Abstract
Objectives
A multicenter, double-blind study was conducted to evaluate the safety, efficacy and pharmacokinetics of balsalazide in pediatric patients with mild-to-moderate UC.
Methods
Sixty-eight patients, 5 to 17 years of age, with mild-to-moderate active UC based on the modified Sutherland UC activity index (MUCAI), were randomized to receive oral balsalazide 2.25 or 6.75 g/day for 8 weeks. The primary endpoint was clinical improvement (reduction of the MUCAI score by ≥3 points from baseline). Clinical remission (MUCAI score of 0 or 1 for stool frequency) and histological improvement after 8 weeks were also assessed. Pharmacokinetic parameters for balsalazide, 5-aminosalicylic acid, and N-acetyl-5-aminosalicylic acid were determined at 2 weeks. Adverse events and laboratory changes were monitored throughout the study.
Results
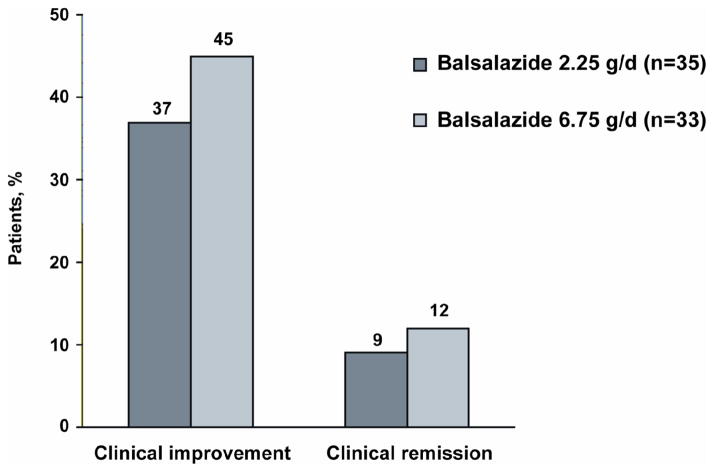
Clinical improvement was achieved by 45% and 37% of patients and clinical remission by 12% and 9% of patients receiving 6.75 and 2.25 g/day, respectively. Improvement in histologic grade was achieved by 8 of 16 (50%) and 3 of 10 (30%) patients receiving 6.75 and 2.25 g/day, respectively. No significant differences were seen in efficacy. Pharmacokinetics in 12 patients were characterized by large inter-subject variability and low systemic exposure. Adverse events were similar between the treatment groups, the most common being headache and abdominal pain. No clinically significant changes were observed in laboratory values, including those indicative of hepatic or renal toxicity.
Conclusions
Balsalazide is well-tolerated and improves the signs and symptoms of mild-to-moderate active UC in pediatric patients 5 to 17 years of age.
Keywords: pharmacokinetics, 5-aminosalicylates, Inflammatory Bowel Disease, Ulcerative colitis, children, adolescents
Introduction
Ulcerative colitis (UC) is an idiopathic, chronic inflammatory disease of the colon that requires acute treatment to induce remission and long-term treatment to maintain remission. Approximately 500,000 people suffer from UC in the United States (US).1, 2 It is more common in adults, but up to 10% of UC cases involve pediatric patients.3 Because of the relatively small number of pediatric UC patients in the US and ethical issues associated with treating pediatric patients with placebo, conducting a large, well-controlled clinical trial in this population can be challenging, and data supporting the safety and efficacy of 5-aminosalicylic acid (5-ASA) in pediatric patients has been lacking.
Oral 5-ASA is the first-line treatment for induction and remission of mild-to-moderate active UC.1, 4–6 The first approved treatment for UC in pediatric patients was sulfasalazine, a pro-drug consisting of 5-ASA conjugated to sulfapyridine.4 Although it has been shown to be efficacious in treating mild-to-moderate UC in pediatric patients, sulfapyridine is absorbed from the colon and can cause serious adverse events including Steven Johnson Syndrome or anaphylaxis in some patients.7–10 Common adverse events seen with sulfasalazine therapy include headache, nausea, vomiting, rash, and neutropenia.
Newer formulations of 5-ASA have been developed to avoid the side effect profile of sulfasalazine and to provide more drug to the colon. These newer formulations contain 5-ASA in a delayed-release tablet or pro-drugs with 5-ASA linked to an azo-bonded carrier other than sulfapyridine or to another 5-ASA molecule. Balsalazide disodium (Colazal®, Salix Pharmaceuticals, Inc., Morrisville, NC, USA) consists of 5-ASA linked via an azo bond to 4-aminobenzoyl-β-alanine. Once enzymatically cleaved, 5-ASA is released to produce an anti-inflammatory effect. Unlike pH-dependent formulations, such as mesalamine, azo-bonded balsalazide delivers more than 99% of its 5-ASA dose to the colon due to enzymatic release.11–16
Balsalazide has a reduced side effect profile relative to that observed with sulfasalazine.9, 10, 17 Following 8 weeks of treatment, balsalazide has been shown to be more effective and more rapid in onset than mesalamine in improving signs and symptoms of acute UC.18 In January, 2007, the Food and Drug Administration (FDA) approved balsalazide for treatment of mild-to-moderate active UC in pediatric patients 5 to 17 years of age. Balsalazide is the only, non-sulfa 5-ASA formulation with a pediatric indication in the US. The objective of this study was to evaluate the safety, efficacy, and pharmacokinetics of two dosing regimens of balsalazide in pediatric patients 5 to 17 of age with mild-to-moderate active UC.
Materials and Methods
This phase 3, randomized, double-blind study was conducted at 23 sites in the United States from August 2004 through March 2006. The protocol and informed consent/assent forms were approved by an Institutional Review Board at each site prior to the start of the study. Children 5 to 17 years of age with the ability to understand the requirements of the study, willingness to comply with all study procedures, documented proctititis, proctosigmoiditis, pancolitis, or indeterminate colitis, and confirmed or suspected active ulcerative colitis were eligible for the study. Patients were diagnosed via colonoscopy within 1 month of screening with mild-to-moderate UC based on a Modified Sutherland UC activity index (MUCAI)19 (Table 1). Mild-to-moderate active UC was defined as a total score between 4 and 10, and a score of at least 1 on the rectal bleeding, mucosal bleeding, and physician’s rating of disease activity scale. The FDA agreed that the MUCAI was an acceptable tool to assess the overall disease activity of each patient and efficacy of the study drug during the study.
