Abstract
Aberrant glycosylation is a fundamental characteristic of progression of diseases such as cancer. Therefore, characterization of glycosylation patterns of proteins from disease tissues may identify changes specific to the disease development and improve diagnostic performance. Thus, analysis strategies with sufficient sensitivity for evaluation of glycosylation patterns in clinical specimens are needed. Here, we describe an analytical strategy for detection and verification of glycosylation patterns. It is based on a two-phase platform including a pattern discovery phase to identify the glycosylation changes using high-density lectin microarrays and a verification phase by developing lectin-based immunosorbent assays using the identified lectins. We evaluated the analytical performance of the platform using the glycoprotein standard, and found that the lectin microarray could detect specific bindings of glycoprotein to lectins at the nanogram level and the lectin-based immunosorbent assay could be used for verification of protein glycosylation. We then applied the approach to the analysis of glycosylation patterns of two glycoproteins, which are highly expressed in prostate cancer in our prior studies, PSA and membrane metallo-endopeptidase (MME), from aggressive (AC) and non-aggressive prostate cancer (NAC) tissues. The observed differences in glycosylation patterns of PSA and MME may represent significant clinical importance, and could used to develop multiplex assays for diagnosis of aggressive prostate cancer.
Introduction
Glycosylation is one of the most common modifications to proteins; its presence affects protein-protein interactions, cell-cell recognition, adhesion, and motility (1–3). Mounting evidence suggests that glycosylation is altered in disease states such as cancer and associated with disease development (4–7). Therefore, analysis of glycosylation patterns of glycoproteins is expected to improve the specificity of disease diagnosis.
Most clinical biomarkers are glycoproteins, but the assays for proteins alone have limited clinical performance (8). For example, since the discovery of PSA, assays that detect this serum biomarker (together with digital rectal exams) have been used for the screening of prostate cancer (9). PSA testing has resulted in early detection and intervention (10–11). However, the PSA protein itself is not specific enough to distinguish AC from latent or NAC (12). The AC is the type of prostate cancer that eventually causes patient death. How to detect the AC is important for cancer management. However, there are no reliable methods to prospectively predict those individuals who will develop AC Therefore, detecting the AC-specific glycosylations of glycoproteins, which are highly expressed in prostate cancer samples, may largely improve the identification of AC from NAC.
Two methods have been used preferentially in analyzing the glycosylation patterns of the glycoproteins. The first and traditional method has been a combination of separation of glycoproteins using gel electrophoresis or chromatography to release glycans from the purified glycoprotein and identification of glycans by mass spectrometry (MS) (13–17). In this method, glycoproteins and glycans are purified before the MS analysis and are used to analyze the protein glycosylation from human cells and body fluids. To date, the method has not been applied to the analysis of glycosylation patterns from individual prostate tissues, mainly due to the large quantity of purified glycoprotein required (μg to mg) and limited sample throughput. The second method is based on the interaction of glycans with different glycan binding proteins such as lectins and antibodies. Several lectin-based detection formats, such as lectin microarray, lectin chromatography, and lectin-based immunosorbent assays (LIA) have been developed to detect the glycosylation patterns (18–21). Using the reported PSA glycosylation, we have shown that Sambucus Nigra Agglutinin (SNA), which specifically is bound to sialylated PSA, can be used to develop LIA assay to investigate PSA sialylation with the sensitivity, reproducibility, and throughput required for the analysis of clinical specimens (19). However, no statistical differences between benign and cancer sera were detected using lectin SNA alone, as additional LIA assays are needed for the detection of prostate cancer, especially for AG. In this context, screening for glycosylation changes of glycoprotein using clinical specimens with similarly high sensitivity is desired because it allows for profiling glycosylation patterns and identifying lectins that detect the glycosylation changes for the development of LIA assays.
Here, we describe a sensitive and high-throughput platform to detect and verify glycosylation changes associated with AG based on a two-phase discovery and verification approach. The discovery phase is based on the recently developed high-density lectin microarray to identify the lectins specific to the altered protein glycosylation associated with disease (22–23). The verification phase is based on the developing LIA to verify the identified lectins (19, 24). Using this two-phase platform, we first analyzed the known glycoprotein marker, PSA, as a standard protein to evaluate the analytical performance. The sensitivities of lectin microarray and LIA both reached at nanogram levels that can be used to profile glycosylation patterns from clinical specimens. We further applied the platform to characterize glycosylations of PSA and membrane metallo-endopeptidase (MME) that are highly expressed in prostate cancer samples in our prior studies (19, 25). Glycosylation changes of the two glycoproteins in AC and NAC tissues were identified and verified using the two-phase platform. The glycosylation patterns and the LIA assays can potentially be used individually or as panel in the validation study to identify AC to improve the clinical management of prostate cancer patients.
