Abstract
Engaged attention, including music listening, has shown mixed results when used as a method for reducing pain. Applying the framework of constructivism, we extend the concept of engagement beyond attention/distraction to include all cognitive and emotional/motivational processes that may be recruited in order to construct an alternative experience to pain and thus reduce pain. Using a music listening task varying in task demand, we collected stimulus evoked potentials (SEP), pupil dilation (PDR) and skin conductance (SCR) responses to noxious electrocutaneous stimulations as indicators of central and peripheral arousal, respectively. Trait anxiety (Spielberger State-Trait Anxiety Inventory) and absorption (Tellegen Absorption Scale) provided indicators of individual differences. One hundred fifty-three healthy normal volunteers participated in a test session in which they received three stimulus intensity levels while listening to background tones (No Task) or performing a music listening task. Linear slopes indicating net engagement (change in stimulus arousal relative to task performance) decreased with increasing task demand and stimulus level for SEP. Slopes for PDR and SCR varied with task demand, anxiety, and absorption, with the largest engagement effect occurring for high anxiety/high absorption participants. Music engagement reduces pain responses, but personality factors like anxiety and absorption modulate the magnitude of effect.
Index words: pain, psychophysiology, music, individual differences, anxiety, absorption
Introduction
Behavioral methods for relieving pain often use engagement in task performance, with graded increases in task demand corresponding to decreasing pain.15 Researchers investigating these methods typically identify distraction, or the shifting of attention away from painful sensations towards competing stimuli, as the primary mechanism of effect. Unfortunately, the results from distraction studies are inconsistent likely due to poor attentional control or differences in pain characteristics.6 Variable effectiveness may also depend on the task’s cognitive demands,35 arousal characteristics14 or differences in personality characteristics.6 For example, anxiety may interfere with task performance and affect pain outcomes.16, 26 Few studies have investigated differences in characteristics of task, personality, or their combined effects in the same study.
Distraction models of pain reduction derive from the construct of attentional resource allocation,18 focusing on resources shared by pain networks and cognitive processes including executive function and memory.5, 25, 46 This conceptual approach restricts itself to cognitive/attentional engagement. We view distraction as one component within an overarching framework that broadens the conceptualization of engagement. In this framework, based on constructivist theory, 44 engagement applies to gradations in a range of contextually rich, dynamic and complex experiences that influence pain perception. The distraction model assumes that pain is a fully formed perception and that a competing perception pulls the subject away from pain. Taking a holistic view of experience, the constructivist position subsumes attentional processes in an array of networks combining cognitive, affective/motivational, memory, sensory, and motor systems. These systems are part of a process in which the brain continuously constructs and re-constructs short-lived perceptual wholes from arrays of incoming information. This view asserts that pain cannot form as a complete perception when the subject is fully engaged in a competing construction.9 The key to successful pain control is not the “pull” of the distractor, but rather the degree of engagement the subject is able to produce.
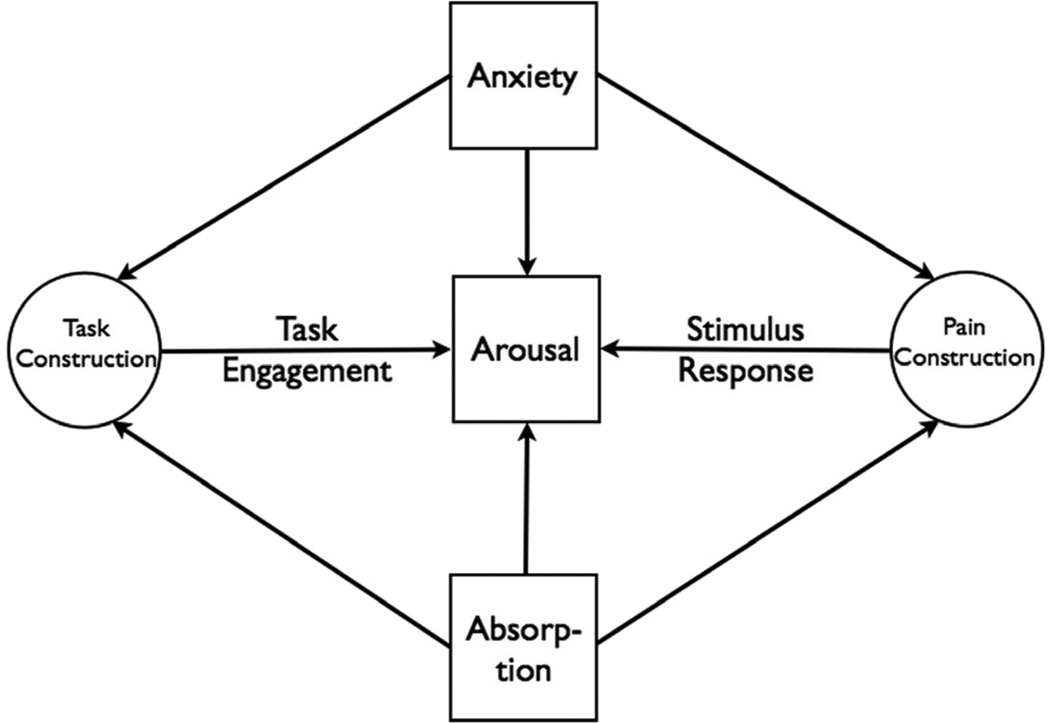
We propose a conceptual model that addresses the combined effects of stimulus and task performance on arousal (Figure 1). Pain and Task are constructions created in the brain that cannot be observed directly but can be inferred from their effects on measures of arousal. Pain effects are evidenced by change in arousal in relation to unit change in stimulus level, represented as Stimulus Response in the model. Task effects are revealed through change in arousal corresponding to unit change in task demand, represented as Task Engagement. Personality characteristics such as anxiety and absorption can affect arousal directly or indirectly through their effects on Task and/or Pain constructions.
Fig. 1.
A model representing the effects of task engagement and stimulus response on arousal. Pain and Task are mental constructions represented as latent (unmeasured) variables whose effects on arousal can be inferred from changes in arousal slopes relative to unit change in stimulus level (Stimulus Response) and task demand (Task Engagement). Anxiety and absorption are personality measures that can affect arousal directly or indirectly.
The present study investigates how engaging in listening to music might affect physiological responses to normally painful stimulation as indicated by changes in a measure of central arousal, stimulus evoked potential (SEP), and peripheral arousal: pupil dilation response (PDR) and skin conductance response (SCR). Furthermore, and critically, the study investigates the extent to which these changes depend on characteristics of the person such as anxiety and absorption. We hypothesized that low anxiety and high absorption would confer an engagement advantage producing large task-mediated reductions in arousal, whereas high anxiety and low absorption should be the least advantageous for engagement. A separate study conducted in our laboratory using a different group of participants (Bradshaw et al., submitted for publication) showed that reductions in SEP and PDR in response to noxious stimulation were linearly related to task engagement. Although the study failed to find significant individual differences on the personality indicators, it was under-powered to detect these effects. Also, SEP and PDR may have been inadequate indicators for detecting these effects. Consequently, the present study improves upon the earlier study by adding SCR as a more sensitive indicator of anxiety-related arousal and by increasing the sample size.
Methods
Participants
We recruited 153 volunteers to participate in the study, 70 females and 83 males, with 143 successfully completing the test session. Ten participants failed to complete due to equipment or software failure. Participants were 18 years or older, healthy and free of chronic pain, not using psychoactive or blood pressure medication, and had normal hearing as confirmed by audiometric testing (Earscan 3 Screening Audiometer, Micro Audiometrics, Murphy, NC). Volunteers consented to participate according to the University of Utah Institutional Review Board approved protocol and in accordance with the ethical standards established in the 1964 Declaration of Helsinki. Participants were compensated for their time spent in the study.
Design
The design for this study comprised multivariate repeated measures having two within subjects manipulated factors, stimulus level and task with three levels of stimulus intensity: low, moderate, and high intensity, and three levels of task condition: No Task, Easy Task, and Hard Task. Task conditions increased in task demand in a graded fashion. Stimulus level was randomized within test blocks and condition order was counterbalanced across subjects.
