Abstract
Purpose
To compare stereoacuity thresholds before and after visual acuity testing in patients with intermittent strabismus and in controls.
Design
Prospective cohort study.
Participants and controls
We prospectively enrolled 88 patients (41 with intermittent strabismus and 47 controls) with measurable stereoacuity on their initial stereoacuity test.
Methods
Stereoacuity was measured prior to and immediately following visual acuity testing using the near Preschool Randot and Distance Randot stereotests. Stereoacuity was transformed to log units for analysis.
Main Outcome Measures
Change in stereoacuity thresholds (log arcsec).
Results
There was no overall deterioration in distance stereoacuity or near stereoacuity thresholds in either the intermittent strabismus or control groups. The mean change for patients with intermittent strabismus was 0.02 log arcsec (95% confidence interval (CI): -0.02 to 0.06) for near stereoacuity and 0.04 log arcsec (95% CI: -0.01 to 0.09) for distance stereoacuity. Control patients demonstrated a mean change of 0.03 log arcsec (95% CI: -0.01 to 0.06) for near stereoacuity and 0.01 log arcsec (95% CI: -0.06 to 0.08) for distance stereoacuity. These mean changes correspond to less than approximately one eighth of an octave. For individual patients, deterioration in stereoacuity beyond previously reported test-retest variability (0.6 log arcsec or greater) was not observed in patients with intermittent strabismus or controls using either test.
Conclusions
Stereoacuity thresholds do not deteriorate following visual acuity testing and therefore measurements of stereoacuity do not need to precede visual acuity measurement or other tests that involve short periods of dissociation.
Most pediatric eye care providers and strabismus specialists measure both visual acuity and stereoacuity on every patient. Changes in stereoacuity have been interpreted as improvement or worsening of strabismus,1, 2 with deterioration of stereoacuity sometimes used to make decisions regarding treatment, including surgery.3 Nevertheless, it is unclear whether testing stereoacuity first, or testing stereoacuity later in the examination, has any influence on the results.
There are very few published data to resolve this controversy. Several ophthalmic texts4, 5 suggest that the most representative measure of stereoacuity is obtained by testing stereoacuity at the beginning of an eye examination before any dissociation. Testing stereoacuity before visual acuity has also been incorporated into the design of several large clinical studies (MEPEDS6, BPEDS7, IXT1 (A Randomized Trial of Bilateral Lateral Rectus Recession versus Unilateral Lateral Rectus Recession with Medial Rectus Resection for Intermittent Exotropia) and IXT2 (A Randomized Clinical Trial of Observation versus Occlusion Therapy for Intermittent Exotropia)- available online at www.pedig.net accessed June 15, 2011). Nevertheless, for practical reasons, in common clinical practice, stereoacuity is often measured following occlusion of each eye for visual acuity testing. It is important to know whether measuring stereoacuity, after measuring visual acuity, yields worse stereoacuity thresholds.
To test the hypothesis that stereoacuity thresholds would be degraded by monocular visual acuity testing, particularly in intermittent strabismus, we prospectively evaluated stereoacuity prior to, and immediately following, visual acuity testing in patients with intermittent strabismus and controls.
Subjects and Methods
Institutional review board approval was obtained and all patients ≥ 18 years of age gave informed, written consent before participating. Children under the age of 18 gave verbal assent, with written consent obtained from either a parent or legal guardian. This research protocol, including data collection and analysis, were conducted in a manner compliant with the Health Insurance Portability and Accountability Act.
Patients
Eighty-eight patients were prospectively enrolled (age range 3 to 80 years, 59% female, 63% children ages 3 to 17 years). All consecutive, eligible patients were offered enrollment over a 10-week period. We excluded patients who, on initial assessment, did not have measurable stereoacuity at distance and near, because they could not, by definition, demonstrate any decrease in stereoacuity thresholds. Similarly, we excluded patients with visual acuity worse than 20/200 in one or both eyes because poor visual acuity is not consistent with measurable random dot stereoacuity.8 We excluded patients with incomitant strabismus (for this study defined as a difference of 5 or more prism diopters (pd) between primary and down gaze or a difference of 5 or more degrees of torsion) due to potential variability in gaze position for near testing. Patients less than 3 years of age were excluded because younger children often do not understand the stereoacuity tests we used.