Methods
Materials
The ninety-four lectins were collected from four commercial sources (22). Human PSA was from Lee BioSolutions, Inc. (St. Louis, MO). The MME protein was from Abnova (Taipei City, Taiwan). Mouse anti-human PSA antibody was from Scripps Laboratories (San Diego, CA). Mouse anti-human MME monoclonal antibody was from Abcam (Cambridge, MA). Rabbit anti-mouse IgG-Alexa Fluor 647 conjugate was from Invitrogen (Eugene, OR). The incubation chamber and holder for the lectin microarray were purchased from Whatman Schleicher & Schuell (Keene, NH). Components for the electrochemiluminescence assays were purchased from Meso Scale Discovery (Gaithersburg, MD). Sodium periodate was from Bio-Rad Laboratories (Hercules, CA). 4-(4-N-maleimidophenyl) butyric acid hydrazide hydrochloride (MPBH) was from Thermo Fisher Scientific, Inc. (Rockford, IL). Biotinylated Jacalin, SNA, GS-II, and MAL-I were from Vector Laboratories (Burlingame, CA). All other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Specimen
Samples and clinical information were obtained with informed consent and performed with the approval of the Institutional Review Board of the Johns Hopkins University. In this study, primary prostate cancers were collected from radical prostatectomy specimens at Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center. Under the NCI-funded Johns Hopkins prostate cancer SPORE project, annual follow-up data has continuously been obtained from consenting subjects, allowing the clinical follow-up data to be used in conjunction with the pathologic data. The four NAC and four AC prostate tissues were selected from cases with known Gleason scores and clinical outcome following each surgery. The NAC tissues were microdisected from primary tissues with Gleason scores of 3+3=6 and without cancer recurrence after 11–15 years of follow-up. The AC tissues were microdissected from primary tissues from cancers with Gleason score of 5+4=9 (3 cases) or 5+3=8 (one case) and either seminal vesicle or pelvic lymph node involvement (or both). Two of the men with AC died of prostate cancer 2 and 6 years after the surgery, one died of myocardial infarction 5 years after surgery with known prostate cancer recurrence, and one is thought to have been surgically cured of AC as he shows no evidence of recurrence 15 years after surgery. Four metastatic prostate cancer tissues that were selected from 33 men who died of metastatic prostate cancer between 1995 and 2004 were used in our recent genomic studies (26). Normal prostate tissues were surgically removed prostates from transplant tissue donors. Collection process was performed with support from the Transplant Resource Center of Maryland (TRCM). These tissue samples are currently being maintained in the laboratory without specific personal information as defined by HIPAA.
About 5 slides of 6-micron section (1cm × 1cm) were collected in to a centrifuge vial from each frozen OCT-embedded tissue. The tissue sections were solubilized with 200 μl of RIPA buffer containing 150 mM NaCl, 1.0% IGEPAL® CA-630, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris, pH 8.0. The samples were sonicated (1min), vortexed (1min), and centrifuged at 13,200 rpm for 5min. The supernatant was collected for glycosylation analysis, and BCA assay was performed to determine the protein concentration.
Determination of sensitivity of the high-density lectin microarray with purified PSA
Lectin microarray was fabricated based on the procedure published previously (22) with the following changes. The lectin microarray was first immersed in 50 mM ethanolamine in borate buffer (pH 8.0) for 1 hour for surface blocking. The slide was washed and dried by spinning at 500 g for 5 min. PSA protein isolated from seminal fluid was diluted into 200 μL using PBST buffer (for concentrations of 0 ng, 0.02 ng, 0.2 ng, 2 ng, 20 ng, and 200 ng). The samples were applied to lectin microarray and incubated at room temperature (RT) for 2 hours. The primary antibody (mouse anti-human PSA antibody) and the secondary antibody (rabbit anti-mouse IgG-Alexa Fluor 647 conjugate) were mixed with 20 mM sodium periodate to oxidize sugar groups at 4°C for 1 hour in the dark. The oxidized glycans of antibodies were then blocked with 1 mM 4-(4-N-Maleimidophenyl)butyric acid hydrazide hydrochloride (MPBH) for 2 hours followed by 1 mM Cys-Gly dipeptide in 4°C overnight (18). Then, 200 μL of 2 μg/mL oxidized mouse anti-human PSA antibody and 200 μL of 2 μg/mL oxidized rabbit anti-mouse IgG-Alexa Fluor 647 conjugate were sequentially hybridized with the microarray for 1 hour. After washing, secondary antibody was added; 200 μL of 2 μg/mL oxidized rabbit anti-mouse IgG-Alexa Fluor 647 conjugate was hybridized for 1 hour with gentle shaking. After washing with PBST buffer, the microarray was removed from the incubation chamber, and washed twice with water. The array was dried by spinning at 500 g for 5 minutes, and scanned using a GenePix 4000B scanner at a wavelength of 647 nm and PMT setting of 800. The slide images were analyzed using GenePix 3.0 software to convert to numerical format (GPR) using a homemade “.GAL” files. The median of the foreground spot intensity, medium of the background spot intensity, and the lectin protein identification were used in this analysis. The signal to noise ratio (the medium of spot foreground intensity relative to the medium of spot background intensity) of each lectin spot was used to determine the LOD of each lectin.
Glycan profiling of glycoproteins PSA and MME extracted from prostate tissues using high-density lectin microarray
The pooled tissue samples for each group were generated by mixing equal amount of tissue proteins (50 μg) from the 4 cases in normal (N), non-aggressive cancer (NAC), aggressive cancer (AC), and metastasis (M) groups.
For protein glycosylation analysis using lectin microarray, PSA and MME proteins were extracted from each tissue group using anti-human total PSA antibody or anti-human MME monoclonal antibody immobilized on 100 μL of magnetic beads. The magnetic beads were incubated with pooled tissue samples at 4°C for 12 hours (27). The PSA and MME proteins were eluted using 100 μL of 100 mM glycine (pH 2.3). The final protein concentration was determined using the Beckman Access® Hybritech PSA assay.
PSA protein (40 ng) and MME protein (50 ng) isolated from each of the clinical samples were incubated with the lectin microarray using the protocol described above. PBST buffer without protein was used as negative control. The signal to noise ratio of PSA and MME proteins divided by the signal to noise ratios of the control was used for data analysis.
