Abstract
Background
The development of disease complications is poorly characterized in pediatric patients with Crohn’s disease (CD).
Methods
We retrospectively determined the cumulative incidence of stricturing and penetrating complications of CD prior to first surgery utilizing data from 989 consecutively enrolled CD patients (age 0–17 years at diagnosis) collected between January 2000 and November 2003 and stored in the Pediatric IBD Consortium Registry.
Results
Mean age at diagnosis was 11.5 ± 3.8 (standard deviation) years. Median follow-up time was 2.8 years. Prior to first surgery, the cumulative incidence of stricturing or penetrating complications was 27% at 5 years and 38% at 10 years from the diagnosis of inflammatory bowel disease. The cumulative incidence of complicated disease was lowest in isolated colonic disease (P = 0.009). Penetrating complications that followed stricturing complications prior to first surgery occurred within 2 years of stricturing complications (cumulative incidence was 13% at 2 years from diagnosis of stricturing disease). Stricturing complications that followed penetrating complications prior to first surgery occurred within 8 years of penetrating complications (cumulative incidence was 26% at 8 years from diagnosis of penetrating complications).
Conclusions
Strictures, abscesses, and fistulas are common in pediatric CD. Earlier aggressive management may be indicated. Prospective study is required to identify genetic and serologic markers that predict a patient’s risk for the development of complicated disease and to determine optimal treatment regimens.
Keywords: abscess, fistula, non-inflammatory disease, complicated disease, children, adolescence, outcomes, database, registry, inflammatory bowel disease
Abscess, stricture, or fistula development is associated with increased risk for first surgery in pediatric patients with Crohn’s disease (CD).1 The risk for development of these complications is greater in patients who are diagnosed with inflammatory bowel disease (IBD) at an older age (6–17 years) compared with a younger age (0–5 years).2 Despite the obvious clinical importance of these disease complications in the pediatric population, few studies describe the evolution of disease in patients diagnosed with CD during childhood and adolescence.
Current classification schemes for IBD categorize disease as penetrating disease as soon as penetrating complications appear, even with coexisting strictures,3 and therefore underestimate the incidence of stricturing disease in patients. Furthermore, reports on the association between disease behavior and future complications are conflicting.4
To improve our understanding of the progression of disease prior to first surgery in pediatric CD, we retrospectively determined the cumulative incidence of stricturing and penetrating complications of disease and studied the association of disease complications with initial disease location in a large cohort of children and adolescents with CD.
PATIENTS AND METHODS
Patient Population
Enrollment into the Pediatric IBD Consortium Registry began in January, 2000. The Pediatric IBD Consortium consists of the following geographically distinct pediatric centers: 1) UCSF Children’s Hospital, San Francisco, and Kaiser Permanente of Northern California; 2) University of Chicago Comer Children’s Hospital; 3) Emory University School of Medicine, Egleston Children’s Hospital, and Scottish Rite Children’s Hospital of Children’s Healthcare of Atlanta; 4) Texas Children’s Hospital, Baylor College of Medicine; 5) Children’s Hospital of Philadelphia; and 6) MassGeneral Hospital for Children, Boston. Each site obtained Institutional Review Board (IRB) approvals for the registry protocol. The registry was established in compliance with the Health Insurance Portability and Accountability Act (HIPAA). Informed consent and assent were obtained from parents and patients prior to entry of patient information into the registry. Appropriate consent as required by each IRB was obtained from all patients enrolled, including assent from patients who were age 7 to 12 years and consent directly from patients who were older than 12 years and from parents for children who were younger than 18 years at the time of enrollment. Details of establishment of the registry, enrollment, and data collection have been previously reported.5,6
Inclusion and Exclusion Criteria
As of November 1, 2003 (data retrieval date), 1736 patients with CD, ulcerative colitis (UC), or indeterminate colitis were enrolled in the Pediatric IBD Consortium Registry. We identified all subjects diagnosed with IBD before 18 years of age who had a diagnosis of CD as of November 1, 2003; diagnosis was established between 1979 and this end date. Clinical history, physical examination, endoscopy, histology, and radiology were used to diagnose the type of IBD, as previously described.5,6
Day 0 was defined as the date of initial diagnosis of IBD. Study end date for surgical patients was defined as date of first intestinal resection. Study end date for nonsurgical patients was defined as date of the most recent clinic visit if this occurred prior to November 1 2003, or November 1 2003 if the date of the latest clinic visit was after this data retrieval date. Surgical patients were excluded from the analysis if study end date (for surgical patients) was prior or equal to Day 0 (date of initial diagnosis of IBD). In all, 989 pediatric patients with CD met these criteria.