Experimental Conditions
Subjects experienced eight blocks of trials, with four blocks of No Task and two blocks each of Easy and Hard Task conditions. To provide a control for habituation effects, No Task blocks alternated with Easy and Hard Task blocks, with paired order, No Task-Easy Task, No Task-Hard Task, presented in a sequence that was randomized across subjects. Each block consisted of a test window during which music played while the subject received a total of 18 stimulus trials. Noxious fingertip stimuli occurred at three intensity levels varying in random order with equal numbers of trials delivered at each intensity over each block. The inter-trial interval for stimuli varied randomly from 8 to 11 seconds.
Operational Definition of Engagement
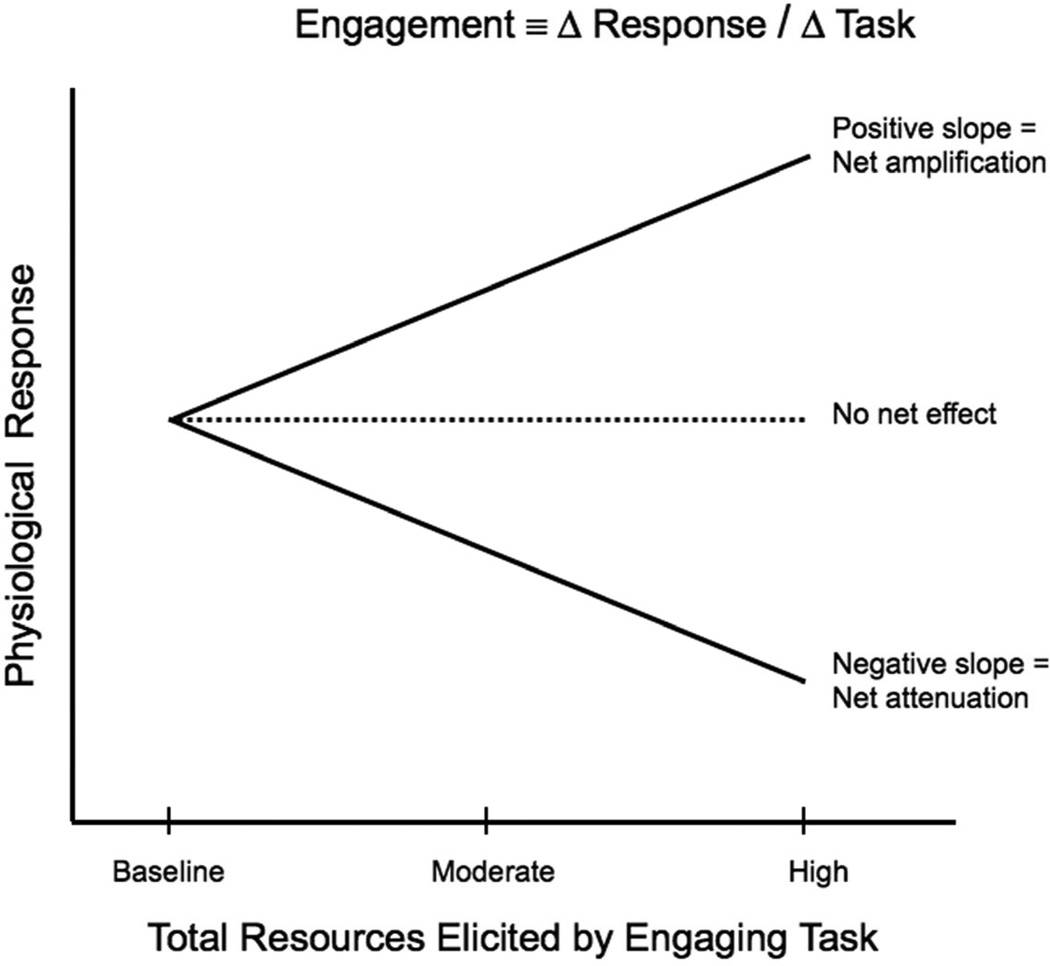
The construct of engagement is not directly observable and measurable, since it is a process inferred from relationships among interacting conditions and variables, in this case, stimulus and task. For purposes of experimental study, we propose the following operational definition: Engagement, as we have operationalized it, is the rate of change observed in the physiological variable measured in response to a stimulus per unit change in task level. Conceptually, its value may increase, decrease, or remain unchanged with increasing task demand, relative to the baseline control condition (No Task). This rate of change value, may vary across people, across different kinds of tasks, and across different choices for levels of the same task. In this experiment, we selected one type of task (error detection in musical passages) with three levels of graded task demand. The units of change in task demand level were necessarily arbitrary, as was the decision to treat the spacing between levels as linear in the construction of slopes. Even so, the slope measure with three points provides a reasonable summary of overall increase or decrease, with magnitudes that are consistent within this particular experiment. Defining engagement with a single summary number, the slope, is highly parsimonious and simplifies interpretation.
Figure 2 illustrates the operational definition. A linear fit defines two parameters, an intercept and slope, for each subject. The intercept corresponds to the response obtained at the baseline control condition in which there is no task demand. The slope defines the rate of change per unit change in task demand. A negative slope signals attenuation, as increasingly demanding tasks reduce the response evoked by the stimulus compared with the control condition. A positive slope indicates amplification corresponding to increasing response with increased task demand relative to the control. Strictly speaking, we refer to net attenuation and net amplification, since the same task may induce competing processes such as heightened arousal relative to the task but reduced arousal relative to the stimulus. Amplification and attenuation combine in a single net observed effect We define a net decrease in arousal with increasing task demand, or net attenuation, as net engagement, reasoning that a decreasing response to the stimulus with increasing task demand indicates greater task engagement.
Fig. 2.
Illustration of the operational definition of engagement as net change in physiological response relative to change in task level.
Music Listening Task
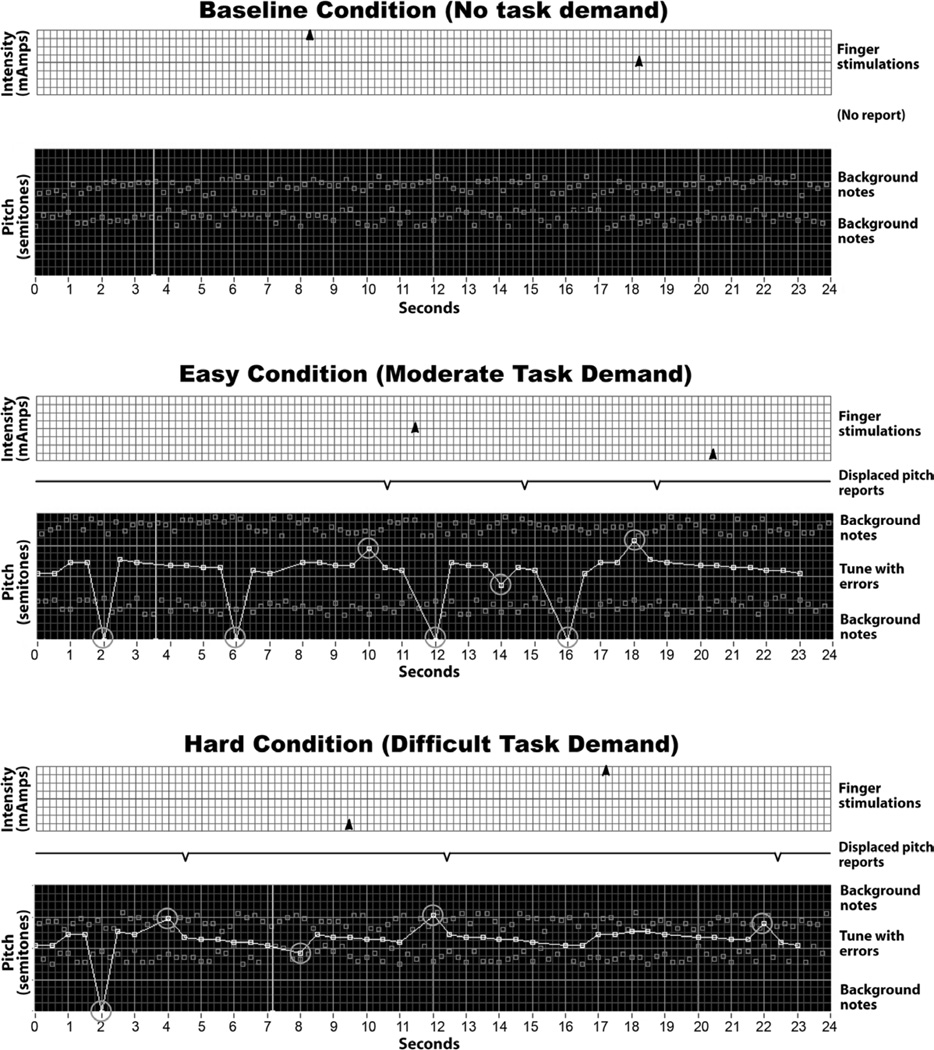
The music listening task demand varied on two dimensions: structural complexity of the auditory signal and difficulty of the listening task (Figure 3). The music listening task conditions consisted of an auditory analog to the familiar visual figure-ground perceptual challenge. The auditory figure comprised a familiar melody (e.g., “Twinkle, twinkle, little star”) presented at a rate of one tone per second. The background comprised two series of random tones varying over a 6 semitone range and sounding above and below the pitch range of the melody. Background tones occurred at twice the rate of the melody tones. Melody tones were selected at random to either deviate by 12 semitones (one octave) from the normally occurring pitch frequency or to be omitted (frequency = 0). The listening task was to track the melody and identify deviant tones while ignoring omitted tones. Adjusting the frequency distance between the melody and background varied the task complexity. Pilot testing found that positioning the background directly adjacent to the frequency range of the melody compared to 12 semitones above and below the melody resulted in consistent and predictable increases in task performance errors, indicating increasing task demand. In the Easy Task condition, subjects heard the melody against the background of random tones sounding ±12 semitones from the melody and in the Hard Task the background tones sounded adjacent to the melody. During the No Task control condition, the background tones were played without a melody and subjects did not perform a task.
