Abstract
BACKGROUND
The relationship between the age at diagnosis and disease course is poorly defined in children with Crohn’s disease (CD). We examined the presentation and course of disease in patients 0–5 compared to 6–17 yr of age at diagnosis.
METHODS
We analyzed uniform data from 989 consecutive CD patients collected between January 2000 and November 2003, and stored in the Pediatric IBD Consortium Registry. The statistical tests account for the length of follow-up of each patient.
RESULTS
In total, 98 patients (9.9%) were of 0–5 yr of age at diagnosis. The mean follow-up time was 5.6 ± 5.0 yr in the younger group and 3.3 ± 2.8 yr in the older group (P < 0.001). Race/ethnicity differed by the age group (P = 0.015); a larger proportion of the younger group was Asian/Pacific Islander or Hispanic, and a larger proportion of the older group was African American. The initial classification as ulcerative colitis or indeterminate colitis was more common among the 0–5 yr of age group (P < 0.001). The 6–17 yr of age patients presented with more abdominal pain (P < 0.001), weight loss (P = 0.001), or fever (P = 0.07), while the 0–5 yr of age patients presented with more rectal bleeding (P = 0.008). The 6–17 yr of age patients were more likely to be treated with antibiotics (P < 0.001), 6-mercaptopurine/azathioprine (P < 0.001), infliximab (P = 0.001), or corticosteroids (P = 0.0006). The 6–17 yr of age patients had a higher cumulative incidence of treatment with 5-aminosalicylates (P = 0.009) or methotrexate (P = 0.04). The risk for developing an abscess (P = 0.001), a fistula (P = 0.02), a stricture (P = 0.05), or a perianal fissure (P = 0.06) was greater in the 6–17 yr of age patients.
CONCLUSIONS
The 6–17 yr of age patients with CD appear to have a more complicated disease course compared to 0–5 yr of age children. The 0–5 yr of age group may represent a unique disease phenotype and benefit from different approaches to management. Long-term prospective studies are required to validate these findings.
INTRODUCTION
Several studies have examined the presentation or course of inflammatory bowel disease (IBD) by age at diagnosis within adult or within combined pediatric and adult populations (1–8). Sparse data on the presentation and course of disease by age at diagnosis within the pediatric population (0–17 yr, inclusive) are available. We reported that older age at diagnosis is associated with an increased risk for surgery in pediatric patients with Crohn’s disease (CD) (9).
Early-onset IBD (age 0–5 yr) may represent a different disease phenotype than later-onset disease (age 6–17 yr) (10–12). Prior reports suggest that early-onset IBD differs epidemiologically and is characterized by predominant colonic involvement and a high positive family history compared to older age of onset (10, 11). Another study reported a high proportion of colonic and perianal disease in early-onset CD (12). We compared the presentation and course of CD by age at diagnosis (age 0–5 yr vs age 6–17 yr) utilizing a large multicenter registry.
MATERIALS AND METHODS
Patient Population
The Pediatric IBD Consortium consists of centers located in six geographically distinct regions in the United States: (a) UCSF Children’s Hospital, San Francisco, and Kaiser Permanente of Northern California; (b) University of Chicago Comer Children’s Hospital; (c) Emory University School of Medicine, Egleston Children’s Hospital and Scottish Rite Children’s Hospital of Children’s Healthcare of Atlanta; (d) Texas Children’s Hospital, Baylor College of Medicine, Houston; (e) Children’s Hospital of Philadelphia; and (f) MassGeneral Hospital for Children, Boston. The institutional review board (IRB) approvals and Health Insurance Portability and Accountability Act (HIPAA) compliance were assured at all sites. The enrollment into the registry began in January 2000. Informed consent and assent were obtained from parents and patients prior to entry of patient information into the registry. Appropriate consent, as required by each IRB, was obtained from all subjects enrolled, including assent from patients of age 7–12 yr and consent directly from patients older than 12 yr and from parents for children under 18 yr of age at the time of enrollment. The details of establishment of the registry, enrollment, and data collection have been previously reported (10).
Inclusion and Exclusion Criteria
As of November 1, 2003 (the data retrieval date), 1,736 patients with CD, ulcerative colitis (UC), or indeterminate colitis (IC) were enrolled into the Pediatric IBD Consortium Registry. Of these 1,736 patients with IBD, we identified all patients diagnosed with the disease before 18 yr of age who had a final classification of CD (9). Patients with a diagnosis of UC or IC as of November 1, 2003, were excluded from the study. Clinical history, physical examination, endoscopy, histology, and radiology were used to diagnose the type of IBD, as previously described (10). Patients who were diagnosed with IBD at age ≥18 yr were excluded from the study.