Description of Variables
Intestinal resection was defined as partial small bowel resection, partial colectomy, or total colectomy. Initial IBD diagnosis was defined as the initial classification of IBD, i.e., CD, UC, or indeterminate colitis.
Location of disease (determined ± 3 months from diagnosis date) was designated as 1) small bowel involvement without colon involvement; 2) small bowel and colon involvement; or 3) colon involvement without small bowel involvement. Disease location was established by abnormal pathology or IBD-compatible radiology. Gastroesophageal involvement was not considered in this analysis.
Disease phenotype was defined as inflammatory, stricturing, or penetrating disease. Inflammatory disease referred to nonstricturing and nonpenetrating disease. Stricturing disease referred to the presence of a constant luminal narrowing diagnosed radiologically, endoscopically, or surgically. Penetrating disease referred to radiographic, endoscopic, surgical, or clinical evidence of an abscess or fistula in any location. Complicated disease referred to stricturing or penetrating disease.
Statistical Methods
Kaplan–Meier survival analyses and log-rank tests were used to determine the cumulative incidence of stricturing and penetrating disease and compare time to outcome distributions by initial disease location. All log-rank P-values cited below are based on the entire length of follow-up. However, the figures show these distributions only up to a maximum of the first 10 years of follow-up, with the cumulative incidence of the outcome of interest graphed on the Y-axis.
Registry data were collected and stored in Microsoft Access 2000. Statistical analyses were performed with SAS, v. 9.1, and STATA, v. 9. Statistical significance was defined as P-values less than or equal to 0.05.
RESULTS
Demographics
In our cohort of 989 patients with CD, the mean age at diagnosis was 11.5 ± 3.8 (standard deviation) years (range 120 days to 17.9 years), 57.2% were male, and 80.2% were Caucasian. Mean follow-up time was 3.6 ± 3.1 years (1 day to 16.7 years). Median follow-up time was 2.8 years (95% confidence interval [CI] = 2.5–3.1 years).
Penetrating and Stricturing Complications (Table 1)
TABLE 1.
Cumulative Incidence of Abscess and Fistula
| Time from Diagnosis | 0 yr | 1 yr | 95% CI | 5 yrs | 95% CI | 10 yrs | 95% CI |
|---|---|---|---|---|---|---|---|
| No. risk for abscess | 989 | 736 | 243 | 41 | |||
| Cumulative incidence of abscess | 4.7% | 3.5%–6.3% | 8.8% | 6.7%–11.4% | 16.1% | 11.6%–22.2% | |
| No. risk for all fistula | 989 | 736 | 234 | 43 | |||
| Cumulative incidence of all types of fistula | 5.5% | 4.2%–7.1% | 12.7% | 10.3%–15.7% | 18.7% | 14.5%–23.9% | |
