Abstract
PURPOSE
To evaluate anterior chamber biometric factors associated with the degree of angle widening and intraocular pressure (IOP) reduction after phacoemulsification.
SETTING
University of California, San Francisco, California, USA.
DESIGN
Case series.
METHODS
Anterior chamber parameters obtained by anterior segment coherence tomography were compared preoperatively and 3 months postoperatively. Measurements included the angle opening distance 500 μm anterior to the scleral spur (AOD500), trabecular–iris space area 500 μm from the scleral spur (TISA500), iris curvature (I-Curv), anterior chamber angle (ACA), trabecular–iris space area, anterior chamber volume, anterior chamber width, and lens vault (LV).
RESULTS
The study enrolled 73 eyes. The mean patient age was 77.45 years ± 7.84 (SD); 65.75% of patients were women. From preoperatively to 3 months postoperatively, the mean AOD500 increased significantly (0.254 ± 0.105 to 0.433 ± 0.108 mm) and the mean IOP decreased significantly (14.97 ± 3.35 to 12.62 ± 3.37 mm Hg) (P < .001). The reduction in IOP was correlated with the increase in AOD500 (r = 0.240, P = .041) and preoperative LV (r = 0.235, P = .045). After adjusting for related factors, AOD500 widening was positively correlated with LV (β = 0.458, P = .044) and I-Curv (β = 0.235, P = .043) and negatively correlated with preoperative TISA500 (β = −0.269, P = .025) and ACA (β = −0.919, P = .027).
CONCLUSIONS
Surgically induced AOD widening was significantly correlated with anterior chamber biometric factors. Preoperative LV appears to be a significant factor in angle widening and IOP reduction after phacoemulsification.
There is increasing evidence that uneventful cataract extraction can result in anterior chamber deepening, iridocorneal angle widening, and intraocular pressure (IOP) reduction in glaucomatous or nonglaucomatous eyes.1–10 Several studies11–14 show that cataract extraction provides the opportunity to restore vision and to eliminate a narrow angle in eyes with acute primary angle closure (PAC) or chronic primary angle-closure glaucoma (PACG).
Our previous study15 confirmed that IOP lowering is related to the angle opening induced by cataract surgery. However, the anatomic predictors of angle widening and an IOP decrease after phacoemulsification with foldable intraocular lens (IOL) implantation have not been fully understood.
Anterior segment optical coherence tomography (AS-OCT) provides objective high-resolution images of the anterior chamber angle (ACA) structures. Examples of ACA parameters that can be quantitatively assessed by AS-OCT include angle opening distance (AOD), iris thickness, anterior chamber depth (ACD), trabecular-iris surface area (TISA), angle recess area (ARA), and lens vault (LV)16–20; these parameters are often assessed using custom software.
The purpose of the present study was to prospectively evaluate the changes in the ACA parameters before and 3 month after cataract surgery in eyes with visually significant cataract. Biometric factors related to angle widening and the IOP reduction after surgery were also evaluated.
PATIENTS AND METHODS
Institutional review board approval was obtained from the University of California San Francisco (UCSF) Committee on Human Research. All participants gave written informed consent. Patients were recruited from the glaucoma service at UCSF from March 2009 to November 2010. Inclusion criteria included visually significant cataract, a corrected distance visual acuity worse than 20/40, and an IOP of 21 mm Hg or less. Exclusion criteria included previous penetrating surgery; complications related to the cataract surgery (eg, posterior capsule rupture, vitreous loss); primary or secondary glaucoma, peripheral anterior synechiae, glaucomatous optic neuropathy, or topical glaucoma therapy; nonadherence to the follow-up regimen; a cup-to-disc ratio greater than 0.6 (vertical meridian), which may be consistent with glaucoma; and substantial corneal abnormality2 (eg, edema, dystrophy, abrasion, marginal degeneration, pterygium). If both eyes were having cataract surgery and qualified, right eyes were used in the analysis; however, left eyes that met inclusion criteria were included if the patient's right eye did not meet the inclusion criteria.
Preoperative Evaluation
The preoperative evaluation included slitlamp examination, visual acuity testing, manual refraction, routine fundus examination, gonioscopy, and IOP determination. The IOP was measured using Goldmann applanation tonometry by the same observer (S.L.). Two values were assessed, and the mean value was used for analysis. If the 2 values differed by more than 2 points, a third value was obtained and the median value was chosen. Gonioscopy was performed using a Zeiss-style 4-mirror gonioscopic lens (model OPDSG, Ocular Instruments, Inc.) by the same glaucoma specialist (S.L.) in a dark room. Angles were graded in all 4 quadrants (superior, nasal, temporal, and inferior) based on the Shaffer method. The axial length (AL) was measured using an IOLMaster device (Zeiss Meditec AG). Fundoscopy was performed with +90.0 diopter (D) and +20.0 D lenses. When fundoscopy was not possible, the posterior segment was evaluated with B-scan ultrasonography.