Developing lectin-based immunosorbent assays and evaluating analytical performances
To verify the glycoprotein-lectin interactions, LIA assays for targeted glycoprotein-lectin interactions were developed. A 384-MSD plate was first coated with 10 μL of 10 μg/mL mouse anti-human PSA or MME antibody overnight at 4°C. The MSD plate was blocked using 50 μL of non-protein blocker by incubation at RT for 1 hour. The plate was washed three times using PBST buffer. To reduce background from lectin-antibody binding, the PSA/MME monoclonal antibody was oxidized using 50 μL of 20 mM sodium periodate in 150 mM NaCl and 100mM sodium acetate (pH 5.5) in 4°C in the dark for 1 hour as previously described by Haab (18). The oxidized glycans of PSA/MME monoclonal antibody were then blocked with 1 mM MPBH for 2 hours followed by 1 mM dipeptide in 4°C overnight. PSA protein extracted from pooled prostate cancer tissue was diluted using PBST buffer to generate a concentration gradient ranging from 1000 ng/mL to 0.24 ng/mL (1000, 250, 62.5, 15.6, 3.9, 0.98, and 0.24ng/mL). MME protein extracted from pooled metastatic prostate cancer tissues was diluted using PBST buffer to generate a concentration gradient ranging from 25ug/mL to 6ng/mL (25, 6.25, 1.56, 0.39, 0.098, 0.024, and 0.006ug/mL) with a four-fold dilution. For each immunosorbent assay, 10 uL of glycoprotein sample was incubated in triplicate in wells of MSD plates coated with monoclonal antibody at RT for 2 hours with gentle shaking. Unbound proteins were removed by washing three times with PBST buffer. Then 10 μL of 20 μg/mL biotinylated lectin (SNA and Jacalin for PSA test, GS-II and MAL-I for MME test) and 5 μg/mL streptavidin S LFO-TAG were added to each well and the plate was incubated at RT for 1 hour with gentle shaking. Unbound lectin and S LFO-TAG were removed by washing three times with PBST buffer. Finally, 50 μL of 1X MSD read buffer was added to each well and plates were immediately analyzed using the MSD SECTOR Imager 2400. The data was analyzed using Prism software.
Verify the identified lectin-glycoprotein interactions using lectin-based immunosorbent assays
Four frozen tissues from each of the four groups of prostate tissue samples (normal, non-aggressive primary prostate cancer, aggressive primary prostate cancer, and metastatic prostate cancer (N, NAC, AC, M)) were analyzed. The protein lysate containing the same amount of PSA (1 μg total PSA) from each individual was used in each PSA lectin-based immunosorbent assay. For the MME analysis, 1ug of total protein, instead of MME protein level, was used for each sample since there is no available assay to measure absolute MME protein in the concentration of ng/mL level. In brief, 10 μL of each sample was added in triplicate into blocked and oxidized PSA or MME antibodies coated 384-MSD plates as described above. Then 10 μL of 20 μg/mL biotinylated Jacalin or SNA for PSA analyses and biotinylated GS-II or MAL-I for MME analyses were mixed with 5 μg/mL streptavidin SMLFO-TAG. Finally, 50 μL of 1x MSD plate read buffer was added to each well and electrochemiluminescence was detected using the MSD SECTOR Imager 2400. The statistic analyses were done using Graphpad Prism V5 software. The significance was set at 0.05.
Results
Glycosylation pattern analysis of glycoprotein using high-density lectin microarrays and evaluation of analytical performance
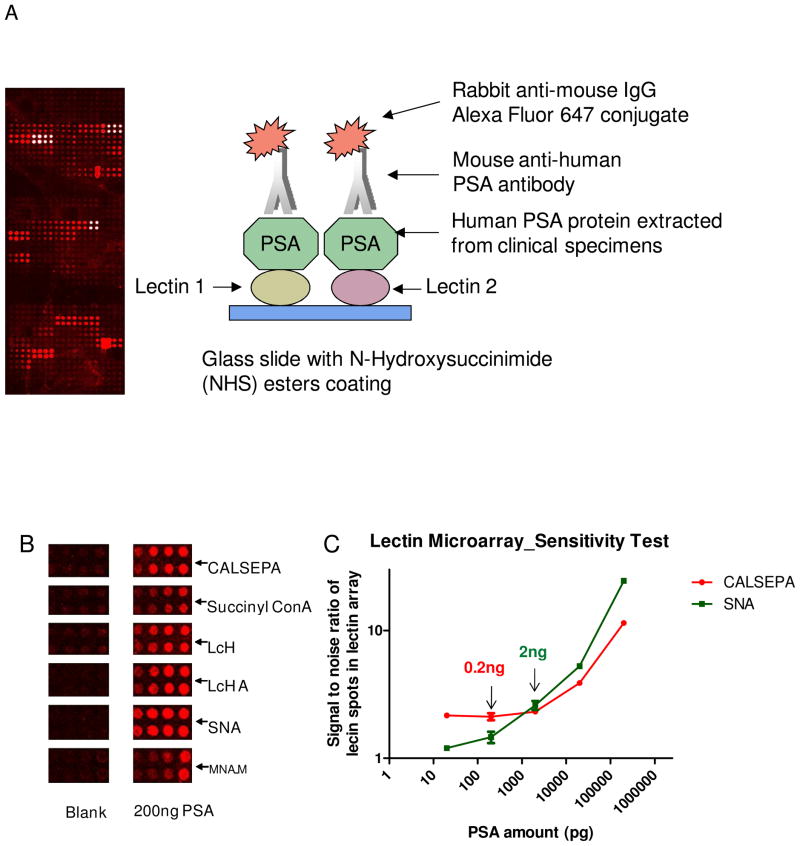
Using this platform, the detectable glycosylation of glycoproteins, the relative abundance of glycans and the different glycosylation patterns between normal and disease states were simultaneously identified (Figure 1A). We first optimized the procedure and evaluated the specificity and the sensitivity of the lectin microarray using PSA protein. To prevent non-specific binding of the primary and secondary antibodies to lectins, the antibodies were treated with sodium periodate to oxidize glycans from antibodies followed by blocking the oxidized glycans with MPBH and dipeptide solutions (18). There was minimal signal from the lectin array without PSA whereas 200 ng PSA produced specific binding to some lectins (Figure 1B).