We hypothesized that if stereoacuity thresholds are degraded by the dissociation necessary for monocular visual acuity testing, the effect would be greatest in patients with intermittent strabismus. Intermittent strabismus, by definition, has a manifest tropia some of the time and orthotropia at other times, and the tropia is more apparent after dissociation. We would expect very different stereoacuity thresholds in tropic and phoric states, because a manifest tropia of greater than 4 pd is not consistent with true random dot stereoacuity.8
Intermittent Strabismus Cohort
Forty-one patients were classified as intermittent strabismus, having intermittent exotropia (n=22), convergence insufficiency (n=2), divergence insufficiency (n=1), or any underlying variable condition (e.g., myasthenia gravis) (n=2). Patients with intermittent diplopia (n=14) in distance straight ahead or reading were also included in this cohort because we reasoned that the intermittency of their diplopia makes them similarly prone to deterioration of stereoacuity following visual acuity testing.
Thirty-nine (95%) of the 41 patients in this cohort had primarily horizontal deviations, 1 (2.5%) had a primarily vertical deviation and 1 (2.5%) was orthophoric (this patient was diagnosed with myasthenia gravis). Of the patients with primarily horizontal deviations, 32 (82%) had exodeviations (up to 55pd at distance and 65pd at near by the prism and alternate cover test (PACT)) and 7 (18%) had esodeviations (up to 4pd at distance and 2pd at near by PACT). Vertical deviations ranged up to 10pd at distance and 8pd at near by PACT.
Control Cohort
We also enrolled 47 patients as controls to evaluate the test-retest reliability of the specific testing protocol we used. We selected these patients because we believed they would be least likely to show deterioration of stereoacuity thresholds immediately following monocular visual acuity testing. These patients had diagnoses of microtropia (n=5), heterophoria (n=22) or orthophoria (n=20). None of the control patients had a measurable tropia exceeding 2pd at distance or near, consistent with our previous report that true stereoacuity is only associated with angles of constant strabismus no greater than 4pd.8
Stereoacuity Tests
Stereoacuity was measured, while wearing habitual refractive correction, either by a certified orthoptist (SRH) or a medical student (SJS) (trained by SRH) using two Polaroid vectograph stereoacuity tests, the Preschool Randot test9 (Stereo Optical, Chicago, IL) and the Distance Randot test10 (Stereo Optical, Chicago, IL). Prior to test administration, patients confirmed that they could recognize the different shapes presented in these vectographs by pointing to, or naming, each shape on a matching card.9
The Preschool Randot test was administered according to a previously described presentation protocol.8, 9, 11, 12 The vectographs were presented at 40 cm, yielding thresholds of 800, 400, 200, 100, 60, and 40 seconds of arc (arcsec), in approximately one-octave steps. To pass each level, patients were required to correctly identify 2 of 3 shapes. Patients unable to identify shapes at the largest disparity (800 arcsec) were assigned nil stereoacuity.
For the intermittent strabismus cohort, 39 (95%) of 41 had measurable near stereoacuity on their initial test, while all 47 patients in the control cohort had measurable near stereoacuity on their initial test, and were therefore included for analysis of near stereoacuity.
The Distance Randot test was administered according to a previously published protocol11-14 at 3m, yielding thresholds of 400, 200, 100, and 60 arcsec, in approximately one-octave steps. Patients were required to correctly identify both shapes at each disparity level to move on to the next level of stereoacuity. Those who were unable to identify shapes at the largest disparity (400 arcsec) were assigned nil stereoacuity.
For the intermittent strabismus cohort, 20 (49%) of 41 had measurable distance stereoacuity on their initial test, while 23 (49%) of 47 in the control cohort had measurable distance stereoacuity on their initial test, and were therefore included for analysis of distance stereoacuity.
