Abstract
Studies with nonhuman primates and rodents, as well as with human children, have suggested that early separations from caregivers are often associated with changes in the functioning of the hypothalamus–pituitary–adrenal (HPA) axis. On the basis of these findings, we designed a relational intervention that was intended to normalize HPA functioning among children in foster care. This paper presents findings from a randomized clinical trial that assessed the effectiveness of a relational intervention (Attachment and Biobehavioral Catch-up [ABC]) with regard to HPA functioning. The ABC intervention was intended to enhance children’s ability to regulate physiology and behavior. The control intervention (Developmental Education for Families) was intended to enhance children’s cognitive skills. A comparison group of children who had never been in foster care was also included. Children’s cortisol production was assessed upon arrival at the lab, and 15 and 30 min following the Strange Situation. Random effects analyses of variance were performed to assess differences in initial values and change between children in the two intervention groups. Children in the ABC intervention and comparison group children showed lower initial values of cortisol than children in the treatment control group, considering arrival at lab as initial values (p < .05). Groups did not differ significantly in change over time. These results suggest that the ABC intervention is effective in helping children regulate biology in ways more characteristic of children who have not experienced early adversity.
A rich nonhuman primate and rodent literature suggests long-term effects of early maternal separations on the regulation of glucocorticoids (CORT; e.g., Mirescu, Peters, & Gould, 2004; Sanchez, Ladd, & Plotsky, 2001). CORT are steroid hormones produced as an end product of the hypothalamus–pituitary–adrenal cortex (HPA) axis. The CORT of particular relevance to early development are cortisol among humans and other primates and corticosterone among rodents. Several recent studies have examined patterns of cortisol production among foster children, a group that may share some conditions in common with animals studied in early separation paradigms. Many preschool-aged children in foster care showed low levels of cortisol across the day, with a smaller number showing high levels (Dozier, Manni, et al., 2006; Fisher, Gunnar, Chamberlain, & Reid, 2000; Gunnar, Fisher, & The Early Experience, Stress, and Prevention Network, 2006) relative to other children. In contrast, many foster infants showed high (rather than low) levels of cortisol production relative to other infants (Dozier, Peloso, et al., 2006). Although infants and preschoolers in foster care were distinguished from one another in their proportion of high and low patterns, both groups differed from children who were not in foster care. These findings of different cortisol production among young children in foster care led us to develop an intervention that specifically targets the regulation of cortisol among these children.
The current paper presents findings from a randomized clinical trial that assesses effects of this intervention on children’s production of cortisol prior to and following the Strange Situation, a laboratory procedure that challenges children by separating them from caregivers (Ainsworth, Blehar, Waters, & Wall, 1978). In the Strange Situation, children experience separations and reunions with their caregivers twice, conditions similar in some respects to paradigms used to elicit glucocorticoid responses among other species. Prior research has examined daytime cortisol production among foster children, with far fewer studies assessing cortisol reactivity to stress among this population.
Functioning of the HPA System
CORT has two major functions that appear to be relatively orthogonal of one another. First, CORT is one of the contributors to the maintenance of circadian patterns of activity, affecting the timing of activities such as waking, sleeping, eating, and social interactions. Other systems, most notably temperature, function in analogous ways to maintain circadian patterns. These systems enhance the likelihood that members of a species are awake at the same time as one another, thus increasing the likelihood of reproductive success.
A cirdadian pattern of cortisol production typically emerges in the first few months of life among humans, with the pattern remaining relatively similar across the life span (Larson, White, Cochran, Donzella, & Gunnar, 1998; Price, Close, & Fielding, 1983). High wake-up levels are followed by a peak in CORT within 30 min of wake-up. A sharp decrease is then seen by midmorning, with relatively low levels throughout the day to a nadir at bedtime (Gunnar & Cheatham, 2003; Gunnar & Donzella, 2002). The primary developmental change in the diurnal pattern beyond infancy is a decrease from morning to afternoon that is not seen predictably until children are about 4 years old (Bruce, Davis, & Gunnar, 2002; Watamura, Donzella, Alwin, & Gunnar, 2003).
Second, and superimposed upon this daily pattern, is the involvement of CORT in mounting a stress response. In mounting a stress response, CORT is released as an end product of the HPA axis. The hypothalamus releases corticotropin-releasing hormone, which signals the pituitary to release adrenocorticotropin hormone (ACTH). ACTH then signals the adrenocortical axis to release CORT. The presence of high levels of CORT increases the organism’s readiness for fight or flight, with resources diverted toward increased oxygen intake, heart rate, and blood pressure, whereas blood flow is diverted away from digestive, reproductive, and immune system processes. Given that a CORT stress response diverts resources away from functions that are critical for long-term survival, it is clear that this system’s stress response evolved as a short-term solution to acute challenges. Indeed, the system has a negative feedback loop, with the production of CORT signaling to the pituitary to stop producing ACTH (Gunnar & Cheatham, 2003).
Among adults, there is convincing evidence of stress reactivity. Tasks that elicit the most robust cortisol elevations involve the combination of social threat and uncontrollability (Dickerson & Kimeny, 2004; Kirschbaum, Pirke, & Hellhammer, 1993). Dickerson and Kimeny argue that this makes sense from an evolutionary perspective because the threat of being excluded from the social group is of such great consequence. Consistent with this model, low-ranking animals that experience frequent stressors show the largest basal levels of cortisol (Abbott et al., 2003). However, the degree of observed cortisol reactivity to stress appears to be highly dependent on development. Newborn human infants show rises in cortisol in response to stressful events within the first few days of life, and stressors such as physical examinations and inoculations elicit strong cortisol elevations in 2-month-olds. However, similar stressors elicit weaker cortisol elevations as children grow older (Gunnar, Broderson, Krueger, & Rigatuso, 1996; Larson et al., 1998; Lewis & Ramsay, 1995).
In childhood, whereas diurnal production of CORT is well characterized, findings with regard to stress reactivity are more mixed. In some studies, rises in cortisol in response to stressors have been observed among young children (Gunnar, Mangelsdorf, Larson, & Hertsgaard, 1989; Spangler & Grossmann, 1993), but there have also been many failures to elicit cortisol reactions among young children under stressful conditions (Gunnar & Donzella, 2002; Gunnar & Quevedo, 2007). These failures may often go unreported because of the difficulty in publishing null results. Gunnar and others (Gunnar & Quevedo, 2007; Gunnar & Vazquez, 2001; Sapolsky & Meaney, 1986) have suggested that young children may undergo a stress hyporesponsive period (SHRP) during which cortisol is not elevated in response to stressors. This period may parallel the SHRP in the rodent that extends from Postnatal Day 4 to Postnatal Day 12 (Gunnar & Quevedo, 2007; Sapolsky & Meaney, 1986). The hyporesponsive period in humans may extend through much of childhood (Gunnar et al., 2006). As Gunnar and Cheatham (2003) point out, it makes sense that the hyporesponsive period may extend further for humans because of the slower maturation of the human brain relative to other species and therefore the extended period of dependence on caregivers.