Fig. 3.
Experimental conditions for music listening tasks and stimulus delivery. Connected white circles indicate tones in the melody being tracked; large circles indicate pitch displacement errors and omitted tones (pitch = 0); dark circles indicate background distraction tones.
Manipulating the loudness of the melody relative to the background provided an effective way to adjust for individual differences in task performance ability. During pre-test training, we optimized the loudness adjustments so that subjects achieved 95% accuracy in reporting pitch deviations in the Easy Task condition and 80% accuracy in the Hard Task. We applied Fletcher-Munson corrections to the frequency amplitudes to assure equal loudness across the entire frequency range (similar to pressing the loudness toggle on a stereo system). Auditory signals were delivered free-field over a loudspeaker positioned in front of the subject. To assure that subjects were familiar with the test melody, could easily recall and track it, and had an expressed preference for it, we asked each subject to select a single favored tune from a list of experimenter provided simple children’s songs.
Dolorimetry
We delivered safe but noxious stimuli to subjects using a modified version of a standard laboratory pain stimulation technique.3 As a stimulation electrode, we inserted a standard prick lancetter (Bayer No. 170400 B03) having a 1mm triangular tip in the stratum corneum of a fingertip on the subject’s non-dominant hand, and as a return electrode, we applied a 2.5 by 4 inch silver/silver chloride flat plate to the volar surface of the forearm of the same arm. A stimulus trial consisted of a 5 msec square wave constant current pulse delivered to the fingertip by a Grass S-44 stimulator (Grass Technologies, West Warwick, RI) and a stimulus isolation unit connected in series. Stimulus levels for testing were established for each subject, based on the subject’s pain threshold and tolerance. To establish pain threshold and tolerance, we stimulated the fingertip with gradually increasing stimulus intensity and asked subjects to indicate the point at which they first felt slight pain and the point at which they did not wish to receive higher intensity stimulation. This procedure repeated,, as needed (usually three repetitions), until subjects reported just detectable pain and maximum pain at within 0.1% deviation in stimulus intensity. We identified pain threshold as the intensity at which the subject first consistently reported slight pain, and pain tolerance as the maximum intensity the subject was willing to receive. Using the range in current values obtained from threshold to tolerance, we set 3 stimulus levels: 20%, 50%, and 80% of pain tolerance for low, medium and high stimulus level, respectively.
Psychophysiological Indicators of Arousal
SEPs indicate perceptual-cognitive processing of nociceptive signals with increasing amplitudes correlated with increasing noxious stimulus intensity and higher pain ratings.37 PDRs correspond to cognitive load with higher amplitudes correlated with increasing task demand.43 Skin conductance response (SCR) is a reliable indicator of autonomic arousal corresponding to changes in emotional states such as anxiety.11 SCR may prove more responsive to detecting differences corresponding to personality indicators of anxiety. Several studies have reported that, although diverting attention away from painful stimuli reduces reported pain, arousal indicators such as SCR nevertheless increase.17 Thus, we predicted that increasing task demand would reduce physiological responses to painful stimulation but recognized that task performance itself may generate an arousal response, particularly in SCR.
Psychophysiological Data Collection
Changes in electroencephalographic (EEG), skin conductance response (SCR), and pupil diameter response (PDR) data streams time-locked with stimulus onsets provided indicators of psychophysiological responses to noxious events. We collected EEG data continuously from a single low impedance silver/silver chloride recording electrode placed at vertex (Cz), with reference clip electrodes affixed to the earlobes and a single ground electrode attached to the left cheek. The conditioned and amplified signal (Grass Model 12 Neurodata Acquisition System, Grass Technologies, West Warwick, RI) was acquired and sampled at 1024 Hz. Single trial epochs were selected from the data stream at 100 ms pre- and 500 ms post-stimulus intervals and low-pass filtered at 20 Hz using zero phase shift inverse fast Fourier transformations (FFT) digital filtering. For each subject, a grand average of all single trials obtained during the session provided pilot latencies for identifying negative (N150) and positive (P250) EEG peaks. Using these pilot latencies as guides, an in-house software routine identified local minimum and maximum peaks and recorded the corresponding amplitudes. The peak-to-peak amplitudes provided single trial stimulus evoked potential (SEP) values for subsequent analysis.
Single trial pupil dilation response (PDR) amplitudes derived from the continuous pupil diameter data collected using an ISCAN RK 416 infrared pupillometry system (ISCAN, Inc., Woburn, MA) sampled at 256 Hz. An in-house software system built in LabView (National Instruments, Austin, TX) identified and removed eye blinks with interpolation. Identifying single trials as the segment beginning 500ms before and continuing 2 sec after a stimulus event, we obtained grand averages for each subject and identified pilot peak latencies corresponding to a baseline latency prior to the response (typically within 500ms after the stimulus) and a peak (usually around 1250 ms after the stimulus). An in-house software routine identified local maximum peaks for single trials and calculated the difference between the peak and baseline amplitude measurements to provide single trial PDR amplitudes for subsequent analysis.
We collected continuous skin conductance measures using two silver/silver chloride recording electrodes filled with electroconductive paste (0.5% saline in a neutral base, EC33 paste, Grass Technologies, West Warwick, RI) placed at the proximal thenar and hypothenar eminences of the palmar surface of the dominant (non-stimulated) hand. A Grass SCA1 system provided isolated constant DC voltage of 0.5 V to the recording electrodes. The conditioned and amplified signal (Grass Model 12 Neurodata Acquisition System) was acquired and sampled at 256 Hz using LabView acquisition hardware and in-house software written in LabView. For each subject, single trials, identified as the segment beginning 500 ms before and continuing 4 sec after a stimulus event, were averaged and manually inspected to identify pilot baseline (usualy around 1 sec post-stimulus) and peak latencies (usually around 3.5 sec post-stimulus). Using the pilot latencies as a guide, an in-house software routine identified local baselines and maximum peaks for single trials. The calculated difference in baseline and peak amplitudes provided the single trial SCR values for subsequent analysis.
Outcome Measures
Psychophysiological arousal indicators served as the primary outcome measures of response to painful stimulation. SEP provided an indicator of central nervous system (CNS) arousal, and SCR and PDR provided indicators of peripheral sympathetic nervous system (SNS) arousal responses. The Tellegen Absorption Scale (TAS), and the Spielberger State-Trait Anxiety Inventory-Trait (STAI-T) instruments yielded secondary outcomes that could potentially clarify individual differences in ability to engage in a task. The TAS is a validated measure assessing openness to absorbing experiences as a personality trait.38 It has proven effective at predicting successful stress-related anxiety reduction using visual imagery.22 The STAI scale is a well validated measure of persistent anxiety as a personality characteristic.36 As a manipulation check for task difficulty, we recorded accuracy (number of correct detections – number of false alarms = task performance accuracy) and response time (latency to report errors for correct responses) measures in each listening task block. We elected not to collect subjective pain ratings following stimulations because providing self-reports of pain would conflict with task conditions. We wished to foster an environment in which participants could perform effective constructions of the task performance that might compete with that of noxious stimulation. Instructions to rate pain might undermine that effort.
