Abstract
Endotoxin tolerance, a key mechanism for suppressing excessive inflammatory cytokine production and attendant toxicity, is induced by prior exposure of macrophages to TLR ligands. Induction of tolerance by endogenous cytokines has not been investigated. We show that prior exposure to TNF induces a tolerant state in macrophages, with diminished cytokine production on LPS challenge and protection from LPS-induced lethality. TNF-induced tolerization was mediated by coordinate action of two inhibitory mechanisms, suppression of LPS-induced signaling and chromatin remodeling. Mechanistically, TNF-induced tolerance was distinct from TLR-induced tolerance as it was dependent on GSK3, which suppressed chromatin accessibility and promoted rapid termination of NF-κB signaling by augmenting negative feedback by A20 and I-κBα. These results reveal an unexpected homeostatic function of TNF and provide a GSK3-mediated mechanism for preventing prolonged and excessive inflammation.
INTRODUCTION
Innate immune cells sense microbial infection or tissue damage and produce key inflammatory cytokines such as TNF and IL-6. These cytokines coordinate inflammatory responses that are important for host defense and tissue remodeling and repair. Among the most potent inducers of inflammatory cytokine production are Toll-like receptors (TLRs) that sense microbial products and transduce inflammatory signals via NF-κB and MAPK pathways1.2. High production of inflammatory cytokines results in excessive inflammation and associated tissue damage, and contributes to pathogenesis of inflammatory disorders. Accordingly, inflammatory cytokine production is tightly regulated by various mechanisms that modulate the intensity of inflammation and promote its eventual resolution and the return of tissue homeostasis3. Many of the mechanisms that dampen inflammation are induced by inflammatory stimuli themselves, and thus function as part of feedback loops that allow fine-tuning of inflammatory responses. For example, the potent anti-inflammatory cytokine IL-10 is induced by TLRs and feeds back to limit TLR-induced inflammatory cytokine production.
One of the most effective protective mechanisms that suppresses inflammatory cytokine production is termed endotoxin tolerance. Endotoxin tolerance is a phenomenon whereby prior exposure of cells or organisms to microbial products, such as TLR ligands, results in strong suppression of inflammatory cytokine production, and protection from toxicity and lethality on subsequent challenge with TLR ligands such as LPS/endotoxin. Monocytes and macrophages are the principal cells involved in endotoxin tolerance in vivo and tolerization of these cells has been extensively studied4. One interesting aspect of endotoxin tolerance is that TLR-induced expression of genes involved in host defense and tissue homeostasis, such as antimicrobial peptides and growth factors, remains intact. Thus, endotoxin tolerance selectively prevents toxicity associated with excessive cytokine production while allowing beneficial TLR-induced responses to proceed. A large body of work has shown that an important mechanism of endotoxin tolerance is suppression of TLR signaling, which is achieved by induction of signaling inhibitors such as SOCS1, IRAK-M and SHIP1, and downregulation of TLR signaling pathway components4. Diminished TLR signaling can contribute to diminished inflammatory cytokine production by tolerized cells, but can not explain selective regulation of different genes or why induction of certain genes, termed non-tolerizable genes, remains intact in tolerized cells. Recent work has clarified that gene-specific regulation in tolerized macrophages is mediated by chromatin modifications, including changes in histone marks and nucleosome remodeling, at selective gene loci5,6. Gene-specific chromatin modifications can explain selective gene regulation7, and repressive changes at the chromatin level can cooperate with diminished signaling to effectively downregulate inflammatory cytokine gene expression.
TNF is well known as a potent pro-inflammatory cytokine. TNF can drive local inflammation by activating tissue and endothelial cells as well as infiltrating immune cells, and can also act systemically, for example by mediating many of the deleterious effects of endotoxin toxicity. The predominant activating role of TNF in innate immunity and host defense, and in chronic inflammation associated diseases such as rheumatoid arthritis, is well established. On the other hand, anti-inflammatory effects of TNF have been described8–13, and TNF plays a role in restraining inflammation in animal models of systemic lupus erythematosus and multiple sclerosis14,15. However, TNF does not induce expression of suppressive cytokines such as IL-10, and induction of feedback inhibition or tolerance-like states by TNF has not been investigated. Thus, in contrast to TLRs, mechanisms by which TNF limits inflammation are minimally understood.