Table 1.
Components of the Modified Sutherland Ulcerative Colitis Activity Index
| 1. Stool Frequency | |
| 0 | ≤ 3 stools/day |
| 1 | 4 to 5 stools/day |
| 2 | 6 to 7 stools/day |
| 3 | ≥ 8 stools/day |
| 2. Rectal Bleeding | |
| 0 | None |
| 1 | Streaks of blood |
| 2 | Obvious blood |
| 3 | Mostly blood |
| 3. Mucosal Appearance | |
| 0 | Intact mucosa with preserved or distorted vessels |
| 1 | Edematous mucosa with granularity and mild friability without ulceration |
| 2 | Pinpoint ulceration and moderate friability |
| 3 | Gross ulceration and spontaneous hemorrhage |
| 4. Physician’s rating of disease activity | |
| 0 | Normal |
| 1 | Mild |
| 2 | Moderate |
| 3 | Severe |
| Patients are assigned a minimum score of 0 and a maximum score of 12. | |
Exclusion criteria included: intolerance or allergy to salicylates; previous intolerance to balsalazide or treatment failure on balsalazide; severe UC (MUCAI > 10); significant bowel distention or tenderness associated with guarding or rebound; infectious, ischemic, or immunologic diseases with gastrointestinal involvement; clinically significant hepatic disease (twice the upper normal limit of serum transaminases); clinically significant renal disease (1.5 times the upper normal limit of serum creatinine or blood urea nitrogen); unstable cardiovascular or pulmonary disease; condition or circumstance that would prevent completion of the study or interfere with analysis of study results, including history of drug or alcohol abuse, mental illness, or noncompliance with treatment or visits; pregnancy or breastfeeding; previous treatment in the study; participation in an investigational drug or device study within 30 days prior to study screening; and active malignancy within the last 5 years, except basal cell carcinoma of the skin, or if female, in situ cervical carcinoma that had been surgically excised. Patients were not permitted to take anticholinergic, antidiarrheal, or 5-ASA products once randomized and throughout the study, provided there was no potential risk associated with the subject not receiving these products. Parental or legal guardian consent and patient assent were obtained prior to the start of the study for each patient.
The dosing regimens selected were based on the minimal systemic absorption and local therapeutic action of balsalazide, as well as a survey of prescribing patterns of pediatric gastroenterologists in the US. The survey revealed that the majority of pediatric gastroenterologists routinely treated their patients with mild-to-moderate active UC with balsalazide 6.75 g/day and then tapered the dose for maintenance. The lower dose was selected for this study because it was associated with improved endoscopic scores and physician’s global assessment of disease when administered to adults with mild-to-moderate active UC.18 A placebo was not selected because of ethical issues associated with using a placebo in pediatric patients with UC.
Patients were randomized in a 1:1 ratio using a computer-generated randomization schedule to receive balsalazide 6.75 g/day or 2.25 g/day. Randomization was stratified into 3 of the following groups based upon age and agreement to participate in the pharmacokinetic (PK) analysis: patients 5 to 8 years of age; patients 9 to 17 years of age and participating in the PK portion of the study; and patients 9 to 17 years of age and not participating in the PK portion of the study. Patients were provided 8 weeks of study medication packaged in 2-week supply cartons. Daily doses, consisting of 3 tablets each, were taken orally 3 times a day for a total of 9 tablets daily, approximately 8 hours a part, for a total of 8 weeks. If patients were unable to swallow the capsules, the parents were instructed to open the capsules and sprinkle the contents on food for immediate consumption. All capsules were identical in appearance and contained either 750 mg of balsalazide or the inert carrier alone. Both investigator and patient were blinded to the study drug assignment throughout the course of the study. Patients were instructed to return used and unused study medication during the 2-week, 4-week, and 8-week follow-up visits to determine compliance with study medication. Patients, parents, or patient guardians were contacted weekly between study visits by phone to assess patient symptoms and to determine compliance.
The following items were obtained prior to randomization: medical history, physical examination, vital signs, urine pregnancy test (for females), record of concomitant medications, routine laboratory studies (chemistry, hematology, serum transaminases, urinalysis, and creatinine clearance test), baseline MUCAI score, and colonoscopy with biopsies. Patients who had a colonoscopy and biopsy within 1 month of the screening visit did not have the procedure repeated provided that adequate documentation diagnosing UC was on file. Patients received diary cards to record the presence of abdominal cramps and fever ≥ 37.8C between visits.
Patients returned for follow-up visits at 2 weeks (± 3 days), 4 weeks (± 3 days), and 8 weeks (± 3 days). The following were performed or obtained at the 2-week and 4-week visit: vital signs, urine pregnancy test (for females), record of concomitant medications, record of adverse events and serum creatinine. Diary cards were also collected during these visits. Plasma concentration measurements for balsalazide, 5-aminosalicylic acid (5-ASA), and N-acetyly-5-aminosalicylic acid (N-Ac-5-ASA) were performed at the 2-week visit. Maximum observed plasma concentration (Cmax), area under the plasma concentration-time curve (AUC), and time to maximum plasma concentration (Tmax) values were determined.
The following were performed or obtained during the 8-week visit: physical examination, vital signs, urine pregnancy test (for females), record of concomitant medications, record of adverse events, routine laboratory studies (chemistry, hematology, serum transaminases, urinalysis, and creatinine clearance test), diary card, MUCAI score, and colonoscopy with biopsy. Three to four colonic biopsies were obtained from the same general regions that were biopsied during the screening period. Histologic assessment was performed and graded on a scale from 0 to 3 (0: no cryptitis or crypt abscesses; 1: cryptitis or abscesses involving < 50% of sampled crypts; 2: cryptitis or abscesses involving ≥ 50% of sampled crypts; 3: active inflammation plus erosions or ulcerations).