Visual Acuity Measurement
Visual acuity was measured by routine methods according to standard practice of individual providers. For children younger than 7 years, visual acuity was primarily measured using HOTV optotypes. For subjects 7 years and older, visual acuity was primarily measured using the electronic Early Treatment Diabetic Retinopathy Study method (e-ETDRS)15 or a Snellen chart. All patients were tested in their habitual spectacle correction. Patients' eyes were occluded, either by patching (pediatric patients) or by an occluder. The time taken to measure visual acuity (duration of occlusion and dissociation), ranged from 1 minute to 8 minutes (median 2 minutes; quartiles: 1 minute and 3 minutes)
Testing Sequence
Prior to visual acuity testing, the Preschool Randot test was administered first, followed by the Distance Randot test. Following visual acuity measurement, testing with the Preschool Randot and Distance Randot was repeated in the same order. In all patients stereoacuity retesting began within 45 seconds of completing the visual acuity test. Binocular viewing was allowed between completion of visual acuity testing and stereoacuity testing, consistent with common practice.
Statistical analysis
Stereoacuity values, ranging from 40 arcsec to 800 arcsec, were transformed to log arcsec for the purpose of analysis, because stereoacuity thresholds are not on a linear scale. As previously reported,12 if patients did not have measurable stereoacuity, the next log level (0.3 log arcsec progression) above the largest disparity for that test was assigned as nil. Hence, nil for the Distance Randot was assigned 2.90 log arcsec, while the Preschool Randot was assigned 3.20 log arcsec. The assignment of the next log level to nil is commonly used in analysis of stereoacuity data and allows for calculations of changes in stereoacuity.16
Changes in stereoacuity, from before visual acuity testing to after visual acuity testing, were calculated for each subject as the first value minus the second value, so that a negative value would indicate a worsening of stereoacuity. Mean changes (log arcsec) were calculated with 95% confidence intervals (CI). Changes were also described as octaves (a doubling of the stereoacuity threshold, for example, 100 arcsec to 200 arcsec) which corresponds to a change of 0.3 log arcsec. Previous studies have found that test-rest variability for an individual patient on sequential examinations is typically 0.6 log arcsec (2 octaves).12 The study by Adams et al12 included patients of similar age to the present study (7 to 76 years vs 3 to 80 years) and Adams et al also found little difference in variability between children (7 to 17 years of age) and adults (18 to 76 years of age). Despite this individual test-retest variability, a study that plans a paired comparison of test and retest, can have sufficient statistical power to detect a meaningful mean change, if such a change exists. Very few studies have attempted to define a meaningful change in mean stereoacuity thresholds across a group of patients, but to draw parallels with visual acuity, some recent clinical studies17, 18 have been powered to detect mean changes of three quarters of a logMAR line, which would be a quarter octave. We therefore initially designed our study to detect a mean change of one quarter octave in stereoacuity threshold, corresponding to approximately a quarter of a step on many stereoacuity tests. We also report the proportion of individual patients that exceed previously accepted thresholds for individual test-retest variability (0.6 log arcsec, or 2 octaves).12
To detect a mean change of one quarter octave between test and retest, would have required a sample size of 175 patients, with 90% power and an alpha of 0.05, based on parametric assumptions and previous estimates of the variability of test-retest differences.12 After collecting data on the first 12 patients, we performed an analysis of the standard deviation of the test-retest differences, to confirm or refute our assumptions for sample size calculations. We found the standard deviation of the test-retest differences was far smaller than we had assumed. The probable reasons for the difference in standard deviation of test-retest differences, between our study and previous studies, are explored in the discussion. Recalculating the sample size, based on our revised estimate of the standard deviation of test-retest differences, yielded a sample size of 30 patients to detect a difference of one eighth of an octave, with 90% power and an alpha of 0.05, based on parametric assumptions. We therefore planned to collect data on 40 patients in each group.
Wilcoxon signed rank tests were used to evaluate change in stereoacuity thresholds from before to after visual acuity testing. Differences between groups (intermittent strabismus versus controls) were evaluated using the Wilcoxon rank sum test. Agreement was also represented using Bland-Altman plots,19 and 95% limits of agreement (LOA) and the 95% confidence intervals around the 95% LOA were calculated. All statistical analyses were run using SAS version 9.1.3.