Day 0 was defined as the date of initial diagnosis of IBD. The study end date was defined as the date of the most recent clinic visit if this occurred prior to November 1, 2003, or November 1, 2003, if the date of the latest clinic visit was after this data retrieval date. For patients who underwent intestinal resection (partial small bowel resection, partial colectomy, or total colectomy), the study end date was defined as the date of intestinal resection. If the length of follow-up was less than 1 day, the patients were excluded from the analysis. In total, 989 pediatric patients with CD met these criteria (9, 13).
Description of Variables
Initial IBD diagnosis was defined as the initial classification of IBD, that is, CD, UC, or IC, in this cohort of children with a final diagnosis of CD.
Age at diagnosis was defined as the age at diagnosis of disease.
Disease location (determined ±3 months from the diagnosis date) was designated as (a) small bowel involvement without colon involvement, (b) small bowel and colon involvement, or (c) colon involvement without small bowel involvement. The disease location was established by an abnormal pathology or IBD-compatible radiology. Gastroesophageal involvement was not considered in this analysis. Granuloma on histology refers to granuloma reported on initial histology (±3 months from the time of diagnosis of disease).
Symptoms at onset of disease were patient- and/or parent-reported and included the presence or absence of abdominal pain, diarrhea, fatigue, fever, joint pain, mouth sores, nausea, perianal disease, poor growth, rectal bleeding, dermatologic manifestations, vomiting, or weight loss.
Disease severity (determined ±1 wk from the diagnosis date) refers to severity of abdominal pain, diarrhea/hematochezia, limitation of activity, abdominal tenderness, perirectal disease, and extraintestinal manifestations. The severity of each of these parameters was rated according to the criteria established in the Pediatric Crohn’s Disease Activity Index (PCDAI) (14) and then further dichotomized into normal or abnormal.
Laboratory parameters (obtained ±1 wk from the date of diagnosis) included serum albumin level, hematocrit (HCT), erythrocyte sedimentation rate (ESR), white blood cell (WBC) count, and platelet count. Serum albumin levels, hematocrit, and ESR were each dichotomized into normal or abnormal. The criteria in the PCDAI (14) were used to define normality for albumin, HCT, and ESR. WBC count and platelet count were dichotomized into elevated or not elevated. An elevated WBC count was defined according to the age-established criteria (Riley Kidometer 2.2 [http://rileychildrenshospital.com/physicians/practice-management/rk/kidometer.jsp]). A platelet count greater than 450,000 per milliliter was categorized as elevated.
Medications included 6-mercaptopurine (6-MP)/ azathioprine, methotrexate, thalidomide, cyclosporine, infliximab, 5-aminosalicylic acid (5-ASA: oral [mesalamine, sulfasalazine, balsalazide, olsalazine], enema [mesalamine], or suppository [mesalamine]), corticosteroids (oral [budesonide, prednisone, prednisolone], intravenous [methylprednisolone], or hydrocortisone foam or enema), antibiotics (clarithromycin, ciprofloxacin, metronidazole, ampicillin, or gentamicin), and tacrolimus. The date of initiation of medication was recorded in the registry.
Complications of disease were dichotomized into present or absent and included the presence or absence of an abscess, aphthous stomatitis, arthritis, clubbing, compression fractures or osteopenia/osteoporosis, erythema nodosum or pyoderma gangrenosum, fistula, renal calculi, sclerosing cholangitis, stricture, perianal fissure, or growth failure. Growth failure was defined as a height for age below the 5th percentile or a decrease in height velocity below the 5th percentile.
Statistical Methods
The Mann–Whitney rank-sum test was used to compare the follow-up time by age at diagnosis. The Fisher exact test was used to compare race/ethnicity, initial classification of disease, disease location, and initial histology by age group. Odds ratios (OR) from univariate logistic regression models were used to assess differences by age group in the prevalence of symptoms at disease onset, severity of symptoms and signs at diagnosis, and abnormal laboratory values at diagnosis. Hazard ratios (HR) from univariate Cox proportional hazards models were used to quantify the risk for treatment with a particular medication or development of a specific complication by age at diagnosis, except for 5-ASA, methotrexate, erythema nodosum/pyoderma gangrenosum, and arthritis because of violations of the underlying assumptions of Cox modeling. Kaplan–Meier survival analysis and log-rank test were used to determine the cumulative incidence of treatment with 5-ASA and methotrexate and the cumulative incidence of erythema nodosum/pyoderma gangrenosum and arthritis and compare the time to outcome distributions by age group. The log-rank P values cited were based on the entire length of follow-up.