| No. risk for perianal fistula | 989 | 751 | 247 | 43 | |||
| Cumulative incidence of perianal fistula | 2.2% | 1.4%–3.3% | 5.0% | 3.5%–7.1% | 9.4% | 6.1%–14.5.% | |
| No. risk for other fistula | 989 | 759 | 250 | 46 | |||
| Cumulative incidence of other fistula | 1.4% | 0.8%–2.4% | 3.6% | 2.4%–5.5% | 4.1% | 2.6%–6.3% | |
| No. risk for ileocolonic fistula | 989 | 763 | 255 | 46 | |||
| Cumulative incidence of ileocolonic fistula | 0.7% | 0.3%–1.5% | 1.7% | 0.9%–3.4% | 2.1% | 1.1%–4.1% | |
| No. risk for ileoileal fistula | 989 | 764 | 256 | 46 | |||
| Cumulative incidence of ileo-ileal fistula | 0.4% | 0.1%–1.1% | 1.0% | 0.5%–2.1% | 1.5% | 0.7%–3.3% | |
| No. risk for rectovaginal fistula | 989 | 762 | 255 | 46 | |||
| Cumulative incidence of rectovaginal fistula | 0.3% | 0.1%–0.9% | 0.5% | 0.2%–1.5% | 1.4% | 0.4%–4.9% | |
| No. risk for enterocutaneous fistula | 989 | 761 | 254 | 46 | |||
| Cumulative incidence of enterocutaneous fistula | 0.5% | 0.2%–1.3% | 1.3% | 0.7%–2.6% | 1.3% | 0.7%–2.6% | |
| No. risk for rectovesical fistula | 989 | 763 | 255 | 46 | |||
| Cumulative incidence of rectovesical fistula | 0.2% | 0.1%–0.9% | 0.5% | 0.1%–1.6% | 1.0% | 0.3%–3.1% | |
| No. risk for ileorectal fistula | 989 | 758 | 253 | 46 | |||
| Cumulative incidence of ileorectal fistula | 0.8% | 0.4%–1.6% | 0.8% | 0.4%–1.6% | 0.8% | 0.4%–1.6% |
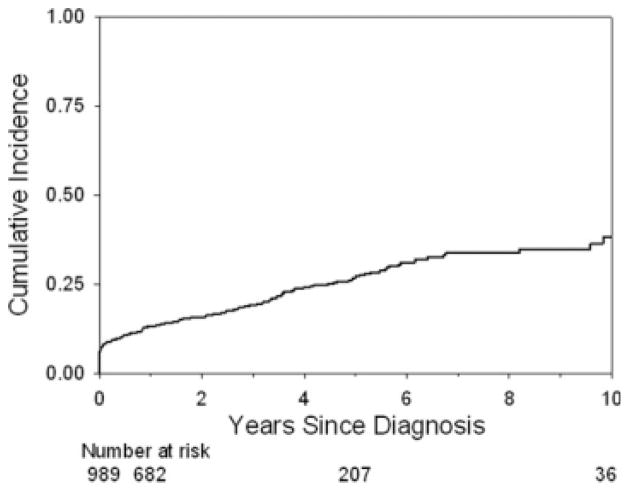
The cumulative incidence of development of an abscess was similar to that of fistula at 10 years from the time of diagnosis of disease (Table 1). The cumulative incidence of development of complicated disease was 13.0% at 1 year, 26.7% at 5 years, and 37.9% at 10 years from diagnosis of disease (Fig. 1).
FIGURE 1.
Cumulative incidence of complicated disease from the time of diagnosis of disease. The Kaplan–Meier curve shows the cumulative incidence of complicated disease from the time of diagnosis of disease. The cumulative incidence of complicated disease behavior at 1, 5, and 10 years from diagnosis was 13.0% (95% CI = 11.0%–15.4%), 26.7% (23.3%–30.5%), and 37.9% (31.8%–44.7%).
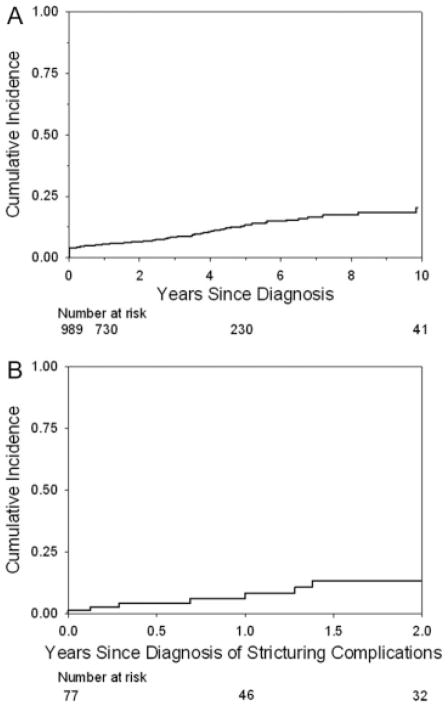
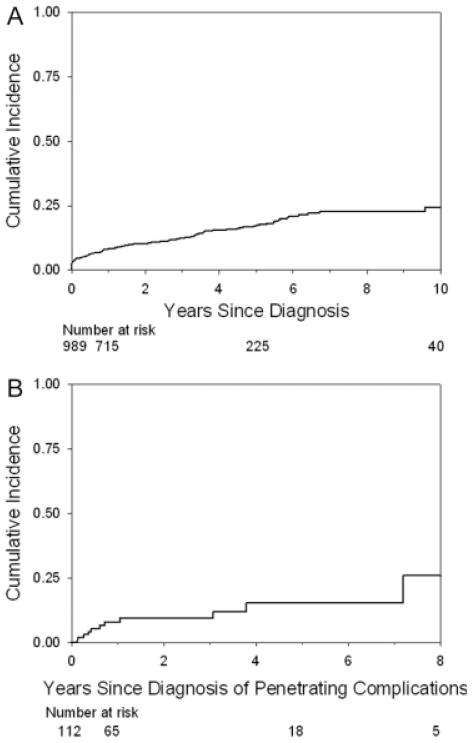
The cumulative incidences of stricturing complications (Fig. 2A) and penetrating complications (Fig. 3A) at 10 years from the time of diagnosis were 20.5% and 24.5%, respectively. The cumulative incidence of penetrating complications 2 years from the diagnosis of stricturing complications was 13.3% (Fig. 2B) and remained stable. The cumulative incidence of stricturing complications 8 years from the diagnosis of penetrating complications was 25.9% (Fig. 3B) and remained stable.