The study subjects could discontinue from the study at any time but were withdrawn if they experienced an increase in stool frequency for 3 consecutive days defined as 2 or more additional stools per day or a clinically relevant increase in rectal bleeding based on the investigator’s assessment of patient diary responses. Regardless of the reason of the withdrawal, all patients were asked to undergo an end-of-therapy evaluation (Week 8 visit).
The primary efficacy endpoint was the proportion of all randomized patients [intention-to-treat (ITT) population] achieving clinical improvement, which was defined as a reduction from baseline in the MUCAI total score by at least 3 points at week 8. A Fisher’s exact test was used to determine any significant difference between the treatment groups. An exact (Clopper-Pearson) two-sided 95% confidence interval to determine the proportion of subjects with clinical improvement was calculated. The same analysis was performed on the protocol population which included patients who did not violate the protocol in any fundamental way and who were at least 70% compliant in taking study medication. An analysis of factors influencing the primary endpoint of clinical improvement was performed in the ITT population. A logistic regression model (Wald chi-sqare test) was used for this statistical analysis with a 2-sided significance level of 0.05. Variables in the initial model included treatment, MUCAI total score at baseline, age, sex, time since colonoscopy with biopsies, and duration of ulcerative colitis.
Secondary measures of efficacy, which was based on the ITT population, included: the proportion of patients achieving remission, as evidenced by a score of 0 or 1 (a score of 1 was only allowed on the stool frequency index) on the MUCAI at week 8; change from baseline to week 8 in the total MUCAI score defined as the individual items (stool frequency, rectal bleeding, mucosal appearance, physician’s rating of disease activity) of the MUCAI, and the pathology classification of the colonic biopsies; and number of days abdominal cramps and/or fever were reported on the individual patient diary card assessment in the 7 days prior to the 4-week and 8-week visits. The following scale was used to assess the categorical change in stool frequency, rectal bleeding, mucosal appearance, physician’s rating of disease activity, and pathology classification:
improved = reduction from baseline to week 8 of MUCAI subscore and pathology classification
unchanged = MUCAI subscore was the same at baseline and at week 8
worsened = increase from baseline to week 8 of the MUCAI subscore.
A Fisher’s exact test was used to determine any significant difference between treatment groups in achieving remission at week 8. A p-value and an approximate 2-sided 95% confidence interval based on the normal distribution for the difference in proportion achieving remission between the 2 groups were calculated. An analysis of covariance (ANCOVA) with treatment group as a factor and baseline value as a covariate was used to evaluate the change from baseline to week 8 in the total MUCAI score. A Wilcoxon rank-sum test was used to evaluate the following secondary endpoints: change from baseline to week 8 in the individual items (stool frequency, rectal bleeding, mucosal appearance, physician’s rating of disease activity) of MUCAI, change from baseline in pathology classification at week 8, and number of days that abdominal cramps and/or fever were reported on individual subject diary cards in the 7 days prior to the 4-week and 8-week visit.
All statistical tests were 2-tailed at the 5% level of significance. Missing data were entered for the ITT population using the last-observation-carried-forward method for the primary and secondary endpoints. Baseline observations were not carried forward if all subsequent measurements were missing.
Pharmacokinetic (PK) analyses were performed for the PK population on plasma concentration-time data, and computed parameters were calculated from this data. The sample size for the study was based on what was considered feasible to obtain rather than standard considerations of statistical power.
Results
Sixty-eight patients were randomized over 19 months and were included in the primary efficacy analysis. Fifty-three patients completed the study and had colonoscopies performed at Screening and Week 8 (Figure 1). Twelve patients, six per study group, 9 to 17 years of age were included in the PK analysis. Twenty-nine patients were included in the protocol population. During the study, 164 protocol deviations were documented in 55 patients. Waivers were granted for 32 of the 164 deviations. Waivers were granted before any deviation occurred and were not granted in retrospect. At least one protocol violation was noted in 39 (57%) of the patients. Protocol violations with the highest incidence included violations of exclusion criteria (n = 19, 28%), < 70% compliant with taking the study medication (n = 19, 28%), MUCAI data not collected between days 42 and 70 (n = 15, 22%), and incomplete MUCAI data at Week 8 (n = 12, 18%).
Figure 1. Patient Disposition.
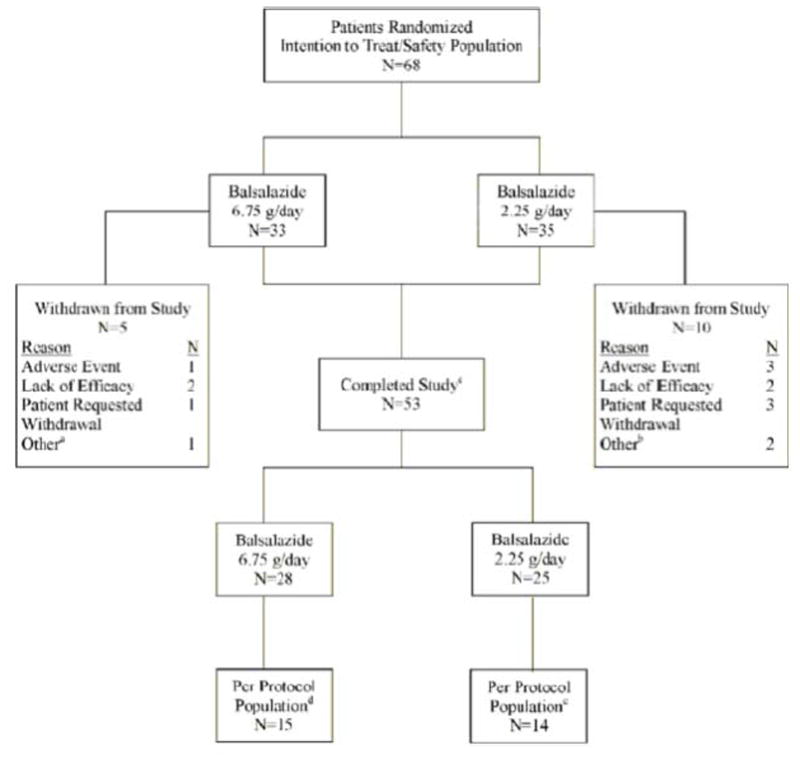
- Patient withdrawn by investigator because rectal biopsy slides from colonoscopy showed mild dysplasia
- One patient did not meet inclusion criteria, another patient was noncompliant
- atients who completed the study had colonoscopies performed at screening and week 8