The registry data were collected and stored in Microsoft Access 2000. The statistical analyses were performed using SAS Version 8.2 (SAS Institute, Cary, NC) and STATA Version 9 (STATA Corporation, College Station, TX). Statistical significance was defined as P values less than or equal to 0.05. The results are expressed as mean ± standard deviation.
RESULTS
Demographics
The mean age at the time of diagnosis of IBD was 11.5 ± 3.8 yr. In total, 98 children (9.9%) were less than 6 yr of age at the time of diagnosis. The mean follow-up time was 3.6 ± 3.1 yr (range 1 day to 16.7 yr). The mean follow-up time in the age group 0–5 yr at diagnosis was 5.6 ± 5.0 yr (range 8 days to 16.7 yr) and in the age group 6–17 yr at diagnosis was 3.3 ± 2.8 yr (range 1 day to 14.5 yr) (P < 0.001). In total, 63.3% of the age 0–5 yr group was male, while 56.6% of the age 6–17 yr group was male (P = 0.24). Race/ethnicity differed by age at diagnosis (P = 0.015) (Table 1). When race/ethnicity was dichotomized into Caucasian (N = 793) and non-Caucasian (N = 196), 17.0% of each age group was non-Caucasian (P = 0.99).
Table 1.
Race/Ethnicity
| Race/Ethnicity | Age 0–5 Yr, N (%) | Age 6–17 Yr, N (%) | Total N (%) |
|---|---|---|---|
| Caucasian | 78 (83.0) | 715 (83.0) | 793 (83.0) |
| African American | 5 (5.3) | 105 (12.2) | 110 (11.5) |
| Asian/Pacific Islander | 5 (5.3) | 16 (1.9) | 21 (2.2) |
| Hispanic | 4 (4.3) | 16 (1.9) | 20 (2.1) |
| Other | 2 (2.1) | 10 (1.2) | 12 (1.3) |
| Total | 94 (100) | 862 (100) | 956* (100) |
| P = 0.015 |
Race/ethnicity was unknown in 33 patients.
Initial Classification of Disease
In this cohort of children with a final classification of CD, the initial classification of IBD differed according to the age group (P < 0.001) (Table 2). A greater proportion of children in the 6–17 yr age group was initially classified as CD compared to the 0–5 yr age group. A greater proportion of children in the 0–5 yr age group with a final diagnosis of CD was initially labeled as UC or IC.
Table 2.
Initial Classification of Disease
| Initial Diagnosis | Age 0–5 Yr, N (%) | Age 6–17 Yr, N (%) | Total N (%) |
|---|---|---|---|
| Crohn’s disease | 83 (84.7) | 857 (96.2) | 940 (95.1) |
| Ulcerative colitis | 6 (6.1) | 22 (2.5) | 28 (2.8) |
| Indeterminate colitis | 9 (9.2) | 12 (1.4) | 21 (2.1) |
| Total | 98 | 891 | 989 |
| P < 0.001 |
Location of Disease and Initial Histology
Data meeting the specified time criteria (within 3 months of diagnosis) for location of disease were available for 600 patients. The proportion of patients presenting with small bowel, no colon involvement was similar in the two age groups. A larger proportion of children presented with colon, no small bowel disease in the younger age group (38.1%) compared to the older age group (22.9%). A larger proportion of the older age group (62.5%) presented with combined small bowel and colon involvement compared to the younger age group (47.6%), although the results did not achieve a statistical significance (P = 0.08) (Table 3).
Table 3.
Location of Disease
| Initial Diagnosis | Age 0–5 Yr, N (%) | Age 6–17 Yr, N (%) | Total N (%) |
|---|---|---|---|
| Small bowel, no colon | 6 (14.3) | 81 (14.5) | 87 (14.5) |
| Small bowel and colon | 20 (47.6) | 349 (62.5) | 369 (61.5) |
| Colon, no small bowel | 16 (38.1) | 128 (22.9) | 144 (24.0) |
| Total* | 42 | 558 | 600 |
| P = 0.08 |
Data were available for 42.9% of the younger age group and 62.7% of the older age group (P < 0.001).
Histology data meeting the specified time criteria were available for 783 patients (64 in the age 0–5 yr group; 719 in the age 6–17 yr group). The presence of granuloma on initial histology did not differ by age group (17.2% in the younger age group compared to 19.9% in the older age group; P = 0.74).