Anterior Segment Ocular Coherence Tomography
The AS-OCT data were collected by 2 experienced operators 4 days before and 3 months after cataract surgery using a Visante AS-OCT device (Carl Zeiss Meditec AG); the operators were masked to the results of the clinical ophthalmic examinations. Standard resolution scans captured temporal and nasal quadrants (nasal–temporal 0 to 180 degrees) in 1 image with patients looking straight ahead and with a good central corneal reflex. All images were taken under the same dark conditions (0 to 1 lux by digital light meter [Easy View model EA30, Extech Instruments, Inc.]) with the patient seated. After several scans were acquired, the operator selected the best image with no motion or image artifacts from the eyelids. Assessment of the superior and inferior quadrants often requires manual manipulation of the eyelids, which may distort the angle. To prevent systematic bias in angle assessment in patients who may have required eyelid manipulation, images of the nasal and temporal quadrants only were included in this study.21
Images were analyzed with the Zhongshan Angle Assessment Program, which has been shown to have good reproducibility for iris measurements.16–19 Initial attention was focused on quality control of the images. Images in which the scleral spur could not be clearly detected were removed from analysis because quantitative evaluation of the anterior chamber parameters by AS-OCT depends on correctly identifying the scleral spur as the landmark.22,23 After the scleral spur is identified, the software calculates various parameters of the iris, cornea, and lens using automated identification of anterior and posterior surfaces of the cornea and iris and the anterior surface of lens. The AOD at 500 μm (AOD500) was defined as the perpendicular distance measured from the trabecular meshwork at 500 μm anterior to the scleral spur to the anterior iris surface; the AOD250 and AOD750 parameters have similar definitions but with their respective distances from the scleral spur. Iris thickness was measured 750 μm (IT750) from the scleral spur. To calculate iris curvature (I-Curv), the software draws a line from the most peripheral to the most central point of the iris pigment epithelium. Next, a perpendicular line is extended from this line to the iris pigment epithelium at the point of greatest convexity.18,19,24 Angle recess area 750 was defined as the area bordered by the anterior surface of the iris surface, corneal endothelium and a line perpendicular to the plane of the trabecular surface to the iris surface from a point 750 μ m anterior to the scleral spur. Trabecular–iris space area 500 (TISA500) is bounded anteriorly by the AOD500 line, posteriorly by a line drawn from the scleral spur perpendicular to the plane of the inner scleral wall to the opposing iris, superiorly by the inner corneoscleral wall, and inferiorly by the iris surface. The ACD was defined as the axial distance from the posterior corneal surface to the lens surface measured at the pupil center. The anterior chamber width (ACW) was measured as a line joining the scleral spurs in the AS-OCT image. The ACA was the angle between the point of the trabecular meshwork 500 μm from the scleral spur and the point on the anterior iris perpendicularly, with the apex at the iris recess. The curvature of the anterior corneal surface (AC-Curv) and posterior corneal surface (PC-Curv) were defined relative to their surfaces between scleral spurs, respectively. Lens vault was defined as the perpendicular distance between the anterior pole of the crystalline lens and a horizontal line drawn between the nasal and temporal scleral spurs. Figure 1 shows a schematic of AOD500, IT750, TISA500, I-Curv, AC-Curv, and PC-Curv. Figure 2 shows the LV and ACW.
Figure 1.
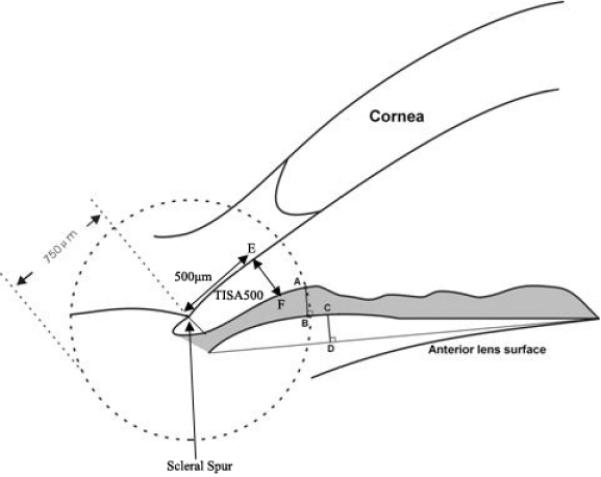
Automated measurement of iris thickness (IT750), angle opening distance (AOD500), I-Curv, trabecular-iris space area (TISA500), corneal curvature of anterior and posterior surface (AC-Curv, PC-Curv). The distance between point A and point B is the iris thickness at 750 μm (IT750). The distance between point E and point F is the AOD 500 μm from scleral spur (AOD500). Iris curvature (I-Curv) is determined by creating a line from the most peripheral to the pupillary end of the iris, and the perpendicular distance from this line to the most convex point along the posterior iris surface (point C to D) is defined as I-Curv. The trabecular–iris space area 500 (TISA500) is an area bounded anteriorly by the AOD 500 line, posteriorly by a line drawn from the scleral spur perpendicular to the plane of the inner scleral wall to the opposing iris, superiorly by the inner corneoscleral wall and inferiorly by the iris surface.
Figure 2.
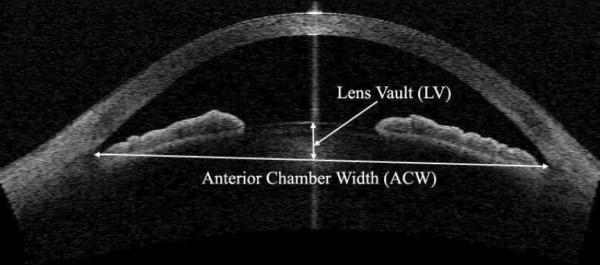
The measurement of LV and ACW (AS-OCT).
The intraobserver and interobserver reproducibility of the iris measurements were assessed using a random subset of 20 images (10 eyes before surgery and 10 eyes after surgery) by 2 examiners (G.H., Y.C.) who were masked to the initial results. The interval of separation for intraobserver assessments was 1 month. Anterior chamber parameters based on AS-OCT imaging and the IOP were compared between preoperatively and 3 months postoperatively.
Surgical Technique
In brief, all operations were performed by the same surgeon (S.L.) using conventional procedures. After a 3.2 mm temporal clear corneal tunnel incision was made, a continuous curvilinear capsulorhexis approximately 5.5 mm in diameter was created with a cystotome and a Utrata forceps. The technique included hydrodissection, hydrodelineation, in-the-bag phacoemulsification using the divide-and-conquer technique, cortical aspiration, and implantation of a single-piece foldable acrylic IOL (Acrysof SA60AT, Alcon Laboratories, Inc.) in the capsular bag. At the end of the operation, the surgeon always confirmed that the IOL was accurately implanted in the capsular bag.