Glycogen synthase kinases 3 (GSK3) α and β are serine/threonine kinases that are broadly expressed and constitutively active in most cells types, including immune cells16–18. GSK3 activity is regulated by various immune receptors such as TLRs, cytokine receptors and antigen receptors, and an important role for GSK3 in regulating immune and inflammatory responses has been established19. GSK3 regulates the activity of various transcription factors important in inflammation and cytokine production, including NF-κB, AP-1, CREB, NFAT, β-catenin and STAT proteins. Although GSK3 typically inactivates its substrates16, its function is context-dependent and GSK3 has been shown to activate or inhibit NF-κB, depending on cell type and experimental conditions19–24. A pro-inflammatory function for GSK3 has been demonstrated in various cell-based functional assays and animal models of disease, and linked to GSK3-mediated regulation of the balance of pro- vs. anti-inflammatory cytokine production16–19. However, there are many clear-cut examples of an anti-inflammatory function of GSK3 that has been linked to suppression of cytokine production19,25–29 and may be related to its differential regulation of NF-κB signaling. There is minimal understanding of the mechanistic basis of the differential and context-dependent regulation of inflammation and NF-κB activity by GSK3.
Investigation of TNF function has focused predominantly on its acute effects on cell activation and inflammation. We reasoned that, similar to other potent inflammatory activating receptors such as TLRs, TNFRs induce feedback inhibitory mechanisms that restrain and fine tune inflammation. Such feedback inhibition would be particularly important to regulate cells, either locally or at a distance, that are not directly contacted by microbial products during infection, and in settings of sterile inflammation (for example, induced by tissue damage or autoimmunity). Several sterile inflammatory conditions associated with TNF production have been suggested to induce a tolerance-like state in monocytes4,30–32, and thus we investigated whether TNF can induce an endotoxin tolerance-like state, i.e. selective hyporesponsiveness and diminished inflammatory cytokine production on secondary TLR challenge. To maximize physiological relevance for human inflammatory conditions, we used primary human monocytes and macrophages that play a key role in human inflammatory diseases and in endotoxin tolerance4,33, and extended our work to a murine system to obtain corroborating genetic evidence and test in vivo importance of our findings. We found that, similar to classical TLR-induced endotoxin tolerance, pretreatment with TNF selectively reduced cytokine production in response to subsequent LPS challenge and a low dose of TNF protected mice from the lethal effects of a subsequent challenge with a high dose of LPS. TNF pretreatment attenuated TLR4-induced signaling and suppressed chromatin remodeling at the IL6 locus. This represents to our knowledge the first report of induction of endotoxin tolerance, including epigenetic regulation that provides transcriptional memory, by an endogenous cytokine. Mechanistically, TNF-induced tolerance was distinct from TLR-induced tolerance as it was strongly dependent on GSK3. TNF increased nuclear expression of GSK3, and GSK3 promoted tolerance by mediating delayed and sustained expression of the signaling inhibitor A20, robust I-κBα resynthesis that rapidly terminated TLR4-induced NF-κB signaling, and by suppressing chromatin remodeling. This preferential coupling of GSK3 to A20- and I-κBα-mediated negative feedback that depends on prior stimulation of cells can explain the context-dependent function of GSK3 in regulating NF-κB and inflammation. Our findings reveal unexpected suppressive functions and mechanisms induced by TNF and implicate GSK3 as a key regulator of macrophage tolerance. These results provide insights that can be exploited to develop new approaches to manipulating TNF and GSK3 activity to fine tune the balance between beneficial and detrimental effects of TNF in inflammatory responses.