Symptoms at Presentation
The older age group more commonly presented with abdominal pain, weight loss, or fever, while the younger age group more commonly presented with rectal bleeding (Table 4).
Table 4.
Prevalence of Symptoms at Presentation
| Symptom | Age 0–5 Yr (N = 98) | Percentage | Age 6–17 Yr (N = 891) | Percentage | Odds Ratio* | 95% CI | P Value |
|---|---|---|---|---|---|---|---|
| Abdominal pain | 24 | 24.5 | 388 | 43.6 | 2.38 | 1.47–3.84 | < 0.001 |
| Weight loss | 8 | 8.2 | 203 | 22.8 | 3.32 | 1.58–6.96 | 0.001 |
| Rectal bleeding | 32 | 32.7 | 185 | 20.8 | 0.54 | 0.34–0.85 | 0.008 |
| Fever | 7 | 7.1 | 122 | 13.7 | 2.06 | 0.93–4.55 | 0.07 |
| Perianal disease | 3 | 3.1 | 68 | 7.6 | 2.62 | 0.81–8.48 | 0.11 |
| Joint pain | 3 | 3.1 | 63 | 7.1 | 2.41 | 0.74–7.82 | 0.14 |
| Nausea | 3 | 3.1 | 51 | 5.7 | 1.92 | 0.59–6.28 | 0.28 |
| Fatigue | 6 | 6.1 | 77 | 8.6 | 1.45 | 0.61–3.42 | 0.40 |
| Diarrhea | 35 | 35.7 | 349 | 39.2 | 1.16 | 0.75–1.79 | 0.51 |
| Poor growth | 9 | 9.2 | 66 | 7.4 | 0.79 | 0.38–1.64 | 0.53 |
| Mouth sore | 2 | 2.0 | 23 | 2.6 | 1.27 | 0.30–5.48 | 0.75 |
| Vomiting | 7 | 7.1 | 61 | 6.9 | 0.96 | 0.42–2.15 | 0.91 |
The younger age group is the reference group.
Laboratory Studies at Diagnosis
The prevalence of hypoalbuminemia, anemia, an elevated WBC count, thrombocytosis, or an elevated ESR at diagnosis did not differ by age group (Table 5).
Table 5.
Laboratory Parameters at Diagnosis
| Laboratory Parameter (N*) | Age 0–5 Yr | Percentage | Age 6–17 Yr | Percentage | Odds Ratio† | 95% CI | P Value |
|---|---|---|---|---|---|---|---|
| Hypoalbuminemia (162) | 6/12 | 50.0 | 96/150 | 64.0 | 1.78 | 0.55–5.78 | 0.34 |
| Anemia (227) | 10/17 | 58.8 | 99/210 | 47.1 | 0.62 | 0.23–1.70 | 0.36 |
| Elevated WBC (191) | 2/12 | 16.7 | 46/179 | 25.7 | 1.73 | 0.37–8.19 | 0.49 |
| Thrombocytosis (180) | 7/13 | 53.9 | 82/167 | 49.1 | 0.83 | 0.27–2.56 | 0.74 |
| Elevated ESR (190) | 12/16 | 75.0 | 130/174 | 74.7 | 0.98 | 0.30–3.21 | 0.98 |
N denotes the number of patients with data available on specific lab parameter within the specified time period.
The younger age group is the reference age group.
Severity of Symptoms and Signs of Disease at Diagnosis
The severity of perirectal disease (P = 0.15), diarrrhea/hematochezia (P = 0.67), extraintestinal manifestations of disease (P = 0.67), abdominal pain (P = 0.99), abdominal tenderness (P = 0.99), and limitation of activity (P = 0.99) within 1 wk of the time of diagnosis of disease did not differ by age group.