FIGURE 2.
(A) Cumulative incidence of stricturing disease from the time of diagnosis of disease. The Kaplan–Meier curve shows the cumulative incidence of stricturing disease from the time of diagnosis of disease. The cumulative incidence of stricturing disease at 1, 5, and 10 years from diagnosis was 5.5% (95% CI = 4.3%–7.2%), 13.6% (10.9%–16.9%), and 20.5% (15.4%–26.9%). (B) Cumulative incidence of penetrating complications from the time of diagnosis of stricturing complications of disease. The Kaplan–Meier curve shows the cumulative incidence of penetrating complications from the time of diagnosis of stricturing complications of disease. The cumulative incidence of penetrating complications at 1 and 2 years from the time of diagnosis of stricturing disease was 6.1% (95% CI = 2.3%–15.6%) and 13.3% (6.4%–26.5%).
FIGURE 3.
(A) Cumulative incidence of penetrating disease from the time of diagnosis of disease. The Kaplan–Meier curve shows the cumulative incidence of penetrating disease from the time of diagnosis of disease. The cumulative incidence of penetrating disease at 1, 5, and 10 years from diagnosis was 8.2% (95% CI = 6.6%–10.2%), 17.1% (14.3%–20.4%), and 24.5% (19.7%–30.3%). (B) Cumulative incidence of stricturing complications from the time of diagnosis of penetrating complications of disease. The Kaplan–Meier curve shows the cumulative incidence of stricturing complications from the time of diagnosis of penetrating complications of disease. The cumulative incidence of stricturing complications at 1, 5, and 8 years from diagnosis of penetrating disease was 8.0% (95% CI = 3.9%–16.1%), 15.3% (7.9%–28.5%), and 25.9% (10.9%–54.2%).
Disease Location
Data on disease location were available for 600 patients. Eighty-seven patients had small bowel disease without colonic involvement (SBNC), 369 patients had small bowel and colonic disease (SB&C), and 144 patients had colonic disease without small bowel involvement (CNSB).
The cumulative incidence of abscess development did not differ by initial disease location (P = 0.12). The cumulative incidence of fistula development did not differ by initial disease location (P = 0.15). The cumulative incidence of penetrating disease did not differ by initial disease location (P = 0.13). The cumulative incidence of stricture development following development of penetrating complications did not differ by initial disease location (P = 0.91). The cumulative incidence of penetrating complications following stricture development did not differ by initial disease location (P = 0.63).