Figure 1.
The detection of glycosylation patterns and identification of lectins that interact with PSA using high-density lectin microarrays. (A) Diagram of lectin microarrays for the analysis of target glycoprotein. (B) Specific binding of lectins on lectin microarrays to PSA. (C) The detection sensitivity of the specific lectin-PSA interactions.
Then, different amounts of purified seminal fluid PSA were analyzed to evaluate the sensitivity of the lectin microarray. To ensure a detectable signal, we required that the ratio of sample (with seminal fluid PSA) to blank (without seminal fluid PSA) for the same lectin spot be greater than 1.5. The limits of detection (LOD) were 0.2ng of PSA for binding to SNA, 2ng of PSA for CALSEPA and 2 ng of PSA for LcH A. The glycan-lectin binding curves of SNA and CALSEPA are shown in Figure 1C.
Glycosylation pattern analysis of glycoproteins extracted from aggressive and non-aggressive prostate cancer using high-density lectin microarrays
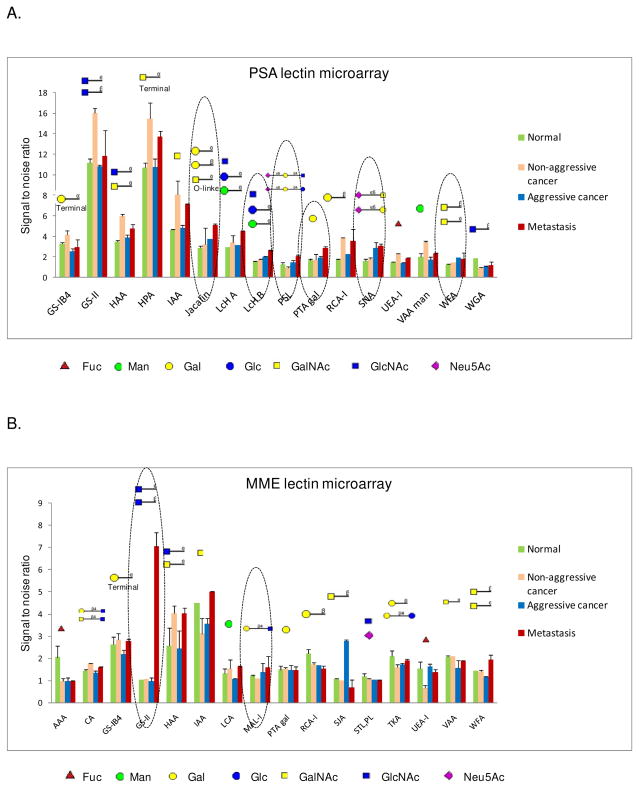
To apply lectin microarrays for comprehensive analysis of glycan structures from complex clinical specimens, PSA and MME proteins were extracted from the four groups of pooled prostate tissues (N, NAC, AC, M) using protein immunoprecipitation. The lectins with detectable signals are defined as: 1) signal to noise of each lectin spot>1.5; 2) (S/N of lectin spots with sample)/(S/N of lectin spots with control) >1.5 for at least one group tissue sample. For PSA protein, 16 lectins demonstrated specific interactions with PSA (Figure 2A and supplementary data 1). These structural features correlated well with known PSA glycan structures (13–15, 28, 29). This is the first quantitative study of PSA glycosylation using a lectin microarray to determine the glycosylation changes in AC tissues compared to NAC prostate cancer tissues, and the result provides the following glycosylation changes in AC and metastatic prostate cancer: (1) increased Neu5Acα2-6 sialylated glycans as evidenced by increased binding on SNA and PSL; (2) galactosylated glycans that were detected by the increased binding to Jacalin and PTA gal; (3) manosylated, glucosylated, or N-acetylglucosaminylated glycans detected by increased binding to lectin LcH B; and (4) increased N-acetylgalactosaminylated glycans that were detected with WFA (Figure 2A and supplementary table 1).
Figure 2.
Identification of lectins interacting with PSA and MME from normal prostate, non-aggressive, aggressive, and metastatic prostate cancer tissues using lectin microarrays. (A) Lectins that interact with PSA from pooled tissue samples of different groups. (B) Lectins that interact with MME from pooled tissue samples of different groups.
For MME protein, which have reported to associate with prostate cancer(25,30–32), lectin microarray analysis provided the following information: (1) the lectins that bound to MME were different from those that interacted with PSA; (2) unlike PSA, most lectins did not show changes in aggressive and metastatic cancer compared to normal and NAC; (3) increased N-acetylglucosaminylated glycans detected by increased binding to lectin GS-II in metastatic prostate cancer; and (4) slightly increased lacNAc motif (Galβ1-4GlcNAc) detected by MAL-I in AC and metastatic prostate cancer (Figure 2B and supplementary data 1).