Initiation of Medication Treatment
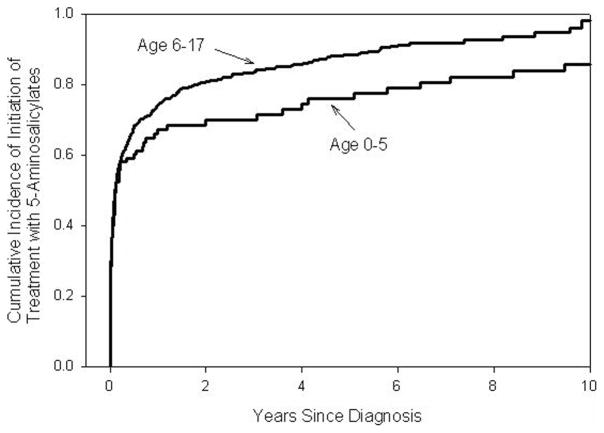
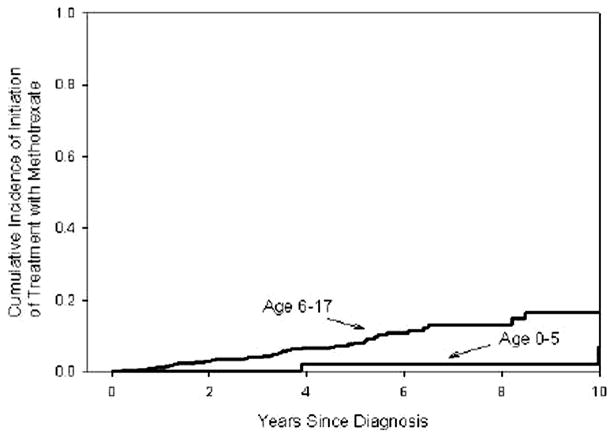
The older age group was more likely to be treated with antibiotics (HR = 1.91, 95% confidence interval [CI] 1.38–2.66, P < 0.001), 6-MP/azathioprine (HR 2.40, 95% CI 1.74–3.32, P < 0.001), infliximab (HR 2.60, 95% CI 1.46–4.64, P = 0.001), or corticosteroids (HR 1.41, 95% CI 1.10–1.81, P = 0.0006) than the younger age group. Treatment with tacrolimus (P = 0.97), thalidomide (P = 0.98), or cyclosporine (P = 0.99) did not differ by age group. The cumulative incidence of initiation of treatment with 5-ASAs (P = 0.009) or methotrexate (P = 0.04) was higher in the 6- to 17-yr-old group compared to the 0- to 5-yr-old group (Figs. 1 and 2).
Figure 1.
Cumulative incidence of initiation of treatment with 5-ASAs from the time of diagnosis by age at diagnosis of disease. The Kaplan–Meier survival curves show the cumulative incidence of initiation of treatment with 5-ASAs from the time of initial diagnosis of IBD by age group. The cumulative incidence of initiation of treatment with 5-ASAs was greater in the older age group than in the younger age group (P = 0.009). The cumulative incidence of initiation of treatment with 5-ASA’s in the 0- to 5-yr-old group at 1, 5, and 10 yr was 66.1% (56.4–75.5%), 75.9% (66.3–84.5%), and 85.9% (76.6–92.9%), respectively. The cumulative incidence of initiation of treatment with 5-ASA’s in the 6- to 17-yr-old group was 74.0% (71.1–77.1%), 88.5% (85.7–90.9%), and 96.1% (91.8–98.5%). Numbers at risk: At the baseline, time of diagnosis: 98 E (early-onset disease: age 0–5 yr), 891 L (later-onset disease: age 6–17 yr); at 1 yr after diagnosis: 28 E, 185 L; at 3 yr after diagnosis 22 E, 86 L; at 5 yr after diagnosis: 17 E, 43 L; at 7 yr after diagnosis 13 E, 13 L; at 9 yr after diagnosis: 10 E, 6 L; at 10 yrs after diagnosis 7 E, 3 L.
Figure 2.
Cumulative incidence of initiation of treatment with methotrexate from the time of diagnosis by age at diagnosis of disease. The Kaplan–Meier survival curves show the cumulative incidence of initiation of treatment with methotrexate from the time of initial diagnosis of IBD by age group. The cumulative incidence of initiation of treatment with methotrexate was greater in the older age group than in the younger age group (P = 0.04). The cumulative incidence of initiation of treatment with methotrexate in the 0- to 5-yr-old group at 1, 5, and 10 yr was 0%, 2.1% (0.3–13.9%), and 6.8% (1.6–26.7%), respectively. The cumulative incidence of initiation of treatment with methotrexate in the 6-to 17-yr-old group was 1.1% (0.6–2.3%), 8.2% (5.8–11.3%), and 16.7% (11.3–24.2%). Numbers at risk: At the baseline, time of diagnosis: 98 E (early-onset: age 0–5 yr), 891 L (later-onset: age 6–17 yr); at 1 yr after diagnosis: 76 E, 681 L; at 3 yr after diagnosis 57 E, 397 L; at 5 yr after diagnosis: 42 E, 205 L; at 7 yr after diagnosis 36 E, 90 L; at 9 yr after diagnosis: 29 E, 38 L; at 10 yr after diagnosis 22 E, 26 L.
