Abstract
RAB25, a member of the rat sarcoma (RAS) family of small GTPase, has been implicated in the pathophysiology of ovarian, breast and other cancers. Its role in endosomal transport and recycling of cell-surface receptors and signaling proteins presents a novel paradigm for the disruption of cellular pathways and promotion of tumor development and aggressiveness. Variations in structure and post-translational modifications control the localization of RAS superfamily proteins to specific subcellular compartments and recruitment of downstream effectors, allowing these small GTPases to function as sophisticated modulators of a complex and diverse range of cellular processes. Here, we review the link between RAB25 and tumor development and current knowledge regarding its possible roles in cancer.
Keywords: breast, cancer, CATX-8, endocytosis, ovarian, Rab25, small GTPases
The rat sarcoma (RAS) oncoprotein small GTPase superfamily contains over 170 members, divided into five subfamilies—RAS, RHO, RAB, RAN and ARF (1). Members of the RAB superfamily play important roles in regulating signal transduction and, subsequently, a diverse range of cellular processes, including differentiation, proliferation, vesicle transport, nuclear assembly and cytoskeleton formation. Among these small G proteins, the Ras subfamily is the most studied, primarily because of its critical roles in human oncogenesis (2,3). Recently, another member of the RAS superfamily RAB25 has been implicated in cancer (4–7).
Rab proteins, first identified as Ras-related genes expressed in rat brain (8), comprise the largest subfamily of small GTPases, with more than 70 putative members in the human genome (1). Studies on Rab GTPases, along with their associated regulators and effectors, have revealed that Rab proteins are major regulators of intracellular vesicular transport and trafficking of proteins between organelles of the endocytic and secretory pathways (9). Here, we review the biology of Rab proteins and the role of RAB25 in cancer.
Biology of RAB25 Proteins
RAB25 (also known as CATX-8) was first isolated from rabbit gastric parietal cells using 3′-rapid amplification of complementary DNA ends with a degenerate primer to the WDTAGQE small GTPase consensus of the GTP-binding sequence (10). In contrast to most RABs, which are ubiquitously expressed, RAB25 expression was confined to the gastrointestinal mucosa, lung and kidney: the highest levels of expression were in the colon and ileal epithelium (10). The ubiquitously expressed Rab11 proteins (Rab11a and Rab11b), which are homologous to the yeast YPT3 protein (10), are the closest homologues to Rab25, forming the Rab11 subfamily.
RAB11 subfamily proteins, like all RAS superfamily proteins, are thought to share a conserved mechanism of regulation (Figure 1). The activity of the protein is determined by the relative amount of GTP-bound (active) versus GDP-bound (inactive) forms. GTP binding induces conformational changes in the switch I and switch II regions, resulting in the modulation of binding affinities that are critical for association with regulatory and effector proteins (11–13). In vivo, the GDP/GTP exchange and GTPase activity are regulated by a complex regulatory network consisting of several classes of proteins, including guanine nucleotide exchange factors (14), which promote dissociation of bound GDP and formation of the active GTP-bound complex (14), whereas GTPase-activating proteins accelerate the intrinsic GTPase activity of the small GTPases to promote formation of the inactive GDP-bound form (15). Rab GTPases are further regulated by guanine nucleotide dissociation inhibitors that inhibit GDP dissociation and promote cytosolic sequestration of these GTPases (16,17). Rab11 and Rab25, detected in the apical recycling endosome (ARE), perinuclear recycling endosome (PRE) and trans Golgi network (TGN) (Figure 1), regulate cellular functions including proliferation, signal transduction, apoptosis, microtubule organization, recruitment of H+K+ ATPase, transferrin receptor recycling, immunoglobulin A transcytosis and integrin trafficking (4,18–25).
Figure 1.
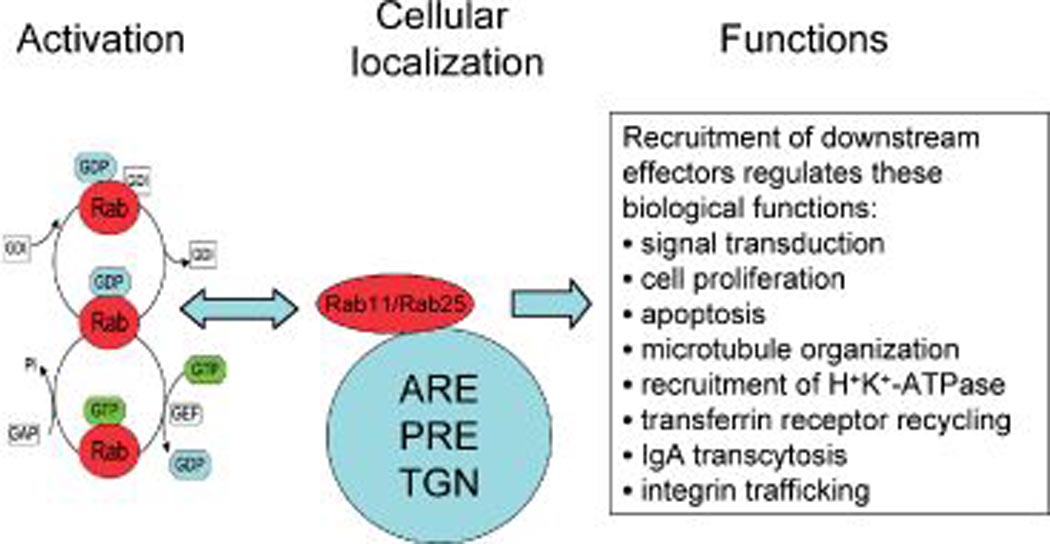
Biological function of RAB11 family. RAB11 and Rab25, located in the ARE, PRE and TGN, are activated by molecular switches cycling between GDP-bound and GTP-bound states through interaction with the GDP dissociation inhibitor (GDI), guanine nucleotide exchange factor (GEF) and GTPase-activating proteins (GAPs). Once activated, RAB11 and RAB25 recruit specific downstream effectors for different biological functions.