Caregiver Involvement and Stress Response
Early in development, human caregivers serve a critical function as behavioral and physiological coregulators for infants in a variety of ways (Gunnar et al., 2006; Gunnar & Donzella, 2002; Hofer, 1994). The caregiver appears central to the infant’s developing a more mature regulation of the HPA system. Gunnar and others (Gunnar, 1998) have suggested that the caregiver serves as a buffer against stress for the infant during the SHRP. Events that would be associated with a cortisol response for younger infants (before the onset of the SHRP) are not associated with such a response when infants are in the presence of responsive caregivers (Gunnar, 1998; Hertsgaard, Gunnar, Erickson, & Nachmias, 1995; Spangler & Grossmann, 1993). There is some evidence that infants and young children show an elevation in cortisol when experiencing stressful events in the presence of insensitive caregivers (Gunnar, Larson, Hertsgaard, Harris, & Brodersden, 1992). The samples sizes are small in these studies, however, and typically only emerge in interactions with other variables. For example, insecure attachment has been associated with cortisol increases following maternal separation, but only among children who were temperamentally inhibited (Nachmias, Gunnar, Mangelsdorf, Parritz, & Buss, 1996) or socially fearful (Gunnar, Brodersen, Nachmias, Buss, & Rigatuso, 1996).
The rodent literature has been especially compelling as a model of developmental stress neurobiology with evidence of the dam’s (i.e., mother’s) role in buffering the pup (i.e., infant) from stress (Caldji et al., 1998; Cirulli, Berry, & Alleva, 2003; Meaney & Szyf, 2005). With time-limited separations from the dam (15 min to 3 hr) during the hyporesponsive period, the pup typically does not show increases in corticosterone. These short separations are thought to reflect those that would occur naturally in the environment and promote stress resilience in the pup. The dam’s behavior in resuming normal behaviors, including licking and grooming, following the separation appears to be key to the pup’s response. In contrast, extended (e.g., 12 hr) separations are associated with both short-term and long-term effects for the pup, including hypercortisolism and increased fearful behavior (Cirulli et al., 2003; Sanchez et al., 2001). These separations are longer than the pup would typically experience in the wild. Thus, they may not be within the range of experiences for which the pup’s system is evolutionarily prepared.
It is of interest that the findings regarding developmental stress neurobiology may have been overstated with regard to the importance of the sensitivity of the caregiver and understated in terms of the salience of the SHRP. More specifically, increases in cortisol are very difficult to elicit among young children, providing rather strong evidence for a SHRP. With the exception of Gunnar and Quevedo (2007), the existence of a SHRP among human young is rarely discussed. In contrast, differences in cortisol reactivity related to normal variations in caregiver availability, such as attachment security and parental sensitivity are often assumed, despite the limited findings regarding such effects (Gunnar, Brodersen, Nachmias, et al., 1996; Gunnar et al., 1989; Hertsgaard et al., 1995; Nachmias et al., 1996).
Adversity and Stress Neurobiology
The lack of a caregiver, the loss of a caregiver, or neglect from a caregiver may pose challenges for the infant in regulating the stress system. These are extreme conditions that may fall outside of those for which the stress system has evolved (Cicchetti & Valentino, 2006). The rodent and nonhuman primate literatures have suggested that effects of severe deprivation (extended separation) may be seen in persistent changes in the animal’s stress responsiveness (Cirulli et al., 2003; Sanchez et al., 2001). Effects of extended early separations are seen in a more biologically and behaviorally hyperreactive adult animal (Caldji et al., 1998; Levine & Mody, 2003; Sanchez et al., 2005).
The human literature has provided more support for effects of early adversity on diurnal functioning than on stress reactivity. Young children who spent extended periods of their early years in orphanages, as well as children who entered foster care or experienced maltreatment, showed high rates of atypical diurnal patterns of cortisol production compared with other children who had not experienced early privation or deprivation (Carlson & Earls, 1997; Cicchetti & Rogosch, 2001; Gunnar, Morison, Chisholm, & Schuder 2001; Gunnar & Vazquez, 2001; Tarullo & Gunnar, 2006).
Our research group (Dozier, Manni, et al., 2006) as well as Fisher and colleagues (Fisher et al., 2000; Gunnar et al., 2006) found differences in daytime patterns of cortisol production among preschool children in foster care compared with children not in foster care. In both studies, about half of the preschoolers in foster care showed atypical patterns of cortisol production, with many showing low levels of cortisol, and a smaller number showing high levels of cortisol production. In contrast, among infants in foster care, we (Dozier, Manni, et al., 2006) found a different pattern. Most foster infants (less than 24 months of age) showed high, rather than low, daytime cortisol production. These differences between infants and preschoolers could well reflect a downregulation of the HPA system over time as the result of initially high levels of circulating CORT (Davies, Sturge-Apple, Cicchetti, & Cummings, 2007).
Intervening With Foster Children
Over the past 15 years, we have been assessing the specific issues that are challenging to young children in foster care, and developing intervention strategies that target these issues. Three issues have been identified as central. First, children in foster care often regulate physiology and behavior differently than do other children (Dozier, Manni, et al., 2006; Dozier, Peloso, et al., 2006). Second, infants and toddlers often behave in ways that push new caregivers away (Stovall & Dozier, 2000; Stovall-McClough & Dozier, 2004). Third, foster parents’ own issues sometimes make it difficult for them to provide nurturance. Whereas birth children can often cope effectively with nonnurturing care, children in foster care often develop disorganized attachments to nonnurturing caregivers, which puts them at risk for a number of problematic outcomes (Dozier, Stovall, Albus, & Bates, 2001). We have developed a 10-session intervention that targeted these three issues (Dozier, Higley, Albus, & Nutter, 2002). The regulation of behavior and physiology is targeted in 5–6 sessions, and the provision of nurturing care is targeted in 4–5 sessions.
We target children’s regulation of behavior and physiology in several ways. First, we borrowed conceptually from intervention strategies that Barnard (1999) and van den Boom (1994, 1995, 1997) had developed for premature and temperamentally difficult children, children who experienced difficulty regulating behavior and physiology for constitutional reasons. These previous interventions targeted self-regulatory capabilities by teaching parents to be very effective, responsive interpersonal partners. Related to this first component, we helped caregivers to be very responsive to their children’s emotions, especially with respect to negative affect (Izard, Fine, Mostow, Trentacosta, & Campbell, 2002; Izard, Trentacosta, King, & Mostow, 2004; Southam-Gerow & Kendall, 2002). In addition, we borrowed from Field’s work on the importance of touch to young children’s developing self-regulatory capabilities (Field et al., 1986, 2004; Field, Hernandez-Reif, Diego, Schanberg, & Kuhn, 2005).