RESULTS
TNF induces endotoxin tolerance in human primary macrophages
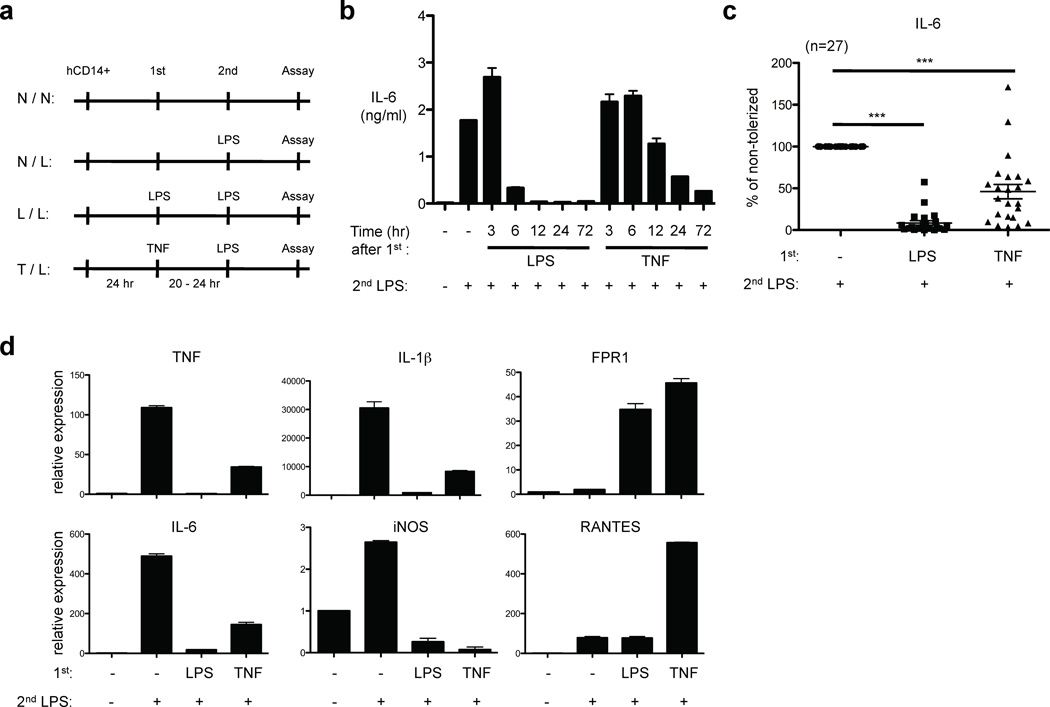
To determine whether TNF can induce endotoxin tolerance, primary human macrophages were pre-treated with LPS or TNF, challenged with LPS, and subsequent cytokine production was measured (experimental design is depicted in Fig. 1a). Consistent with previous studies describing LPS-induced endotoxin tolerance, LPS pre-treatment nearly completely inhibited IL-6 production after secondary LPS challenge in primary human macrophages; a 6 hour pretreatment with LPS was sufficient to strongly suppress subsequent IL-6 production (Fig. 1b). Surprisingly, TNF pre-treatment also blunted IL-6 production in response to secondary LPS stimulation in a time-dependent manner (Fig. 1b). A 24 hr pretreatment with TNF was required to substantially block IL-6 production in response to secondary LPS challenge; thus, TNF-induced attenuation of the LPS challenge developed more slowly than attenuation induced by primary LPS stimulation. TNF-induced attenuation of LPS-induced IL-6 production was highly reproducible in experiments with more than 25 independent blood donors (p < 0.0001), and macrophages pretreated with TNF produced 42.5% as much IL-6 as did control non-pretreated macrophages (Fig. 1b and 1c); significant toxicity or increases in cell death in TNF-treated monocytes were not observed. TNF pretreatment also attenuated macrophage cytokine production in response to subsequent stimulation of TLR2 by Pam3Cys (Supplementary Fig. 1). TNF pretreatment was more effective in attenuating subsequent IL-6 induction than were other members of the TNF family such as CD40L and RANKL (Supplementary Fig. 2), and in this study we focused on analysis of TNF.
Figure 1.
TNF pretreatment suppresses induction of pro-inflammatory cytokines on secondary challenge by LPS. (a) Experimental design. (b) Human primary macrophages were stimulated with LPS (10 ng/ml) or TNF (10 ng/ml) for the indicated times and challenged with 10 ng/ml LPS for 24 hr. IL-6 in culture supernatants was measured by ELISA. (c) Percent tolerization of IL-6 was calculated by dividing IL-6 production of tolerized (LPS- or TNF-pretreated) cells by that of non-tolerized cells. Data are a summary of 27 independent donors. P values were calculated by paired Student’s t-test. ***, P < 0.0001 (d) Human primary macrophages were stimulated with LPS (10 ng/ml) or TNF (10 ng/ml) for 24 hr and challenged with 10 ng/ml LPS for 1 hr (TNF, IL-1β, iNOS and RANTES) or 3 hr (IL-6 and FPR1). Real-time PCR was used to measure mRNA levels. Data are representative of four independent donors (mean ± s.d. of triplicate determinations).