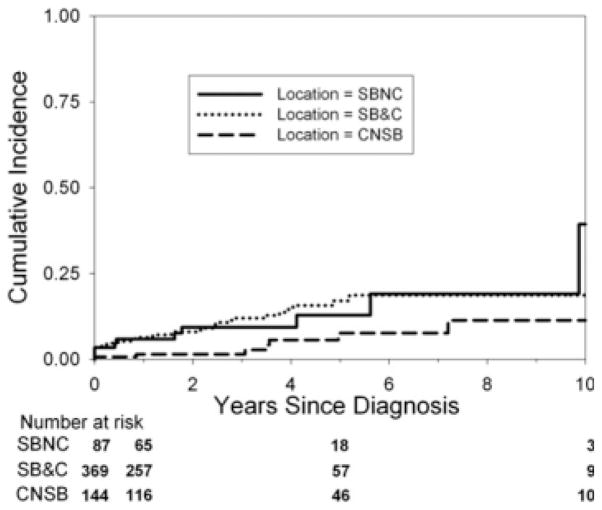
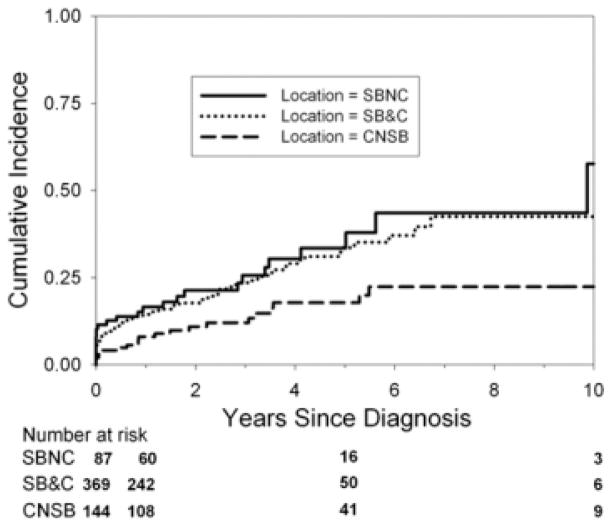
The cumulative incidence of stricture development (Fig. 4) differed by initial disease location. The cumulative incidence of complicated disease differed by initial disease location (Fig. 5).
FIGURE 4.
Cumulative incidence of stricture development by initial disease location. The Kaplan–Meier curve shows the cumulative incidence of stricture development from the time of diagnosis by initial disease location. The cumulative incidence of stricture development differs by location (P = 0.02). The cumulative incidence of stricture development in small bowel (no colon) disease at 1, 5, and 10 years from the time of diagnosis was 5.9% (95% CI = 2.5%–13.6%), 12.9% (6.1%–25.9%), and 39.3% (14.1%–80.6%). The cumulative incidence of stricture development in small bowel and colon disease at 1, 5, and 10 years from the time of diagnosis was 6.4% (95% CI = 4.2%–9.6%), 17.1% (12.1%–23.8%), and 18.7% (13.1%–26.3%). The cumulative incidence of stricture development in colon (no small bowel) disease at 1, 5, and 10 years from the time of diagnosis was 1.5% (95% CI = 0.4%–5.9%), 7.7% (3.4%–17.1%), and 11.4% (4.9%–25.0%). Small bowel no colon (SBNC); small bowel and colon (SB&C); colon, no small bowel (CNSB).
FIGURE 5.
Cumulative incidence of the development of complicated disease by initial disease location. The Kaplan-Meier curve shows the cumulative incidence of the development of complicated disease from the time of diagnosis by initial disease location. The cumulative incidence of development of complicated disease differs by location (P = 0.009). The cumulative incidence of development of complicated disease in small bowel (no colon) disease at 1, 5, and 10 years from the time of diagnosis was 16.6% (95% CI = 10.2%–26.4%), 33.5% (22.6%–47.7%), and 57.7% (33.5%–83.6%). The cumulative incidence of development of complicated disease in small bowel and colon disease at 1, 5, and 10 years from the time of diagnosis was 14.5% (95% CI = 11.2%–18.7%), 33.6% (26.9%–41.4%), and 42.5% (32.9%–53.7%). The cumulative incidence of development of complicated disease in colon (no small bowel) disease at 1, 5, and 10 years from the time of diagnosis was 8.1% (95% CI = 4.6%–14.1%), 17.8% (11.4%–27.1%), and 22.4% (14.4%–33.8%). Small bowel no colon (SBNC); small bowel and colon (SB&C); colon, no small bowel (CNSB).
DISCUSSION
In our study, one of the largest pediatric cohorts reported to date, the cumulative incidence of the development of complicated disease in pediatric patients with CD was 13.0%, 26.7%, and 37.9% at 1, 5, and 10 years, respectively, from the time of diagnosis of disease. Our study illustrates that evolution to complicated disease in a pediatric cohort is common.
Few reports describe the evolution of disease in the pediatric population.7–12 It is difficult to make direct comparisons of these reports to our study because of different disease classification schemes and approaches to statistical analysis. Regardless, taken together, our studies indicate that the development of stricturing and penetrating disease are important complications of CD diagnosed during childhood and adolescence.