The issues addressed in the additional four to five sessions concerned attachment security rather than self-regulatory capabilities. However, given that attachment can be seen to serve the purpose of providing the child with expectations regarding parental availability, breakdowns (or disorganization) in attachment are likely relevant to regulatory capabilities. Consistent with this are findings that children who show breakdowns in organization of their attachments are at risk for a host of internalizing, externalizing, and dissociative symptoms (Carlson, 1998; Lyons-Ruth, Alpern, & Repacholi, 1993). Thus, we consider it likely that sessions aimed at promoting attachment security also enhance children’s regulatory capabilities.
Preliminary evidence has suggested that Attachment and Biobehavioral Catch-up (ABC) is effective in changing young children’s daytime patterns of cortisol production (Dozier, Manni, et al., 2006; Dozier, Peloso, et al., 2006). Children in the ABC showed lower patterns of daytime cortisol production than children in the control intervention. Our results are consistent with results of Fisher et al. (2000), suggesting that daytime cortisol patterns can be modified with a social intervention. Prior to the present study, we did not have evidence regarding differences in stress reactivity among children in foster care. The present study assesses children from the two intervention groups, as well as comparison children, under stressful conditions. Of interest are differences in initial values of cortisol production, as well as changes in cortisol production in response to challenge.
Method
Overview
The primary sample included 46 children who completed the ABC intervention, and 47 children who completed the educational intervention. An additional 48 children were included who were not in the foster care system. These comparison children did not receive intervention services. For children to participate, both foster parent and birth parent (or proxy) consent were required. Foster parents consented to their own participation, and birth parents (or proxies) consented to children’s participation. The university’s institutional review board approved all procedures.
After enrollment, children in the foster care groups were randomly assigned to one of the two intervention groups (ABC or Developmental Education for Families [DEF]). Foster parents and birth parents were blind to condition, as were researchers responsible for entering data, assaying cortisol samples, and analyzing data.
Participants
Children ranged in age at the time they participated in the Strange Situation from 15 to 24 months. Differences between the three groups in age did not approach significance (p > .10).
Although children were randomly assigned to intervention group, there were 59% girls in the ABC group, compared with 43% girls in the DEF group and 44% in the comparison group of children who had not been in foster care. These represented statistically significant differences (p < .05). In addition, more of the children in the two intervention groups (ABC and DEF) were from underrepresented minorities compared with children in the comparison group (see Table 1). Child age, gender, and ethnicity were examined as possible control variables.
Table 1.
Age and ethnicity of children in ABC and DEF intervention groups and a comparison group
| Variable | Group
|
||
|---|---|---|---|
| ABC | DEF | Comparison | |
| Child age in months (SD) | 20.0 (5.98) | 19.5 (5.6) | 19.5 (3.8) |
| Child ethnicity n (%) | |||
| African American | 38 (81) | 31 (66) | 19 (35) |
| Hispanic | 1 (2) | 2 (5) | 3 (5) |
| Asian American | 0 (0) | 0 (0) | 2 (4) |
| White, non-Hispanic | 8 (17) | 14 (29) | 30 (56) |
Household income for foster children in THE ABC intervention groups was $37,600 and $39,100 (p > .10). Information regarding income was not available for comparison parents.
Procedures
All children participated in the Strange Situation (Ainsworth et al., 1978), an assessment of infants’ attachment quality, in one of three laboratories affiliated with the University of Delaware. The labs were unfamiliar to the children prior to the experimental session. In each of the labs, the primary experimental room resembled a waiting room at a doctor’s office. There were several chairs along the wall and a number of attractive toys on the floor. The waiting room was viewable through a one-way mirror from an observation room. Children and their parents were immediately taken to the “waiting room” when they arrived at the lab. Parents were asked to take a saliva sample immediately from their child, as described more fully below.
Strange Situation
The Strange Situation (Ainsworth et al., 1978) consists of seven episodes that are designed to increasingly stress the child and elicit attachment behavior. In the first episode, the infant and caregiver enter the waiting room, where the mother takes a seat and invites the child to play with toys that are on the floor. In the second episode, a “stranger” (always a female) enters the room and takes a seat beside the mother. After a 1-min period without any interaction, the stranger interacts with the mother for 1 min and then with the child for 1 min. In the fourth episode, the parent leaves the room, such that the child is alone in the room with the stranger. In the fifth episode, the parent returns to the room; the stranger leaves unobtrusively when possible. In the sixth episode, the parent again leaves, this time leaving the child alone in the room. In the seventh episode, the stranger returns and in the eighth episode the parent returns. Episodes are typically of 3-min duration, although separations are shortened when children show extreme behavioral distress.
Saliva sampling
The procedures used for collecting and assaying cortisol carefully followed established protocol (e.g., Gunnar & White, 2001). Experimenters trained parents to collect saliva samples. Parents collected saliva samples from children when they first arrived at the lab, 15 min after completion of the Strange Situation, and 30 min after completion of the Strange Situation. In addition, a subset of foster parents collected salivary cortisol samples prior to leaving home for the lab, and 2 hr after completion of the Strange Situation (after returning home).
Caregivers were instructed to moisten the end of the dental cotton roll by placing it briefly in the child’s mouth. The cotton was then dipped in a vial containing 0.8 g of flavored beverage crystals (Pathmark cherry-flavored drink mix) to promote salivation. The roll was placed back in the child’s mouth and the child was encouraged to mouth the roll until it was wet with saliva.
Although flavored drink mixes and other sweeteners have been reported to affect values on the radioimmunoassay (Schwartz, Granger, Susman, Gunnar, & Laird, 1998), recent controlled studies in our lab and in Gunnar’s lab have indicated that the ELIZA enzyme-immunoassay designed specifically for the measurement of salivary cortisol that we use (Salimetrics, Inc., State College, PA, High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit) is affected little by very low levels of the crystals (Gordon, Peloso, Auker, & Dozier, 2005; Talge, Donzella, Kryzer, Gierens, & Gunnar, 2005). In fact, in rigorous testing of the effect of the beverage crystals on cortisol measurement in adult volunteers, sample cortisol values varied less than 15% across all samples, and no significant differences were found between those samples collected with and those samples without the use of the drink mix (Gordon et al., 2005).
Caregivers were asked to indicate whether the child was teething, whether the child had had anything to eat or drink in the 30 min prior to sampling, and several other issues known to affect cortisol levels. Values were excluded when any of these conditions was indicated. Caregivers were also asked to indicate whether the child was sick or having other acute physical problems before beginning to take samples. If the child was experiencing such problems, assessments were delayed for one week or until the child’s condition improved.