Statistical Analysis
Statistical analysis was performed using SPSS for Windows software (version 13.0, SPSS, Inc.). All data were reported as means ± standard deviations (SD). The differences in compared statistically using the paired t test. The Pearson correlation was used to assess the associations between changes in IOP, ACD, AOD500, iris curvature, and LV. Linear regression analysis (age- and sex-adjusted linear regression model), adjusted for age and sex, was performed to assess the effect of each variable on AOD500 widening and ACD deepening postoperatively. Multivariate linear regression models were further constructed with increases in the AOD500 and ACD as the dependent variables and the relevant predictive factors as covariates. Factors significant at a level of P < .25 in age-adjusted and sex-adjusted linear regression models were included in multivariate regression analysis adjusted for pupil diameter. The Pearson correlation was used to assess the associations between preoperative LV and the changes in AOD500 or ACD. Intraobserver agreement was performed to assess the repeatability of AS-OCT images. Probability values less than 0.05 were considered statistically significant.
RESULTS
Of 86 eyes (86 consecutive patients) that had uneventful phacoemulsification, 13 eyes (15.12%) were excluded because the scleral spur could not be properly identified. With the exclusions, the study comprised 73 eyes of 73 patients. Twenty-nine eyes (39.73%) had Shaffer grades of 2 or less in 3 or all quadrants by gonioscopy, and 44 eyes (60.27%) had Shaffer grades of 3 to 4 in 3 or all quadrants. Table 1 shows the patients' demographics and baseline data.
Table 1.
Baseline demographics of patients.
| Characteristic | Value |
|---|---|
| Sex, n (%) | |
| Male | 25 (34.2) |
| Female | 48 (65.8) |
| Eye, n (%) | |
| Right | 38 (52.1) |
| Left | 35 (47.9) |
| Age (y) | |
| Mean ± SD | 77.45 ± 7.84 |
| Range | 57, 93 |
| Axial length (mm) | |
| Mean ± SD | 23.69 ± 1.26 |
| Range | 20.71, 26.84 |
| Lens vault (mm) | |
| Mean ± SD | 0.58 ± 0.38 |
| Range | −0.29, 1.39 |
| Preop IOP(mm Hg) | |
| Mean ± SD | 14.97 ± 3.35 |
| Range | 8.00, 23.00 |
| Anterior corneal surface curvature (mm) | |
| Mean ± SD | 7.35 ± 0.50 |
| Range | 5.87, 8.43 |
| Posterior corneal surface curvature (mm) | |
| Mean ± SD | 6.48 ± 0.39 |
| Range | 5.20, 7.39 |
| Central corneal thickness (μm) | |
| Mean ± SD | 554.00 ± 31.00 |
| Range | 482.00, 638.00 |
IOP = intraocular pressure
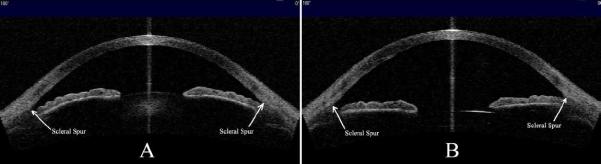
The mean AOD500, TISA500, ACD, ARA, anterior chamber volume (ACV), and ACA values increased significantly after phacoemulsification with IOL implantation (Table 2); however, IOP decreased significantly. The iris was significantly flatter after surgery, as shown by the decrease in the curvature of the posterior iris surface (P < .001). No significant difference was found for IT750, ACW, and pupil width. Figure 3 shows representative preoperative and postoperative AS-OCT images.
Table 2.
Comparison of biometric parameters between preoperatively and 3 months postoperatively.
| Mean±SD | ||||
|---|---|---|---|---|
| Mean Difference (95% CI) | ||||
| Parameter | Preop | Postop | P Value | |
| IOP (mm Hg) | 14.97 ± 3.35 | 12.62 ± 3.37 | 1.99, 2.70 | <.001 |
| AOD500 (mm) | 0.254 ± 0.105 | 0.433 ± 0.108 | −0.207, −0.151 | <.001 |
| ACW (mm) | 11.62 ± 0.55 | 11.62 ± 0.52 | −0.106, 0.004 | .069 |
| ACD (mm) | 2.52 ± 0.43 | 3.84 ± 0.29 | −1.384, −1.241 | <.001 |
| Pupil (mm) | 3.74 ± 0.95 | 3.67 ± 0.96 | −0.020, 0.148 | .131 |
| ACV (mm3) | 132.06 ± 32.69 | 181.49 ± 33.67 | −56.64, 42.21 | <.001 |
| ACA (degree) | 19.33 ± 3.97 | 26.12 ± 6.80 | −8.46, −5.12 | <.001 |
| I-Curv (mm) | 0.235 ± 0.130 | 0.183 ± 0.100 | 0.017, 0.086 | .004 |
| TISA500 (mm2) | 0.105 ± 0.035 | 0.159 ± 0.035 | −0.063, −0.045 | <.001 |
| ARA750 (mm2) | 0.234 ± 0.104 | 0.343 ± 0.093 | −0.130, −0.087 | <.001 |
| IT750 (mm) | 0.444 ± 0.091 | 0.443 ± 0.096 | −0.020, 0.023 | .871 |
ACA = anterior chamber angle; ACD = anterior chamber depth; ACV = anterior chamber volume; ACW = anterior chamber width; AOD500 = angle opening distance 500 μm anterior to the scleral spur; ARA750 = angle recess area 750 μm anterior to the scleral spur; CI = confidence interval; I-Curv = iris curvature; I0P = intraocular pressure; IT750 = iris thickness measured at 750 μm; TISA500 = trabecular-iris space area 500 μm from sclera spur
Figure 3.
A: Anterior chamber configuration before cataract surgery. B: Anterior chamber configuration after cataract surgery. Note the widening of the anterior chamber, deepening of ACD, and relative flattening of the iris profile postoperatively (AS-OCT).
In the age- and sex-adjusted linear regression model, changes in the AOD after surgery were positively correlated with the preoperative LV and iris curvature and negatively correlated with the preoperative AOD500, ACD, TISA500, ACV, ARA, and ACA (Table 3). Changes in the ACD after surgery were positively correlated with the preoperative LV and negatively correlated with the preoperative ACD, AOD500, TISA500, ARA, ACA, ACW, ACV, and axial length (AL) (Table 4).