Developing lectin-based immunosorbent assays
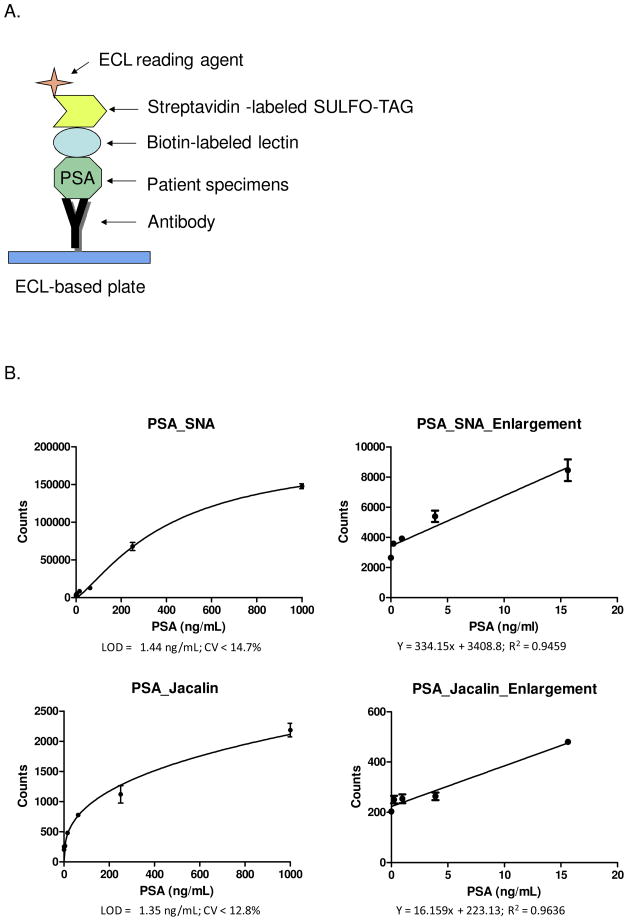
The identified lectins can be used to develop LIA to verify the lectins for the analysis of different glycosylation isoforms of glycoproteins. PSA protein was first used to optimize the LIA procedure. PSA was captured by a PSA monoclonal antibody immobilized on electrochemiluminescence (ECL) plates. The glycans of PSA were detected with biotinylated lectins and streptavidin conjugated ECL-detection agent (Figure 3A). Six biotinylated lectins with elevated binding to PSA from AC/metastasis cancer in lectin microarrays, including Jacalin, LcH B, PSL, PTA gal, SNA, and WFA, were used to develop LIA assays. PSA extracted from metastatic prostate cancer tissues was diluted using PBST buffer to 0.244ng/mL to 1000ng/mL in triplicates. We were able to successfully establish two LIAs using biotinylated SNA and Jacalin, in which the electrochemiluminescent signals increased with corresponding PSA concentrations (Figure 3B). The LODs (Limit of Detection) of SNA and Jacalin binding were 1.44ng/mL and 1.35ng/mL, respectively. The coefficients of variation (CVs) of the SNA and Jacalin LIAs were 14.7% and 12.8% (n=3) at the concentration of LODs, respectively. The assay linear range for PSA_SNA was 1.5 – 400 ng/mL and linear range for PSA_Jacalin was 1.5 – 200 ng/mL (Figure 3B).
Figure 3.
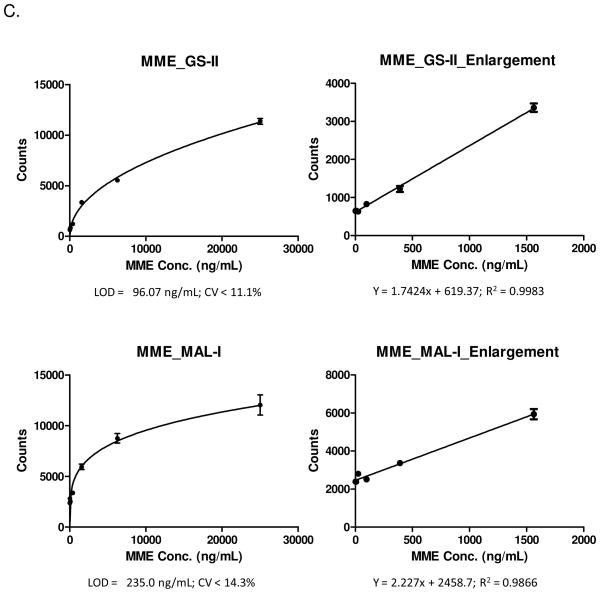
Method and analytical performances of lectin-based immunosorbent assays. (A) Diagram of a lectin-based immunosorbent assay. (B) PSA_SNA and PSA_Jacalin assays and the expended portions of the calibration curves. (C) MME_GS-II and MME_MAL-I assays and the expanded portions.
The MME protein showed different glycosylation from lectin microarray analysis. To verify the glycosylation of MME and develop LIA assays, similar procedure was used to establish LIAs. The LIAs for GS-II and MAL-I were successfully developed. The analytical performances were determined using the calibration curve with the LODs for lectins GS-II and MAL-I as 96.07ng/mL and 235.0ng/mL with CV of 11.1% and 14.3% respectively at the concentration of LODs. MME proteins within the 100 – 6250 ng/mL concentration were in the quantitative range for MME_GS-II assay and 250 – 1560 ng/mL concentration were in the quantitative range for MME_MAL-1 (Figure 3C). The analytical performances for both PSA and MME indicate that the developed LIAs using the identified lectins from lectin microarray can be used to analyze glycosylation patterns of glycoproteins from clinical specimens.