Results
Intermittent Strabismus
There was no mean deterioration of near stereoacuity thresholds following visual acuity testing in the 39 intermittent strabismus patients with initial measurable near stereoacuity (mean change 0.02 log arcsec (95% CI: -0.02 to 0.06, p = 0.7)). This small mean change corresponds to 0.05 octaves (95% CI: -0.08 to 0.19 octaves), and was not distinguishable from zero. Similarly, there was no deterioration of distance stereoacuity in the 20 intermittent strabismus patients with initial distance stereoacuity (mean change 0.04 log arcsec (95% CI: -0.01 to 0.09, p = 0.25)). This small mean change corresponded to 0.14 octaves (95% CI: -0.02 to 0.29 octaves), and was not distinguishable from zero.
No individual patient (0%, 95% CI: 0 to 8.6%) in the intermittent strabismus group had a reduction in stereoacuity following visual acuity testing that would be consistent with a true deterioration in stereoacuity (≥ 0.6 log arcsec, which is 2 octaves or more) based on published test-retest data for both tests.12
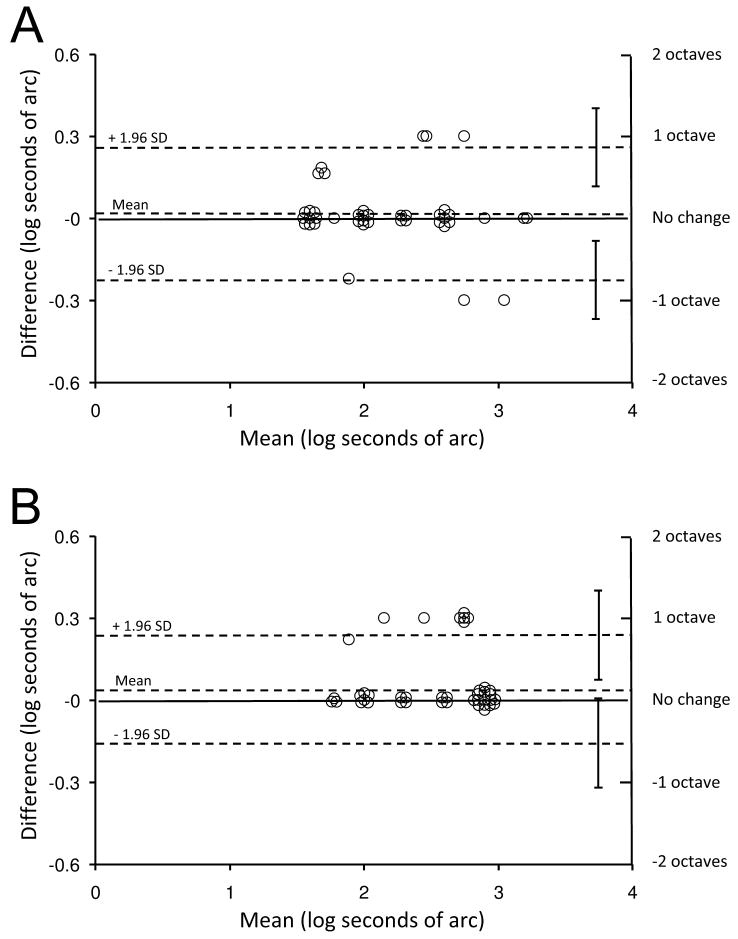
Test-retest differences are also presented on Bland-Altman plots (Figure 1), which show that variability falls well within previously published data on stereoacuity test-retest variability for the Preschool Randot and Distance Randot tests.12 Half-widths of the 95% limits of agreement for the Preschool Randot test and Distance Randot test were less than 1 octave for both tests (Preschool Randot: 0.24 log arcsec, 95% CI: 0.11 to 0.38; Distance Randot: 0.20 log arcsec, 95% CI: 0.04 to 0.36).