Table 3.
Linear regression analysis of the association between preoperative biometric parameters and changes in AOD.
| Anterior Chamber Widening (mm) | ||||
|---|---|---|---|---|
| Age-Sex Model* | Multivariate Model† | |||
| Characteristic | β (95% CI) | P Value | β (95% CI) | P Value |
| Age (y) | 0.231 (0.000, 0.007) | .049 | 0.059 (−0.002, 0.004) | .589 |
| Sex, female | 0.041 (−0.047, 0.067) | .729 | −0.068 (−0.070, 0.036) | .527 |
| Preop AOD500 (mm) | −0.535 (−0.814, −0.369) | <.001 | −0.239 (−0.765, 0.227) | .282 |
| Preop iris curvature (mm) | 0.380(0.144, 0.533) | .001 | 0.235 (0.007, 0.412□ | .043 |
| Preop TISA500 (mm2) | −0.443 (−2.169, −0.749) | <.001 | −0.269 (−1.65, −0.117□ | .025 |
| Preop IT750 (mm) | 0.067 (−0.222, 0.392) | .581 | – | |
| Preop ARA750 (mm2) | −0.441 (−0.728, −0.254□ | <.001 | −0.263 (−0.803, 0.216) | .254 |
| Preop ACA (degree) | −0.347 (−0.017, −0.004) | .003 | −0.919 (−0.051, −0.003□ | .027 |
| Preop ACD (mm) | −0.274 (−0.135, −0.012) | .019 | −1.108(0.022, 0.573) | .034 |
| Preop LV (mm) | 0.378 (0.048, 0.182) | .001 | 0.458 (−0.015, 0.293) | .044 |
| Preop AC-Curv (mm□ | −0.053 (−0.068, 0.044) | .659 | – | |
| Preop PC-Curv (mm) | −0.178 (−0.120, 0.015) | .128 | −0.126 (−0.153, 0.071) | .466 |
| CCT (μm) | 0.020 (−0.001, 0.001) | .870 | – | |
| Preop pupil (mm) | −0.127 (−0.046, 0.015) | .306 | 0.153 (−0.020, 0.059□ | .330 |
| Preop ACW (mm) | −0.096 (−0.070, 0.029) | .417 | – | |
| Preop ACV (mm3) | −0.359 (−0.002, 0.000) | .020 | 2.237 (−0.004, 0.021) | .202 |
| Preop IOP(mm Hg) | 0.020 (−0.007, 0.009) | .864 | – | |
| Axial length (mm) | −0.210 (−0.032, 0.002) | .086 | −0.075 (−0.023, 0.012) | .544 |
ACA = anterior chamber angle; AC-Curv = curvature of anterior corneal surface; ACD = anterior chamber depth; ACV = anterior chamber volume; ACW = preoperative anterior chamber width; AOD500 = angle opening distance 500 μm anterior to the scleral spur; ARA = preoperative angle recess area; CCT = central corneal thickness; CI = confidence interval; IOP = intraocular pressure; IT750 = iris thickness measured at 750 μm; LV = lens vault; PC-Curv = curvature of posterior corneal surface; TISA500 = trabecular-iris space area 500 μm from scleral spur
Adjusted for age and sex
Adjusted for age, sex, pupil, axial length, preoperative AOD500, preoperative iris curvature, preoperative TISA500, preoperative ARA750, preoperative ACA, preoperative ACD, preoperative LV, preoperative PC-Curv, preoperative ACV, and axial length (adjusted R2 = 0.373)
Table 4.
Linear regression analysis of the association between preoperative biometric parameters and changes in ACD.
| Anterior Chamber Widening (mm) | ||||
|---|---|---|---|---|
| Age-Sex Model* | Multivariate Model† | |||
| Characteristic | β (95% CI) | P Value | β (95% CI) | P Value |
| Age (y) | 0.324 (0.004, 0.022) | .005 | −0.066 (−0.009, 0.004) | .440 |
| Sex, female | 0.139 (−0.054, 0.233) | .220 | 0.034 (−0.079, 0.122) | .670 |
| Preop AOD500 (mm) | −0.408 (−1.814, −0.582) | <.001 | 0.030 (−0.850, 1.020) | .856 |
| Preop iris curvature (mm) | 0.124 (−0.249, 0.834) | .285 | – | |
| Preop TISA500 (mm2) | −0.322 (−4.705, −0.927□ | .004 | −0.182 (−4.421, 1.402) | .303 |
| Preop IT750 (mm) | −0.072 (−1.018, 0.535□ | .537 | – | |
| Preop ARA750 (mm2) | −0.289 (−1.496, −0.208) | .010 | 0.132 (−0.557, 1.292) | .429 |
| Preop ACA (degree) | −0.641 (−0.063, −0.036) | <.001 | −0.577 (−0.249, 0.164) | .680 |
| Preop ACD (mm) | −0.684 ((−0.604, −0.371) | <.001 | −0.207 (−0.785, 0.505) | .665 |
| Preop LV (mm) | 0.747 (0.478, 0.723) | <.001 | 0.792(0.499, 0.710) | <.001 |
| Preop AC-Curv (mm□ | −0.142 (−0.231, 0.056) | .228 | −0.188 (−0.290, 0.056) | .181 |
| Preop PC-Curv (mm) | −0.211 (−0.338, 0.008) | .062 | 0.071 (−0.254, 0.368) | .714 |
| CCT (μm) | 0.089 (−0.001, 0.003) | .453 | – | |
| Pupil (mm) | 0.029 (−0.068, 0.087) | .808 | 0.076 (−0.047, 0.096) | .497 |
| Preop ACW (mm) | −0.264 (−0.264, −0.018) | .025 | −0.152 (−0.164, −0.008□ | .031 |
| Preop ACV (mm3) | −0.579 (−0.007, −0.004) | <.001 | 0.560 (−0.016, 0.027) | .640 |
| Preop IOP (mm Hg) | 0.111 (−0.010, 0.031□ | .326 | – | |
| Axial length (mm) | −0.286 (−0.097, −0.012) | .013 | 0.036 (−0.026, 0.040) | .687 |
ACA = anterior chamber angle; AC-Curv = curvature of anterior corneal surface; ACD = anterior chamber depth; ACV = anterior chamber volume; ACW = preoperative anterior chamber width; AOD500 = angle opening distance 500 μm anterior to the scleral spur; ARA = preoperative angle recess area; CCT = central corneal thickness; CI = confidence interval; IOP = intraocular pressure; IT750 = iris thickness measured at 750 μm; LV = lens vault; PC-Curv = curvature of posterior corneal surface; TISA500 = trabecular-iris space area 500 μm from scleral spur
Adjusted for age and sex
Adjusted for age, sex, pupil, axial length, preoperative AOD, preoperative TISA500, preoperative ARA750, preoperative ACA, preoperative ACD, preoperative LV, preoperative corneal curvature, preoperative ACW, preoperative ACV, and axial length (adjusted R2 = 0.687)