Cortisol assay
The saliva samples were stored in a freezer until they were assayed in our laboratory. Assays were performed using the Salimetrics, Inc. High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit. All samples from a child were run in duplicate on the same assay plate. In addition, a collection of saliva from several different donors was pooled and frozen as a control. Samples of the control saliva were included on each assay plate. All samples were within an acceptable pH range, as demonstrated by an absence of color change when indicator (as part of the assay dilutant) was added. All values measured < 4 μg/dl, and no pairs of samples differed by more than 15%. Inter- and intraassay coefficients of variation for this study were below 7 and 4%, respectively.
Interventions
For both interventions, parent trainers had bachelor’s or master’s degrees in psychology or social work and at least 5 years’ clinical experience. They conducted 10 training sessions according to a structured training manual. All sessions were videotaped, allowing assessments of fidelity to the manual. Sessions took place in foster parent homes. To the extent possible, the format, duration, and frequency of the interventions were kept similar across the two interventions.
Relational intervention: ABC intervention
The ABC Intervention is designed to help children develop regulatory capabilities. It helps caregivers learn to (a) provide an environment that helps children develop regulatory capabilities, (b) reinterpret children’s alienating behaviors, and (c) help caregivers override their own issues that interfere with providing nurturing care. The intervention is manualized, with the same themes introduced across the 10 sessions, regardless of child age. Intervention principles are held constant, but specific activities are varied to be appropriate for children of different ages or issues.
Control intervention: DEF
The DEF session is of the same duration (10-hr long sessions) and frequency (weekly) as the ABC intervention. The educational intervention was borrowed partly from the home visitation component of the early intervention program developed by Ramey and colleagues (Ramey, McGinness, Cross, Collier, & Barrie-Blackley, 1982; Ramey, Yeates, & Short, 1984). This intervention was designed to enhance cognitive, and especially linguistic, development. The intervention has been successful in improving intellectual functioning when provided intensively and for a long duration in day care settings (Brooks-Gunn, Klebanov, Liaw, & Spiker, 1993). Components that involve parental sensitivity to child cues were excluded in our version of the intervention so as to keep the interventions distinct. Although the intervention is manualized, specific activities take into account children’s developmental level.
Results
Data analytic strategy
Group differences in initial cortisol values and changes in cortisol values from before and after the Strange Situation were analyzed using hierarchical linear modeling (HLM; Raudenbush & Bryk, 2002, see also Snijders & Bosker, 1999). HLM allows separate estimates of Level 1 (within-subjects) and Level 2 (between-subjects) variation, thereby accounting for the non-independence of within-person observations over time. HLM allows for “missingness” on outcome variables assuming that the data are missing at random (MAR; Schafer & Graham, 2002). Within the context of this study, MAR refers to the situation in which missingness is not related to future unobserved measurements of cortisol but is related to previous measurements of cortisol or other measured covariates.
The dependent variable was the log-transformed cortisol value measured across each of three time points, measured in micrograms per deciliter (μg/dl). First, initial values were assessed. Second, the basic model assessed whether cortisol systematically changes from its initial value to after children participated in the Strange Situation. After within-subject change was characterized, individual difference predictors at the between subject level were considered. Group status was dummy coded to allow for comparisons among members of any two groups against a selected reference group.
Gender was coded as 0 for boys or 1 for girls, ethnicity was coded as 0 for nonminority and 1 for minority, and age was recorded in months. Analyses at Level 2 considered whether individual differences in intercepts and slopes were related to individual differences in group status, gender, ethnicity, or age.
Primary analyses included the three time points that were obtained during the laboratory session. Secondary analyses included the five time points (one time prior to leaving home and one time after returning home) that were available for a subset of participants. Multilevel models were conducted using symbolism consistent with Raudenbush and Bryk (2002) and of the following form:
- Level 1 (within individual):
-
Level 2 (between individual):
where, at Level 1, log cortti represents the log of child i’s cortisol level at time t, β0i represents child i’s estimated log cort at the initial time point, β1i represents the amount of linear change in log cort over time for child i, (time)ti represents elapsed time coded in minutes from the initial lab cortisol assessment, and rti represents the within-person residual in child i’s log cort at time t that cannot be accounted for by initial estimated log cort and linear change in log cort over time. Only linear change was examined in these analyses because there were only three repeated measures for each child.
At Level 2, the pair of equations specify estimation of the β0i and β1i terms that represent individual differences in initial log cort and linear change in log cort over time. The term γ00 represents the average estimated initial level of log cort for the comparison group, γ01 is the difference between the comparison group and the group coded 1 in the first group dummy code (DUM1), γ02 represents the difference between the comparison group and the group coded 1 in the second group dummy code (DUM2), and ε0i is the between-child individual differences that are unexplained by the Level 2 grouping variable. Similar equations were used to model between-child differences in linear change over time.
Initial cortisol levels
We explored individual difference predictors of initial values of cortisol, including group status (ABC and comparison coded as 1, and DEF as 0), ethnicity, gender and age. These analyses allowed comparisons between the reference group (DEF) and the other two groups (ABC and nonfoster comparison group). The only comparisons not allowed by these analyses were between ABC and the nonfoster comparison group. Additional analyses were conducted with ABC as the reference group to allow these comparisons.
Initial cortisol values differed significantly between children in the ABC treatment group and the DEF (treatment control) group, and between children in the comparison group and children in the DEF (treatment control) group (see Table 2 and Figure 1). Subsequent analyses including the ABC group as the reference group indicated that the ABC group did not differ significantly from the comparison group (see Table 3). Gender, age, and minority status did not account for significant variability in cortisol levels (see Tables 2 and 3). Analyses excluding these variables result in comparable effects, as can be seen in Tables 4 and 5.
Table 2.
Multilevel modeling coefficients of treatment effects for salivary cortisol with DEF (treatment control) as reference group
| Effect | Salivary Cortisol
|
||||
|---|---|---|---|---|---|
| Coefficient | SE | t | df | p | |
| Intercept, B00 | −.62 | .18 | −3.30 | 135 | .00 |
| Gender, B01 | .02 | .09 | 0.21 | 135 | .83 |
| Minority, B02 | .02 | .09 | 0.23 | 135 | .82 |
| Age, B03 | −.00 | .01 | −0.06 | 135 | .95 |
| ABC, B04 | −.27 | .10 | −2.54 | 135 | .01 |
| Comparison, B05 | −.27 | .11 | −2.54 | 135 | .01 |
| Slope, B10 | −.06 | .06 | −0.98 | 135 | .33 |
| Gender, B01 | −.01 | .03 | −0.19 | 135 | .85 |
| Minority, B02 | .00 | .03 | −0.13 | 135 | .90 |
| Age, B03 | −.00 | .00 | −0.03 | 135 | .54 |
| ABC, B04 | .06 | .04 | 1.62 | 135 | .11 |
| Comparison, B05 | .05 | .04 | 1.27 | 135 | .21 |
Figure 1.