Complications of Disease
The risk for the development of an abscess (HR 7.66, 95% CI 2.36–24.9, P = 0.001), a fistula (HR 2.67, 95% CI 1.15–6.15, P = 0.02), a stricture (HR 21.5, 95% CI 0.99–4.69, P = 0.05), or a perianal fissure (HR 2.24, 95% CI 0.97–5.19, P = 0.06) was greater in the older age group compared to the younger age group. The risk for the development of compression fractures/osteopenia/osteoporosis (P = 0.22), growth failure (P = 0.28), autoimmune hepatitis (P = 0.42), aphthous stomatitis (P = 0.59), or clubbing (P = 0.63) did not differ by age at diagnosis. The cumulative incidence of erythema nodosum/pyoderma gangrenosum (P = 0.16) or arthritis (P = 0.88) did not differ by age at diagnosis.
DISCUSSION
We describe the presentation and course of CD in early- (age 0–5 yr) versus later (age 6–17 yr)-onset disease in the pediatric population. As expected, the average length of follow-up was longer in age 0–5 yr group compared to the age 6–17 yr group because of transition to adult care as the children get older. However, the statistical analyses account for the total length of follow-up.
Clinical data on the early-onset group are sparse because this age group represents a smaller proportion of the pediatric IBD population. Sawczenko and Sandhu (15) reported on 739 incident IBD cases of age less than 16 yr diagnosed between June 1, 1998 and June 30, 1999. In total, 431 were classified as CD. Nine CD cases (2.1%) were of age less than 5 yr at diagnosis. Four percent of patients were less than 5 yr of age at diagnosis (N = 503) in Griffiths’ (16) report on children with CD diagnosed from 1980 to 1999. In our cohort of children with CD, 9.9% of our population was of age 0–5 yr at the time of diagnosis.
The differences in the presentation and course of disease may indicate a unique disease phenotype in the 0–5 yr age group, which may affect our approach to management in this age group. Paul et al. (17) analyzed data from 413 consecutive pediatric IBD outpatients (1995–2000) and compared children presenting before and after the age of 5 yr (5–15 yr). CD was diagnosed in 254 children, of which 17 patients were diagnosed before the age of 5 yr. In the early-onset group (age less than 5 yr), no children had proximal and ileal disease, 34% had ileocolic disease, and 76% had isolated colonic disease. In contrast, 26% in the later-onset group had proximal and ileal disease, 48% had ileocolic disease, and 26% had isolated colonic disease. The early-onset group showed a predominantly isolated colonic disease distribution, while the children presenting over the age of 5 yr showed a predominantly ileocolic pattern. In our cohort, both age groups had predominantly combined small bowel and colonic disease. A larger proportion of our younger age group had colonic disease without small bowel involvement compared to the older age group. As with our cohort, Paul et al. found no significant differences in the blood parameters (hemoglobin, albumin, and ESR) between the age groups (17).
Sawczenko and Sandhu (15) reported a significantly greater proportion of children of Asian origin among the children under the age of 5 yr in their cohort of 739 children with IBD. Asian refers to those reported to be of Indian, Pakistani, or Bangladeshi origin in their cohort. In our population of children with CD, we found a larger proportion of the age 0–5 yr group was Asian/Pacific Islander or Hispanic, while a larger proportion of the age 6–17 yr group was African American.
In our cohort of children with a final diagnosis of CD, a higher proportion of the younger age group was initially classified as UC or IC. The younger age group also had a higher proportion of rectal bleeding and colonic disease without small bowel involvement that may explain the initial classification as UC/IC before the ultimate diagnosis of CD was established. The children in the older age group presented with more varied symptoms: abdominal pain, weight loss, or fever were more prevalent in the 6- to 17-yr-olds. Although the higher prevalence of fever noted in older children did not meet statistical significance (P = 0.07), the point estimate of 2.06 and the range of large HRs consistent with these data (95% CI 0.93–4.55) suggests clinically important results. Furthermore, the older age group was at higher risk for the development of an abscess, a fistula, a stricture, or a perianal fissure. Although the higher risk for the development of a perianal fissure noted in older children did not meet statistical significance (P = 0.06), the point estimate of 2.24 and the range of large HRs consistent with these data (95% CI 0.97–5.19) suggests clinically important results. These differences in the presentation and course of disease suggest a more complicated disease pattern in the 6- to 17-yr-old age group compared to the 0- to 5-yr-old age group. These findings may explain the greater use of 5-ASAs, antibiotics, corticosteroids, 6-MP/azathioprine, methotrexate, and infliximab in children of age 6–17 yr at diagnosis compared to the younger group. The treatment differences may also reflect the differences in the initial diagnosis between the two age groups. The risk for treatment with tacrolimus, cyclosporine, and thalidomide did not differ by age group; this may be because these medications are less commonly used in pediatric CD overall.