In the multivariate regression model of changes in AOD500, age, sex, AL, and other significant systemic factors were included as covariates (Table 3). Widening of AOD500 postoperatively was positively correlated with the preoperative LV and the preoperative iris curvature and negatively correlated with the preoperative ACD, TISA500, and ACA. In the multivariate regression model of changes in ACD, age, sex, AL, and other significant systemic factors were included as covariates (Table 4); changes in ACD after surgery were positively correlated with the preoperative LV and negatively correlated with the preoperative ACW. When AOD750 and AOD250 were entered in both univariate regression and multivariate modeling as a representation of AOD, the correlations were roughly the same as for AOD500 (results not shown).
Pearson correlation analysis of the association between preoperative LV and changes in AOD500 or ACD before surgery and after surgery showed that preoperative LV was positively related to the changes in AOD500 (r = 0.358, P = .002) and ACD (r = 0.787, P < .001). Figure 4 shows the associations between preoperative LV and the changes in AOD500 and in ACD.
Figure 4.
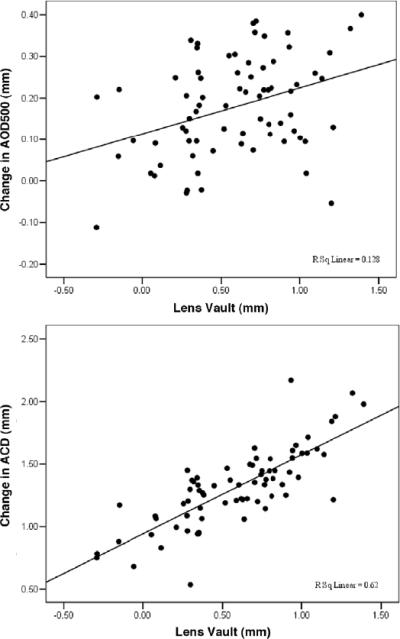
Correlation between the changes in AOD500 and preoperative LV (r = .358, P = .002). B: Correlation between the changes in ACD and preoperative LV (r = 0.787, P < .001) (ACD = anterior chamber depth; AOD500 = angle opening distance 500 μm anterior to the scleral spur).
Pearson correlation analysis of the association between changes in IOP, ACD, AOD500, iris curvature, and LV showed that the reduction in IOP was significantly related to an increase in AOD500 (r = 0.240, P = .041) and ACD (r = 0.233, P = .047) and reduction in IOP was positively related to preoperative IOP (r = 0.245, P = .036) and preoperative LV (r = 0.235, = .045). When AOD500 was controlled for, the increase in LV was not significantly related to the reduction in IOP (r = 0.164, P = .168). No significant correlation was found between IOP reduction and preoperative iris curvature (r = 0.154, P = .193) or changes in iris curvature before surgery and after surgery (r = 0.130, P = .274).
Table 5 shows intraobserver and interobserver reproducibility of anterior chamber parameters in the nasal quadrants. All the parameters showed excellent reproducibility in the intraobserver tests and interobserver tests. The table also shows the means, differences, and confidence intervals for the differences between measurements.
Table 5.
Intraclass correlation coefficients for anterior chamber parameters in a random subset of 20 eyes.
| Intraobserver | Interobserver | |||||
|---|---|---|---|---|---|---|
| Parameter | ICC (95% CI) | Overall Mean | Difference | ICC (95% CI) | Overall Mean | Difference |
| AOD500 | 0.967 (0.917, 0.987) | 0.316 | 0.004 | 0.926(0.814, 0.971) | 0.334 | −0.042 |
| TISA500 | 0.941 (0.852, 0.977) | 0.124 | 0.001 | 0.921 (0.801, 0.969) | 0.129 | −0.013 |
| IT750 | 0.946 (0.862, 0.978) | 0.425 | −0.005 | 0.974 (0.934, 0.990) | 0.431 | −0.008 |
| ARA750 | 0.967 (0.916, 0.987) | 0.254 | 0.011 | 0.925 (0.812, 0.970) | 0.265 | −0.034 |
| ACA | 0.989 (0.972, 0.996) | 24.404 | −0.342 | 0.989 (0.973, 0.996) | 24.409 | −0.352 |
| ACD | 0.934 (0.833, 0.974) | 3.568 | −0.054 | 0.934 (0.833, 0.974) | 3.569 | −0.057 |
| ACW | 0.993 (0.983, 0997) | 11.745 | 0.009 | 0.990 (0.975, 0.996) | 11.699 | 0.081 |
| ACV | 0.991 (0.978, 0.997) | 157.191 | 0.956 | 0.992 (0.980, 0.997) | 157.256 | −1.086 |
| LV | 0.997 (0.988, 0.999) | 658.148 | 18.464 | 0.986 (0.950, 0.996) | 633.587 | 67.585 |
ACA = anterior chamber angle; ACD = anterior chamber depth; ACV = anterior chamber volume; ACW = anterior chamber width; AOD500 = angle opening distance 500 μm anterior to the scleral spur; ARA750 = angle recess area 750 μm anterior to the scleral spur; CI = confidence interval; ICC = intraclass correlation coefficient; IT750 = iris thickness measured at 750 μm; LV = lens vault; TISA500 = trabecular–iris space area at 500 μm from scleral spur
DISCUSSION
Based on quantitative assessment of AS-OCT imaging, the present study confirms previous reports of an increase in the anterior chamber angle opening and anterior chamber deepening after phacoemulsification with foldable IOL implantation for cataract. Furthermore, our results suggest that after adjusting for other known biometric parameters, LV, I-Curv, ACD, TISA, and ACA are independent predictors of a wider anterior ACA opening and LV and ACW are independent predictors of greater ACD after phacoemulsification for visually significant cataract. More important, we found that preoperative LV was related to IOP reduction after surgery; thus, LV could be a preoperative predictor in AOD widening and IOP reduction after cataract surgery. We are unaware of previous reports of this finding and could find no reference to it in a computerized search of the PubMed database.