Analyses of clinical specimens with the lectin-based immunosorbent assays
We then applied the developed LIAs to the analyses of glycosylation patterns of PSA and MME from normal prostate, NAC, AC, and metastatic prostate cancer tissues with 4 individual tissues in each group with three analyses for each tissue specimen.
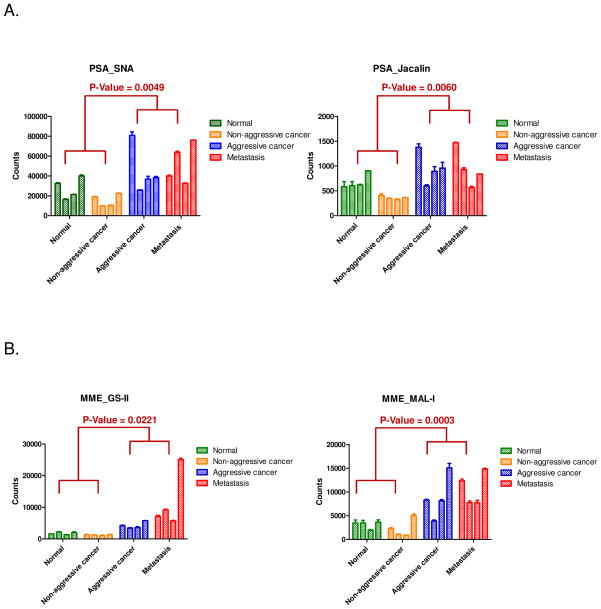
From the analyses of PSA from four groups of prostate tissues using the developed SNA and Jacalin LIAs, we found that: (1) the relative abundances of PSA_SNA and PSA_Jacalin in each group were correlated reasonably well with the lectin microarray signals from the pooled tissue specimens from each group; (2) there are considerable variation of glycosylations for PSA detected by SNA and Jacalin for each individuals in each group; but (3) signals from tissues with aggressive prostate cancer and metastatic prostate cancer are elevated compared to normal and NAC prostate cancer tissues for both PSA_SNA (p=0.0049) and PSA_Jacalin (p=0.0060) (Figure 4A).
Figure 4.
Analyses of glycosylated PSA and MME in individual tissue specimens from normal, non-aggressive, aggressive, and metastatic prostate cancers using developed immunosorbent assays. (A) PSA assays. (B) MME assays.
Similar results were observed for MME_GS-II and MME_MAL-I assays. For MME_GS-II assay, the significance between AC/metastatic prostate cancer tissues to normal and NAC tissues is 0.0221. For MME_MAL-I assay, the significance between the AC/metastatic prostate cancer tissues and normal/NAC prostate cancer tissues is 0.0003 (Figure 4B).
Discussion
Glycosylation is a common modification on proteins found in extracellular environments, including membrane proteins, cell-surface proteins, and secreted proteins (33, 34). These proteins are easily accessible for diagnostic purposes (35). The analyses of the glycosylation patterns have potential to identify glycosylation isoforms to increase the clinical performance of glycoproteins for AC diagnosis. However, here are several issues to analyze glycosylation patterns in clinical specimens. Most relevant markers are often present at low abundance. The analytical sensitivity of conventional glycosylation analysis platforms including chromatography, mass spectrometry, or electrophoresis methods is not sufficient for discovery and validation of the glycosylation changes between different disease states in clinical samples. Moreover, to verify the discovered glycosylation changes, an independent assay is required to further investigate the global screening result. The data which are consistent in both discovery and verification have great potential to be able to use for large scale validation to determine the clinical performance of the identified glycosylation isoforms.
In this study, we established a two-phase analytical platform that included a discovery phase using high-density lectin microarrays and a verification phase using lectin-based immunosorbent assays to investigate glycosylation patterns in individual clinical specimens. To identify the lectins that can interact with glycoproteins and determine the glycosylation changes in different disease states, a large number of lectins immobilized in a microarray were first probed with PSA and MME isolated from pooled prostate tissues from normal prostate, NAC, AC, and metastatic prostate cancer tissues. The identified lectins were then used to develop LIAs for quantitative analysis of lectin-glycan interactions for individuals in each group. Using this platform, we have developed PSA_SNA, PSA_Jacalin, MME_GS-II, and MME_MAL-I LIAs that have potential for diagnosis of AC. This two-phase analytical platform is able to screen and verify disease-related glycosylation changes of the protein in the cancer cells in a sensitive, reproducible, and high-throughput fashion. If successfully validated, biomarkers capable of distinguishing AC from NAC would prevent patients with NAC from overtreatment, and could allow patients with AC to receive appropriate treatment earlier in the course of their disease.
Due to the low abundance of marker proteins in clinical samples, the detection sensitivity is one of the most critical challenges for glycosylation analysis. To increase the sensitivity, we optimized the experimental procedure. First, the glycoproteins were extracted from clinical samples prior to lectin microarray analysis using a covalently immobilized antibody to avoid the interference of antibody glycans to lectin microarrays.
Second, an antibody was used to detect the target glycoprotein bound to the lectin microarray, increasing detection sensitivity and specificity. Third, for LIAs, oxidation reaction was used to reduce the background binding of glycans from antibodies to lectins (18). Fourth, we used ECL detection in the lectin-based immunosorbent assays as ECL was more sensitive compared to colorimetric detection (data is not shown). We were able to detect PSA and MME at nanogram levels in both lectin microarray and the LIAs.