- Patients were excluded from per-protocol population for one or more of the following reasons: Violations of inclusion/exclusion criteria (n = 12); <70% compliant (n = 7); > 30 days between colonoscopy and start of study medication (n = 3); used prohibited medications (n = 2); did not provide complete week 8 MUCAl data (n = 4); week 8 MUCAI data not collected between days 42 and 70 (n = 5)
- Patients were excluded from per-protocol population for one or more of the following reasons. Violations of inclusion/exclusion criteria (n = 8); <70% compliant (n = 12); did not meet baseline MUCAI requirements (n = 2); >30 days between colonoscopy and start of study medication (n = 5); used prohibited medications (n = 2); did not provide complete week 8 MUCAI data (n = 8); week 8 MUCAI data not collected between days 42 and 70 (n = 10)
The study population was predominantly Caucasian with more females than males (Table 2). The average age was 13 ± 3.43 years. Nine subjects were 5 to 8 (mean 6.6 ± 1.24) years of age, and 59 subjects were 9 to 17 (mean 14 ± 2.44) years of age. The median duration of disease prior to study enrollment was 79 (range 1 to 1117) days in the Colazal 6.75 g/day group and 121 (range 1 to 1769) days in the Colazal 2.25 g/day group. Twenty-three (70%) subjects in the 6.75 g/day and twenty (57%) subject in the 2.25g/day group had been previously diagnosed and treated for UC with 5-ASA therapy. The mean baseline MUCAI total score was 5.7 ± 1.6 in the balsalazide 6.75 g/day group and 5.9 ± 1.5 in the balsalazide 2.25 g/day group.
Table 2.
Patient Demographics and Baseline Characteristics
| Characteristic | Balsalazide 6.75 g/day (n=33) |
Balsalazide 2.25 g/day (n=35) |
Total (n=68) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 12.8 (3.56) | 13.2 (3.35) | 13.0 (3.43) |
| Gender (n,%) | |||
| Male | 13 (39%) | 10 (29%) | 23 (34%) |
| Female | 20 (61%) | 25 (71%) | 45 (66%) |
| Race (n,%) | |||
| Asian | 2 (6%) | 1 (3%) | 3 (4%) |
| Black or African-American | 2 (6%) | 3 (9%) | 5 (7%) |
| White | 29 (88%) | 31 (89%) | 60 (88%) |
| Mean MUCAI Score at Baseline (SD) | 5.7 (1.6) | 5.9 (1.5) | 5.8 (1.5) |
MUCAI = modified Sutherland ulcerative colitis activity index; SD = Standard Deviation
At week 2 and 4, the proportion of patients with ≥ 70% compliance was higher in the balsalazide 6.75 g/day group and than in the balsalazide 2.25 g/day group (91% vs. 83%, at week 2; 82% vs. 66% at week 4). Compliance was similar in the 2 treatment groups at week 8 (79% vs. 80%).
The proportion of patients with clinical improvement (defined as a reduction from baseline in the MUCAI total score by at least 3 points at week 8) was 45% (95% confidence interval: 28.1% – 63.6%) and 37% (95% confidence interval: 21.5% – 55.1%) for the 6.75 g/day and 2.25 g/day group, respectively (Figure 2). Analysis using the per-protocol population (n=29) revealed no significant difference: 53% in the balsalazide 6.75 g/day and 50% in the balsalazide 2.25 g/day groups showed clinical improvement. Clinical improvement also did not differ between the two dosing regimens (Figure 2). An analysis of factors influencing clinical improvement among both the ITT population and the ITT population of subjects who did not terminate from the study early, revealed that the MUCAI total score at baseline was a significant (P = 0.01) factor in influencing clinical improvement. Treatment, age, sex, time since last colonoscopy with biopsies and duration of disease were not significant factors in influencing clinical improvement.
Figure 2.
Percentage of Patients Who Achieved Clinical Improvement and Clinical Remission After 8 Weeks of Treatment with Balsalazide
No statistically significant differences between treatment groups were noted in the remission rate or mean change from baseline to week 8 in MUCAI total score; mean scores decreased by 2.6 points in the 6.75 g/day group and 2.4 points in the 2.25 g/day group (Table 3). Mean MUCAI individual items scores for stool frequency, rectal bleeding, mucosal appearance, and physician’s rating of disease activity decreased in both groups (Table 3). No significant differences were observed between the 2 treatment groups in mean change from baseline to week 8 in any of the MUCAI individual items.
Table 3.
Change from Baseline to Week 8 in MUCAI Individual Items
| Balsalazide 6.75 g/day | Balsalazide 2.25 g/day | |
|---|---|---|
| Stool Frequency | ||
| Baseline Mean (SD)* | 0.8 (0.74) | 0.8 (0.86) |
| Week 8/Final Mean (SD)† | 0.5 (0.87) | 0.3 (0.47) |
| Change from Baseline Mean (SD)‡ | −0.2 (0.95) | −0.3 (0.66) |
| Rectal Bleeding | ||
| Baseline Mean (SD)* | 1.6 (0.71) | 1.6 (0.77) |
| Week 8/Final Mean (SD) † | 0.6 (0.69) | 0.6 (0.88) |
| Change from Baseline Mean (SD) ‡ | −1.0 (0.98) | −0.9 (0.95) |
| Mucosal Appearance | ||
| Baseline Mean (SD)* | 1.9 (0.70) | 2.0 (0.86) |
| Week 8/Final Mean (SD) † | 1.1 (0.94) | 1.1 (0.72) |
| Change from Baseline Mean (SD) ‡ | −0.9 (0.88) | −0.9 (0.91) |
| Physician’s Rating of Disease Activity | ||
| Baseline Mean (SD)* | 1.4 (0.56) | 1.4 (0.61) |
| Week 8/Final Mean (SD) † | 0.9 (0.74) | 1.0 (0.73) |
| Change from Baseline Mean (SD) ‡ | −0.5 (0.78) | −0.4 (0.80) |
SD = Standard Deviation
At Baseline n = 33 for 6.75 g/day and n = 35 for 2.25 g/day
At Week 8/Final n = 27 for 6.75 g/day and n = 29 for 2.25 g/day
P-value not significant (> 0.05) based on a Wilcoxon rank-sum test comparing treatment groups
When analyzed categorically, the majority of subjects had improvements in rectal bleeding and mucosal appearance from baseline to week 8 (Table 4). Approximately one third of all subjects had improvement in stool frequency and physician’s rating of disease activity.