Studies focusing on primarily adult populations also illustrate that evolution of disease is common in CD.3,4,13 Cosnes et al14 reported that the probability of having inflammatory disease (without complications) at 5 and 20 years after diagnosis was 48% ± 1% and 12% ± 1%, respectively. In contrast, in our pediatric cohort the cumulative probability of remaining free of stricturing or penetrating disease at 5 and 10 years after diagnosis was 73.3% (95% CI = 69.5%–76.8%) and 62.1% (95% CI = 55.3%–68.2%), respectively. In the Cosnes et al cohort, at 5 and 20 years after diagnosis, the risk of stricturing disease alone (not associated with a penetrating complication), was 12% and 18%, respectively. The cumulative incidence of stricturing disease in our pediatric cohort was 13.6% and 20.5% at 5 and 10 years after diagnosis, respectively. It is difficult to make direct comparisons of the development of stricturing disease in these 2 cohorts, as we did not exclude patients from this group who also had a penetrating complication. In the Cosnes et al population, the cumulative incidence of penetrating disease at 5 and 20 years after diagnosis was 40% and 70%, respectively. In our pediatric population, the cumulative incidence was 17.1% at 5 years and 24.5% at 10 years. The cumulative incidence of development of penetrating complications of disease in the Cosnes et al study cohort was higher than our pediatric cohort, possibly reflecting differing phenotypes of disease diagnosed in adulthood compared with childhood and adolescence.
Louis et al3 reported that 78% of patients with stricturing disease remained stricturing (median follow-up 6.5 years; range 1–32 years). We found that in patients with stricturing disease, patients who had penetrating complications prior to first surgery developed penetrating disease within 2 years of the stricturing complication. The cumulative incidence of penetrating disease prior to first surgery was 13.3% at 2 years from the time of diagnosis of stricturing disease. Furthermore, Louis et al3 reported that the rate of change from nonstricturing, nonpenetrating disease to stricturing or penetrating disease was stable at approximately 25%–33% every 5 years. In contrast, the cumulative incidence of development of complicated disease in our cohort increased from 26.7% at 5 years to 37.9% at 10 years from the time of diagnosis of disease.
Disease Location
Studies have reported associations between small bowel disease and stricturing complications13,14 and between colonic disease and penetrating complications.3,15 Others have reported an association between stricturing/penetrating disease complications and small bowel CD and between inflammatory disease and colonic CD.4,12 Smith et al4 reported that progression to more severe disease was associated with small bowel disease (irrespective of associated colonic lesions), while, conversely, colonic disease was associated with the least progression to more severe disease. Similarly in our cohort, the cumulative incidence at 1, 5, and 10 years of complicated disease was lower in patients with colonic disease compared with patients with small bowel disease or small bowel disease combined with colonic disease.
In a cohort of 169 patients with CD diagnosed between 1970 and 1993, Schwartz et al16 found the cumulative incidence of at least 1 fistula (any location) after 1 year was 21%, after 5 years was 26%, after 10 years was 33%, and after 20 years was 50%. The cumulative incidence at 10 years of any fistula in our cohort was 18.7%, lower than the Schwartz et al population. Schwartz et al reported that the cumulative risk of at least 1 perianal fistula was 12% after 1 year, 15% after 5 years, 21% after 10 years, and 26% after 20 years. In contrast, in our cohort the cumulative incidence of perianal fistula was 2.2% at 1 year, 5.0% at 5 years, and 9.4% at 10 years. These observed differences in the incidence of any fistula and perianal fistula in the Schwartz et al population compared with ours may reflect that the Schwartz et al population included both adult and pediatric patients, while our population was a defined pediatric cohort. Schwartz et al reported that 9% of fistulizing episodes were rectovaginal fistulas and 3% were enterovesical. Although we cannot make direct comparisons to our study because of different approaches to statistical analysis, the cumulative incidence of rectovaginal fistula and rectovesical fistula in our cohort was low: 1.4% and 1.0%, respectively, at 10 years from the time of diagnosis.
Classification of Disease
The Montreal classification system allows for disease diagnosed in patients less than age 16 years to be classified as a separate category.17 Studies in pediatric patients have suggested early onset disease (age 0–5 years) may represent a different disease phenotype.5,18,19 In a previous study, we found that children who are older at diagnosis (6–17 years) are at higher risk for the development of complicated disease compared with children who are younger at diagnosis (0–5 years).2 Therefore, adjusting the classification system further to create an age category for patients diagnosed at 0–5 years may be beneficial.