Numerous studies1–10 have shown that cataract surgery plays a specific role in reducing IOP in glaucomatous eyes and in nonglaucomatous eyes. The present study confirmed those results; the mean IOP decreased from 14.97 ± 3.35 mm Hg preoperatively to 12.55 ± 3.30 mm Hg 3 months postoperatively (P < .001). Studies1,25–27 have also focused on changes in angle configuration 1 to 12 months after surgery based on anterior segment imaging by Scheimpflug devices, ultrasound biomicroscopy, and AS-OCT and support our finding that phacoemulsification can result in significantly deepening of the ACD and widening of the AOD. Several studies3,7,28,29 report that a surgically induced reduction in IOP was significantly related to preoperative IOP and ACD deepening after cataract surgery. Our previous study15 confirmed that the postoperative reduction in IOP is proportional to the increase in angle width in narrow-angle eyes and open-angle eyes after phacoemulsification with IOL implantation for cataract. We found that each 0.1 mm of increase in AOD500 corresponded to a mean IOP decrease of 0.42 ± 0.18 mm Hg in narrow-angle eyes (P < .001) and of 0.32 ± 0.16 mm Hg in open-angle eyes (P = .046). The current study included 29 eyes (39.73%) with Shaffer grades of 2 or less in 3 or all quadrants by gonioscopy and 44 eyes (60.27%) with Shaffer grades of 3 to 4 in 3 or all quadrants.
The purpose of the current study was to understand the predictors related to angle opening and anterior chamber deepening as well a decrease in IOP 3 months after phacoemulsification for visually significant cataract. We found that LV was significantly related to AOD widening and AOD deepening and to IOP reduction. In terms of surgically induced ACD deepening, we found that changes in the ACD after surgery were positively correlated with LV (β = 0.792, P < .001) and negatively correlated with ACW (β = −0.152, P < .031). This may not be a surprising finding because a study by Nongpiur et al.30 found that LV to be independently associated with angle closure. The authors hypothesize that an increased LV likely increases the amount of iridolenticular contact, leading to a more pronounced iris curvature, pupillary block, and angle crowding. Nongpiur et al.20 found a smaller ACW in eyes with a shallower ACD in a population-based study. In the present study, AOD500 was significantly increased (from 0.254 ± 0.105 mm to 0.433 ± 0.108 mm) from preoperatively to 3 months after surgery (P < .001). In the multivariate regression model adjusted for other significant factors, the increase in AOD500 was positively correlated with LV and iris curvature and inversely correlated with ACD, TISA, and ACA. Increased lens thickness and forward movement of the anterior lens surface may result in forward shift of the iris, thus decreasing the ACD, AOD, and ACV.31–33 In a clinic-based study, Cheon et al.34 confirmed that ACA parameters, including AOD, ARA, and TISA, showed significantly negative slopes with aging. Theoretically, however, cataract surgery can correct the age-related tendency toward a decrease in ACD and AOD by removing the crystalline lens, which continuously thickens with age.
In the current study, patients with PAC or PACG were excluded, although further study would help us better understand the determinants of angle opening induced by cataract surgery in cases of PACG as well as the association between IOP reduction and angle opening. The relationship between LV and IOP reduction (P = .045) is likely mediated through the angle opening because adjustment for AOD500 resulted in an insignificant correlation value (P = .168). Refractive lens exchange (RLE) for PAC is being advocated by some,35 and our findings may help provide a basis for preoperative assessment of anterior segment features that could help predict IOP control for RLE in eyes with angle-closure glaucoma.
Limitations of this study include unavailability of data on lens thickness and relative lens position. This would have provided further information about the predictors of angle widening before cataract surgery and refractive outcomes. In addition, a longer follow-up would allow evaluation of the long-term associations between ACD deepening, AOD widening, and systemic and ocular parameters.
In summary, eyes with higher LV, deeper I-Curv, narrower TISA, shallower ACD, and narrower ACA are more likely to achieve greater angle opening after cataract removal. Preoperative LV was the only factor among these that correlated with greater IOP reduction. Its relationship is likely mechanistically mediated by the wider angle opening associated with cataract surgery. These findings can have clinical significance for patients with IOP control issues.
Eyes with higher lens vault, deeper iris curvature, a narrower trabecular–iris space, a shallower ACD, and a narrower anterior chamber angle were more likely to achieve greater angle opening and greater IOP reduction after cataract removal.
Acknowledgments
Supported by core grant EY002162, National Eye Institute, Bethesda, Maryland, That Man May See, Inc., San Francisco, California, and Research to Prevent Blindness, New York, New York, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: No author has a financial or proprietary interest in any material or method mentioned.