Table 4.
Categorical Change from Baseline to Week 8 in MUCAI Individual Items and Pathology Classification
| Balsalazide 6.75 g/day | Balsalazide 2.25 g/day | |
|---|---|---|
| N=33 | n=35 | |
| Stool Frequency* | ||
| Improved† | 11 (33%) | 8 (23%) |
| Unchanged‡ | 14 (42%) | 17 (49%) |
| Worsened§ | 4 (12%) | 2 (6%) |
| Rectal Bleeding* | ||
| Improved | 21 (64%) | 19 (54%) |
| Unchanged | 6 (18%) | 5 (14%) |
| Worsened | 2 (6%) | 3 (9%) |
| Mucosal Appearance* | ||
| Improved | 20 (61%) | 16 (46%) |
| Unchanged | 7 (21%) | 10 (29%) |
| Worsened | 2 (6%) | 1 (3%) |
| Physician’s Rating of Disease Activity* | ||
| Improved | 13 (39%) | 11 (31%) |
| Unchanged | 14 (42%) | 14 (40%) |
| Worsened | 2 (6%) | 2 (6%) |
| Pathology Classification* | ||
| Improved | 8 (24%) | 3 (9%) |
| Unchanged | 5 (15%) | 5 (14%) |
| Worsened | 3 (9%) | 2 (6%) |
p-value > 0.05 based on Cochran-Mantel-Haenszel Row Mean Scores test comparing the association between treatment
and changed from baseline status
Improved: reduction from baseline to week 8 of the MUCAI score
Unchanged: MUCAI subscore was the same at baseline and at week 8
Worsened: Increase from baseline to week 8 of the MUCAI subscore
Histologic assessments were available for 26 subjects during both the screening period and at week 8. Mean pathology scores decreased similarly in both groups (Table 5). Among these patients, 8 (50%) and 3 (30%) patients in the 6.75 g/day and 2.25 g/day group, respectively, had improvement in pathology classification (Table 4).
Table 5.
Change from Baseline to Week 8 in Pathology Classification of Inflammation in Colonic Biopsies*
| Balsalazide 6.75 g/day N = 33 |
Balsalazide 2/25 g/day N = 35 |
Total N=68 |
|
|---|---|---|---|
| Baseline | N = 21 | N=13 | N=34 |
| Mean (SD) | 1.8 (0.93) | 1.5 (0.78) | 1.7 (0.87) |
| Week 8/Final | N = 26 | N=26 | N=52 |
| Mean (SD) | 1.5 (0.76) | 1.5 (0.86) | 1.5 (0.80) |
| Change from Baseline | N = 16 | N = 10 | N=26 |
| Mean (SD)† | −0.3 (0.79) | −0.2 (0.92) | −0.3 (0.83) |
Mucosal pathology was graded on a scale of 0 to 3 (0: no cryptitis or abscess; 1: cryptitis or abscesses involving < 50% of sampled crypts; 2: cryptitis or abscesses involving ≥50% of sampled crypts; 3: active inflammation plus erosions or ulcerations)
P-value not significant (> 0.05) based on a Wilcoxon rank-sum test comparing treatment groups
More numeric days of patients reporting abdominal cramps and fever during the 7 days prior to the 4-week and 8-week visit were documented in the 2.25 g/day compared with the 6.75 g/day group. Abdominal cramps were reported at a mean of 2.6 days at week 4 and 8 for the 6.75 g/day group and 3.2 and 3.1 days at week 4 and 8, respectively, for the 2.25 g/day group. Fever was not reported in the 6.75 g/day group while it was reported 0.3 and 0.4 days at week 4 and 8, respectively, for the 2.25 g/day group.
Forty-two (62%) patients reported adverse events during the study (23 in the 6.75 g/day group and 19 in the 2.25 g/day group). Sixteen (23%) patients, 7 in the 6.75 g/day group and 9 in the 2.25 g/day group, reported adverse events that were considered by the investigator to be related to treatment. Overall the most common adverse events were headache (15%) and upper abdominal pain (13%) (Table 6).
Table 6.
Treatment-Emergent Adverse Events Reported >5% of Patients Receiving Balsalazide
| Adverse Event | Balsalazide 6.75 g/day N = 33 |
Balsalazide 2.25 g/day N = 35 |
Total N = 68 |
|---|---|---|---|
| Headache | 5 (15%) | 5 (14%) | 10 (15%) |
| Abdominal Pain Upper | 3 (9%) | 6 (17%) | 9 (13%) |
| Abdominal Pain | 4 (12%) | 4 (11%) | 8 (12%) |
| Vomiting | 1 (3%) | 6 (17%) | 7 (10%) |
| Diarrhea | 2 (6%) | 4 (11%) | 6 (9%) |
| Ulcerative Colitis | 2 (6%) | 2 (6%) | 4 (6%) |
| Nasopharyngitis | 3 (9%) | 1 (3%) | 4 (6%) |
| Pyrexia | 0 (0%) | 4 (11%) | 4 (6%) |
Most adverse events were mild or moderate in severity as judged by the investigator. Four subjects (2 in each treatment group) experienced serious adverse events, none of which was considered to be related to the study drug by the investigator. Four subjects (1 in the 6.75 g/day group and 3 in the 2.25 g/day group) discontinued treatment because of adverse events; 1 subject in each treatment group discontinued balsalazide due to events considered to be related to study drug. One patient experienced abdominal pain and urticaria which resolved after discontinuing balsalazide. The second patient was treated with balsalazide 2.25 g/day for 32 days and developed frequent bowel movements, which did not resolve following withdrawal from the study. No cardiovascular or hepatic adverse events and no cases of pancreatitis, gastritis, or cholecystitis were reported in the study.