A hierarchy for disease behavior exists for stricturing and penetrating complications.3 If a penetrating complication is present, disease is classified as penetrating even in the presence of coexisting strictures. Reports on the association between disease behavior and future complications are conflicting.4 Our data show that development of stricturing disease commonly follows development of penetrating disease. It may be helpful to revise the classification system such that hierarchical rules are not in place, and classification as stricturing or penetrating disease is not mutually exclusive.
Limitations
The main limitations of this study include those that are inherent to conducting a retrospective, secondary database analysis in tertiary care referral centers and affiliated centers. Although our study is not population-based, almost all pediatric patients with chronic disease including CD are followed by pediatric specialists in major regional medical centers such as those represented by the Consortium. Inaccurate codings are inherent in data collection and storage and can influence the results; however, these limitations will most likely bias our results toward the null hypothesis secondary to nondifferential misclassification. Complete data on disease location are missing for some of our patients. Although our findings regarding associations between the development of disease complications and initial disease location are similar to previous reports in the literature, prospective longitudinal studies that minimize missing data are needed. While the total number of subjects declines with time, primarily due to transition to adult care, the statistical models take this into account, emphasizing the earlier part of the models when the numbers are larger. Furthermore, the sample size of our cohort is one of the largest populations of pediatric patients with CD reported to date.
SUMMARY AND CONCLUSIONS
Development of complicated disease prior to first surgery is common in pediatric patients with CD. Small bowel disease and small bowel/colonic disease are more commonly associated with the development of complicated disease behavior than isolated colonic disease. The cumulative incidence of the development of stricturing and penetrating complications of disease is similar. The high rate of stricturing disease (prior to first surgery) in the first 1–2 years following the diagnosis of penetrating disease and penetrating disease in the first 1–2 years (prior to first surgery) following the diagnosis of stricturing disease suggests that more aggressive management at the time of diagnosis of the first complication should be considered. Adjusting current classification schemes to create additional age categories within the pediatric age group and to exclude hierarchical rules for categorization of disease complications may improve characterization of disease diagnosed in the pediatric population. Future prospective studies are required to identify genetic and serologic markers that predict a patient’s increased risk for the development of these complications and to develop optimal treatment strategies.
Acknowledgments
The authors thank Joel Cutler for his vision and leadership in raising awareness and support for issues relevant to children with inflammatory bowel disease. The registry was supported by the indispensable efforts of a group of local study coordinators: Jennifer Cooper, Elizabeth Garnett, Catherine Geraci, Rachel Kreh, and Amy York. Advice and guidance pertaining to database development and management was provided by Traci Clemens, PhD, Emmes Corp., Rockville, MD. We are grateful for local support, including generous support from the Wallace Family (to H.S.W.), Jim Brooks (to H.S.W.), the Joel Barnett Family (to B.S.K.), John Fullerton and family (to M.B.H.), and the Marcus Foundation (to S.A.C.). The authors would like to acknowledge the generous collaboration of the following associates who referred patients for our registry: Jeffrey Blumental, Tim Buie, Robert Cannon, Conrad Cole, Michael Durant, Mark Gilger, Ranjana Gokhale, Stefano Guandalini, Colleen Hadigan, Stephen Hardy, Jay Hochman, Alison Hoppin, Sandy Hwang, Esther Israel, Crain Jensen, Seiji Kitagawa, Ronald Kleinman, William Klish, Jeffrey Lewis, Larry Glen Lewis, Carlos Lifshitz, Petar Mamula, Marjorie McCracken, William Meyers, Kathleen Motil, William Mow, Anthony Olive, Dinesh Patel, David Piccoli, Edith Pilzer, Rene Romero, Philip Rosenthal, Gary Russell, Larry Saripkin, Bess Schoen, Robert Shulman, John Snyder, Gayathri Tenjalra, Ritu Verma, Xavier Villa, and Qian Yuan.
Supported in part by National Institutes of Health (NIH) grants DK060617 (to M.B.H.), DK53708 (to B.D.G.), DK006544 (to B.D.G.), DK007762 (to M.B.H., N.G.), and DK077734 (to N.G.); Children’s Digestive Health and Nutrition Foundation/Crohn’s and Colitis Foundation of America (CCFA) Award for New Investigators (to N.G.); CCFA Career Development Award (to N.G.); NIH/NCRR UCSF-CTSI Grant No. UL1 RR024131; and the CCFA.