REFERENCES
- 1.Hayashi K, Hayashi H, Nakao F, Hayashi F. Changes in anterior chamber angle width and depth after intraocular lens implantation in eyes with glaucoma. Ophthalmology. 2000;107:698–703. doi: 10.1016/s0161-6420(00)00007-5. [DOI] [PubMed] [Google Scholar]
- 2.Tai M-C, Chien K-H, Lu D-W, Chen J-T. Angle changes before and after cataract surgery assessed by Fourier-domain anterior segment optical coherence tomography. J Cataract Refract Surg. 2010;36:1758–1762. doi: 10.1016/j.jcrs.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Shin HC, Subrayan V, Tajunisah I. Changes in anterior chamber depth and intraocular pressure after phacoemulsification in eyes with occludable angles. J Cataract Refract Surg. 2010;36:1289–1295. doi: 10.1016/j.jcrs.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Dooley I, Charalampidou S, Malik A, Loughman J, Molloy L, Beatty S. Changes in intraocular pressure and anterior segment morphometry after uneventful phacoemulsification cataract surgery. [Accessed August 2, 2011];Eye. 2010 24:519–526. doi: 10.1038/eye.2009.339. CME quiz 527. Available at: http://www.nature.com/eye/journal/v24/n4/pdf/eye2009339a.pdf. [DOI] [PubMed] [Google Scholar]
- 5.Cho YK. Early intraocular pressure and anterior chamber depth changes after phacoemulsification and intraocular lens implantation in nonglaucomatous eyes; comparison of groups stratified by axial length. J Cataract Refract Surg. 2008;34:1104–1109. doi: 10.1016/j.jcrs.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Uçakhan Ö Ö , Özkan M, Kanpolat A. Anterior chamber parameters measured by the Pentacam CES after uneventful phacoemulsification in normotensive eyes. [Accessed August 3, 2011];Acta Ophthalmol (Oxf) 2009 87:544–548. doi: 10.1111/j.1755-3768.2008.01305.x. Available at: http://onlinelibrary.wiley.com/doi/10.1111/j.1755-3768.2008.01305.x/pdf. [DOI] [PubMed] [Google Scholar]
- 7.Poley BJ, Lindstrom RL, Samuelson TW, Schulze R., Jr. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes; evaluation of a causal relationship between the natural lens and open-angle glaucoma. J Cataract Refract Surg. 2009;35:1946–1955. doi: 10.1016/j.jcrs.2009.05.061. [DOI] [PubMed] [Google Scholar]
- 8.Mathalone N, Hyams M, Neiman S, Buckman G, Hod Y, Geyer O. Long-term intraocular pressure control after clear corneal phacoemulsification in glaucoma patients. J Cataract Refract Surg. 2005;31:479–483. doi: 10.1016/j.jcrs.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 9.Lee SJ, Lee CK, Kim WS. Long-term therapeutic efficacy of phacoemulsification with intraocular lens implantation in patients with phacomorphic glaucoma. J Cataract Refract Surg. 2010;36:783–789. doi: 10.1016/j.jcrs.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Kurimoto Y, Park M, Sakaue H, Kondo T. Changes in the anterior chamber configuration after small-incision cataract surgery with posterior chamber intraocular lens implantation. Am J Ophthalmol. 1997;124:775–780. doi: 10.1016/s0002-9394(14)71694-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhuo Y-H, Wang M, Li Y, Hao Y-T, Lin M-K, Fang M, Ge J. Phacoemulsification treatment of subjects with acute primary angle closure and chronic primary angle-closure glaucoma. J Glaucoma. 2009;18:646–651. doi: 10.1097/IJG.0b013e31819c4322. [DOI] [PubMed] [Google Scholar]
- 12.Tham CCY, Kwong YYY, Leung DYL, Lam SW, Li FCH, Chiu TYH, Chan JCH, Lam DSC, Lai JSM. Phacoemulsification vs phacotrabeculectomy in chronic angle-closure glaucoma with cataract; complications. Arch Ophthalmol. 2010;128:303–311. doi: 10.1001/archophthalmol.2010.12. correction, 1128. [DOI] [PubMed] [Google Scholar]
- 13.Tarongoy P, Ho CL, Walton DS. Angle-closure glaucoma: the role of the lens in the pathogenesis, prevention, and treatment. Surv Ophthalmol. 2009;54:211–225. doi: 10.1016/j.survophthal.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Lai JSM, Tham CCY, Chan JCH. The clinical outcomes of cataract extraction by phacoemulsification in eyes with primary angle-closure glaucoma (PACG) and co-existing cataract; a prospective case series. J Glaucoma. 2006;15:47–52. doi: 10.1097/01.ijg.0000196619.34368.0a. [DOI] [PubMed] [Google Scholar]
- 15.Huang G, Gonzalez E, Peng P-H, Lee R, Leeungurasatien T, He M, Porco T, Lin S. Anterior chamber depth, iridocorneal angle width and intraocular pressure changes after phacoemulsification: narrow versus open iridocorneal angles. In press, Arch Ophthalmol. 2011 doi: 10.1001/archophthalmol.2011.272. [DOI] [PubMed] [Google Scholar]
- 16.Wang B, Sakata LM, Friedman DS, Chan Y-H, He M, Lavanya R, Wong T-Y, Aung T. Quantitative iris parameters and association with narrow angles. Ophthalmology. 2010;117:11–17. doi: 10.1016/j.ophtha.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Quigley HA, Silver DM, Friedman DS, He M, Plyler RJ, Eberhart CG, Jampel H, Ramulu P. Iris cross-sectional area decreases with pupil dilation and its dynamic behavior is a risk factor in angle closure. J Glaucoma. 2009;18:173–179. doi: 10.1097/IJG.0b013e31818624ce. [DOI] [PubMed] [Google Scholar]