Routine laboratory tests revealed minor increases and decreases from baseline to week 8 in both treatment groups. In both treatment groups, a slight median increase was observed in aspartate aminotransferase, while a median decrease was observed in alanine aminotransferase and random serum glucose levels. All shifts in individual laboratory values were only minimally out of range and were not considered clinically meaningful.
The pharmacokinetics of balsalazide, 5-ASA, and N-Ac-5-ASA were characterized by large inter-subject variability (Table 7). Balsalazide appeared to exhibit dose-independent (e.g. dose-linear) kinetics, with both Cmax and AUC0–8 increasing in a dose-proportional pattern when comparing the mean values for the 6.75 g/day and 2.25 g/day doses. The systemic exposure parameters for the active metabolites, 5-ASA and N-Ac-5-ASA, increased in less than a dose-proportional manner when comparing the mean values for the 6.75 g/day versus the 2.25 g/day doses. All PK parameters are presented in Table 7.
Table 7.
Pharmacokinetic Parameters for Balsalazide, 5-ASA, and N-Ac-5-ASA
| Balsalazide 6.75 g/day | N | Balsalazide 2.25 g/day | N | |
|---|---|---|---|---|
| Mean Cmax, ng/mL ± SD | ||||
| Balsalazide | 452 ± 400 | 6 | 211 ± 156 | 6 |
| 5-ASA | 344 ± 352 | 6 | 317 ± 268 | 6 |
| N-Ac-5-ASA | 815 ± 782 | 6 | 686 ± 423 | 6 |
| Mean AUC0–8, ng.mL± SD | ||||
| Balsalazide | 2031 ± 1964 | 6 | 625 ± 474 | 6 |
| 5-ASA | 1931 ± 2236 | 6 | 2043 ± 1664 | 6 |
| N-Ac-5-ASA | 4721 ± 4613 | 6 | 4582 ± 2812 | 6 |
| Mean Tmax h (range) | ||||
| Balsalazide | 1.54 (1.0–6.0) | 6 | 1.00 (1.0–2.0) | 6 |
| 5-ASA | 2.5 (1.0–6.0) | 4 | 3.00 (2.0–6.0) | 5 |
| N-Ac-5-ASA | 1.96 (1.0–6.0) | 6 | 2.99 (2.0–8.0) | 6 |
| % Fluctuation ± SD | ||||
| Balsalazide | 172 ± 49 | 6 | 252 ± 56.3 | 6 |
| 5-ASA | 105 ± 93,3 | 4 | 46.5 ± 12.4 | 5 |
| N-Ac-5-ASA | 95.2 ± 65.3 | 6 | 59.3 ± 60.7 | 6 |
SD = Standard Deviation; 5-ASA = 5-aminosalicylic acid; N-Ac-5-ASA = N-acetyl-5-aminosalicylic acid
Discussion
The findings from this study indicate that balsalazide at doses of 2.25 g/day and 6.75 g/day is effective in treating mild-to-moderate active UC in pediatric patients 5 to 17 years of age. No significant dose response was observed in this study. Clinical improvement was seen in 45% of the 6.75 g/day group and 37% of the 2.25 g/day group. Clinical remission was noted in 12% and 9% in the 6.75 g/day group and 2.25 g/day groups, respectively. Stool frequency, rectal bleeding, mucosal appearance, and physician’s rating of disease activity were comparable in both groups (Table 3). In the subgroup that had a histologic assessment performed, no significant differences were noted between both dosing regimens.
A similar number of patients in each treatment group were excluded from the per-protocol analysis. The percentage of patients meeting the definition of clinical improvement in the per-protocol population compared to the ITT population was slightly higher (53% for 6.75 g/day group and 50% for the 2.25 g/day group), but were not significantly different between treatment groups. It is possible that the primary and secondary efficacy outcomes, including remission rate, would have been higher had more patients completed the study or with increased compliance. It is unknown how much the variables including sample size, number of protocol violations, compliance rate, or patient age affected the study outcome. The design of this study makes it difficult to compare the results to previous studies evaluating the use of balsalazide for treatment mild-to-moderate UC in adult patients.
The side effect profile for both treatment groups was similar. Although 62% of patients reported adverse events, only 23% of patients reported adverse events related to the study medication. Many of the adverse events may have been related to underlying disease, since pediatric UC is more extensive and more likely to be associated with severe acute exacerbations compared with adults.20
The reported adverse events were generally considered mild or moderate by the investigators, but 4 patients discontinued treatment due to adverse events. Of these 4 adverse events, only 1 event of frequent bowel movements was considered related to the study medication by the investigator. The event did not resolve upon discontinuation of the study medication. Whether this was a side effect of balsalazide or a symptom of progressive disease in this patient is unclear. Frequent bowel movements and diarrhea have been reported previously in adult patients with UC treated with balsalaizde.21
Balsalazide is a prodrug that is enzymatically cleaved to release 5-ASA, which produces a local anti-inflammatory effect in the colon of patients with ulcerative colitis.11, 13 Compared with sulfasalazine, balsalazide has an inert carrier molecule, 4-aminobenzoyl-β-alanine, instead of sulfapyridine, that facilitates high-dose delivery of 5-ASA to the colonic mucosa without the side effects associated with sulfasalazine.13 The pharmacokinetics of balsalazide were measured to analyze the rate and extent of absorption of balsalazide and key metabolites, 5-ASA and N-Ac-5-ASA. In theory, lower systemic absorption is associated with more drug delivered to the colon and fewer systemic side effects.