To examine whether TNF pre-treatment altered LPS responses at the level of gene expression, we measured mRNA using real time quantitative PCR. TNF pre-treatment, similar to LPS pre-treatment, suppressed subsequent LPS-induced expression of IL-6, TNF, IL-1β and iNOS mRNA (Fig. 1d). Similar to gene-specific regulation observed in LPS-induced endotoxin tolerance4,5, FPR1 and RANTES (also termed CCL5) mRNAs were induced by secondary LPS challenge in TNF-pretreated cells. Thus, similar to LPS-tolerized macrophages, expression of only a subset of LPS-inducible genes, including pro-inflammatory cytokine genes, was selectively suppressed in TNF-pretreated human macrophages. Blocking TNF signaling using a soluble TNF receptor (etanercept) restored LPS-induced cytokine production in TNF-pretreated cells (Supplementary Fig. 3). These results indicate that the TNF-induced effects were mediated by TNF and not by contaminating LPS amounts that were negligible (endotoxin levels were below limit of detection and less than 1 pg/ml), as contaminating LPS would induce tolerance directly rather than requiring autocrine TNF4 (see also below). Overall, the results suggest that TNF pretreatment induces a state resembling LPS-induced endotoxin tolerance, with selective suppression of TLR-induced inflammatory cytokine gene expression, in human macrophages.
TNF-induced tolerance prevents excessive inflammatory responses in mice
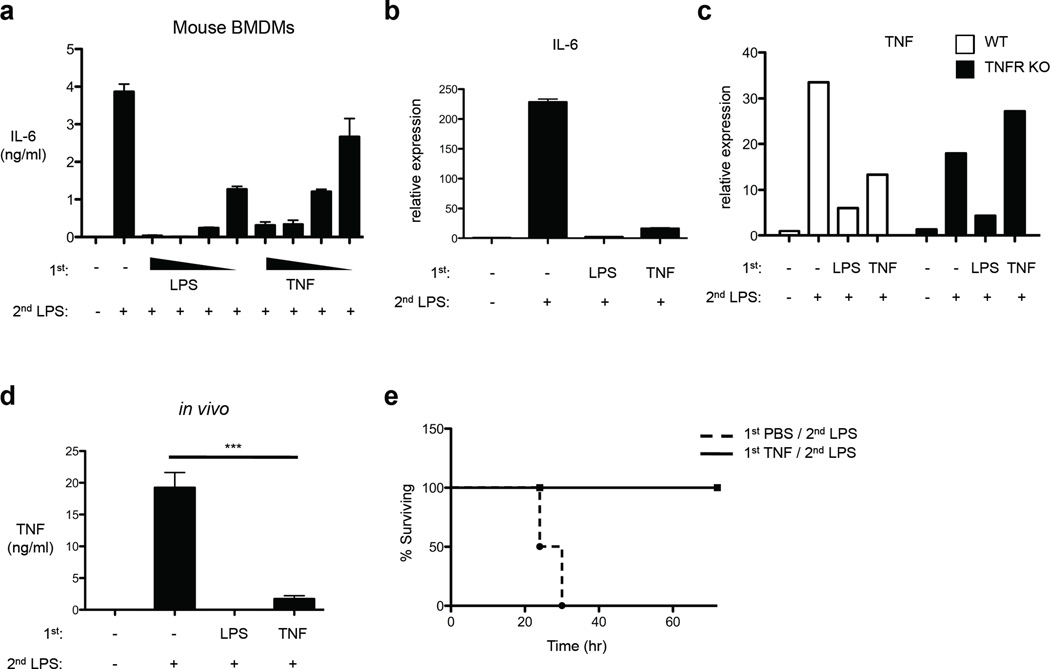
We next used bone marrow-derived macrophages (BMDMs) to extend our investigation of TNF-induced tolerance. TNF pre-treatment of BMDMs induced tolerization of IL-6 production in a dose-dependent manner (Fig. 2a); induction of IL-6 protein and mRNA were suppressed in TNF-tolerized BMDMs (Fig. 2a and 2b). When BMDMs from mice deficient in TNFRs were used, LPS, but not TNF, induced tolerance (Fig. 2c). These results indicate that, consistent with the literature4, LPS-induced tolerance was not dependent on autocrine TNF, and establish that TNF-induced tolerance is mediated by TNFRs. To examine the physiological relevance of TNF-induced tolerance in vivo, mice were intravenously pretreated with 2 µg TNF and after 1 day were challenged with 200 µg LPS by intraperitoneal injection and serum concentrations of TNF were measured. LPS-induced TNF production in vivo was diminished by 91% in mice that had been pretreated with TNF (p = 0.0004) (Fig. 2d); TNF pretreatment was almost as effective as LPS pretreatment in suppressing TNF production on subsequent in vivo LPS challenge (Fig. 2d). Because TNF mediates LPS-induced lethality, we next determined whether a low dose of TNF confers protection against the lethal effects of a subsequent high dose of LPS. All control mice that had been pretreated with PBS (vehicle control) died within 30 hr after high dose LPS challenge, while mice pre-treated with TNF survived (Fig. 2e). Thus, similar to LPS, TNF-induced tolerance suppresses inflammatory cytokine production and confers protection from high dose LPS toxicity in vivo.