Interestingly, analysis of the GTP-binding domain consensus sequence (WDTAGQE) of Ras family members revealed that Rab25 has a unique glutamine (Q) to leucine (L) substitution (WDTAGLE) (10). This substitution is commonly observed in oncogenic mutant versions of small GTPases, such as the homologous Q61L mutation in H-Ras, with reduced GTPase activity, resulting in a dominant constitutively GTP-bound active conformation and increased transforming activity (26). This suggests that Rab25 likely exists naturally in a preferentially GTP-bound active state. Early in vitro studies have suggested that rabbit Rab25 expresses GTPase activity that can be induced with gastric parietal mucosal cytosolic extracts despite the Q-to-L substitution (19). However, this observation should be interpreted with caution because the use of a bacterial expression system in the study by Casanova et al. (19) may have led to a different post-translational modification. Therefore, human Rab25 GTPase activity deserves further evaluation.
Ras proteins undergo post-translational modifications, such as farnesylation, prenylation and geranylgeranylation, which mediate membrane attachment, subcellular localization protein–protein interactions and inhibit proteolytic degradation, enabling additional mechanisms of functional regulation (27). Similarly, Rab subfamily proteins terminate in a distinct set of cysteine-containing C-terminal motifs (CC, CXC, CCX, CCXX or CCXXX; C = cysteine, X = any amino acid), allowing modification by geranylgeranyltransferase II that targets Rab GTPases to different membrane locations (28).
Rab25 and Cancer
Based on the expression of RAB25 in normal colonic epithelium, Goldenring et al. (10) demonstrated increased RAB25 expression in colon cancer cell lines HT-29 and LIM1215, although it remains unclear whether this was a consequence of the tissue-specific expression pattern of RAB25 or critical to oncogenesis.
Using high-resolution array comparative genomic hybrid- ization profiling, we demonstrated that the minimum region of recurrent amplification in the 1q22 amplicon contained RAB25 in 54% of ovarian cancers (4). Analysis of all open-reading frames in this region revealed that RAB25 messenger RNA (mRNA) levels were selectively increased in ovarian cancer. Furthermore, ovarian cancer patients with RAB25 DNA amplification or increased RNA levels (> 2 × normal ) had poor prognoses, indicating an important role for RAB25 in tumor progression and aggressiveness. In vivo studies also support a role for RAB25 in tumor growth and aggressiveness in ovarian cancer in that expression of RAB25 increases tumor development, while downregulation of RAB25 by RNA interference (RNAi) transfection significantly inhibits ovarian cancer growth (4,5). Together, these data strongly implicate RAB25 in ovarian tumorigenesis and aggressiveness, and in keeping with these results, RAB25 overexpression has been suggested to be a marker of serous ovarian-peritoneal cancer, the most aggressive form of ovarian cancer (29).
Like ovarian cancer, breast cancer exhibits 1q gains in approximately 50% of tumors (4,30); however, in breast cancer, in contrast to the specific 1q22 overamplification that occurs in ovarian cancer, the broader 1q amplification in breast cancer has impeded identification of its putative drivers. Based on the results from our previous study of ovarian cancer (4), we explored the role of RAB25 in the breast cancer 1q amplification. RAB25 gene amplification and mRNA overexpression, measured by array comparative genomic hybridization expression arrays and quantitative polymerase chain reaction, respectively, are correlated with shorter overall survival in breast cancer [(4); unpublished reanalysis of data from Ref. (30)]. In addition, RAB25 mRNA and protein appear to exhibit a subtype-specific pattern of expression with high levels of expression in estrogen receptor (ER)- and Her2-positive tumors, intermediate expression in basal tumors and low or absent expression in metaplastic breast cancers (unpublished data). This mirrors the recent findings of Cheng et al. (6), who observed high Rab25 expression in 92% (11 of 12) of ER-positive samples and apparent loss of expression in 83% (5 of 6) of ER-negative and progesterone receptor-negative samples, because the latter group likely comprises basal- and triple-negative tumors (6). This suggests that Rab25 may have different roles in breast cancer, depending on the subtype.
Korkola et al. (31) profiled 74 testicular germ cell tumors, the most common solid tumors in young adult men, and found RAB25 amplified in 45% of cases. RAB25 and APOA2 were the only two genes in the 1q amplicon exhibiting at least a fourfold increase in expression in tumors relative to normal testes. RAB25, therefore, represents a candidate oncogenic driver in testicular germ cell tumors.
Chromosome 1q gain is also a feature of Wilms tumors. In a series of Wilms tumor samples, 1q gain was observed in 55% of patients whose disease relapsed versus 20% of those whose disease did not, providing a significant association between decreased rates of relapse-free survival and gain of 1q (32). The amplicon, initially defined as extending from 1q22 to 1q25, was remapped using a chromosome 1 tiling path array to 1q22–23.1, which encompasses RAB25. While RAB25 was overexpressed, neither its expression nor that of the other genes in the amplicon tested showed significant correlation with gene copy number, suggesting that an alternative mechanism mediates increased RAB25 expression. Therefore, it is currently unclear what role RAB25 and the 1q22–23.1 amplicon play in Wilms tumors.
Increased levels of RAB25 mRNA expression have been reported in 11 early-stage transitional cell carcinomas of the bladder compared with normal levels of expression in the urothelium (33). Also, increased RAB25 expression was observed in a case series of 11 hepatocellular carcinomas and a cholangiohepatoma along with dysregulated expression of a number of other Rab proteins (RAB1B, RAB4B, RAB10, RAB22A and RAB24) (34). In contrast, Ray et al. (35) found, by immunohistochemistry, loss of RAB25 with increasing dysplasia in a series of 60 endoscopic biopsies in patients with Barrett's esophagus.