The pharmacokinetics of balsalazide were characterized by very large interpatient variability, similar to data reported in adults.11 Balsalazide appears to exhibit dose-independent kinetics with mean Cmax and mean AUC0–8 increasing in a dose proportional manner when comparing the 6.75 g/day and 2.25 g/day doses. Compared with adults who have received balsalazide 6.75 g/day, the absolute magnitude of these parameters was much greater. The mean Cmax and mean AUC0–8 in this pediatric study was 26% and 102%, respectively, greater than those previously observed in adults receiving balsalazide 6.75 g/day. In contrast, the mean Cmax and AUC0–8 for 5-ASA and N-Ac-5-ASA increased in a less dose-proportional manner when comparing the 6.75 g/day and 2.25 g/day doses. The absolute magnitude of these parameters was decreased in comparison to data seen in adults receiving balsalazide 6.75 g/day. For 5-ASA, the mean Cmax and AUC0–8 were 67% and 64%, respectively, lower than those observed in adults receiving balsalazide 6.75 g/day. For N-Ac-5-ASA, the Cmax and AUC0–8 were 68% and 55%, respectively, lower than those observed in adults receiving balsalazide 6.75g/day. Although the clinical efficacy after balsalazide administration was presumably due to the local effects of 5-ASA on the colonic mucosa, the systemic exposure of 5-ASA may not have been the most important metric of efficacy. The effectiveness of balsalazide appeared to be best demonstrated from the efficacy and safety endpoints of this study.
As noted previously, studies evaluating the efficacy of 5-ASA in pediatric patients with mild-to-moderate UC are difficult to conduct because of the relatively small numbers of children with UC meeting specified entry criteria at single centers. Hence, a multi-center study is necessary to recruit enough patients for accurate data analysis. In a pediatric study comparing the safety and efficacy of sulfasalazine and olsalazine, 270 patients were identified in a 2-year period from 13 centers. A total of 188 patients had moderate-to-severe disease and were not eligible for the study. This left 82 patients with mild-to-moderate disease eligible for the study. From this number, a total of 59 patients from 13 centers were enrolled over 2 years. This pediatric study took 19 months to enroll 68 pediatric patients from 23 centers in the United States. Significant differences were not noted between treatment groups. A larger study may be required to detect a dose response.
An obvious limitation of this study is the use of a scale that is not validated in pediatric patients to assess efficacy of the study drug. At the time that this study was conducted, a validated scale to assess the extent of disease in pediatric UC patients did not exist, and the FDA was consulted when the study was designed. Therefore, the MUCAI was developed for determining clinical efficacy as this study evolved from a written request by the FDA to Salix Pharmaceuticals. The FDA accepted the MUCAI to assess overall disease activity and efficacy of the study drug in each patient. The FDA also agreed that a 3-point decline in the total MUCAI scale was clinically meaningful.
Recently, a non-invasive Pediatric Ulcerative Colitis Activity Index to assess disease severity has been developed and validated.22 This Index was not available during the time this study was conducted. As with the MUCAI, this scale is based on subjective criteria such as the presence of abdominal pain, rectal bleeding, stool consistency, and assessment of activity level. A post-hoc analysis using this newer assessment tool unfortunately could not be performed due to lack of some required data.
Another obvious limitation of the study is the absence of a placebo arm. In this patient population, the investigators believed it would be unethical to treat children with UC with placebo. A lack of placebo complicates the design and results of the study. We did include two doses and dummy pills to assess a relatively low dose with a standard dose and found little difference between the two dosing regimens. Our data were deemed to be sufficient by the FDA to support a pediatric indication for balsalazide for the treatment of mild-to-moderate UC in children 5 to 17 years of age in the US. It is well known that 5-ASA and prodrugs of 5-ASA are consistently used in pediatric patients for treatment of mild-to-moderate UC.
In conclusion, this study confirms that 8 weeks of treatment with balsalazide either 2.25 g/day or 6.25 g/day is well-tolerated and improves the signs and symptoms of mild-to-moderate ulcerative colitis in pediatric patients 5 to 17 years of age. This conclusion is further supported by the minimal systemic absorption of balsalazide and its metabolites. Further studies with larger patient numbers are needed to confirm these results.
Acknowledgments
This study was funded by Salix Pharmaceuticals, Inc.
Study Support
The study and writing of this manuscript was funded and supported by Salix Pharmaceuticals, Inc. Specific information regarding disclosure, conflict of interest, and contributions to the paper is presented below for each author.
Footnotes
Author Support
J. Antonio Quiros, MD, was involved in patient recruitment and acquisition of the data, analysis of the data, and drafting of the manuscript. He is a consultant to UCB Pharma and Prometheus Labs. He receives research support from Boston Scientific and Santarus Inc. Dr. Quiros has approved the final draft of this manuscript.
Melvin B. Heyman, MD, MPH, was involved in the planning and conduct of the study, analysis of the data, and drafting of the manuscript. He was supported in part by NIH grant DK060617 and from a research grant from Salix Pharmaceuticals, Inc. Dr. Heyman has approved the final draft of this manuscript.
John F. Pohl, MD, was involved in the conception and design of the study, acquisition of the data, drafting of the manuscript and critical revisions for important intellectual content, and administrative and technical support. He has received research support from Salix Pharmaceuticals, Inc. Dr. Pohl has approved the final draft of this manuscript.
Thomas M. Attard, MD was involved in the conception and design of the study, acquisition of the data, analysis and interpretation of the data, and drafting of the manuscript. He has no relevant financial interest in this manuscript. Dr. Attard has approved the final draft of the manuscript.
Henry J. Pieniaszek, PhD, was involved in the analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, and pharmacokinetic analysis and interpretation. Dr. Pieniaszek provides consulting and pharmaceutical development services to Salix Pharmaceuticals, Inc. Dr. Pieniaszek has approved the final draft of this manuscript.
Enoch Bortey, PhD, was involved in the analysis and interpretation of the data, critical revisions of the manuscript for intellectual content, and statistical analysis. He is an employee of Salix Pharmaceuticals, Inc. Dr. Bortey has approved the final draft of this manuscript.
Kelli Walker, PharmD, MS, was involved in the analysis of the data, drafting of the manuscript and critical revisions of the manuscript for intellectual content, and administrative and technical support. She is a consultant for Salix Pharmaceuticals, Inc, and has no relevant financial interests in this manuscript. Dr. Walker has approved the final draft of the manuscript.