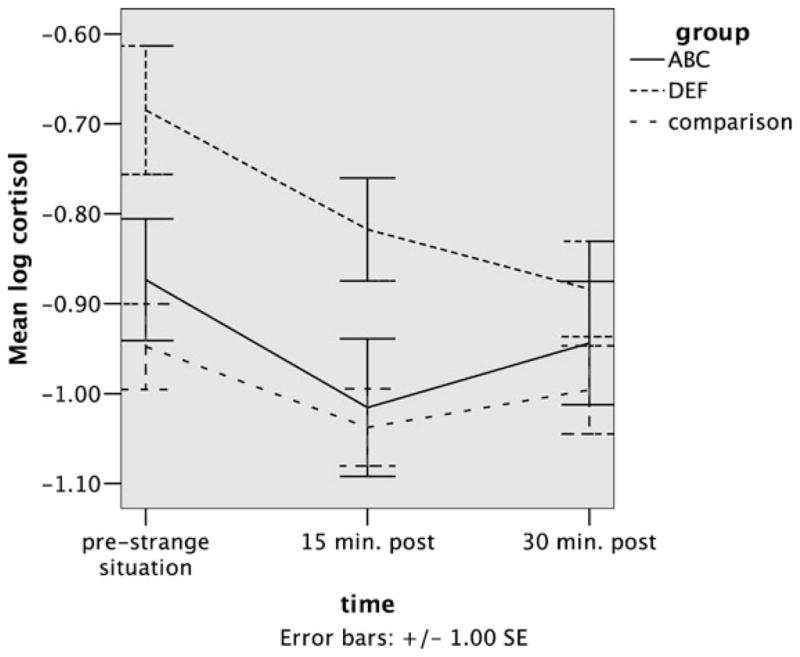
Mean cortisol values (and standard errors) as a function of intervention group and time.
Table 3.
Multilevel modeling coefficients of treatment effects for salivary cortisol with ABC (experimental treatment) as reference group
| Effect | Salivary Cortisol
|
||||
|---|---|---|---|---|---|
| Coefficient | SE | t | df | p | |
| Intercept, B00 | −.89 | .22 | −4.01 | 135 | .00 |
| Gender, B01 | .01 | .08 | 0.23 | 135 | .82 |
| Minority, B02 | .02 | .09 | 0.26 | 135 | .82 |
| Age, B03 | −.00 | .01 | −0.06 | 135 | .95 |
| DEF, B04 | .27 | .11 | 2.54 | 135 | .01 |
| Comparison, B05 | .00 | .11 | 0.03 | 135 | .97 |
| Slope, B10 | −.00 | .07 | −0.02 | 135 | .98 |
| Gender, B01 | −.01 | .03 | −0.19 | 135 | .85 |
| Minority, B02 | −.00 | .03 | −0.13 | 135 | .90 |
| Age, B03 | −.00 | .00 | −0.70 | 135 | .54 |
| ABC, B04 | −.06 | .04 | −1.62 | 135 | .11 |
| Comparison, B05 | −.01 | .04 | −0.37 | 135 | .71 |
Table 4.
Multilevel modeling coefficients of treatment effects for salivary cortisol with DEF (treatment control) as reference group
| Effect | Salivary Cortisol
|
||||
|---|---|---|---|---|---|
| Coefficient | SE | t | df | p | |
| Intercept, B00 | −.61 | .07 | −8.23 | 138 | .00 |
| ABC, B01 | −.27 | .10 | −2.56 | 138 | .01 |
| Comparison, B02 | −.27 | .10 | −2.72 | 138 | .01 |
| Slope, B10 | −.10 | .03 | −3.76 | 138 | .00 |
| ABC, B01 | .06 | .04 | 1.62 | 138 | .11 |
| Comparison, B02 | .05 | .04 | −1.27 | 138 | .21 |
Table 5.
Multilevel modeling coefficients of treatment effects for salivary cortisol with ABC (experimental treatment) as reference group
| Effect | Salivary Cortisol
|
||||
|---|---|---|---|---|---|
| Coefficient | SE | t | df | p | |
| Intercept, B00 | −.87 | .07 | −11.95 | 138 | .00 |
| DEF, B01 | .27 | .10 | 2.56 | 138 | .01 |
| Comparison, B02 | −.01 | .10 | −0.10 | 138 | .92 |
| Slope, B10 | −.04 | .03 | −1.48 | 138 | .14 |
| DEF, B01 | −.06 | .04 | −1.62 | 138 | .11 |
| Comparison, B02 | −.01 | .04 | −0.39 | 138 | .70 |
Response to Strange Situation
None of the three groups showed a significant increase in cortisol in response to the Strange Situation. Indeed, the slopes for all groups were in the negative direction (−.04, −.11, and −.05, for ABC, DEF, and comparison groups, respectively). The slope was not significantly different from zero in the case of the ABC group, but was significantly negative (p < .05) for the other two groups. Differences between slopes did not reach significance (see Tables 2 and 3).
Discussion
In the present study, children whose caregivers had participated in a relational intervention, ABC, showed lower initial levels of cortisol upon arrival to the lab for the Strange Situation procedure than did children in the control intervention group. Children in the comparison group, who had never been in foster care, showed lower levels of cortisol than the foster children in the control intervention group, but levels that were not significantly different from the foster children in the ABC group. These results are exciting because they suggest that a relational intervention can affect the biology of infants and toddlers in foster care. The ABC intervention is a time-limited parent training program that appears to affect cortisol production such that foster children show more normative patterns.
In a prior preliminary study, we assessed the daytime production of cortisol among infants and toddlers whose caregivers had completed the ABC intervention compared with children in the treatment control group and with children never in foster care (Dozier, Peloso, et al., 2006). In this prior study, cortisol levels were assessed at wake-up and bedtime over 2 consecutive days at home. Children in the ABC intervention group showed similar levels of cortisol to children never in foster care, but lower levels than children in the control intervention group. This pattern of results mirrors the findings of the present study in that children in the control intervention group showed higher levels of cortisol than both children in the ABC group and children never in foster care. We speculate that the differences in initial cortisol values seen in the present study reflect differences in daytime cortisol levels rather than differences in cortisol reactivity to stress. Both our current and prior findings (Dozier, Peloso, et. al., 2006) suggest that the daytime patterns of cortisol production among infants and toddlers in foster care are characterized by higher rather than lower values compared to infants and toddlers never placed in foster care.