Calvo et al. (36) suggested that RAB25 expression may be implicated in prostate cancer pathogenesis and aggressiveness. Using the transgenic C3(1)/tag-prostate cancer mouse model, which develops prostate intraepithelial neoplasia (PIN) that progresses to invasive carcinoma with systemic metastases, they derived one cell line each from low-grade PIN, high-grade PIN, and primary invasive prostatic carcinoma and two cell lines from lung metastases, which in vitro retained a characteristic progression with increasing tumorigenicity. RAB25 was differentially overexpressed in parallel with increasing aggressiveness of the cell lines. To our knowledge, there are no published reports specifically on whether RAB25 expression is increased in human prostate cancers, although expression profiles in the Oncomine research edition version 3.6 (http://www.oncomine.org) datasets suggest that RAB25 levels in prostate cancer may be similar to those in breast and ovarian cancers. In addition, Rab-interacting proteins such as Rab11FIP1/RCP, identified as a RAB11/RAB25 interacting protein (37), are also frequently aberrant in cancer, being both amplified and overexpressed in breast cancer, e.g. Refs. (30,38). While we have investigated the potential functional role of RAB25 in ovarian and breast cancers, its tumorigenic role in other epithelial cancers remains unclear, and investigation of the role of RAB25 in these cancers may be fruitful.
Rab25 and the Hallmarks of Cancer
Studies on the physiological function of Rab25 in gastric parietal cells and the polarized Madin-Darby canine kidney cell line suggest that Rab25 regulates apical but not basolateral endosomal recycling. This is based on Rab25′s localization to the recycling endosomes in association and similarity with Rab11a, segregation from the ER/Golgi soluble NSF (NEM-sensitive factor) attachment protein receptor (SNARE), protein-containing endosomes, binding to myosin Vb, and ability to alter immunoglobulin A apical recycling and transcytosis but not basolateral recycling (19–22). Maintenance of appropriate recycling of membrane receptors and proteins is essential for regulating cellular polarity and cell signaling. Deregulated expression of endosomal proteins has been implicated in oncogenesis through mechanisms such as enhancement of growth factor signaling (39) and will be discussed in greater detail below.
Growth, proliferation and apoptosis
It has been demonstrated that the expression of RAB25 increases tumor development while downregulation of RAB25 by RNAi transfection significantly inhibits ovarian cancer growth in vitro and in vivo xenograft models (4,5). In addition, engineered Rab25 overexpression in ovarian cancer cells (A2780, HEY, DOV13, IOSE80) increased proliferation, invasion, migration, and in vivo tumorigenicity while concomitantly reducing apoptosis in response to various stimuli (4). These results have been independently confirmed by Fan et al. (5) using short hairpin RNA (shRNA) to knockdown RAB25 expression in ovarian A2780 cells, such that RAB25 shRNA-transfected cells exhibited slower proliferation, increased apoptosis and decreased tumor growth in mouse xenografts than empty vector or untransfected parental cells.
In contrast, a recent study suggested that Rab25 may instead have a tumor suppressor function in basal- and triple-negative breast tumors (6). Using RAO-3 cells (transformed by Q61L mutant H-RAS) as a model, expression of Rab25 decreased proliferation and anchorage-independent growth (6). In vivo, RAO-3 cells exclusively formed spindle cell tumors, possibly equivalent to metaplastic tumors, which appeared to have low RAB25 expression. In addition, while RAS expression has been reported to be increased in breast cancers, RAS mutations are rare (< 5%); thus, the Rao-3 cells may not be representative of most epithelial breast cancers but may instead be more akin to sarcomatous metaplastic tumors. Thus, the effects of Rab25 on the transformed phenotype may be context dependent, with differing roles in metaplastic and epithelial tumors.
Invasion and migration
Using a breast cancer metastasis model with a well characterized nonmetastatic (MTC) and metastatic (MTLn3) rat tumor cell pair derived from the same original rat mammary adenocarcinoma, retaining their relative metastatic phenotypes after prolonged culture, Wang et al. (40) attempted to identify genes associated with metastasis as determined by complementary DNA microarray analyses. Comparative differential gene expression analysis of metastatic and nonmetastatic tumors and their progenitor cells, both in culture and in live primary tumors, revealed 70 genes known to be involved in cancer invasion and metastasis (40). RAB25 expression was among the highest, with at least a sixfold increase in metastatic MTLn3 cells in vivo, suggesting RAB25 might regulate metastasis (40). However, the molecular mechanism by which Rab25 participates in tumor cell migration remained unclear until a recent study demonstrated a recycling role of integrin by Rab25.
Caswell et al. (7) have demonstrated that RAB25 promotes ovarian cancer cell invasive migration in part by altering the trafficking of integrin α5β1 within a three-dimensional matrix that is characterized by the extension of long pseudopodia at the cell front as cells migrate. RAB25 promotes localization of vesicles that deliver integrin to the plasma membrane at the pseudopodia tips as well as the retention of a pool of cycling α5β1 integrin at the cell front (7). This indicates that Rab25 could contribute to tumor progression by directing the localization of integrin recycling vesicles, thereby enhancing the ability of tumor cells to directionally invade the extracellular matrix (ECM) and, hence, metastasize. Further study has revealed that the increased recycling of integrin α5β1 requires the association of Rab11FIP/RCP at the tips of the long pseudopods; thus, this Rab11 effector represents a key component of an integrin recycling system that controls an α5β1-mediated migration mode (41). Although colocalization of RAB25 and RAB11FIP1/RCP at the pseudopodia tip has not been examined in these studies, it is reasonable to argue that Rab25 interacts with Rab11FIP1/RCP in controlling integrin α5β1 trafficking, as Rab11FIP1/RCP was found to bind Rab25 (37). Furthermore, RAB11 has been shown to control recycling of α6β4 integrin in breast cancer cells in a way that may contribute to hypoxia-induced invasive migration (25). Together, these studies implicate the importance of the RAB11 subfamily in controlling cell migration and invasion.