- 18.Console JW, Sakata LM, Aung T, Friedman DS, He M. Quantitative analysis of anterior segment optical coherence tomography images: the Zhongshan Angle Assessment Program. Br J Ophthalmol. 2008;92:1612–1616. doi: 10.1136/bjo.2007.129932. [DOI] [PubMed] [Google Scholar]
- 19.Wang B-S, Narayanaswamy A, Amerasinghe N, Zheng C, He M, Chan Y-H, Nongpiur ME, Friedman DS, Aung T. Increased iris thickness and association with primary angle closure glaucoma. [Accessed August 2, 2011];Br J Ophthalmol. 2011 95:46–50. doi: 10.1136/bjo.2009.178129. Available at: http://bjo.bmj.com/content/95/1/46.full.pdf. [DOI] [PubMed] [Google Scholar]
- 20.Nongpiur ME, Sakata LM, Friedman DS, He M, Chan Y-H, Lavanya R, Wong TY, Aung T. Novel association of smaller anterior chamber width with angle closure in Singaporeans. Ophthalmology. 2010;117:1967–1973. doi: 10.1016/j.ophtha.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Radhakrishnan S, See J, Smith SD, Nolan WP, Ce Z, Friedman DS, Huang D, Li Y, Aung T, Chew PTK. Reproducibility of anterior chamber angle measurements obtained with anterior segment optical coherence tomography. [Accessed August 2, 2011];Invest Ophthalmol Vis Sci. 2007 48:3683–3688. doi: 10.1167/iovs.06-1120. Available at: http://www.iovs.org/cgi/reprint/48/8/3683. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Li H, Dorairaj S, Cheung CYL, Rousso J, Liebmann J, Ritch R, Lam DSC, Leung CKS. Assessment of scleral spur visibility with anterior segment optical coherence tomography. J Glaucoma. 2010;19:132–135. doi: 10.1097/IJG.0b013e3181a98ce4. [DOI] [PubMed] [Google Scholar]
- 23.Sakata LM, Lavanya R, Friedman DS, Aung HT, Seah SK, Foster PJ, Aung T. Assessment of the scleral spur in anterior segment optical coherence tomography images. [Accessed August 2, 2011];Arch Ophthalmol. 2008 126:181–185. doi: 10.1001/archophthalmol.2007.46. Available at: http://archopht.ama-assn.org/cgi/reprint/126/2/181. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa H, Liebmann JM, Ritch R. Quantitative assessment of the anterior segment using ultrasound biomicroscopy. Curr Opin Ophthalmol. 2000;11:133–139. doi: 10.1097/00055735-200004000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Tham CCY, Leung DYL, Kwong YYY, Li FCH, Lai JSM, Lam DSC. Effects of phacoemulsification versus combined phaco-trabeculectomy on drainage angle status in primary angle closure glaucoma (PACG) J Glaucoma. 2010;19:119–123. doi: 10.1097/IJG.0b013e31819d5d0c. [DOI] [PubMed] [Google Scholar]
- 26.Pereira FA, Cronemberger S. Ultrasound biomicroscopic study of anterior segment changes after phacoemulsification and foldable intraocular lens implantation. Ophthalmology. 2003;110:1799–1806. doi: 10.1016/S0161-6420(03)00623-7. [DOI] [PubMed] [Google Scholar]
- 27.Nolan WP, See JL, Aung T, Friedman DS, Chan Y-H, Smith SD, Zheng C, Huang D, Foster PJ, Chew PTK. Changes in angle configuration after phacoemulsification measured by anterior segment optical coherence tomography. J Glaucoma. 2008;17:455–459. doi: 10.1097/IJG.0b013e3181650f31. [DOI] [PubMed] [Google Scholar]
- 28.Issa SA, Pacheco J, Mahmood U, Nolan J, Beatty S. A novel index for predicting intraocular pressure reduction following cataract surgery. [Accessed August 2, 2011];Br J Ophthalmol. 2005 89:543–546. doi: 10.1136/bjo.2004.047662. Available at: http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1772653&blobtype=pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kashiwagi K, Kashiwagi F, Tsukahara S. Effects of small-incision phacoemulsification and intraocular lens implantation on anterior chamber depth and intraocular pressure. J Glaucoma. 2006;15:103–109. doi: 10.1097/00061198-200604000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Nongpiur ME, He M, Amerasinghe N, Friedman DS, Tay W-T, Baskaran M, Smith SD, Wong TY, Aung T. Lens vault, thickness, and position in Chinese subjects with angle closure. Ophthalmology. 2011;118:474–479. doi: 10.1016/j.ophtha.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 31.Fontana ST, Brubaker RF. Volume and depth of the anterior chamber in the normal aging human eye. Arch Ophthalmol. 1980;98:1803–1808. doi: 10.1001/archopht.1980.01020040655013. [DOI] [PubMed] [Google Scholar]
- 32.Markowitz SN, Morin JD. The ratio of lens thickness to axial length for biometric standardization in angle-closure glaucoma. Am J Ophthalmol. 1985;99:400–402. doi: 10.1016/0002-9394(85)90005-4. [DOI] [PubMed] [Google Scholar]
- 33.He M, Huang W, Li Y, Zheng Y, Yin Q, Foster PJ. Refractive error and biometry in older Chinese adults: the Liwan Eye Study. [Accessed August 2, 2011];Invest Ophthalmol Vis Sci. 2009 50:5130–5136. doi: 10.1167/iovs.09-3455. Available at: http://www.iovs.org/content/50/11/5130.full.pdf. [DOI] [PubMed] [Google Scholar]
- 34.Cheon MH, Sung KR, Choi EH, Kang SY, Cho JW, Lee S, Kim JY, Tchah HW, Kook MS. Effect of age on anterior chamber angle configuration in Asians determined by anterior segment optical coherence tomography; clinic-based study. Acta Ophthalmol (Oxf) 2010;88(6):e205–e210. doi: 10.1111/j.1755-3768.2010.01960.x. [DOI] [PubMed] [Google Scholar]
- 35.Thomas R, Walland MJ, Parikh RS. Clear lens extraction in angle closure glaucoma. Curr Opin Ophthalmol. 2011;22:110–114. doi: 10.1097/ICU.0b013e3283437bdc. [DOI] [PubMed] [Google Scholar]