Findings from two other studies of daytime cortisol production among preschoolers in foster care suggest that children who have experienced early adversity show distinct daytime patterns of cortisol production across development (Fisher et al., 2000; Dozier, Manni, et al., 2006). In these studies, preschoolers tended to show lower daytime levels of cortisol production compared to children never in foster care more often than they showed higher or normative levels. These results are contrary to our findings reported here and elsewhere (Dozier, Peloso, et al., 2006) regarding infants and toddlers in foster care. Infants in foster care appear to show elevated daytime cortisol production, relative to comparison infants, whereas preschoolers show low daytime cortisol production, relative to comparison preschool children. As suggested by Gunnar and Vazquez (2001), the low cortisol levels seen among preschool-aged foster children may reflect downregulation of the HPA axis following extended periods of high cortisol production during infancy and early childhood. Future longitudinal studies of diurnal cortisol production among children in foster care are needed to adequately address this question.
Stress reactivity in infancy
Several previous studies (Hertsgaard et al., 1995; Spangler & Grossmann, 1993) have provided evidence that infants show stress reactivity in the Strange Situation. Increases in cortisol production following the Strange Situation were not observed for any of the three groups included in the present study, however. The lack of stress reactivity could reflect inadequate power or problems with procedures in the current design, or could reflect differences in toddlers’ response to the Strange Situation when compared with infants. On the other hand, the understanding of stress reactivity in early childhood has changed significantly over the last decade (e.g., Fries, Hesse, Hellhammer, & Hellhammer, 2005; Gunnar & Vazquez, 2001), and the present findings are consistent with current expectations. To fully understand these findings, it is critical to place these results in context.
Two studies have reported differences in cortisol reactivity related to attachment (Hertsgaard et al., 1995; Spangler & Grossmann, 1993). Specifically, Spangler and Grossman (1993) found that 12-month-olds from a middle-class, normative sample who were classified as insecurely attached showed higher cortisol levels following the Strange Situation compared to securely attached infants. In the second study, among a high-risk group of 19-month-old infants, Hertsgaard et al. (1995) found the association between cortisol reactivity and attachment to hold only for infants classified as disorganized. These studies have been cited widely even though the sample sizes of both were small, with only 41 and 38 children included in the two studies, respectively. Further, studies that have included larger sample sizes have shown that cortisol levels increase in response to the Strange Situation only among children who are both insecurely attached to caregivers and temperamentally inhibited or fearful (Gunnar, Brodersen, Nachmias, et al., 1996; Nachmias et al., 1996).
Although adolescents and adults have been found to show cortisol reactivity under conditions of social threat, there is mounting evidence that infants and young children do not show reactivity to highly stressful events in terms of their production of cortisol (Gunnar & Quevedo, 2007; Kudielka, Hellhammer, & Kirschbaum, 2007; McRae et al., 2006). Indeed, there may be important changes in cortisol reactivity across development, such that infants and young children go through a SHRP. Results from the previously described studies on attachment and cortisol reactivity are largely consistent with the idea that a hyporesponsive period exists among humans as part of the normative development of the HPA system. Further, Gunnar et al. (2006) have found that it remains difficult to find laboratory stressors that elicit a large cortisol response throughout childhood. Among preschoolers, for instance, a laboratory paradigm involving going to the dentist’s office and seeing several sharp dental instruments failed to provoke an increase in cortisol (Fisher report to Gunnar and Early Experience Network, Feb, 2005).
The existence of a hyporesponsive period to stress during childhood can be seen as having evolutionary significance. The human brain is highly plastic during early development (e.g., Cicchetti & Curtis, 2006; Howe, Cicchetti, & Toth, 2006; Kreppner et al., 2007). Similarly, among rodents, considerable brain development occurs within the first 2 weeks of life, a period that parallels the first 2 decades of life for humans. This first 2-week period is characterized as a SHRP in the rodent because stressors do not readily result in high levels of glucocorticoid production (Sapolsky & Meaney, 1986). Chronically high levels of circulating CORT during this 2-week time period are associated with damaging and long-lasting effects on brain development in regions such as the hippocampus, amygdala, cingulate gyrus, and prefrontal cortex (Gunnaret al., 2006; Sapolsky & Meaney, 1986). Among humans, a hyporesponsive period in cortisol reactivity to stress lasting from infancy through childhood may protect the developing brain from the possibly adverse effects of high levels of circulating CORT.
Limitations
There are a number of challenges in assessing stress reactivity among young children. First, given that stress reactivity is assessed in the context of a diurnal pattern, the time of day should be kept constant, or should be controlled. Although controlling for time of day did not alter findings, it would have been preferable if time of day had been held constant.
Second, the “event” (whether or not it is stressful) likely starts prior to when the experiment is thought to begin (Gunnar & Donzella, 2002; Larson, Gunnar, & Hertsgaard, 1991). For example, the child is likely to experience the day as different than usual when the parent prepares to depart from the house. For example, Larson et al. (1991) found that children show reactions to events (in the form of decreased levels of cortisol) when they first came to the lab, and indeed, even when they were still in their carseats on their ride from home to the lab. Collecting salivary cortisol samples prior to leaving home is optimal (Gunnar & Donzella, 2002). We collected these data among a subset of children in the foster care sample. We chose not to report those data here because home values were not available for any of the comparison children, and data were missing for a larger percentage of foster children than preferred. Nonetheless, among foster children, these differences reflected the same pattern seen for the laboratory assessments, and were statistically significant (in terms of intercept, with home as intercept value).
Third, although children were randomly assigned to the two intervention groups, the groups differed in their gender composition. The effect of gender was evaluated as a possible Level 1 variable, but still, this was not optimal.
Conclusions and Future Directions
The results of this study are important in providing support for changing the biology of vulnerable children via a relational intervention. A first intervention component targets children’s regulatory capabilities very specifically, by helping parents take children’s lead, using techniques that have proved successful behaviorally in other contexts (Barnard, 1999; van den Boom, 1995). A second intervention component helps caregivers provide nurturing care. Although this component was included to enhance attachment security rather than to enhance regulatory capabilities, we expect that it also had important effects on children’s regulatory capabilities. At some point, “unpacking” the intervention will provide additional information about the effects of intervention components.
Second, the findings here have implications for stress neurobiology more generally. Over the last decade, Gunnar and colleagues (Gunnar & Donzella, 2002; Gunnar & Vazquez, 2001) have suggested that, like rodents, human infants may experience a SHRP during which stressors rarely result in increases in cortisol production. The findings here are consistent with this notion, and are at odds with two earlier findings suggesting that the Strange Situation would elicit a stress response in infants. The weight of the evidence suggests that infants and young children are designed so as not to mount a stress response. Despite these assertions, a number of investigations set out to show differences in stress reactivity among children.