Study Procedures
Before testing, participants practiced the music listening task and set stimulus levels. Participants selected their favored familiar melody and were instructed to track the melody and report aloud saying “Bad” when detecting a deviant tone but not to respond when detecting an omitted tone. Participants were to report as quickly and accurately as possible. During music task training, participants practiced the task (without background tones) until achieving 100% accuracy. Easy Task background tones were then introduced and relative sound levels of the melody and background were adjusted and participants practiced the task until achieving 95% performance. Next, participants practiced the Hard Task until reaching 80% accuracy. If necessary, we adjusted sound levels for both task levels until subjects performed at the prescribed performance level. For most participants the training process required three or fewer attempts at each task level and no participants failed to reach the performance criteria.
Data Analysis
To test the conceptual model representing effects of task engagement and stimulus response on arousal, we rely on the operational definition of engagement as the linear rate of change in response per increase in music task level. Changes in linear response trend with respect to task level as a function of other experimental or measured variables correspond to engagement effects. Changes with respect to stimulus intensity, modeled as a quantitative factor with three levels, correspond to stimulus response effects. The statistical model represented trait anxiety and absorption as linear fixed effects that interacted with the level and rate of change of the response, allowing response trend lines to vary with every unique combination of trait and absorption. Exploratory analyses revealed large systematic individual differences in rates of change in the responses with respect to stimulus and trial index, so the formal analysis modeled this variability as random subject effects about the fixed population average effects. To control further for lack of independence in repeated measures on the same individuals, we also included a random subject intercept effect, which was allowed to correlate with the other three random subject effects. We controlled for habituation or sensitization occurring over the course of the experiment and multiple stimulations, using the logarithm (based on exploratory graphs of the time trend) of trial index as a quantitative covariate.
For each response variable, the full statistical model was
Yiabt = Baselinei + (Stimulus Effect)i + (Trial Effect)i + (Task Effect|a, b) + (error) μabi + β1iSit ln(t) + β3abXit + εiabt, [μabi, β1i,β2i]'~MVN([μab, β1,β2]',Σ), εiabt~N(0,σ2)
Where Yiabt is the response observation for subject i, with Anxiety a and Absorption b, on trial t
μabi is the Expected Value of Y for Anxiety a, Absorption b, Stimulus (S) = 0, and Music Task (X) = 0.
Sit =0,1,2 is the stimulus level received by Subject i on trial t
β1i is the Stimulus regression coefficient for Subject i
β2i is the habituation/sensitization regression coefficient for Subject i
Xit = 0,1,2 is the music task level for Subject i on trial t
β3ab is the Music Task regression coefficient for Anxiety a and Absorption b
εiabt is the error for subject i, Anxiety a, Absorption b, on trial t, and
Σ is the unrestricted covariance matrix of the random effects.
The mixed effects model provides standard errors and hypothesis tests that correctly adjust for dependence over repeated measures. The fixed effect estimates for factors and covariates can be interpreted similarly to those from ordinary analysis of variance and regression.
The resulting mixed effects models were analyzed with SPSS 18 (SPSS Inc., Chicago, IL) and SAS 9.2 Proc Mixed (SAS Institute, Inc., Cary, NC), under a modified backwards elimination strategy. The initial model included all random effects and all interactions among the fixed effects. After removing any nonsignificant random effect variances, we removed the least significant fixed effect, one step at a time, until all remaining fixed and random effects were significant, retaining all components of any significant interaction term. The p-values for fixed effects were evaluated under maximum likelihood using F-tests and Satterthwaite denominator degrees of freedom. Hypothesis tests for random effect variance components were evaluated in likelihood ratio tests between nested models.
Results
Participants’ ages ranged from 18 to 55 with a mean of 29 (Table 1). All but one participant fell within the reported normal range for absorption as measured by the TAS.32 A total of 15 participants had STAI-Trait scores at or above 45, a reported threshold for high anxiety.19
Table 1.
Description of participants by age and personality factors.
| Mean | Minimum | Maximum | S.D. | |
|---|---|---|---|---|
| Age | 28.98 | 18 | 55 | 6.94 |
| STAI-Trait | 34.31 | 20 | 62 | 7.86 |
| TAS | 19.85 | 3 | 34 | 7.06 |
Abbreviations: S.D. = standard deviation; STAI = Spielberger State-Trait Anxiety Inventory; TAS = Tellegen Absorption Scale
We provide means and standard deviations (s.d.) for the unadjusted response data (Table 2) but caution that these summary values average over potentially important interactions. A key hypothesis in this study is that relationships change as a function of the individual characteristics of absorption and anxiety. Also, the response may vary strongly as a function of stimulus intensity, and may manifest habituation or sensitization with repeated stimulation. We provide appropriate controls for these interacting factors in the primary analysis models, but these cannot be simultaneously portrayed in an intelligible and simple summary of the raw data. Inspection of detailed exploratory descriptive plots suggested that linear approximations well characterized both stimulus and task effects. In general, any departures from linearity fell within the confidence limits. As argued above, the operational definition of engagement as a linear slope conveys the concept consistently and parsimoniously in a single number, greatly simplifying analysis and interpretation.
Table 2.
Means and standard deviations for physiological responses by stimulus level and task condition, and task performance.
| Measure | Stimulus level |
Task condition |
||||
|---|---|---|---|---|---|---|
| Low | Medium | High | No Task | Easy Task |
Hard Task |
|
| SEP (µVolts) | 13.49 a (22.05) | 23.86 a (25.04) | 33.01 a (27.44) | 25.58b (26.79) | 21.50b (25.82) | 21.15b (24.95) |
| PDR (mm) | 0.053 a (0.30) | 0.117 a (0.31) | 0.239 a (0.37) | 0.140 (0.34) | 0.136 (0.34) | 0.130 (0.34) |
| SCR (mMhos) | 0.223 a (0.34) | 0.293 a (0.39) | 0.466 a (0.48) | 0.294 (0.41) | 0.396 (0.44) | 0.381 (0.44) |
| Task Performance | ||||||
| Response Time (ms) | 555 c (0.08) | 573 c (0.09) | ||||
| Accuracy (%) | 90.5 c 13.6) | 86.9 c (13.9) | ||||
Main effects for stimulus level p<0.0005
Main effect for task condition p<0.0005
Main effect for task condition p<0.01
Abbreviations: SEP = stimulus evoked potential; PDR = pupil dilation response; SCR = skin conductance response.