Growth factor receptor signaling
Characterization of one half of the known Rab GTPases has not only revealed the complexity of membrane trafficking circuits but has also shown that Rab GTPases are critical for mediating signaling transduction propagation ’along‘ the endocytic pathway, in part via the formation of endosome-specific signaling complexes (38,42,43). For instance, epidermal growth factor receptor (EGFR) is frequently overexpressed in human tumors, including breast and lung cancers, glioblastoma, head and neck cancer, bladder carcinoma, colorectal cancer and prostate cancer (44). EGFR is also overexpressed in up to 75% of primary ovarian cancers, in which activation of the EGFR-signaling pathway increased cell proliferation, angiogenesis and metastasis, decreased apoptosis, and was associated with advanced-stage disease and poor prognosis (45). Indeed, prolonged retention of the EGFR on the plasma membrane, induced by impairing clathrin-mediated endocytosis via expression of dynamin K44A, led to reduced activity of intracellular signaling pathways, such as mitogen-activated protein kinases (MAPKs) ERK1/2 or the p85 subunit of phosphatidylinositol 3-kinase (PI3K) (46). Additional biochemical evidence documenting the role of endosomes in epidermal growth factor (EGF)- and platelet-derived growth factor (PDGF)-signal propagation has been obtained by using specific reversible inhibitors of EGFR and PDGF-receptor tyrosine kinases to cause internalization of ligands bound to inactive nonphosphorylated receptors (47,48). These complexes were then specifically activated in endosomes on inhibitor washout and were able to recruit signaling molecules and elicit biological responses. Interestingly, studies describing selective isolation of internalized EGFR by use of reversibly biotinylated antibodies (42) revealed that although both plasma membrane and endosomal pools of EGFR remained active, some downstream signaling molecules were preferentially associated with one of these pools, such as Grb2 and Eps8, which were associated with cell surface and endosomal EGFR pools, respectively.
Recent studies have implicated RAB5 in EGFR signal transduction via endocytosis and RAB5 effector proteins APPL1 (Adaptor protein, phosphotyrosine interaction, PH domain and leucine zipper containing 1) and 2, which are localized to a subpopulation of Rab5-positive endosomes that appear segregated from the well-characterized canonical early endosomes marked by another Rab5 effector, EEA1 (Early endosome antigen 1) (49). The APPL-harboring endosomes selectively acquire endocytic cargo, such as the EGFR, but not transferrin receptors, and represent a specialized endosomal compartment devoted to mediating critical signaling events. Further analysis of APPL1 has demonstrated that its intracellular distribution is dynamic and changes in response to extracellular stimuli, such as EGF or oxidative stress (50). In addition, Rab5 regulates EGFR trafficking dependent on the presence of Eps8, which binds RN-tre, a specific Rab5 GTPase-activating protein, through its SH3 domain and inhibits Rab5-dependent functions, including internalization of the EGFR (51). Therefore, endosomes can function as intracellular platforms for active signal propagation, enabling a precise spatial and temporal control of cellular responses (52). Using immunofluorescence staining analysis, we have observed colocalization of both EGFR and RAB5 proteins after EGF stimulation of ovarian A2780 RAB25-transfected cells (Figure 2A). To investigate the role of Rab25 protein in EGFR trafficking, we have measured cell-surface EGFR level by flow cytometry using a fluorescein isothiocyanate-conjugated antibody to the extracellular EGFR domain RAB25-overexpressing ovarian A2780 and control (empty vector-transfected) cells were treated with 100 ng/mL of EGF for times ranging from 1 min to 24 h. As expected, the cell-surface EGFR level was decreased in both RAB25-overexpressing ovarian A2780 and parental cells after the addition of EGF (Figure 2B). Interestingly, the pattern of change in the EGFR level was different in these two cells (Figure 2B). In the Rab25-expressing cells, EGF treatment caused the cell-surface EGFR level to be lower than that in control cells between 15 and 40 min but higher after 60 min, suggesting a possible role of Rab25 in regulating EGFR trafficking.
Figure 2.
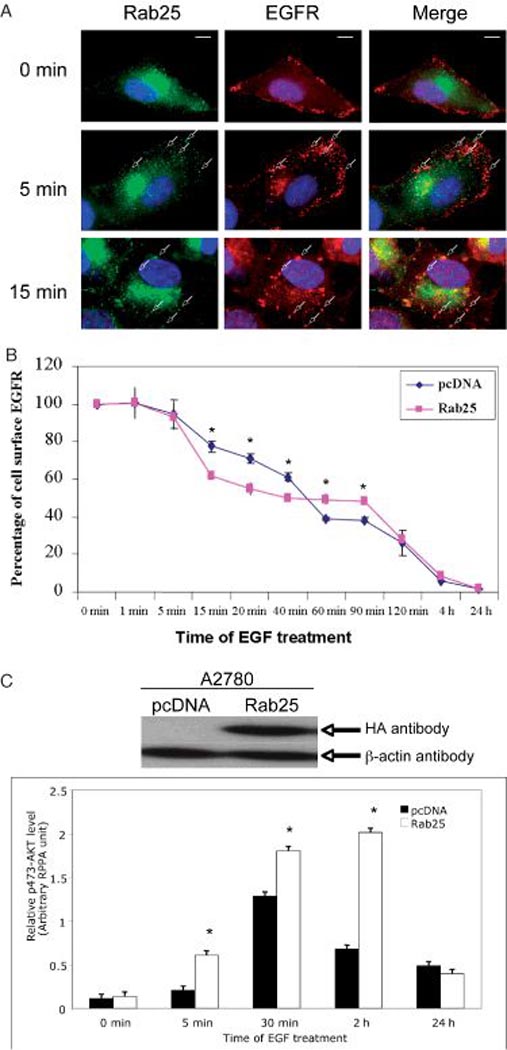
Rab25 regulates EGF signaling. A) Immunofluorescence staining of EGFR (red) and Rab25 (green) in ovarian A2780 cells. Colocalization of EGFR and Rab25 (i.e. yellow) is indicated by arrows. Cells were cultured in complete media, then serum starved for 24 h (0 min), followed by addition of EGF to the final concentration of 100 ng/mL for indicated times. Single scale bar, 10 µm. B) Cell-surface EGFR level detection. Ovarian cancer A2780 cells stably expressing RAB25 or control cells were treated with 100 ng/mL EGF for different time-points. The EGFR level was measured by flow cytometry using a fluorescein isothiocyanate (FITC)-conjugated antibody specific to the extracellular EGFR. *p < 0.05. C) Rab25 expression alters EGFR-induced AKT activity. Ovarian cancer A2780 cells stably expressed Rab25 and control (pcDNA-transfected) cells, detected by western blotting analysis (upper panel), were stimulated with EGF 100 ng/mL for indicated times before total protein isolation and measured by reverse-phase protein lysate array (lower panel). *p < 0.01.