Our findings support the hypothesis that the 0–5 yr age group represents a unique disease phenotype. Prospective studies incorporating serial PCDAI at each visit would be helpful to gauge the differences in disease severity by age group not only at the presentation but also over the course of disease. The incorporation of serial Tanner staging and bone age would allow more accurate interpretation of growth patterns at different stages of development. Investigation of bone density by dual energy x-ray absorptiometry (DEXA) scans obtained at diagnosis and serial time points would improve our understanding of the impact of age at diagnosis on the development of osteopenia/osteoporosis. Ideally, the date of symptom onset would be helpful to assess time to diagnosis to determine whether time to diagnosis varies by age group and impacts future course of the disease. However, determination of the date of symptom onset is problematic, especially when the presenting symptoms and signs (e.g., growth delay) are subtle. Future multicenter prospective studies are needed to improve our understanding of the differences in presentation, management, and outcome by age at diagnosis of CD.
STUDY HIGHLIGHTS.
What Is Current Knowledge
Later-onset pediatric Crohn’s disease (CD) increases the risk for surgery.
Early-onset IBD shows predominant colonic involvement compared to later-onset IBD
Early-onset IBD is associated with higher positive family history than later-onset IBD.
What Is New Here
A higher proportion of early-onset CD patients are initially classified as ulcerative colitis or indeterminate colitis compared to later-onset CD patients.
Among pediatric patients with CD, early-onset CD has a less variable presentation compared to later-onset CD.
Among pediatric patients with CD, early onset CD is associated with a less complicated disease course than later-onset CD.
Acknowledgments
The authors want to thank Joel Cutler for his vision and leadership in raising awareness and support for issues relevant to children with inflammatory bowel disease. The registry was supported by the indispensable efforts of a group of local study coordinators, including Jennifer Cooper Cockayne, Elizabeth Garnett, and Catherine Geraci. Advice and guidance pertaining to database development and management was provided by Traci Clemens, Ph.D., Emmes Corporation, Rockville, MD. We are grateful for local support, including generous support from the Wallace Family (H.S.W.), Jim Brooks (H.S.W.), the Joel Barnett Family (B.S.K.), the Nathan Cummings Foundation (B.S.K.), John Fullerton and family (M.B.H.), and the Marcus Foundation (S.A.C.). The authors would like to acknowledge the generous collaboration of their associates for referring patients for our registry, including Jeffrey Blumenthal, Tim Buie, Robert Cannon, Conrad Cole, Michael Durant, Mark Gilger, Ranjana Gokhale, Stefano Guandalini, Colleen Hadigan, Stephen Hardy, Jay Hochman, Alison Hoppin, Esther Jacobowitz Israel, Craig Jensen, Seiji Kitagawa, Ronald Kleinman, William Klish, Jeffrey Lewis, Carlos Lifshitz, Petar Mamula, Marjorie McCracken, William Meyers, Kathleen Motil, William Mow, Anthony Olive, Dinesh Patel, Edith Pilzer, Rene Romero, Philip Rosenthal, Gary Russell, Larry Saripkin, Bess Schoen, Olga Sherrod, Robert Shulman, John Snyder, Gayathri Tenjarla, Ritu Verma, Xavier Villa, and Qian Yuan.
Financial support: This study was supported by grants from the National Institutes of Health DK077734 (N.G.), DK060617 (M.B.H.), DK53708 (B.D.G.), DK007762 (M.B.H./N.G.), the Children’s Digestive Health and Nutrition Foundation/Crohn’s and Colitis Foundation of America Award for New Investigators (N.G.), the Crohn’s and Colitis Foundation of America Career Development Award (N.G.), and the Crohn’s and Colitis Foundation of America.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Neera Gupta, M.D., M.A.S. and Melvin B. Heyman, M.D., M.P.H.