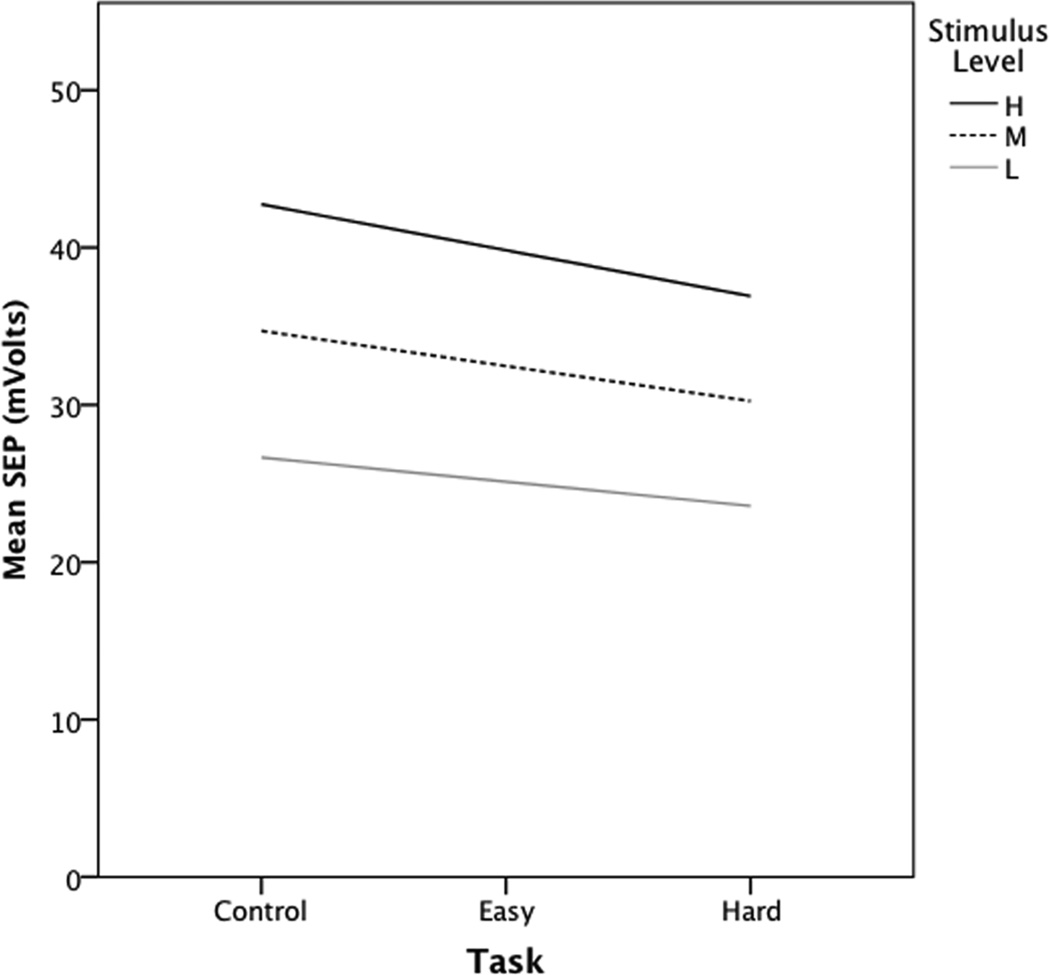
Following the backwards elimination strategy outlined above (Data Analysis), we found that a mixed effects model that included stimulus level, task condition, and their interaction and that allowed for random effects of habituation/sensitization provided the best fit to the SEP data. All effects were significant (p<0.0005). Inspection of the intercepts and rates of change in SEP for unit increases in task condition revealed highly significant (p<0.0005) negative slopes (i.e., decreasing response with increasing task demand), with greater rates of reduction in SEP (larger negative slopes) with increasing stimulus level (p<0.0005, see Figure 4). Personality measures for anxiety and absorption failed to contribute significantly to the SEP model.
Fig. 4.
Change in stimulus evoked potential responses by task condition and stimulus level. Slopes (standard errors) for linear estimates of change in SEP for unit increases in task demand at each stimulus level: L= −1.54 (0.24), M= −2.23 (0.16), H= −2.92 (0.25). Abbreviations: SEP= stimulus evoked potential; L= low, M= moderate, H= high stimulus level; s.e.= standard error.
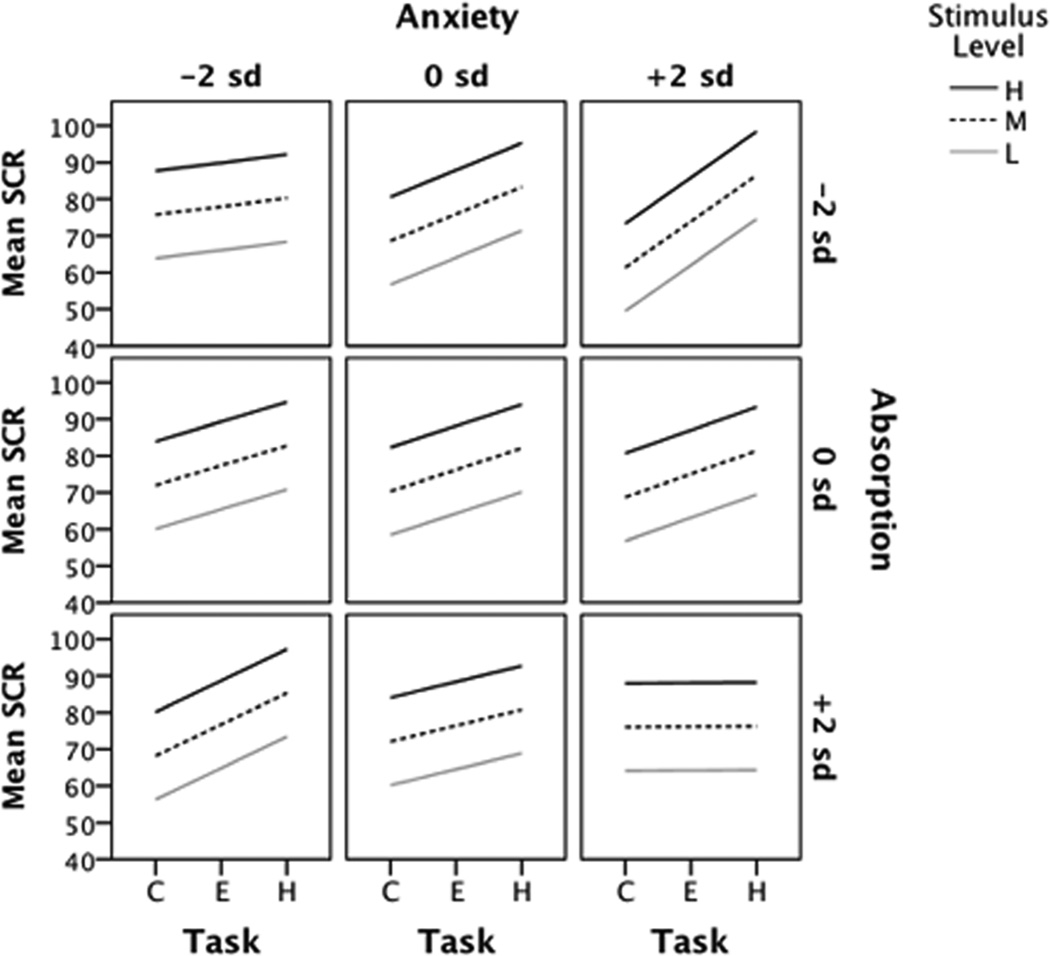
The mixed effects models providing the best fit to both the PDR and SCR data revealed significant three-way interactions between task condition and personality measures of trait anxiety and absorption (p<0.0005) as well as significant effects for habituation/sensitization (p<0.0005). These interactions indicated that the net engagement effects differed for each unique combination of absorption and anxiety scores. A main effect for stimulus level emerged (p<0.0005) but no interactions involving stimulus level proved significant.