AKT, a serine and threonine kinase also known as protein kinase B, is a key molecule involved in the transduction of many cell survival signals. Our previous study showed that overexpression of RAB25 in ovarian cancer cells resulted in increased AKT phosphorylation, an indication of activation of the PI3K/AKT pathway (4). With the aid of reverse-phase protein lysate array technology (53), we observed an increase in AKT activity in Rab25-expressing A2780 cells after EGF stimulation, as indicated by the level of serine 473 phosphorylation (Figure 2C), suggesting a potential role of Rab25 in the recycling of EGFR and/or mediating of EGFR signal transduction.
Rab25 interactome
Expression of RAB25 profoundly alters cellular functions, including the ability of cancer cells to proliferate, survive, migrate and invade (4,5). Thus, a gain or loss of function of RAB25 proteins, resulting from gene amplification and expression (4) or from the recruitment of downstream effectors, could profoundly impact tumorigenesis. Protein–protein interaction mapping represents a generic strategy to elucidate protein function in general. A protein interaction network for Rab25 (Figure 3A) was constructed using information obtained from the I2D database (54), literature and yeast 2 hybrid data (unpublished observations). In addition to the putative Rab25 interactions with EGFR, the network highlights a number of novel proteins and pathways. When Smad4 or transforming growth factor-β (TGF-β) receptor type I was used as bait in a novel high-throughput luminescence-based mammalian interactome (LUMIER) mapping approach, Rab25 selectively bound both Smad4 and TGFβR1 with a high affinity, indicative of a role in TGF-β signaling (55). Our preliminary data suggest that the Rab25 and TGF-β interaction is potentially functional, as expression of Rab25 in A2780 cells completely inhibited TGF-β-induced p21CIP1 but not in the empty vector-transfected cells (Figure 3B). Furthermore, Rab25 markedly inhibited TGF-β-induced activation of the Smad-binding sequences, CAGA boxes promoter and did not alter activation of the plasminogen activator inhibitor -1 (PAI-1) promoter (Figure 3B), indicating selectivity of Rab25 in conducting TGF-β signals.
Figure 3.
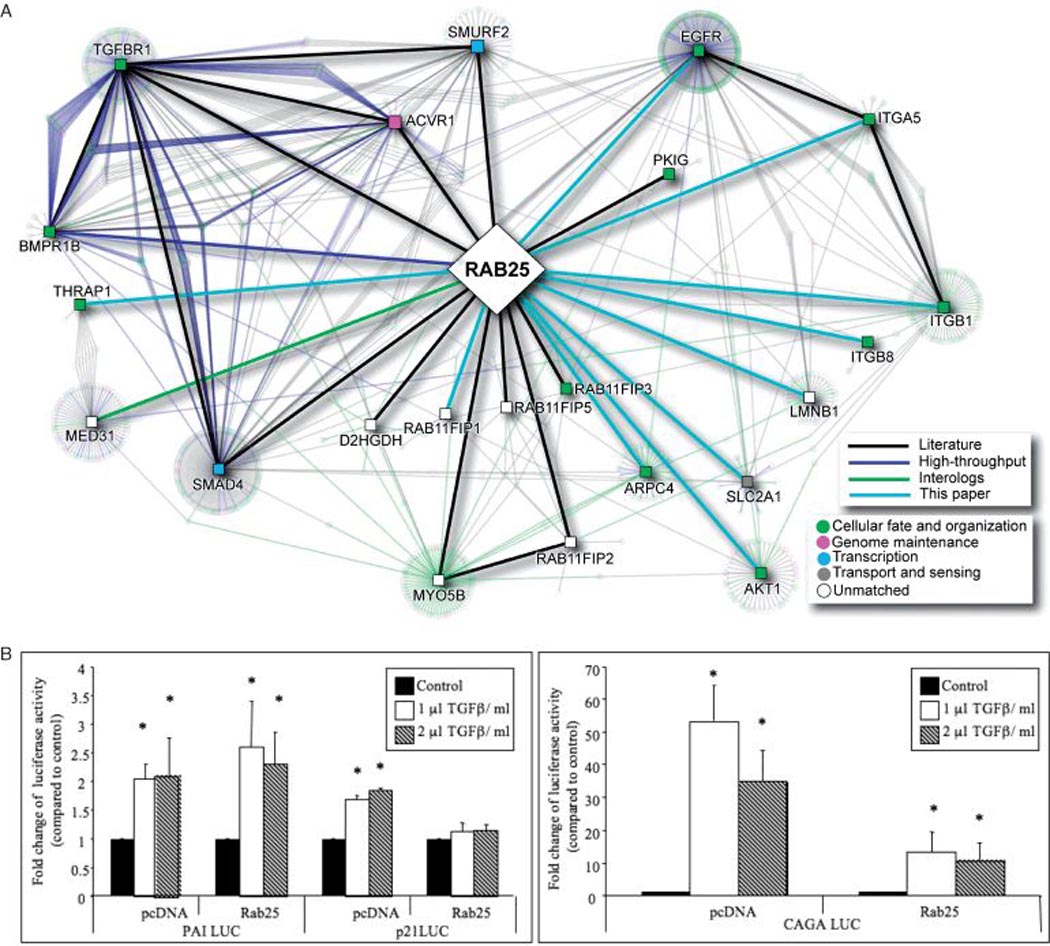
Rab25 Networks. A) Rab25 protein–protein interaction network. The network was constructed by querying the I2D database version 1.71 (http://ophid.utoronto.ca/i2d). Only direct, physical interactions were included, covering human curated, high-throughput, and interologous interactions (further details about interaction sources are present at http://ophid.utoronto.ca/ophidv2.201/statistics.jsp). The core network comprises 23 proteins (square-shape nodes) connected by 29 direct interactions (thick edges). Including interactions among the 23 core proteins results in the network with 1077 proteins and 1421 interactions, which are known (n = 1023), determined by high throughput (HTP) experiments (n = 246) or predicted interologs (n = 142). Individual interaction sources are listed at http://ophid.utoronto.ca/ophidv2.201/statistics.jsp. Importantly, known interactions connect 806 of the 1077 proteins. Node color corresponds to Gene Ontology biological function and edge color represents interaction source, according to legends. Network visualization was done in NAViGaTOR version 2.015 (http://ophid.utoronto.ca/navigator). B) Expression of Rab25 regulates TGF-β-induced promoter activity. P21cip, PAI and CAGA promoter luciferase constructs were transiently transfected in ovarian cancer A2780 stably expressing RAB25 or control (pcDNA) cells. TGF-β (1 µL: 100 ng/mL; 2 µL: 200 ng/mL) was added 24 h post-transfection. Luciferase activity was assayed 48 h post-transfection. *p < 0.01 versus control.