References
- 1.Gupta N, Cohen SA, Bostrom AG, et al. Risk factors for initial surgery in pediatric patients with Crohn’s disease. Gastroenterology. 2006;130:1069–1077. doi: 10.1053/j.gastro.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Gupta N, Bostrom AG, Kirschner BS, et al. Presentation and disease course in early compared to later onset pediatric Crohn’s disease. Am J Gastroenterol. 2008;103:1–7. doi: 10.1111/j.1572-0241.2008.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis E, Collard A, Oger AF, et al. Behavior of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith BR, Arnott ID, Drummond HE, et al. Disease location, anti-Saccharomyces cerevisiae antibody, and NOD2/CARD15 genotype influence the progression of disease behavior in Crohn’s disease. Inflamm Bowel Dis. 2004;10:521–528. doi: 10.1097/00054725-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Heyman MB, Kirschner BS, Gold BD, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr. 2005;146:35–40. doi: 10.1016/j.jpeds.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 6.Gupta N, Bostrom AG, Kirschner BS, et al. Gender differences in presentation and course of disease in pediatric patients with Crohn’s disease. Pediatrics. 2007;120:e1418–e1425. doi: 10.1542/peds.2007-0905. [DOI] [PubMed] [Google Scholar]
- 7.Kugathasan S, Judd RH, Hoffmann RG, et al. Wisconsin Pediatric Inflammatory Bowel Disease Alliance. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. J Pediatr. 2003;143:525–531. doi: 10.1067/s0022-3476(03)00444-x. [DOI] [PubMed] [Google Scholar]
- 8.Dubinsky MC, Lin YC, Dutridge D, et al. Western Regional Pediatric IBD Research Alliance. Serum immune responses predict rapid disease progression among children with Crohn’s disease: immune responses predict disease progression. Am J Gastroenterol. 2006;101:360–367. doi: 10.1111/j.1572-0241.2006.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman HJ. Comparison of longstanding pediatric-onset and adult-onset Crohn’s disease. J Pediatr Gastroenterol Nutr. 2004;39:183–186. doi: 10.1097/00005176-200408000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Shaoul R, Karban A, Reif S, et al. Disease behavior in children with Crohn’s disease: the effect of disease duration, ethnicity, genotype, and phenotype. Dig Dis Sci. 2009;54:142–150. doi: 10.1007/s10620-008-0326-7. [DOI] [PubMed] [Google Scholar]
- 11.Vernier-Massouille G, Balde M, Salleron J, et al. Natural history of pediatric Crohn’s disease: a population-based cohort study. Gastroenterology. 2008;135:1106–1113. doi: 10.1053/j.gastro.2008.06.079. [DOI] [PubMed] [Google Scholar]
- 12.Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008;135:1114–1122. doi: 10.1053/j.gastro.2008.06.081. [DOI] [PubMed] [Google Scholar]
- 13.Louis E, Michel V, Hugot JP, et al. Early development of stricturing or penetrating pattern in Crohn’s disease is influenced by disease location, number of flares, and smoking but not by NOD2/CARD15 genotype. Gut. 2003;52:552–557. doi: 10.1136/gut.52.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Nos P, Hinojosa J, Mora J, et al. Validation of a simplified clinical index to predict evolving patterns in Crohn’s disease. Eur J Gastroenterol Hepatol. 2002;14:847–851. doi: 10.1097/00042737-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz DA, Loftus EV, Jr, Tremaine WJ, et al. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875–880. doi: 10.1053/gast.2002.32362. [DOI] [PubMed] [Google Scholar]
- 17.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravikumara M, Sandu BK. Epidemiology of inflammatory bowel diseases in childhood. Indian J Pediatr. 2006;73:717–721. doi: 10.1007/BF02898451. [DOI] [PubMed] [Google Scholar]
- 19.Mamula P, Telega GW, Markowitz JE, et al. Inflammatory bowel disease in children 5 years of age and younger. Am J Gastroenterol. 2002;97:2005–2010. doi: 10.1111/j.1572-0241.2002.05915.x. [DOI] [PubMed] [Google Scholar]