Third, developmental differences in diurnal production of cortisol appear critical to consider. Whereas preschoolers who have experienced adversity often show low levels of cortisol, infants who have experienced adversity may show relatively high levels of cortisol across the day. These developmental differences may reflect a down-regulation of the HPA axis overtime. Downregulation refers to adjustments with the HPA system that reduce the production of cortisol. Nonetheless, it will be important to follow the same children longitudinally to demonstrate that high early levels of cortisol production are associated with downregulation as children become older.
Despite significant progress in the past several decades in the field of stress neurobiology, it is critical to remember that there is much we do not know. Effects of experimental interventions on the functioning of stress system will contribute to this growing body of knowledge.
Acknowledgments
Support for this research was provided by NIMH R01 52135 and NIMH K02 74374 to the first author, and by NIMH Network Grant 65046 (Gunnar and Fisher, co-Principal Investigators). We acknowledge the support of the Philadelphia Department of Human Services and Delaware Division of Family Services and the caseworkers, foster families, and children at both agencies.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, et al. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Hormones and Behavior. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Ainsworth MDS, Blehar MC, Waters E, Wall S. Patterns of attachment: A psychological study of the strange situation. Hillsdale, NJ: Erlbaum; 1978. [Google Scholar]
- Barnard K. Beginning rhythms: The emerging process of sleep wake behaviors and self-regulation. Seattle, WA: University of Washington, NCAST; 1999. [Google Scholar]
- Brooks-Gunn J, Klebanov PK, Liaw F, Spiker D. Enhancing the development of low birth weight, premature infants: Changes in cognition and behavior over the first three years. Child Development. 1993;64:736–753. doi: 10.1111/j.1467-8624.1993.tb02940.x. [DOI] [PubMed] [Google Scholar]
- Bruce J, Davis EP, Gunnar MR. Individual differences in children’s cortisol response to the beginning of the new school year. Psychoneuroendocrinology. 2002;27:635–650. doi: 10.1016/s0306-4530(01)00031-2. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of behavioral fearfulness in adulthood in the rat. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson EA. A prospective longitudinal study of attachment disorganization/disorientation. Child Development. 1998;69:1107–1128. [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Curtis WJ. The developing brain and neural plasticity: Implications for normality, psychopathology, and resilience. In: Cicchetti D, Cohen D, editors. Developmental psychopathology: Vol. 2. Developmental neuroscience. 2. New York: Wiley; 2006. pp. 1–64. [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Valentino K. An ecological–transactional perspective on child maltreatment: Failure of the average expectable environment and its influence on child development. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Vol. 3. Risk, disorder, and adaptation. 2. Hoboken, NJ: Wiley; 2006. pp. 129–201. [Google Scholar]
- Cirulli F, Berry A, Alleva E. Early disruption of the mother–infant relationship: Effects on brain plasticity and implications for psychopathology. Neuroscience and Biobehavioral Reviews. 2003;27:73–82. doi: 10.1016/s0149-7634(03)00010-1. [DOI] [PubMed] [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D, Cummings EM. The role of child adrenocortical functioning in pathways between interparental conflict and child maltreatment. Developmental Psychology. 2007;43:918–930. doi: 10.1037/0012-1649.43.4.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kimeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dozier M, Higley E, Albus KE, Nutter A. Intervening with foster infants’ caregivers: Targeting three critical needs. Infant Mental Health Journal. 2002;23:541–554. [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, et al. Foster children’s diurnal production of cortisol: An exploratory study. Child Maltreatment. 2006;11:189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Dozier M, Peloso E, Lindhiem O, Gordon K, Manni M, Sepulveda S, et al. Developing evidence-based interventions for foster children: An example of a randomized clinical trial with infants and toddlers. Journal of Social Issues. 2006;62:767–785. [Google Scholar]
- Dozier M, Stovall KC, Albus KE, Bates B. Attachment for infants in foster care: The role of caregiver state of mind. Child Development. 2001;72:1467–1477. doi: 10.1111/1467-8624.00360. [DOI] [PubMed] [Google Scholar]
- Field T, Hernandez-Reif M, Diego M, Feijo L, Vera Y, Gil K. Massage therapy by parents improves early growth and development. Infant Behavior & Development. 2004;27:435–442. [Google Scholar]
- Field T, Hernandez-Reif M, Diego M, Schanberg S, Kuhn C. Cortisol decreases and serotonin and dopamine increase following massage therapy. International Journal of Neuroscience. 2005;115:1397–1413. doi: 10.1080/00207450590956459. [DOI] [PubMed] [Google Scholar]
- Field T, Schanberg SM, Scafidi F, Bauer CR, Vega-Lahr N, Garcia R, et al. Tactile/kinesthetic stimulation effects on preterm neonates. Pediatrics. 1986;77:654–658. [PubMed] [Google Scholar]
- Fisher PA, Gunnar MR, Chamberlain P, Reid JB. Preventative intervention for maltreated pre-school children: Impact on children’s behavior, neuroendocrine activity, and foster parent functioning. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:1356–1364. doi: 10.1097/00004583-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Peloso E, Auker A, Dozier M. The effect of flavored beverage crystals on salivary cortisol enzyme-immunoreactive assay measurements. Developmental Psychobiology. 2005;47:189–195. doi: 10.1002/dev.20081. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Quality of early care and buffering of neuroendocrine stress reactions: Potential effects on the developing human brain. Preventive Medicine: An International Journal Devoted to Practice and Theory. 1998;27:208–211. doi: 10.1006/pmed.1998.0276. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Krueger K, Rigatuso J. Dampening of adrenocortical responses during infancy: Normative changes and individual differences. Child Development. 1996;67:877–889. [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Nachmias M, Buss K, Rigatuso J. Stress reactivity and attachment security. Developmental Psychobiology. 1996;29:191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Cheatham CL. Brain and behavior interface: Stress and the developing brain. Infant Mental Health Journal. 2003;24:195–211. [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Fisher PA The Early Experience, Stress, and Prevention Network. Bringing basic research on early experience and stress neurobiology to bear on preventative interventions for neglected and maltreated children. Development and Psychopathology. 2006;18:651–677. [PubMed] [Google Scholar]
- Gunnar MR, Larson MC, Hertsgaard L, Harris ML, Brodersen L. The stressfulness of separation among nine-month-old infants: Effects of social context variables and infant temperament. Child Development. 1992;63:290–303. [PubMed] [Google Scholar]