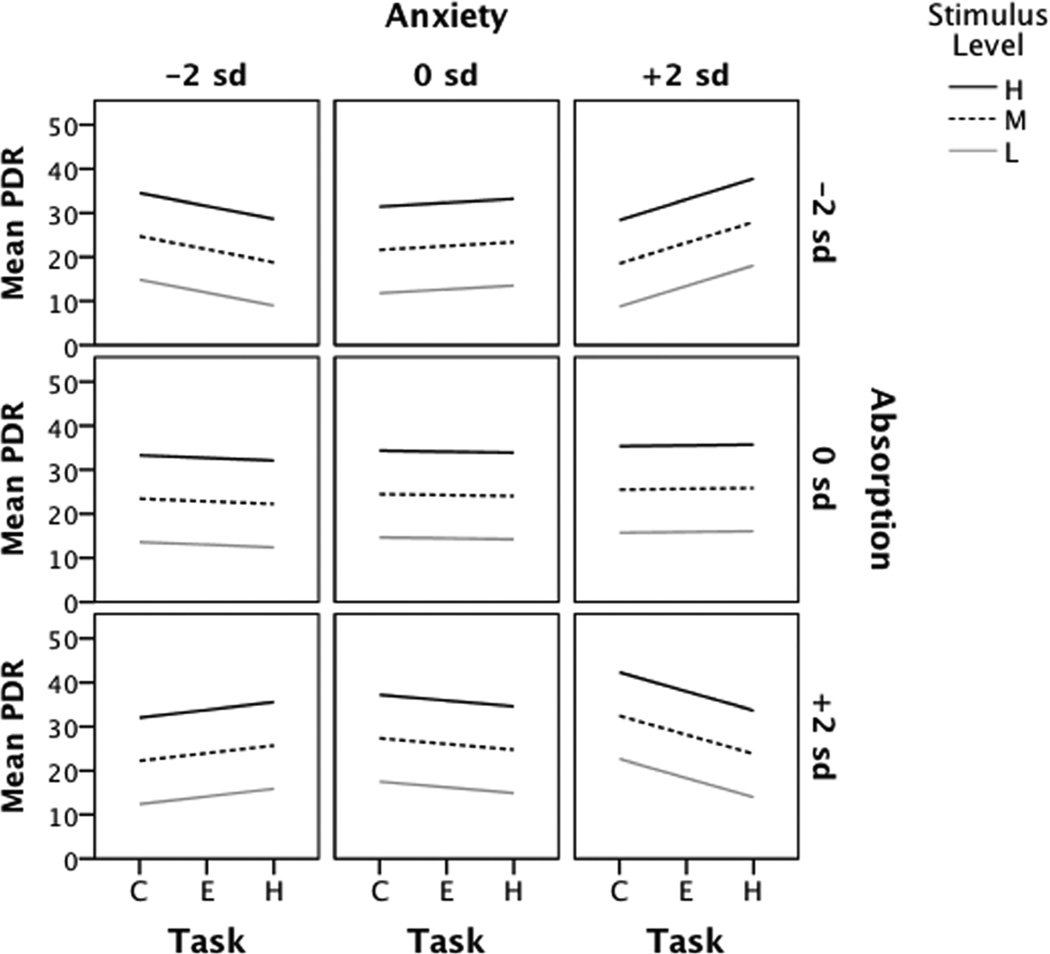
Because net engagement, operationally defined as the slope of the linear trend in the measure with respect to task demand, differed as a function of absorption and anxiety, we used model-based specific comparisons at key covariate values to describe the changing values of these regression coefficients. We inspected intercepts and slopes for rates of change in PDR and SCR for unit increases in task condition at multiple values of trait anxiety and absorption. For illustrative purposes, we show only the more extreme and mid-range values of trait anxiety and absorption (Table 3). The rate of change in PDR decreased as absorption increased such that persons having high anxiety and low absorption had increasing PDR with increasing task level whereas high anxiety persons who also scored high on absorption had decreasing PDR with increasing task level. PDR slopes for high anxiety persons with moderate absorption scores fell between these two. Interestingly, the opposite effect was seen for low anxiety persons: if also low absorbers, their PDR slopes decreased with increasing task level whereas if high absorbers their PDR slopes increased, with moderate absorbers falling between. These results were contrary to the prediction that low anxiety/high absorption would produce the highest net engagement (largest negative slope). Similar to high anxiety persons, persons with moderate anxiety had PDR slope patterns that decreased with increasingly higher absorption. By comparison, low anxiety/low absorbers showed a much reduced PDR response with increasing task. Figure 5 presents the patterns of change in slope for each combination of anxiety × absorption. As there was no interaction with stimulus level, slopes by stimulus level at each anxiety × absorption values tested are parallel, differing only by the initial value (intercept), with intercepts increasing with increasing stimulus level.
Table 3.
Rates of change in pupil and skin conductance responses by task condition at three levels of trait anxiety and absorption
| Anxiety | |||||||
|---|---|---|---|---|---|---|---|
| Low | Moderate | High | |||||
| PDR | Intercept (S.E.) |
Slope (S.E.) |
Intercept (S.E.) |
Slope (S.E.) |
Intercept (S.E.) |
Slope (S.E.) |
|
| Absorption | Low | 34.52 (3.50) | −2.95 (1.54) | 31.45 (2.20) | 0.87 (0.67) | 28.37 (4.05) | 4.70 (1.88) |
| Moderate | 33.28 (2.20) | −0.60 (0.67) | 34.31 (1.85) | −0.21 (0.29) | 35.34 (2.22) | 0.18 (0.71) | |
| High | 32.05 (3.33) | 1.75 (1.44) | 37.18 (2.17) | −1.30 (0.65) | 42.31 (3.20) | −4.34 (1.37) | |
| SCR | |||||||
| Absorption | Low | 87.73 (8.03) | 2.18 (1.76) | 80.54 (5.80) | 7.36 (0.79) | 73.34 (9.25) | 12.54 (2.22) |
| Moderate | 83.93 (5.80) | 5.38 (0.78) | 82.30 (5.24) | 5.85 (0.35) | 80.66 (5.84) | 6.33 (0.81) | |
| High | 80.12 (7.90) | 8.57 (1.70) | 84.05 (5.79) | 4.34 (0.78) | 87.98 (7.68) | 0.11 (1.63) | |
All values adjusted for habituation/sensitization; intercepts calculated at stimulus level = high. Anxiety values obtained at −2 (Low), 0 (Moderate), and +2 (High) standard deviations in Spielberger State-Trait Anxiety Inventory-Trait measures; Absorption values obtained at −2 (Low), 0 (Moderate), and +2 (High) standard deviations in Tellegen Absorption Scale measures.
Abbreviations: PDR= pupil dilation response; SCR= skin conductance response; S.E.= standard error
Fig. 5.
Change in pupil response by task, trait anxiety, and absorption at three stimulus levels. Adjusted mean slopes and intercepts obtained at three values of Anxiety and Absorption: low (−2 s.d.), moderate (0 s.d.) and high (+2 s.d.). Abbreviations: L= low, M= moderate, H= high stimulus levels; C= control, E= easy, H= hard task conditions; s.d.= standard deviation.
Patterns of change in slopes for SCR by each anxiety × absorption pair appear in Figure 6. Overall, SCR tended to increase with increasing task demand, however, the rate of change varied widely depending on anxiety and absorption values. The rate of increase in SCR was highest for high anxiety/low absorbers (slope= 12.54, s.e.= 2.22) and lowest for high anxiety/high absorbers (slope= 0.11, s.e.= 1.63)(Table 3). Thus, for high anxiety participants, if they were also low absorbers, they experienced the highest increases in SCR arousal with increasing task demand; if high absorbers, they experienced the lowest rates of arousal. Slopes for high anxiety/moderate absorbers fell between these two, suggesting a continuum on absorption. No interaction with stimulus was found, such that slopes varied only by level, with intercepts increasing by increasing stimulus level.
Fig. 6.
Change in skin conductance by task, trait anxiety, and absorption at three stimulus levels. Adjusted mean slopes and intercepts obtained at three values of Anxiety and Absorption: low (−2 s.d.), moderate (0 s.d.) and high (+2 s.d.). Abbreviations: SCR= skin conductance response; L= low, M= moderate, H= high stimulus levels; C= control, E= easy, H= hard task conditions; s.d.= standard deviation.
Discussion
We investigated whether engaging in a music listening task varying in task demand produced a net reduction in physiological measures of central and peripheral arousal. Our conceptual development led to an operational definition of net engagement as a slope coefficient in the regression of the response on music task demand. We tested whether the magnitudes of these slope coefficients (net engagement proxies) varied as a function of absorption and anxiety, controlled for stimulus level and trial habituation/sensitization effects. Statistical evaluation of this question depends on testing and scaling the three-way interaction among task, absorption, and anxiety to address the question: Do personal characteristics interact with task demand to alter the engagement response? Although many studies treat high-order interactions as nuisances, this becomes a scientific question central to the present study and thus cannot be simplified.
We found that central arousal reliably decreased with increasing task demand. The SEP slopes varied with stimulus intensity with the largest reduction in SEP over task level occurring at the highest stimulus intensity. Indicators of peripheral arousal, PDR and SCR, suggested a more complex story understood only by considering personality factors. The three-way interactions with anxiety and absorption for PDR and SCR revealed very different engagement responses depending upon individual personality characteristics. We predicted that low anxiety/high absorbers would show the largest arousal decreases with increasing task demand, reasoning that high absorption would allow more task engagement and low anxiety would predict little interference from anxious thoughts and feelings. In fact this group showed the opposite effect: high anxiety/low absorbers had the lowest net engagement. Rather, high anxiety/high absorbers showed the greatest net engagement. These findings imply that arousal from anxiety may improve engagement, rather than interfering as predicted, especially for persons with high absorption. In fact, low anxiety appears to diminish the ability to engage, even for high absorbers.
Although these interactions present a complex pattern of relationships, we believe that the consistency in slopes for the combination of anxiety/absorption found for both PDR and SCR argues for a fairly simple and parsimonious explanation when understood as describing the combination of stimulus-related arousal and task-related arousal resulting in an indication of net engagement. That is, stimulus-evoked arousal decreases with increasing task-evoked arousal if engagement is effective but increases with task if the task fails to engage.