Figure 1.
Test-retest variability for patients with intermittent strabismus represented as Bland-Altman plots. (A) Preschool Randot test (half width of the 95% limits of agreement [LOA] = 0.24 log arcsec; 95% confidence interval [CI]: 0.11 to 0.38) (B) Distance Randot test (half width of the 95% LOA = 0.20 log arcsec; 95% CI: 0.04 to 0.36). Upper and lower dotted lines show 95% limits of agreement. SD = standard deviation.
Controls
There was no mean deterioration of near stereoacuity thresholds following visual acuity testing in the 47 control patients with initial measurable near stereoacuity (mean change 0.03 log arcsec (95% CI: -0.01 to 0.06, p = 0.3)). This small mean change corresponds to 0.09 octaves (95% CI: -0.03 to 0.20 octaves), and was not distinguishable from zero. Similarly, there was no deterioration of distance stereoacuity in the 23 control patients with initial distance stereoacuity (0.01 log arcsec (95% CI: -0.06 to 0.08, p = 1.0)). This small mean change corresponded to, 0.03 octaves (95% CI: -0.20 to 0.27 octaves) and was not distinguishable from zero.
No individual patient (0%, 95% CI: 0 to 7.5%) in the control group had a reduction in stereoacuity following visual acuity testing that would be consistent with a true deterioration in stereoacuity (≥ 0.6 log arcsec, which is 2 octaves or more) based on published test-retest data for both tests.12
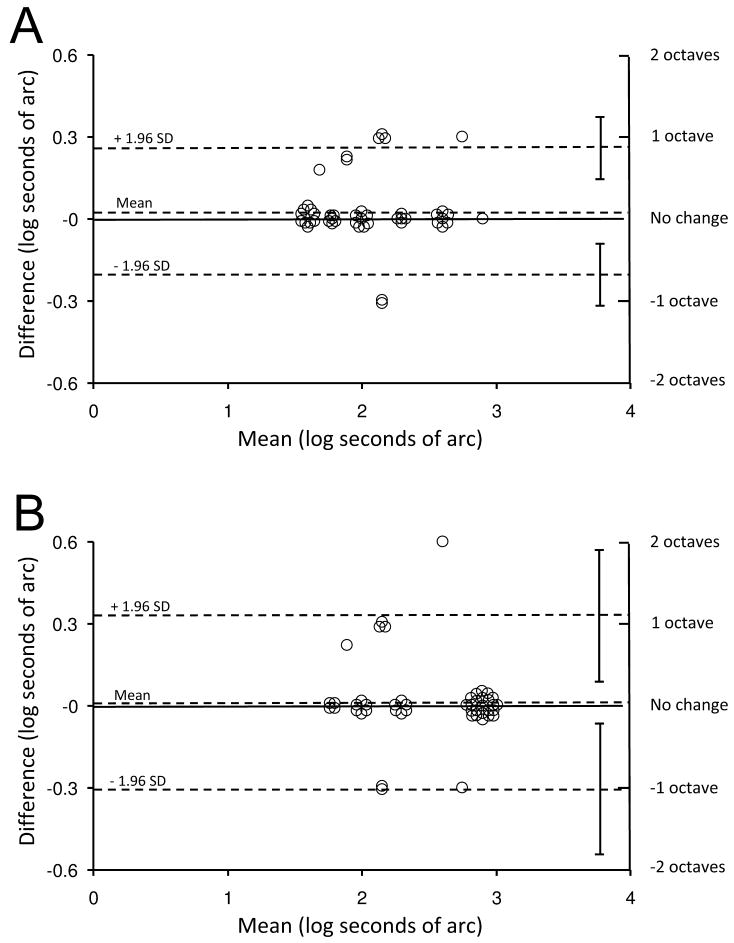
Test-retest differences are also presented on Bland-Altman plots (Figure 2), which show that variability falls well within previously published data on stereoacuity test-retest variability for the Preschool Randot and Distance Randot tests.12 Half-widths of the 95% limits of agreement for the Preschool Randot test and Distance Randot test were less than 1 octave for the Preschool Randot (0.23 log arcsec, 95% CI: 0.12 to 0.35) and approximately 1 octave for the Distance Randot (0.32 log arcsec, 95% CI: 0.08 to 0.56).