Throughput is another important issue for clinical utility. Using lectin microarrays, we were able to profile hundreds of lectins in a single experiment. Thus, the microarray is an excellent tool for global screening to identify glycoprotein interacting lectins and determine the glycosylation changes. Once the glycosylation changes and the detection lectins are identified, the LIAs can be developed to verify the screening results with increased throughput using individual patient samples from each group (19). LIA has a format similar to conventional ELISA, which is used routinely in clinical diagnostics. In LIA, the glycoprotein is first captured from a complex clinical sample onto antibody-coated plates; afterwards, the glycosylation of the target glycoprotein is directly detected using lectins. Overall, this two-phase analytical platform allows sensitive and high-throughput analysis for glycosylation patterns in clinical specimens.
Using the developed platform, we investigated the glycosylation patterns of PSA and a new glycoprotein biomarker, MME, for detection of aggressive prostate cancer. The increased bindings of PSA to SNA and Jacalin are due to the glycosylation changes of PSA since the same amount (1ug) of PSA was used. However, due to the lack of reliable assays for direct quantification of MME protein, the changes in MME bindings to GS2 and MAL-1 could have been caused by either the changes in MME protein or glycosylation. MME has been studied as aa cell surface peptidase, and its expression has been shown to be associated with cancer (25, 30–32, 36) while some groups reported that MME protein could be up-regulated in tissue with higher Gleason Score (37). In our study, we observed the higher signals for MME_GS-II and MME_MAL-I from AC and metastasis cancer tissues. This result provided additional data to support glycosylation of MME might act as potential biomarker for AC. Our data has provided strong evidence and argument for need to develop glycosylation isoform-specific biomarkers to improve diagnostic performance.
In conclusion, our study shows that the sensitive lectin microarray and immunosorbent assay platform to the analysis of NAC and aggressive prostate cancer tissues in order to identify glycosylation changes of aggressive prostate cancer. These assays can be further used in validation studies to determine the clinical utilities of different glycosylation isoforms for the early detection of aggressive prostate cancer.
Supplementary Material
Acknowledgments
This work was supported by federal funds from the Early Detection Research Network (NIH/NCI/EDRN by grants U01CA152813 and U24CA115102) and the United States Department of Defense, by grant PC081386. We gratefully acknowledge the discussion of Dr. Zhen Zhang for data analysis and the technical assistance of Xiaer Sun for tissue sample preparation.
Abbreviations
- PSA
prostate specific antigen
- MME
membrane metallo-endopeptidase
- ECL
electrochemiluminescence
- N
normal control
- NAC
non-aggressive cancer
- AC
aggressive cancer
- M
metastatic prostate cancer
- SNA
Sambucus Nigra Agglutinin
- Jacalin
Jackfruit lectin
- MAL-I
Maackia Amurensis Lectin I
- GS-II
Griffonia Simplicifolia Lectin II
References
- 1.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 2.Roth J. Chem Rev. 2002;102:285–303. doi: 10.1021/cr000423j. [DOI] [PubMed] [Google Scholar]
- 3.Lowe JB. Immunol Rev. 2002;186:19–36. doi: 10.1034/j.1600-065x.2002.18603.x. [DOI] [PubMed] [Google Scholar]
- 4.Orntoft TF, Vestergaard EM. Electrophoresis. 1999;20:362–71. doi: 10.1002/(SICI)1522-2683(19990201)20:2<362::AID-ELPS362>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 5.Dennis JW, Granovsky M, Warren CE. Biochim Biophys Acta. 1999;1473:21–34. doi: 10.1016/s0304-4165(99)00167-1. [DOI] [PubMed] [Google Scholar]
- 6.Gorelik E, Galili U, Raz A. Cancer Metastasis Rev. 2001;20:245–77. doi: 10.1023/a:1015535427597. [DOI] [PubMed] [Google Scholar]
- 7.Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Cancer Sci. 2004;95:377–84. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sokoll LJ, Chan DW. Abeloff’s clinical oncology. 4. Elsevier Inc; Philadelphia, PA: 2008. [Google Scholar]
- 9.Chan DW, Bruzek DJ, Oesterling JE, Rock RC, Walsh PC. Clin Chem. 1987;33:1916–20. [PubMed] [Google Scholar]
- 10.Presti JC., Jr Nat Clin Pract Urol. 2007;4:505–11. doi: 10.1038/ncpuro0887. [DOI] [PubMed] [Google Scholar]
- 11.Keetch DW, Catalona WJ, Smith DS. J Urol. 1994;151:1571–4. doi: 10.1016/s0022-5347(17)35304-1. [DOI] [PubMed] [Google Scholar]