What can we conclude from these results about the effects of engagement on pain? Since we elected not to collect pain ratings in order to avoid interfering with engagement effects, we did not have subjective pain report as an indicator. Our results indicate that task performance reduced SEP and that these effects were consistent across individuals. Some literature suggests that SEP can provide a reliable surrogate for reported pain 37, or perhaps only the affective but not sensory aspects of pain.45 However, this interpretation remains controversial.10 Donaldson et al. have identified a higher-order coordinated pattern of physiological responses that characterize a pain defense response and argue that a failure to form this higher-order pattern corresponds to reduced pain.13 The finding that PDR and SCR response patterns differed substantially from that for SEP suggest that the conditions for forming a pain defense response did not arise during the music listening task
The increased peripheral arousal with task level found for some subjects might indicate correspondingly greater experienced pain. However, whereas physiological arousal corresponds to emotional intensity, emotion labeling depends upon the object to which it is attributed.23 Thus, if subjects associated increased arousal to effort on task rather than to stimulations, they would not necessarily experience increased pain, even in high anxiety/low absorption subjects having the largest task-related arousal increases. Anecdotally, when interviewed after completing the test session, most subjects reported less pain during task performance.
Current thinking about anxiety differentiates anxious arousal (AR) (threat-evoked hyper-arousal and somatic tension) and anxious apprehension (AA) (threat-induced ruminative thoughts).31 In this view, AR high anxiety participants might show greater emotional arousal evidenced by higher SCR, whereas AA participants might display greater cognitive arousal and higher PDR. The STAI-Trait measure typically performs better identifying AA.31 This suggests that high anxiety/low absorbers’ ruminative thoughts about threat would interfere with their ability to engage in the task whereas high anxiety/high absorbers might engage well, even benefiting by having a task on which to direct their thoughts. High anxiety participants may have experienced task anxiety as well and this may have been the source of anxiety reflected in their arousal measures. For high absorbers, performance anxiety may actually enable more effective engagement.
Anxiety, particularly when coupled with pain catastrophizing, may interfere with pain distraction techniques rendering them ineffective.4, 7, 40 Studies have shown that catastrophizing contributes to failure to disengage attention from pain cues.34, 39 Brain imaging research confirms these findings, showing that anxiety biases attentional networks (e.g., amygdala-prefrontal circuits) towards activation of threat-related representations and contributes to under-activation of alternative non-threat-related representations.2 However, emerging work suggests an initial anxiety-provoked attentional bias may be followed by a defensive response that activates attentional orientation towards positive events that present a defense mechanism reducing negative anxiety-associated mood states. This defensive response mechanism seems particularly robust in high anxiety individuals.29 Our results indicate that ability to absorb in a task may improve one’s chances for reducing pain by engagement, even, or especially, if one has a strong tendency for anxious rumination. This conclusion is further supported by work showing that anxiety can have pain-reducing effects, particularly if attention is directed toward an anxiety provoking (but non-painful) object.17
Several studies have investigated effects of task performance on pain and effects of pain on task performance in the same experiment. These studies consistently show that pain interferes with task performance, particularly if the threat value of pain is high.12, 14 These findings may explain the low engagement found with high anxiety/low absorbers, suggesting that anxiety interfered with task engagement more readily due to low capacity for absorption.
For this study we selected a music listening task because it provided several characteristics essential for effective engagement for pain reduction: 1) acoustic properties of music activate sensory pathways that compete with nociceptive pathways; 2) music listening, especially when done actively, requiring complex cognitive/attentional processes; 3) motor assembly processes to deliver speeded responses; and 4) affective/motivational processes associated with performance expectations and properties of the music that may innately and automatically activate emotional associations.20 Finally, this activity could be maintained over an extended time period (most test sessions lasted 30 minutes or more) with consistent performance. According to constructivist theory, an activity that is sufficiently engaging to compete with pain must be contextually rich, dynamic, and complex enough to be engaging intellectually and meaningful enough to be engaging emotionally and motivationally. Music listening meets these requirements for most people.21 Music has been widely used for pain relief but a review of its efficacy found only small benefit.8 Music’s effects have been attributed to distraction30 or its ability to evoke emotion.33 Most studies fail to appreciate the important contributions of preference,30 familiarity,28 and emotional connection.27 The relevance of a goal and the motivation for pursuing it may play a significant role in the capacity of a task to modulate pain.24, 41 The importance of motivation has been demonstrated for behavioral pain relief interventions.42 Music listening can be intrinsically motivating, and when combined with a task and monitored performance, particularly compelling.
Limitations, Implications, and Future Research
This study lacked a no-stimulus task condition providing task performance baseline arousal measures that could then be compared with arousal during stimulus-no task and stimulus-task conditions. The study as designed allowed only extrapolations from responses to noxious stimulation under different task conditions to impute changes in arousal to stimulation or task conditions but not direct measures of each. Noxious stimulation produced phasic physiological changes that were easily detected in this study; responses to musical events prove more difficult to detect. In preliminary work, our attempts to identify responses to musical events failed largely because signal changes were small and easily swamped by the larger responses to noxious stimuli. Future studies must synchronize events carefully to assure ability to detect and separate responses from different modalities. Analytical methods that can disambiguate convolved signals may prove effective for this purpose.1 Finally, corroborating these results with subjective pain report that only minimally interferes with engagement is warranted.
We limited the auditory stimuli used for the listening task to simple melodies to assure that subjects knew them well, could easily generate and track memory representations to detect errors. Although background tones provided a context for manipulating task difficulty, the result admittedly did not provide a pleasing listening environment. A listening activity that provides a more pleasant emotional experience might provide more effective engagement.
The question of music engagement’s ability to relieve pain has no simple answer. In this study, trait anxiety and ability to absorb in a task contributed significantly to how well music engagement reduced stimulus arousal. Our results suggest that engaging activities like music listening may be most effective for reducing pain in high anxiety persons who can easily become absorbed in activities. These findings build on results from studies showing anxiety, fear of pain, catastrophizing, and motivation influence effectiveness of behavioral interventions for pain relief. The interaction of anxiety and absorption is a new finding and suggests that these personality characteristics should be considered when recommending engagement for pain relief. Since this study did not assess fear of pain or catastrophizing in participants this limitation precludes drawing any conclusions regarding potential influences of these personality characteristics on the potential effectiveness of engagement.
Perspective.
Engaging in music listening can reduce responses to pain, depending on the person: people who are anxious and can become absorbed in activities easily may find music listening especially effective for relieving pain. Clinicians should consider patients’ personality characteristics when recommending behavioral interventions like music listening for pain relief.