William P. Forbes, PharmD, was involved in the conception and design of the study, analysis and interpretation of the data, critical revisions of the manuscript for important intellectual content, and supervision of the study. Dr. Forbes is the guarantor of this manuscript. He is an employee and stockholder of Salix Pharmaceuticals, Inc. Dr. Forbes has approved the final draft of the manuscript.
Reference List
- 1.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371–1385. doi: 10.1111/j.1572-0241.2004.40036.x. [DOI] [PubMed] [Google Scholar]
- 2.Loftus CG, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Tremaine WJ, Melton LJ, III, Sandborn WJ. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940–2000. Inflamm Bowel Dis. 2007;13:254–261. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- 3.Bousvaros A, Sylvester F, Kugathasan S, Szigethy E, Fiocchi C, Colletti R, Otley A, Amre D, Ferry G, Czinn SJ, Splawski JB, Oliva-Hemker M, Hyams JS, Faubion WA, Kirschner BS, Dubinsky MC. Challenges in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:885–913. doi: 10.1097/01.mib.0000228358.25364.8b. [DOI] [PubMed] [Google Scholar]
- 4.Sutherland L, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006:CD000543. doi: 10.1002/14651858.CD000543.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Lim WC, Hanauer SB. Controversies with aminosalicylates in inflammatory bowel disease. Rev Gastroenterol Disord. 2004;4:104–117. [PubMed] [Google Scholar]
- 6.Bremner AR, Griffiths DM, Beattie RM. Current therapy of ulcerative colitis in children. Expert Opin Pharmacother. 2004;5:37–53. doi: 10.1517/14656566.5.1.37. [DOI] [PubMed] [Google Scholar]
- 7.Ferry GD, Kirschner BS, Grand RJ, Issenman RM, Griffiths AM, Vanderhoof JA, Fiedorek SC, Winter HS, Hassall EG, Watkins JB. Olsalazine versus sulfasalazine in mild to moderate childhood ulcerative colitis: results of the Pediatric Gastroenterology Collaborative Research Group Clinical Trial. J Pediatr Gastroenterol Nutr. 1993;17:32–38. doi: 10.1097/00005176-199307000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Loftus EV, Jr, Kane SV, Bjorkman D. Systematic review: short-term adverse effects of 5-aminosalicylic acid agents in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2004;19:179–189. doi: 10.1111/j.0269-2813.2004.01827.x. [DOI] [PubMed] [Google Scholar]
- 9.Mansfield JC, Giaffer MH, Cann PA, McKenna D, Thornton PC, Holdsworth CD. A double-blind comparison of balsalazide, 6. 75 g, and sulfasalazine, 3 g, as sole therapy in the management of ulcerative colitis. Aliment Pharmacol Ther. 2002;16:69–77. doi: 10.1046/j.1365-2036.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 10.Green JR, Mansfield JC, Gibson JA, Kerr GD, Thornton PC. A double-blind comparison of balsalazide, 6. 75 g daily, and sulfasalazine, 3 g daily, in patients with newly diagnosed or relapsed active ulcerative colitis. Aliment Pharmacol Ther. 2002;16:61–68. doi: 10.1046/j.1365-2036.2002.01150.x. [DOI] [PubMed] [Google Scholar]
- 11.Product Information for Colazal® (balsalazide disodium) Capsules, Salix Pharmaceuticals, Inc.
- 12.Chan RP, Pope DJ, Gilbert AP, et al. Studies of two novel sulfasalazine analogs, ipsalazide and balsalazide. Dig Dis Sci. 1983;28:609–615. doi: 10.1007/BF01299921. [DOI] [PubMed] [Google Scholar]
- 13.Prakash A, Spencer CM. Balsalazide. Drugs. 1998;56:83–89. doi: 10.2165/00003495-199856010-00008. [DOI] [PubMed] [Google Scholar]
- 14.Product Information for Pentasa® (mesalamine) Controlled-Release Capsules 250mg, Shire US Incorporated.
- 15.Product Information for Asacol® (mesalamine) Delayed-Release Tablets, Procter & Gamble Pharmaceuticals.
- 16.Product Information for Lialda™ (mesalamine) Delayed-Release Tablets, Shire US Inc.
- 17.McIntyre PB, Rodrigues CA, Lennard-Jones JE, Barrison IG, Walker JG, Baron JH, Thornton PC. Balsalazide in the maintenance treatment of patients with ulcerative colitis, a double-blind comparison with sulphasalazine. Aliment Pharmacol Ther. 1988;2:237–243. doi: 10.1111/j.1365-2036.1988.tb00693.x. [DOI] [PubMed] [Google Scholar]
- 18.Levine DS, Riff DS, Pruitt R, Wruble L, Koval G, Sales D, Bell JK, Johnson LK. A randomized, double blind, dose-response comparison of balsalazide (6.75 g), balsalazide (2.25 g), and mesalamine (2. 4 g) in the treatment of active, mild-to-moderate ulcerative colitis. Am J Gastroenterol. 2002;97:1398–1407. doi: 10.1111/j.1572-0241.2002.05781.x. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland LR, Martin F, Greer S, Robinson M, Greenberger N, Saibil F, Martin T, Sparr J, Prokipchuk E, Borgen L. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987;92:1894–1898. doi: 10.1016/0016-5085(87)90621-4. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths AM. Specificities of inflammatory bowel disease in childhood. Best Pract Res Clin Gastroenterol. 2004;18:509–523. doi: 10.1016/j.bpg.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Pruitt R, Hanson J, Safdi M, Wruble L, Hardi R, Johanson J, Koval G, Riff D, Winston B, Cross A, Doty P, Johnson LK. Balsalazide is superior to mesalamine in the time to improvement of signs and symptoms of acute mild-to-moderate ulcerative colitis. Am J Gastroenterol. 2002;97:3078–3086. doi: 10.1111/j.1572-0241.2002.07103.x. [DOI] [PubMed] [Google Scholar]