Specific author contributions: Neera Gupta: initial concept, data management, study design, statistical analyses, data interpretation, initial manuscript preparation, and manuscript editing and revising and finalized submission; Alan G. Bostrom: data management, study design, statistical analyses, data interpretation, and manuscript editing and revising; Barbara S. Kirschner, Stanley A. Cohen, Oren Abramson, George D. Ferry, Benjamin D. Gold, Harland S. Winter, and Robert N. Baldassano: patient enrollment, study design, and manuscript editing and revising; Terry Smith: data management, study design, and manuscript editing and revising; and Melvin B. Heyman: patient enrollment, initial concept, study design, data interpretation, and manuscript editing and revising and finalized submission.
All authors have approved the final version of the manuscript.
Potential competing interests: None.
References
- 1.Card T, Hubbard R, Logan RF. Mortality in inflammatory bowel disease: A population-based cohort study. Gastroenterology. 2003;125:1583–90. doi: 10.1053/j.gastro.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 2.Thia KT, Luman W, Jin OC. Crohn’s disease runs a more aggressive course in young Asian patients. Inflamm Bowel Dis. 2006;1:57–61. doi: 10.1097/01.mib.0000195390.11645.7d. [DOI] [PubMed] [Google Scholar]
- 3.Beaugerie L, Seksik P, Nion-Larmurier I, et al. Predictors of Crohn’s disease. Gastroenterology. 2006;130:650–6. doi: 10.1053/j.gastro.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Jess T, Loftus EV, Jr, Harmsen WS, et al. Survival and cause-specific mortality in patients with inflammatory bowel disease: A long-term outcome study in Olmsted county, Minnesota, 1940–2004. Gut. 2006;55:1248–54. doi: 10.1136/gut.2005.079350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagtmans MJ, Verspaget HW, Lamers CBHW, et al. Crohn’s disease in the elderly: A comparison with young adults. J Clin Gastroenterol. 1998;27:129–33. doi: 10.1097/00004836-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Wagtmans MJ, Verspaget HW, Lamers CBHW, et al. Clinical aspects of Crohn’s disease of the upper gastrointestinal tract: A comparison with distal Crohn’s disease. Am J Gastroenterol. 1997;92:1467–71. [PubMed] [Google Scholar]
- 7.Polito JM, II, Childs B, Mellits ED, et al. Crohn’s disease: Influence of age at diagnosis on site and clinical type of disease. Gastroenterology. 1996;1111:580–6. doi: 10.1053/gast.1996.v111.pm8780560. [DOI] [PubMed] [Google Scholar]
- 8.Harper PC, McAuliffe TL, Beeken WL. Crohn’s disease in the elderly. A statistical comparison with younger patients matched fro sex and duration of disease. Arch Intern Med. 1986;146:753–5. doi: 10.1001/archinte.146.4.753. [DOI] [PubMed] [Google Scholar]
- 9.Gupta N, Cohen SA, Bostrom AG, et al. Risk factors for initial surgery in pediatric patients with Crohn’s disease. Gastroenterology. 2006;130:1069–77. doi: 10.1053/j.gastro.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Heyman MB, Kirschner BS, Gold BD, et al. Children with early-onset inflammatory bowel disease (IBD): Analysis of a pediatric IBD consortium registry. J Pediatr. 2005;146:35–40. doi: 10.1016/j.jpeds.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 11.Ravikumara M, Sandhu BK. Epidemiology of inflammatory bowel diseases in childhood. Indian J Pediatr. 2006;73:717–21. doi: 10.1007/BF02898451. [DOI] [PubMed] [Google Scholar]
- 12.Mamula P, Telega GW, Markowitz JE, et al. Inflammatory bowel disease in children 5 years of age and younger. Am J Gastroenterol. 2002;97:2005–10. doi: 10.1111/j.1572-0241.2002.05915.x. [DOI] [PubMed] [Google Scholar]
- 13.Gupta N, Bostrom AG, Kirschner BS, et al. Gender differences in presentation and course of disease in pediatric patients with Crohn’s disease. Pediatrics. 2007;120:e1418–25. doi: 10.1542/peds.2007-0905. [DOI] [PubMed] [Google Scholar]
- 14.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–47. [PubMed] [Google Scholar]
- 15.Sawczenko A, Sandhu BK. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch Dis Child. 2003;88:995–1000. doi: 10.1136/adc.88.11.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths AM. Specificities of inflammatory bowel disease in childhood. Best Pract Res Clin Gastroenterol. 2004;18:509–23. doi: 10.1016/j.bpg.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Paul T, Birnbaum A, Pal DK, et al. Distinct phenotype of early childhood inflammatory bowel disease. J Clin Gastroenterol. 2006;40:583–6. doi: 10.1097/00004836-200608000-00004. [DOI] [PubMed] [Google Scholar]