stimulated EGR-1 expression in raw 264.7 macrophages - contribution to enhanced tumor necrosis factor alpha production. J. Biol. Chem. 2002;277:14777–14785. doi: 10.1074/jbc.M108967200. [DOI] [PubMed] [Google Scholar]
- 146.Cormode DP, Skajaa T, van Schooneveld MM, Koole R, Jarzyna P, Lobatto ME, Calcagno C, Barazza A, Gordon RE, Zanzonico P, Fisher EA, Fayad ZA, Mulder WJM. Nanocrystal core high-density lipoproteins: a multimodality contrast agent platform. Nano Lett. 2008;8:3715–3723. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]