Targeting Rab Small GTPases in Cancer
Several strategies to therapeutically target members of the Ras superfamily in cancer are being developed and were reviewed in Konstantinopoulos et al. (27). Historically, some of the first agents to enter clinical trials were phosphorothioate-based antisense therapies to H-RAS (ISIS2503) and c-RAF1 (ISIS5132); however, despite promising activity in preclinical studies and a relative lack of toxicity in phase I studies, these therapies did not show any significant activity in phase II studies (56). Inhibition of prenylation, which is required for localization of RAS superfamily proteins to the plasma membrane and efficient signaling, has also been targeted. This can be achieved by inhibition of the mevalonate pathway by statins, bisphosphonates, farnesyltransferase inhibitors and geranylgeranyltransferase inhibitors. Despite in vitro ability of farnesyltransferase inhibitors (such as SCH115777) to reverse the transformed phenotype and impressive in vivo activity in animal models, they did not show significant activity in solid tumors as single agents in phase III trials (57,58). This is thought to be because of cross-prenylation of multiple Ras superfamily members by geranylgeranyltransferase in the context of farnesyltransferase inhibitors, as well as the presence of non-RAS targets of these drugs. The development of specific Rab-targeted agents, therefore, remains a challenge because of the following: (i) Ras superfamily GTPases and their regulatory proteins within each subfamily are highly homologous; (ii) regulatory proteins may target more than one Ras superfamily member and (iii) each Ras superfamily GTPase may in turn be regulated by multiple regulatory proteins, making the identification of specific inhibitors difficult. Therefore, improved understanding of the molecular mechanisms of individual regulatory proteins and GTPases in different tumor types and stages of disease, together with cellular contexts, will be required to enable effective targeting of the Rab small GTPases, including Rab25, for anticancer therapy.
Conclusion
Given the importance of Rab GTPases in regulating many cellular functions, it is not surprising that altered expression or mutation of Rab proteins and their interacting partners may cause human diseases. That Rab25 has a role in cancer has been established by its frequent dysregulation in a number of tumor types, functional studies of its impact on cell growth, proliferation, apoptosis, migration and invasion, in vivo tumorigenicity in mouse models, and association with clinical outcomes. With widespread usage of high-throughput genomic technology, increasing association between human cancer and aberrant Rab gene expression is expected to be revealed in the future. These studies represent an important chapter in cancer research. It is possible that expression profiling of some RAB genes might become useful prognostic and/or diagnostic markers. Progress in this area is likely to improve our understanding of not only the role of RAB GTPase in the biology of cancer but also the essential role of cellular trafficking in the maintenance of a number of normal physiological processes. As the underlying mechanism of Rab-mediated tumorigenesis comes to light, it is certain that Rab proteins and their interaction molecules will represent a novel therapeutic target.
Acknowledgments
The work was supported by grants from the National Cancer Institute (PO1CA099031) and Cancer Center Support Grant (P30CA16672) to G. B. M., the Department of Defense Breast Cancer Ideal Award (W81XWH-06-1-0488) to K. W. C. and a Cancer Research UK Clinician Scientist award (C2757/A5902) to R. A. I. J. was supported in part by Genome Canada via Ontario Genome Institute and IBM.