Acknowledgments
Funding for this study was provided by a grant from the National Institutes of Health/ National Cancer Institute (R01CA74249) awarded to co-author Chapman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Bach DR, Flandin G, Dolan RJ. Time-series analysis for rapid event-related skin conductance responses. Journal of neuroscience methods. 2009;184:224–234. doi: 10.1016/j.jneumeth.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Bromm B, Meier W. The intracutaneous stimulus: a new pain model for algesimetric studies. Methods and findings in experimental and clinical pharmacology. 1984;6:405–410. [PubMed] [Google Scholar]
- 4.Buck R, Morley S. A daily process design study of attentional pain control strategies in the self-management of cancer pain. Eur J Pain. 2006;10:385–398. doi: 10.1016/j.ejpain.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Buhle J, Wager TD. Performance-dependent inhibition of pain by an executive working memory task. Pain. 2010;149:19–26. doi: 10.1016/j.pain.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bushnell MC, Villemure C, Duncan GH. Psychophysical and neurophysiological studies of pain modulation by attention. In: Price DD, Bushnell MC, editors. Psychological methods of pain control: Basic science and clinical perspectives. Seattle, WA: IASP Press; 2004. pp. 99–116. [Google Scholar]
- 7.Campbell CM, Witmer K, Simango M, Carteret A, Loggia ML, Campbell JN, Haythornthwaite JA, Edwards RR. Catastrophizing delays the analgesic effect of distraction. Pain. 2010;149:202–207. doi: 10.1016/j.pain.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cepeda M, Carr D, Lau J, Alvarez H. Music for pain relief. Cochrane Database Syst Rev:CD004843. 2006 doi: 10.1002/14651858.CD004843.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Chapman CR, Nakamura Y. Hypnotic analgesia: a constructivist framework. Int J Clin Exp Hypn. 1998;46:6–27. doi: 10.1080/00207149808409987. [DOI] [PubMed] [Google Scholar]
- 10.Chen AC, Arendt-Nielsen L, Plaghkl L. Laser-evoked potentials in human pain: I. Use and possible misuse. Pain Forum. 1998;7:174–184. [Google Scholar]
- 11.Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci. 2000;20:3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crombez G, Eccleston C, Baeyens F, Eelen P. Attentional disruption is enhanced by the threat of pain. Behav Res Ther. 1998;36:195–204. doi: 10.1016/s0005-7967(97)10008-0. [DOI] [PubMed] [Google Scholar]
- 13.Donaldson GW, Chapman CR, Nakamura Y, Bradshaw DH, Jacobson RC, Chapman CN. Pain and the defense response: structural equation modeling reveals a coordinated psychophysiological response to increasing painful stimulation. Pain. 2003;102:97–108. doi: 10.1016/s0304-3959(02)00351-2. [DOI] [PubMed] [Google Scholar]
- 14.Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychological bulletin. 1999;125:356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- 15.Hodes RL, Howland EW, Lightfoot N, Cleeland CS. The effects of distraction on responses to cold pressor pain. Pain. 1990;41:109–114. doi: 10.1016/0304-3959(90)91115-Y. [DOI] [PubMed] [Google Scholar]
- 16.James JE, Hardardottir D. Influence of attention focus and trait anxiety on tolerance of acute pain. Br J Health Psychol. 2002;7:149–162. doi: 10.1348/135910702169411. [DOI] [PubMed] [Google Scholar]
- 17.Janssen SA, Arntz A. Anxiety and pain: attentional and endorphinergic influences. Pain. 1996;66:145–150. doi: 10.1016/0304-3959(96)03031-x. [DOI] [PubMed] [Google Scholar]
- 18.Johnson MH. How does distraction work in the management of pain? Curr Pain Headache Rep. 2005;9:90–95. doi: 10.1007/s11916-005-0044-1. [DOI] [PubMed] [Google Scholar]
- 19.Kajimura N, Kato M, Sekimoto M, Watanabe T, Takahashi K, Okuma T, Mizuki Y, Yamada M. A polysomnographic study of sleep patterns in normal humans with low- or high-anxiety personality traits. Psychiatry and clinical neurosciences. 1998;52:317–320. doi: 10.1046/j.1440-1819.1998.00401.x. [DOI] [PubMed] [Google Scholar]
- 20.Koelsch S, Siebel WA. Towards a neural basis of music perception. Trends Cogn Sci. 2005;9:578–584. doi: 10.1016/j.tics.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Kohut H, Levarie S. On the enjoyment of listening to music. The Psychoanalytic Quarterly. 1950;19:64–87. [Google Scholar]
- 22.Kwekkeboom K, Huseby-Moore K, Ward S. Imaging ability and effective use of guided imagery. Res Nurs Health. 1998;21:189–198. doi: 10.1002/(sici)1098-240x(199806)21:3<189::aid-nur2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological review. 1990;97:377–395. [PubMed] [Google Scholar]
- 24.Legrain V, Damme SV, Eccleston C, Davis KD, Seminowicz DA, Crombez G. A neurocognitive model of attention to pain: behavioral and neuroimaging evidence. Pain. 2009;144:230–232. doi: 10.1016/j.pain.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Legrain V, Crombez G, Verhoeven K, Mouraux A. The role of working memory in the attentional control of pain. Pain. 2011;152:453–459. doi: 10.1016/j.pain.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 26.Lykken DT, Macindoe I, Tellegen A. Perception: autonomic response to shock as a function of predictability in time and locus. Psychophysiology. 1972;9:318–333. doi: 10.1111/j.1469-8986.1972.tb03215.x. [DOI] [PubMed] [Google Scholar]
- 27.McCaffrey R. Music listening: its effects in creating a healing environment. Journal of psychosocial nursing and mental health services. 2008;46:39–44. doi: 10.3928/02793695-20081001-08. [DOI] [PubMed] [Google Scholar]
- 28.McCaffrey RG, Good M. The lived experience of listening to music while recovering from surgery. J Holist Nurs. 2000;18:378–390. doi: 10.1177/089801010001800408. [DOI] [PubMed] [Google Scholar]
- 29.Mercado F, Carretie L, Hinojosa JA, Penacoba C. Two successive phases in the threat-related attentional response of anxious subjects: neural correlates. Depress Anxiety. 2009;26:1141–1150. doi: 10.1002/da.20608. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell LA, MacDonald RA. An experimental investigation of the effects of preferred and relaxing music listening on pain perception. J Music Ther. 2006;43:295–316. doi: 10.1093/jmt/43.4.295. [DOI] [PubMed] [Google Scholar]
- 31.O'Hare AJ, Dien J. The Fear Survey Schedule as a measure of anxious arousal: evidence from ERPs. Neuroscience letters. 2008;441:243–247. doi: 10.1016/j.neulet.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Platt RD, Lacey SC, Iobst AD, Finkelman D. Absorption, dissociation, and fantasy-proneness as predictors of memory distortion in autobiographical and laboratory-generated memories. Applied Cognitive Psychology. 1998;12:S77–S89. [Google Scholar]
- 33.Roy M, Peretz I, Rainville P. Emotional valence contributes to music-induced analgesia. Pain. 2008 doi: 10.1016/j.pain.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain. 2006;120:297–306. doi: 10.1016/j.pain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Seminowicz DA, Davis KD. Interactions of pain intensity and cognitive load: the brain stays on task. Cereb Cortex. 2007;17:1412–1422. doi: 10.1093/cercor/bhl052. [DOI] [PubMed] [Google Scholar]
- 36.Spielberger CD. Manual for the state-trait anxiety inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press, Inc.; 1970. [Google Scholar]
- 37.Stowell H. Event related brain potentials and human pain: a first objective overview. Int J Psychophysiol. 1984;1:137–151. doi: 10.1016/0167-8760(84)90034-5. [DOI] [PubMed] [Google Scholar]
- 38.Tellegen A, Atkinson G. Openness to absorbing and self-altering experiences ("absorption"), a trait related to hypnotic susceptibility. Journal of abnormal psychology. 1974;83:268–277. doi: 10.1037/h0036681. [DOI] [PubMed] [Google Scholar]
- 39.Van Damme S, Crombez G, Eccleston C, Koster EH. Hypervigilance to learned pain signals: a componential analysis. J Pain. 2006;7:346–357. doi: 10.1016/j.jpain.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Van Damme S, Crombez G, Van Nieuwenborgh-De Wever K, Goubert L. Is distraction less effective when pain is threatening? An experimental investigation with the cold pressor task. Eur J Pain. 2008;12:60–67. doi: 10.1016/j.ejpain.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Van Damme S, Legrain V, Vogt J, Crombez G. Keeping pain in mind: a motivational account of attention to pain. Neurosci Biobehav Rev. 2010;34:204–213. doi: 10.1016/j.neubiorev.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Verhoeven K, Crombez G, Eccleston C, Van Ryckeghem DM, Morley S, Van Damme S. The role of motivation in distracting attention away from pain: an experimental study. Pain. 2010;149:229–234. doi: 10.1016/j.pain.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 43.Verney SP, Granholm E, Dionisio DP. Pupillary responses and processing resources on the visual backward masking task. Psychophysiology. 2001;38:76–83. [PubMed] [Google Scholar]
- 44.Wadsworth BJ. Piaget's Theory of Cognitive and Affective Development: Foundations of Constructivism. New York: Longman Publishing; 1995. [Google Scholar]
- 45.Zaslansky R, Sprecher E, Katz Y, Rozenberg B, Hemli JA, Yarnitsky D. Pain-evoked potentials: what do they really measure? Electroencephalogr Clin Neurophysiol. 100:384–391. 199. [PubMed] [Google Scholar]
- 46.Veldhuijzen DS, Kenemans JL, de Bruin CM, Olivier B, Volkerts ER. Pain and attention: attnetional disruption of distraction? J Pain. 2006;7:11–20. doi: 10.1016/j.jpain.2005.06.003. [DOI] [PubMed] [Google Scholar]