Figure 2.
Test-retest variability for controls represented as Bland-Altman plots. (A) Preschool Randot test (half width of the 95% limits of agreement [LOA] = 0.23 log arcsec; 95% confidence interval [CI]: 0.12 to 0.35) (B). Distance Randot test (half width of the 95% LOA = 0.32 log arcsec; 95% CI: 0.08 to 0.56). Upper and lower dotted lines show 95% limits of agreement. SD = standard deviation.
Comparison of changes between intermittent strabismus and controls
We compared the intermittent strabismus and control cohorts and found no differences in magnitude of change (Preschool Randot: mean 0.02 log secarc vs 0.03, p = 0.8; Distance Randot: 0.04 vs 0.01, p = 0.5).
Discussion
Patients with intermittent strabismus would be considered most at risk for deterioration of stereoacuity following short periods of dissociation such as during monocular visual acuity testing, since, by definition, such patients are tropic at one moment and phoric at another, and the tropia is often elicited by using dissociative examination methods. Nevertheless, in our study, we found no deterioration in distance or near stereoacuity thresholds following the dissociation associated with routine visual acuity testing in these patients. Our negative finding should be viewed in the context of statistical power to detect a mean difference of one-eighth of an octave, while using common stereotests that have primarily one-octave steps.
There are few previous data on the topic of dissociation and stereoacuity thresholds. Jenkins and coauthors20 prospectively evaluated the effect of dissociation on stereoacuity in 96 patients, 56 with strabismus and 40 without. Deterioration of stereoacuity post-dissociation (defined in their study as a drop of 1 level) was observed in only 3 of the 56 (5.4%) strabismic patients, with 22 of the 56 (39.3%) showing improvement. Their non-strabismic patients showed no deterioration in 39 of the 40 (97.5%) cases. Potential limitations of this previous study include evaluation of their patients with a cover-uncover test prior to the initial measurement of stereoacuity, potentially influencing the initial stereoacuity threshold. In addition, they used the Titmus test and the Randot 1 test, but the Titmus test is prone to monocular clues.21 Jenkins and coauthors did not analyze patients with intermittent strabismus separately and they classified each patient as improved, unchanged, or deteriorated, without evaluating mean change. In our study we used random dot stereoacuity tests that are more sensitive to subtle changes in stereoacuity22 and we specifically studied a patient population that would be most prone to deterioration in stereoacuity following dissociation. Using these tests, in this at-risk population, we did not find a mean deterioration in stereoacuity thresholds following visual acuity testing.
Our study did not find any systematic deterioration of stereoacuity, and therefore provides additional data on test-retest variability. Adams et al12 used data from sequential office visits in a cohort of patients with constant strabismus (of similar age to the present study) and found changes of less than 2 octaves should be considered within test-retest variability for the Preschool Randot (95% LOA: 0.59 log secarc; 1.95 octaves) and the Distance Randot (95% LOA: 0.46 log secarc; 1.52 octaves). The study by Adams and coauthors was specifically designed to evaluate test-retest variability in a clinically relevant context of sequential office visits. Consequently, retest stereoacuity thresholds in their study were obtained a week to a year after the initial measurement, with different testers in some cases. Test-retest variability in the present study was markedly lower than in the study by Adams and coauthors for both the Preschool Randot and Distance Randot tests.12 We speculate that our lower variability was because our second stereoacuity measurement was obtained within 10 minutes of the first measurement in all cases. In addition, test and retest were always obtained by the same tester, and 97% of the tests were administered by one individual (SJS).
Fawcett and Birch16 also evaluated test-retest variability of the Preschool Randot test in a cohort of 102 consecutive children (75 patients and 27 normals) who were orthotropic at distance and near at the time of testing.16 Their 95% limit of agreement (0.32 log secarc; 1.07 octaves) was only slightly greater than ours for the Preschool Randot test for either our intermittent strabismus or control cohorts. The similarity in test-retest variability between Fawcett's study and our study is likely because stereoacuity was retested within an hour in Fawcett's study, and within 10 minutes in our study.