References
- 1.Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;250:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 3.Repasky GA, Chenette EJ, Der CJ. Renewing the conspiracy theory debate: does Raf function alone to mediate Ras oncogenesis? Trends Cell Biol. 2004;14:639–647. doi: 10.1016/j.tcb.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Cheng KW, Lahad JP, Kuo WL, Lapuk A, Yamada K, Auersperg N, Liu J, Smith-McCune K, Lu KH, Fishman D, Gray JW, Mills GB. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med. 2004;10:1251–1256. doi: 10.1038/nm1125. [DOI] [PubMed] [Google Scholar]
- 5.Fan Y, Xin XY, Chen BL, Ma X. Knockdown of RAB25 expression by RNAi inhibits growth of human epithelial ovarian cancer cells in vitro and in vivo. Pathology. 2006;38:561–567. doi: 10.1080/00313020601024037. [DOI] [PubMed] [Google Scholar]
- 6.Cheng JM, Ding M, Aribi A, Shah P, Rao K. Loss of RAB25 expression in breast cancer. Int J Cancer. 2006;118:2957–2964. doi: 10.1002/ijc.21739. [DOI] [PubMed] [Google Scholar]
- 7.Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, Mills GB, Humphries MJ, Messent AJ, Anderson KI, McCaffrey MW, Ozanne BW, Norman JC. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Touchot N, Chardin P, Tavitian A. Four additional members of the ras gene superfamily isolated by an oligonucleotide strategy: molecular cloning of YPT-related cDNAs from a rat brain library. Proc Natl Acad Sci U S A. 1987;84:8210–8214. doi: 10.1073/pnas.84.23.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 10.Goldenring JR, Shen KR, Vaughan HD, Modlin IM. Identification of a small GTP-binding protein, Rab25, expressed in the gastrointestinal mucosa, kidney, and lung. J Biol Chem. 1993;268:18419–18422. [PubMed] [Google Scholar]
- 11.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 12.Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann C. Ras-effector interactions: after one decade. Curr Opin Struct Biol. 2003;13:122–129. doi: 10.1016/s0959-440x(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 14.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 15.Bernards A, Settleman J. GAP control: regulating the regulators of small GTPases. Trends Cell Biol. 2004;14:377–385. doi: 10.1016/j.tcb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Seabra MC, Wasmeier C. Controlling the location and activation of Rab GTPases. Curr Opin Cell Biol. 2004;16:451–457. doi: 10.1016/j.ceb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 17.DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Cheng KW, Lahad JP, Gray JW, Mills GB. Emerging role of RAB GTPases in cancer and human disease. Cancer Res. 2005;65:2516–2519. doi: 10.1158/0008-5472.CAN-05-0573. [DOI] [PubMed] [Google Scholar]
- 19.Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, Altschuler Y, Ray GS, Goldenring JR. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calhoun BC, Goldenring JR. Two Rab proteins, vesicle-associated membrane protein 2 (VAMP-2) and secretory carrier membrane proteins (SCAMPs), are present on immunoisolated parietal cell tubulovesicles. Biochem J. 1997;325:559–564. doi: 10.1042/bj3250559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Kumar R, Navarre J, Casanova JE, Goldenring JR. Regulation of vesicle trafficking in Madin-Darby canine kidney cells by Rab11a and Rab25. J Biol Chem. 2000;275:29138–29146. doi: 10.1074/jbc.M004410200. [DOI] [PubMed] [Google Scholar]
- 22.Lapierre LA, Kumar R, Hales CM, Navarre J, Bhartur SG, Burnette JO, Provance DW, Jr, Mercer JA, Bähler M, Goldenring JR. Myosin vb is associated with plasma membrane recycling systems. Mol Biol Cell. 2001;12:1843–1857. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoekstra D, Tyteca D, Van IJzendoorn SC. The subapical compartment: a traffic center in membrane polarity development. J Cell Sci. 2004;117(Pt 11):2183–2192. doi: 10.1242/jcs.01217. [DOI] [PubMed] [Google Scholar]
- 24.Seachrist JL, Ferguson SS. Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci. 2003;74:225–235. doi: 10.1016/j.lfs.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Yoon SO, Shin S, Mercurio AM. Hypoxia stimulates carcinoma invasion by stabilizing microtubules and promoting the Rab11 trafficking of the alpha6beta4 integrin. Cancer Res. 2005;65:2761–2719. doi: 10.1158/0008-5472.CAN-04-4122. [DOI] [PubMed] [Google Scholar]
- 26.Haubruck H, McCormick F. Ras p21: effects and regulation. Biochem Biophys Acta. 1991;1072:215–229. doi: 10.1016/0304-419x(91)90015-d. [DOI] [PubMed] [Google Scholar]
- 27.Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Posttranslational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov. 2007;6:541–555. doi: 10.1038/nrd2221. [DOI] [PubMed] [Google Scholar]
- 28.Pfeffer S, Aivazian D. Targeting Rab GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol. 2004;5:886–896. doi: 10.1038/nrm1500. [DOI] [PubMed] [Google Scholar]
- 29.Davidson B, Zhang Z, Kleinberg L, Li M, Flørenes VA, Wang TL, Shih IM. Gene expression signatures differentiate ovarian/peritoneal serous carcinoma from diffuse malignant peritoneal mesothelioma. Clin Cancer Res. 2006;12:5944–5950. doi: 10.1158/1078-0432.CCR-06-1059. [DOI] [PubMed] [Google Scholar]
- 30.Chin K, DeVries S, Fridlyand J, Spellman PT, Roydasgupta R, Kuo WL, Lapuk A, Neve RM, Qian Z, Ryder T, Chen F, Feiler H, Tokuyasu T, Kingsley C, Dairkee S, et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10:529–541. doi: 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Korkola JE, Heck S, Olshen AB, Reuter VE, Bosl GJ, Houldsworth J, Chaganti RS. In vivo differentiation and genomic evolution in adult male germ cell tumors. Genes Chromosomes Cancer. 2008;47:43–55. doi: 10.1002/gcc.20504. [DOI] [PubMed] [Google Scholar]
- 32.Natrajan R, Williams RD, Hing SN, Mackay A, Reis-Filho JS, Fenwick K, Iravani M, Valgeirsson H, Grigoriadis A, Langford CF, Dovey O, Gregory SG, Weber BL, Ashworth A, Grundy PE, et al. Array CGH profiling of favourable histology Wilms tumours reveals novel gains and losses associated with relapse. J Pathol. 2006;210:49–58. doi: 10.1002/path.2021. [DOI] [PubMed] [Google Scholar]