Figure 2.
TNF suppresses cytokine production in vivo and protects mice from LPS-induced lethality. (a) Mouse BMDMs were stimulated with increasing doses of LPS (1–100 ng/ml) or TNF (1–80 ng/ml) for 24 hr and then challenged with 10 ng/ml LPS. IL-6 in culture supernatants was measured by ELISA. (b) LPS or TNF-pretreated BMDMs as in (a) were stimulated with 10 ng/ml LPS for 1 hr. Real-time PCR was used to measure IL-6 mRNA levels (mean ± s.d. of triplicate determinations). (c) BMDMs from mice lacking TNFR1 and TNFR2 (TNFR KO) or genetically matched controls (WT) were stimulated with LPS (100 ng/ml) or TNF (40 ng/ml) for 24 hr and then challenged with 10 ng/ml LPS for 1 hr. TNF mRNA levels were determined by real-time PCR. Data are representative of three independent experiments. (d) Age- and sex-matched C57/BL6J mice received intraperitoneal (IP) or intravenous (IV) injection of LPS (100 µg) or of TNF (2 µg), respectively. After 24 hr, secondary LPS challenge (200 µg) was given by IP injection and after 90 min serum TNF was determined by ELISA (n=4 mice per group). P value was calculated by unpaired Student’s t-test. ***, P = 0.0004. Similar results were obtained in an additional 2 experiments using different TNF dosing regimens. (e) Mice were injected intravenously with TNF (2 µg), followed by injection of 500 µg of LPS after 24 hr. Survival rates were scored every 6 h for 4 days (n=4 mice per group). Similar results were obtained in an additional 3 experiments using different TNF pretreatment doses.
TNF inhibits TLR signaling and induces delayed expression of A20 that contributes to tolerance
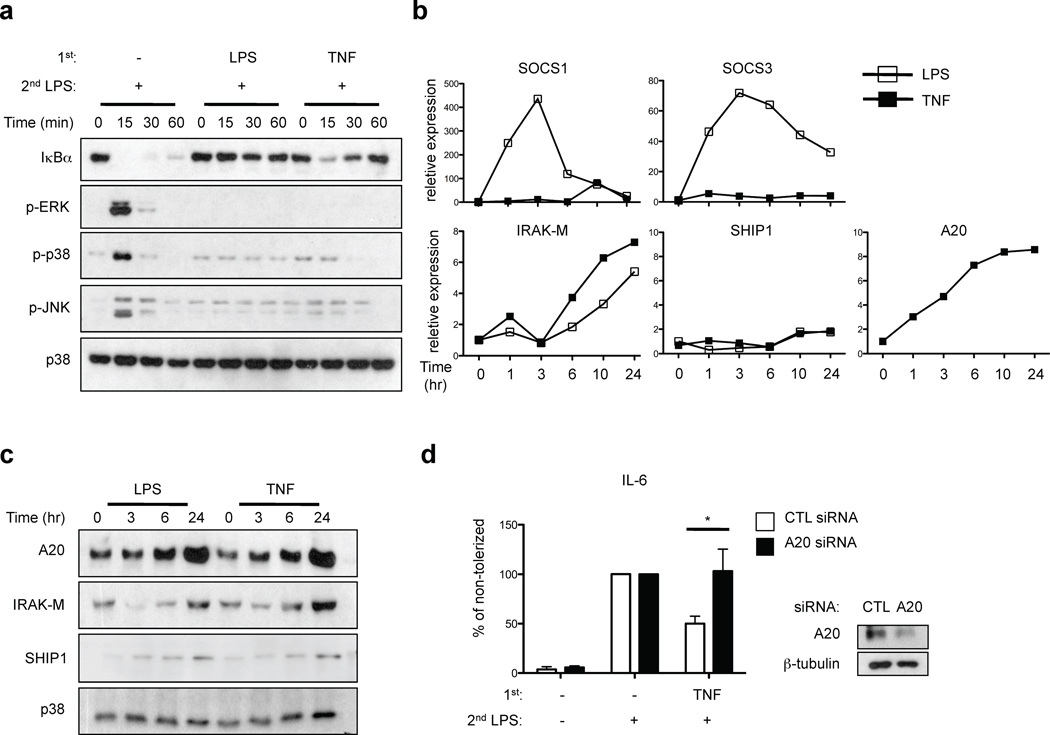