- 12.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O’Brien B, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer BS, Izmirlian G, Miller AB, Pinsky PF, Prorok PC, Gohagan JK, Berg CD PLCO Project Team. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peracaula R, Tabarés G, Royle L, Harvey DJ, Dwek RA, Rudd PM, de Llorens R. Glycobiology. 2003;13:457–70. doi: 10.1093/glycob/cwg041. [DOI] [PubMed] [Google Scholar]
- 14.Ohyama C, Hosono M, Nitta K, Oh-eda M, Yoshikawa K, Habuchi T, Arai Y, Fukuda M. Glycobiology. 2004;14:671–9. doi: 10.1093/glycob/cwh071. [DOI] [PubMed] [Google Scholar]
- 15.Tabarés G, Radcliffe CM, Barrabés S, Ramírez M, Aleixandre RN, Hoesel W, Dwek RA, Rudd PM, Peracaula R, de Llorens R. Glycobiology. 2006;16:132–45. doi: 10.1093/glycob/cwj042. [DOI] [PubMed] [Google Scholar]
- 16.Wada Y, Azadi P, Costello CE, Dell A, Dwek RA, Geyer H, Geyer R, Kakehi K, Karlsson NG, Kato K, Kawasaki N, Khoo KH, Kim S, Kondo A, Lattova E, Mechref Y, Miyoshi E, Nakamura K, Narimatsu H, Novotny MV, Packer NH, Perreault H, Peter-Katalinic J, Pohlentz G, Reinhold VN, Rudd PM, Suzuki A, Taniguchi N. Glycobiology. 2007;17(4):411–22. doi: 10.1093/glycob/cwl086. [DOI] [PubMed] [Google Scholar]
- 17.Nwosu CC, Seipert RR, Strum JS, Hua SS, An HJ, Zivkovic AM, German BJ, Lebrilla CB. J Proteome Res. 2011;6;10(5):2612–24. doi: 10.1021/pr2001429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, LaRoche T, Hamelinck D, Bergsma D, Brenner D, Simeone D, Brand RE, Haab BB. Nat Methods. 2007;4(5):437–44. doi: 10.1038/nmeth1035. [DOI] [PubMed] [Google Scholar]
- 19.Meany DL, Zhang Z, Sokoll LJ, Zhang H, Chan DW. J Proteome Res. 2009;8:613–9. doi: 10.1021/pr8007539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng Z, Hincapie M, Pitteri SJ, Hanash S, Schalkwijk J, Hogan JM, Wang H, Hancock WS. Anal Chem. 2011;83(12):4845–54. doi: 10.1021/ac2002802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng S, Yang N, Pennathur S, Goodison S, Lubman DM. Anal Chem. 2009;81(10):3776–83. doi: 10.1021/ac900085k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao SC, Li Y, Zhou J, Qian J, Schnaar RL, Zhang Y, Goldstein IJ, Zhu H, Schneck JP. Glycobiology. 2008;18:761–9. doi: 10.1093/glycob/cwn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuno A, Kato Y, Matsuda A, Kaneko MK, Ito H, Amano K, Chiba Y, Narimatsu H, Hirabayashi J. Mol Cell Proteomics. 2009;8:99–108. doi: 10.1074/mcp.M800308-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Simeone DM, Brenner DE, Anderson MA, Shedden KA, Ruffin MT, Lubman DM. J Proteome Res. 2009;8:483–492. doi: 10.1021/pr8007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Sokoll LJ, Rush J, Zou N, Chan DW, Zhang H. Proteomics-Clinical Applications. 2009;3:597–608. [Google Scholar]
- 26.Liu W, Laitinen S, Khan S, Vihinen M, Kowalski J, Yu G, Chen L, Ewing CM, Eisenberger MA, Carducci MA, Nelson WG, Yegnasubramanian S, Luo J, Wang Y, Xu J, Isaacs WB, Visakorpi T, Bova GS. Nat Med. 2009;15:559–65. doi: 10.1038/nm.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou Z, Ibisate M, Zhou Y, Aebersold R, Xia Y, Zhang H. Anal Chem. 2008;80:1228–34. doi: 10.1021/ac701950h. [DOI] [PubMed] [Google Scholar]
- 28.Tajiri M, Ohyama C, Wada Y. Glycobiology. 2008;18:2–8. doi: 10.1093/glycob/cwm117. [DOI] [PubMed] [Google Scholar]
- 29.Fukushima K, Satoh T, Baba S, Yamashita K. Glycobiology. 2010;20:452–60. doi: 10.1093/glycob/cwp197. [DOI] [PubMed] [Google Scholar]
- 30.Zheng R, Iwase A, Shen R, Goodman OB, Jr, Sugimoto N, Takuwa Y, Lerner DJ, Nanus DM. Oncogene. 2006;25:5942–52. doi: 10.1038/sj.onc.1209586. [DOI] [PubMed] [Google Scholar]
- 31.Osman I, Dai J, Mikhail M, Navarro D, Taneja SS, Lee P, Christos P, Shen R, Nanus DM. Cancer. 2006;107:2628–36. doi: 10.1002/cncr.22312. [DOI] [PubMed] [Google Scholar]
- 32.Dall’Era MA, True LD, Siegel AF, Porter MP, Sherertz TM, Liu AY. BMC Urol. 2007;7:3. doi: 10.1186/1471-2490-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Loriaux P, Eng J, Campbell D, Keller A, Moss P, Bonneau R, Zhang N, Zhou Y, Wollscheid B, Cooke K, Yi EC, Lee H, Peskind ER, Zhang J, Smith RD, Aebersold R. Genome Biol. 2006;7:R73. doi: 10.1186/gb-2006-7-8-r73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian Y, Kelly-Spratt KS, Kemp CJ, Zhang H. J Proteome Res. 2010;9:5837–47. doi: 10.1021/pr1006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Chan DW. Cancer Epidemiol Biomarkers Prev. 2007;16:1915–7. doi: 10.1158/1055-9965.EPI-07-0420. [DOI] [PubMed] [Google Scholar]
- 36.Tawfic S, Niehans GA, Manivel JC. Hum Pathol. 2003;34:450–6. doi: 10.1016/s0046-8177(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 37.Fleischmann A, Schlomm T, Huland H, Köllermann J, Simon P, Mirlacher M, Salomon G, Chun FH, Steuber T, Simon R, Sauter G, Graefen M, Erbersdobler A. Clin Cancer Res. 2008;14:7838–42. doi: 10.1158/1078-0432.CCR-08-1432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.