One patient in our control cohort demonstrated an improvement in stereoacuity of 2 octaves using the Distance Randot test, which falls outside test-retest variability.12 Although the stereoacuity change in this patient exceeded the 95% LOA published by Adams et al,12 we would expect 1 patient in 88 to fall outside the 95% limits of agreement based on the definition of this metric. It is noteworthy that no patient in our study had a deterioration of stereoacuity greater than 1 octave in either group using either test.
We hypothesized that patients with intermittent strabismus would be most prone to deterioration of stereoacuity following visual acuity testing, yet we found no such deterioration. It appears that even in patients with the most fragile fusion, a period of binocular viewing lasting approximately 45 seconds between the conclusion of visual acuity testing and the beginning of stereoacuity assessment is enough time to regain fusion and achieve stereoacuity thresholds at baseline levels.
This study is not without limitations. It is possible that a learning effect contributed to the lower variability than previously reported.12 Nevertheless, all patients were asked to report only the shapes they saw in each booklet, not what they remembered from the previous test. In addition, testing order of the shapes was varied between test and retest for both the Preschool Randot and the Distance Randot tests. Additionally, a few patients initially measured 800 arcsec on the Preschool Randot test or 400 arcsec on the Distance Randot test. These thresholds would not allow a measurable deterioration of 2 octaves. Nevertheless, only 1 patient in the intermittent strabismus cohort and 1 patient in the control cohort failed to achieve their initial threshold at retest, dropping to nil stereoacuity. Another limitation is the possibility that a particularly fragile patient, not represented in our study, might demonstrate a significant reduction in stereoacuity thresholds following visual acuity testing. Our sample size was not sufficient to rule out the possibility of such a rare event. The 95% CI of 0 events in 41 cases of intermittent strabismus is 0 to 8.6%. Finally, our results cannot be generalized to patients with intermittent esotropia, because our patients with intermittent strabismus had primarily intermittent exotropia or fragile fusion.
In summary, we found no mean deterioration in distance or near stereoacuity following visual acuity testing in patients with intermittent strabismus. In addition, no individual patient had a decrease in stereoacuity greater than previously reported test-retest variability. These results suggest it may be unnecessary to measure stereoacuity prior to tests that involve short periods of dissociation, and providers can be confident in stereoacuity thresholds measured after visual acuity testing.
Acknowledgments
Financial support: Supported by National Institutes of Health Grants RR24152 (SJS) and EY018810 (JMH), Research to Prevent Blindness, New York, NY (JMH as Olga Keith Weiss Scholar and an unrestricted grant to the Department of Ophthalmology, Mayo Clinic), and Mayo Foundation, Rochester, MN.
None of the funding organizations had any role in the design or conduct of this research.