- 33.Mor O, Nativ O, Stein A, Novak L, Lehavi D, Shiboleth Y, Rozen A, Berent E, Brodsky L, Feinstein E, Rahav A, Morag K, Rothenstein D, Persi N, Mor Y, et al. Molecular analysis of transitional cell carcinoma using cDNA microarray. Oncogene. 2003;22:7702–7710. doi: 10.1038/sj.onc.1207039. [DOI] [PubMed] [Google Scholar]
- 34.He H, Dai F, Yu L, She X, Zhao Y, Jiang J, Chen X, Zhao S. Identification and characterization of nine novel human small GTPases showing variable expressions in liver cancer tissues. Gene Expr. 2002;10:231–242. doi: 10.3727/000000002783992406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ray GS, Lee JR, Nwokeji K, Mills LR, Goldenring JR. Increased immunoreactivity for Rab11, a small GTP-binding protein, in low-grade dysplastic Barrett's epithelia. Lab Invest. 1997;77:503–511. [PubMed] [Google Scholar]
- 36.Calvo A, Xiao N, Kang J, Best CJ, Leiva I, Emmert-Buck MR, Jorcyk C, Green JE. Alterations in gene expression profiles during prostate cancer progression, functional correlations to tumorigenicity and down-regulation of selenoprotein-P in mouse and human tumors. Cancer Res. 2002;62:5325–5335. [PubMed] [Google Scholar]
- 37.Hales CM, Griner R, Hobdy-Henderson KC, Dorn MC, Hardy D, Kumar R, Navarre J, Chan EK, Lapierre LA, Goldenring JR. Identification and characterization of a family of Rab11-interacting proteins. J Biol Chem. 2001;276:39067–39075. doi: 10.1074/jbc.M104831200. [DOI] [PubMed] [Google Scholar]
- 38.Garcia MJ, Pole JC, Chin SF, Teschendorff A, Naderi A, Ozdag H, Vias M, Kranjac T, Subkhankulova T, Paish C, Ellis I, Brenton JD, Edwards PA, Caldas C. A 1 Mb minimal amplicon at 8p11–12 in breast cancer identifies new candidate oncogenes. Oncogene. 2005;24:5235–5245. doi: 10.1038/sj.onc.1208741. [DOI] [PubMed] [Google Scholar]
- 39.Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8:835–850. doi: 10.1038/nrc2521. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Wyckoff JB, Frohlich VC, Oleynikov Y, Hüttelmaier S, Zavadil J, Cermak L, Bottinger EP, Singer RH, White JG, Segall JE, Condeelis JS. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res. 2002;62:6278–6288. [PubMed] [Google Scholar]
- 41.Caswell PT, Chan M, Lindsay AJ, McCaffrey MW, Boettiger D, Norman JC. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol. 2008;183:143–155. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burke P, Schooler K, Wiley HS. Regulation of epidermal growth factor receptor signaling by endocytosis and intracellular trafficking. Mol Biol Cell. 2001;12:1897–1910. doi: 10.1091/mbc.12.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- 44.Salomon D, Brandt R, Ciardiello F. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 45.Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958–2970. [PubMed] [Google Scholar]
- 46.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Pennock S, Chen X, Wang Z. Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival. Mol Cell Biol. 2002;22:7279–7290. doi: 10.1128/MCB.22.20.7279-7290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Pennock SD, Chen X, Kazlauskas A, Wang Z. Platelet-derived growth factor receptor-mediated signal transduction from endosomes. J Biol Chem. 2004;279:8038–8046. doi: 10.1074/jbc.M311494200. [DOI] [PubMed] [Google Scholar]
- 49.Lanzetti L, Rybin V, Malabarba MG, Christoforidis S, Scita G, Zerial M, Di Fiore PP. The Eps8 protein coordinates EGF receptor signalling through Rac and trafficking through Rab5. Nature. 2000;408:374–377. doi: 10.1038/35042605. [DOI] [PubMed] [Google Scholar]
- 50.Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 51.Lanzetti L, Rybin V, Malabarba MG, Christoforidis S, Scita G, Zerial M, Di Fiore PP. The Eps8 protein coordinates EGF receptor signalling through Rac and trafficking through Rab5. Nature. 2000;408:374–377. doi: 10.1038/35042605. [DOI] [PubMed] [Google Scholar]
- 52.Miaczynska M, Pelkmans L, Zerial M. Not just a sink: endosomes in control of signal transduction. Curr Opin Cell Biol. 2004;16:400–406. doi: 10.1016/j.ceb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Agarwal R, Gonzalez-Angulo AM, Myhre S, Carey M, Lee JS, Overgaard J, Alsner J, Stemke-Hale K, Lluch A, Neve RM, Kuo WL, Sorlie T, Sahin A, Valero V, Keyomarsi K, et al. Integrative analysis of cyclin protein levels identifies cyclin b1 as a classifier and predictor of outcomes in breast cancer. Clin Cancer Res. 2009;15:3654–3662. doi: 10.1158/1078-0432.CCR-08-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown KR, Jurisica I. Unequal evolutionary conservation of human protein interactions in interologous networks. Genome Biol. 2007;8:R95. doi: 10.1186/gb-2007-8-5-r95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barrios-Rodiles M, Brown KR, Ozdamar B, Bose R, Liu Z, Donovan RS, Shinjo F, Liu Y, Dembowy J, Taylor IW, Luga V, Przulj N, Robinson M, Suzuki H, Hayashizaki Y, et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science. 2005;307:1621–1625. doi: 10.1126/science.1105776. [DOI] [PubMed] [Google Scholar]
- 56.Coudert B, Anthoney A, Fiedler W, Droz JP, Dieras V, Borner M, Smyth JF, Morant R, De Vries MJ, Roelvink M, Fumoleau P. European Organization for Research and Treatment of Cancer (EORTC). Phase II trial with ISIS 5132 in patients with small-cell (SCLC) and non-small cell (NSCLC) lung cancer. A European Organization for Research and Treatment of Cancer (EORTC) Early Clinical Studies Group report. Eur J Cancer. 2001;37:2194–2198. doi: 10.1016/s0959-8049(01)00286-6. [DOI] [PubMed] [Google Scholar]
- 57.End DW, Smets G, Todd AV, Applegate TL, Fuery CJ, Angibaud P, Venet M, Sanz G, Poignet H, Skrzat S, Devine A, Wouters W, Bowden C. Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res. 2001;61:131–137. [PubMed] [Google Scholar]
- 58.Rao S, Cunningham D, De Gramont A, Scheithauer W, Smakal M, Humblet Y, Kourteva G, Iveson T, Andre T, Dostalova J, Illes A, Belly R, Perez-Ruixo JJ, Park YC, Palmer PA. Phase III double-blind placebo-controlled study of farnesyltransferase inhibitor R115777 in patients with refractory advanced colorectal cancer. J Clin Oncol. 2004;22:3950–3957. doi: 10.1200/JCO.2004.10.037. [DOI] [PubMed] [Google Scholar]