- Gunnar MR, Mangelsdorf S, Larson M, Hertsgaard L. Attachment, temperament, and adrenocortical activity in infancy: A study of psychoendocrine regulation. Developmental Psychology. 1989;25:355–363. [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol in children adopted from Romanian orphanages. Development and Psychopathology. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, White BP. Salivary cortisol measures in infant and child assessment. In: Singer LT, Zeskind PS, editors. Biobehavioral assessment of the infant. New York: Guilford Press; 2001. [Google Scholar]
- Hertsgaard L, Gunnar M, Erickson MF, Nachmias M. Adrenocortical responses to the strange situation in infants with disorganized/disoriented attachment relationships. Child Development. 1995;66:1100–1106. [PubMed] [Google Scholar]
- Hofer MA. Hidden regulators in attachment, separation, and loss. Mongraphs of the Society for Research in Child Development. 1994;59:192–207. [PubMed] [Google Scholar]
- Howe ML, Cicchetti D, Toth SL. Children’s basic memory processes, stress, and maltreatment. Development and Psychopathology. 2006;18:759–769. doi: 10.1017/s0954579406060378. [DOI] [PubMed] [Google Scholar]
- Izard CE, Fine S, Mostow A, Trentacosta C, Campbell J. Emotion processes in normal and abnormal development and preventative intervention. Development and Psychopathology. 2002;14:761–787. doi: 10.1017/s0954579402004066. [DOI] [PubMed] [Google Scholar]
- Izard CE, Trentacosta CJ, King KA, Mostow AJ. An emotion-based prevention program for Head Start children. Early Education and Development. 2004;15:407–422. [Google Scholar]
- Kirschbaum C, Pirke K, Hellhammer DH. The “Trier Social Stress Test”: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kreppner JM, Rutter M, Beckett C, Castle J, Colvert E, Groothues C, et al. Normality and impairment following profound early institutional deprivation: A longitudinal follow-up into early adolescence. Developmental Psychology. 2007;43:931–946. doi: 10.1037/0012-1649.43.4.93. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C. Ten years of research with the Trier Social Stress Test—Revisited. New York: Guilford Press; 2007. [Google Scholar]
- Larson M, Gunnar MR, Hertsgaard L. The effects of morning naps, car trips, and maternal separation on adrenocortical activity in human infants. Child Development. 1991;62:362–372. [PubMed] [Google Scholar]
- Larson M, White BP, Cochran A, Donzella B, Gunnar MR. Dampening of the cortisol response to handling at 3-months in human infants and its relation to sleep, circadian cortisol activity, and behavioral distress. Developmental Psychobiology. 1998;33:327–337. [PubMed] [Google Scholar]
- Levine S, Mody T. The long-term psychobiological consequences of intermittent postnatal separation in the squirrel monkey. Neuroscience and Biobehavioral Reviews. 2003;27:83–89. doi: 10.1016/s0149-7634(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Lewis M, Ramsay DS. Developmental changes in infant responses to stress. Child Development. 1995;66:657–670. doi: 10.1111/j.1467-8624.1995.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Alpern L, Repacholi B. Disorganized infant attachment and maternal psychsocial problems as predictors of hostile–aggressive behavior in the preschool classroom. Child Development. 1993;64:572–585. doi: 10.1111/j.1467-8624.1993.tb02929.x. [DOI] [PubMed] [Google Scholar]
- McRae AL, Saladin ME, Brady KT, Upadhyaya H, Back SE, Timmerman MA. Stress reactivity: Biological and subjective responses to the cold pressor and trier social stressors. Human Psychopharmacology: Clinical and Experimental. 2006;21:377–385. doi: 10.1002/hup.778. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends in Neurosciences. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nature Neuroscience. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH. Behavioral inhibition and stress reactivity: The moderating role of attachment security. Child Development. 1996;67:508–522. [PubMed] [Google Scholar]
- Price DA, Close GC, Fielding BA. Age of appearance of circadian rhythm in salivary cortisol values in infancy. Archives of Disease in Childhood. 1983;58:454–456. doi: 10.1136/adc.58.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramey CT, McGinness GD, Cross L, Collier AM, Barrie-Blackley S. The Abecedarian approach to social competence: Cognitive and linguistic intervention for disadvantaged preschoolers. In: Borman K, editor. The social life of children in a changing society. Hillsdale, NJ: Erlbaum; 1982. pp. 14–174. [Google Scholar]
- Ramey CT, Yeates KO, Short EJ. The plasticity of intellectual development: Insights from preventative intervention. Child Development. 1984;55:1913–1925. [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models. 2. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, et al. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biological Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research Reviews. 1986;11:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69:1503. [PubMed] [Google Scholar]
- Shafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Snijders TAB, Bosker RJ. An introduction to basic and advanced multilevel modeling. London: Sage; 1999. Multilevel analysis. [Google Scholar]
- Southam-Gerow MA, Kendall PC. Emotion regulation and understanding: Implications for child psychopathology and therapy. Clinical Psychology Review. 2002;22:189–222. doi: 10.1016/s0272-7358(01)00087-3. [DOI] [PubMed] [Google Scholar]
- Spangler G, Grossman KE. Biobehavioral organization in securely and insecurely attached infants. Child Development. 1993;64:1439–1450. doi: 10.1111/j.1467-8624.1993.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Stovall KC, Dozier M. The development of attachment in new relationships: Single subject analyses for ten foster infants. Development and Psychopathology. 2000;12:133–156. doi: 10.1017/s0954579400002029. [DOI] [PubMed] [Google Scholar]
- Stovall-McClough KC, Dozier M. Forming attachments in foster care: Infant attachment behaviors in the first two months of placement. Development and Psychopathology. 2004;16:253–271. [PubMed] [Google Scholar]
- Talge NM, Donzella B, Kryzer E, Gierens A, Gunnar MR. It’s not that bad: Error introduced by oral stimulants in salivary cortisol research. Developmental Psychobiology. 2005;4:369–376. doi: 10.1002/dev.20097. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- van den Boom DC. The influence of temperament and mothering on attachment and exploration: An experimental manipulation of sensitive responsiveness among lower-class mothers with irritable infants. Child Development. 1994;65:1457–1477. doi: 10.1111/j.1467-8624.1994.tb00829.x. [DOI] [PubMed] [Google Scholar]
- van den Boom DC. Do first-year intervention effects endure? Follow-up during toddlerhood of a sample of Dutch irritable infants. Child Development. 1995;66:1798–1816. [PubMed] [Google Scholar]
- van den Boom DC. Sensitivity and attachment: Next steps for developmentalists. Child Development. 1997;68:592–594. [PubMed] [Google Scholar]
- Watamura SE, Donzella B, Alwin J, Gunnar MR. Morning-to-afternoon increases in cortisol concentrations for infants and toddlers at child care: Age differences and behavioral correlates. Child Development. 2003;74:1006–1020. doi: 10.1111/1467-8624.00583. [DOI] [PubMed] [Google Scholar]