Footnotes
No authors have any financial/conflicting interests to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fu VL, Stager DR, Birch EE. Progression of intermittent, small-angle, and variable esotropia in infancy. Invest Ophthalmol Vis Sci. 2007;48:661–4. doi: 10.1167/iovs.06-0717. [DOI] [PubMed] [Google Scholar]
- 2.Stathacopoulos RA, Rosenbaum AL, Zanoni D, et al. Distance stereoacuity: assessing control in intermittent exotropia. Ophthalmology. 1993;100:495–500. doi: 10.1016/s0161-6420(93)31616-7. [DOI] [PubMed] [Google Scholar]
- 3.O'Neal TD, Rosenbaum AL, Stathacopoulos RA. Distance stereo acuity improvement in intermittent exotropic patients following strabismus surgery. J Pediatr Ophthalmol Strabismus. 1995;32:353–7. doi: 10.3928/0191-3913-19951101-06. [DOI] [PubMed] [Google Scholar]
- 4.Mehta A. Chief complaint, history, and physical examination. In: Rosenbaum AL, Santiago AP, editors. Clinical Strabismus Management: Principles and Surgical Techniques. Philadelphia, PA: Saunders; 1999. pp. 3–21. [Google Scholar]
- 5.Karlsson VC. A Systematic Approach to Strabismus. 2nd. Thorofare, NJ: SLACK; 2009. p. 112. [Google Scholar]
- 6.Varma R, Deneen J, Cotter S, et al. Multi-Ethnic Pediatric Eye Disease Study Group. The Multi-Ethnic Pediatric Eye Disease Study: design and methods. Ophthalmic Epidemiol. 2006;13:253–62. doi: 10.1080/09286580600719055. [DOI] [PubMed] [Google Scholar]
- 7.Friedman DS, Repka MX, Katz J, et al. Prevalence of decreased visual acuity among preschool-aged children in an American urban population: the Baltimore Pediatric Eye Disease Study, methods, and results. Ophthalmology. 2008;115:1786–95. doi: 10.1016/j.ophtha.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leske DA, Holmes JM. Maximum angle of horizontal strabismus consistent with true stereopsis. J AAPOS. 2004;8:28–34. doi: 10.1016/j.jaapos.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Birch E, Williams C, Hunter J, Lapa MC ALSPAC “Children in Focus” Study Team. Random dot stereoacuity of preschool children. J Pediatr Ophthalmol Strabismus. 1997;34:217–22. doi: 10.3928/0191-3913-19970701-08. [DOI] [PubMed] [Google Scholar]
- 10.Fu VL, Birch EE, Holmes JM. Assessment of a new Distance Randot stereoacuity test. J AAPOS. 2006;10:419–23. doi: 10.1016/j.jaapos.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Odell NV, Hatt SR, Leske DA, et al. The effect of induced monocular blur on measures of stereoacuity. J AAPOS. 2009;13:136–41. doi: 10.1016/j.jaapos.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams WE, Leske DA, Hatt SR, Holmes JM. Defining real change in measures of stereoacuity. Ophthalmology. 2009;116:281–5. doi: 10.1016/j.ophtha.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laird PW, Hatt SR, Leske DA, Holmes JM. Stereoacuity and binocular visual acuity in prism-induced exodeviation. J AAPOS. 2007;11:362–6. doi: 10.1016/j.jaapos.2007.01.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Hatt SR, O'Connor AR, et al. Final version of the Distance Randot Stereotest: normative data, reliability, and validity. J AAPOS. 2010;14:142–6. doi: 10.1016/j.jaapos.2009.12.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the Early Treatment of Diabetic Retinopathy Study testing protocol. Am J Ophthalmol. 2003;135:194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 16.Fawcett SL, Birch EE. Interobserver test-retest reliability of the Randot Preschool Stereoacuity test. J AAPOS. 2000;4:354–8. doi: 10.1067/mpa.2000.110340. [DOI] [PubMed] [Google Scholar]
- 17.Pediatric Eye Disease Investigator Group. Pharmacological plus optical penalization treatment for amblyopia: results of a randomized trial. Arch Ophthalmol. 2009;127:22–30. doi: 10.1001/archophthalmol.2008.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pediatric Eye Disease Investigator Group Writing Committee. A randomized trial comparing Bangerter filters and patching for the treatment of moderate amblyopia in children. Ophthalmology. 2010;117:998–1004. doi: 10.1016/j.ophtha.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 20.Jenkins PF, Vengco VJ, Rosby G, Osunsanya V. The effect of dissociation on stereoacuity. Am Orthopt J. 1998;48:94–6. [Google Scholar]
- 21.Holmes JM, Leske DA. Monocular clues in tests of stereoacuity. In: Pritchard C, editor. Orthoptics in focus: visions for the new millennium. Transactions IX International Orthoptic Congress. Nuremberg, Germany: Berufsverbandes der Orthoptistinnen Deutchlands; 1999. pp. 103–6. [Google Scholar]
- 22.Leske DA, Birch EE, Holmes JM. Real depth vs randot stereotests. Am J Ophthalmol. 2006;142:699–701. doi: 10.1016/j.ajo.2006.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]