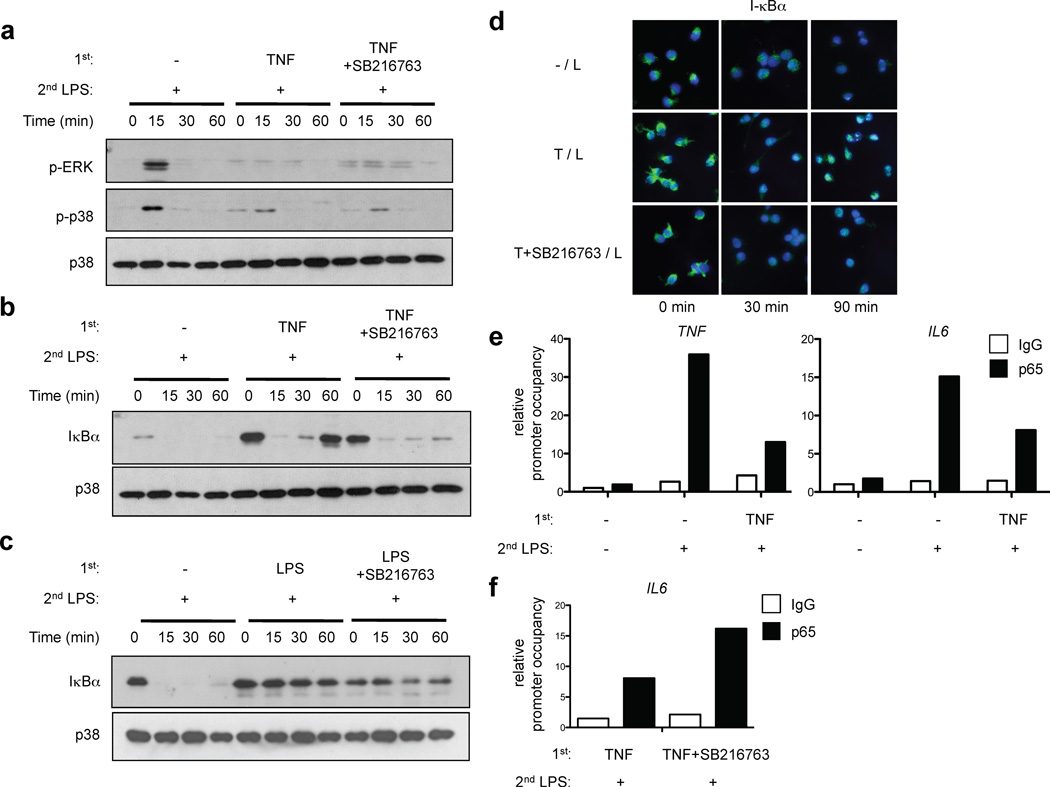
One characteristic of LPS-induced tolerance is suppression of TLR-induced signaling. Thus, we compared the effects of LPS and TNF pretreatment on signal transduction elicited by subsequent LPS stimulation. Non-tolerized primary human macrophages responded to LPS stimulation with robust and prolonged degradation of I-κBα and transient phosphorylation of p38, ERK and JNK MAPKs (Fig. 3a, lanes 1–4). As expected in macrophages tolerized with moderate concentrations of LPS (10 ng/ml), LPS-induced degradation of I-κBα was essentially completely abrogated and activation of MAPKs was strongly diminished (Fig. 3a, lanes 5–8). In TNF-tolerized cells, activation of MAPKs was diminished in a similar manner to LPS-tolerized cells. In contrast to LPS-tolerized cells, in TNF-tolerized macrophages the initial phase of LPS-induced I-κBα degradation was nearly intact (Fig. 3a, lanes 9–12 vs. 5–8). However, in contrast to control cells (Fig. 3a, lanes 1–4), I-κBα protein amounts were rapidly restored to prestimulation levels in TNF-tolerized macrophages (Fig. 3a, lanes 9–12). In eight independent experiments with different blood donors, I-κBα levels rapidly increased to return to baseline levels 60 min after LPS stimulation of tolerized macrophages, whereas there was minimal I-κBα protein expression at the 60 min time point in control non-tolerized macrophages. Thus, in TNF-tolerized macrophages the initial LPS-induced signal for I-κBα degradation was mostly intact, but NF-κB signaling was terminated very quickly by the well established I-κBα-mediated postactivation repression mechanism34 (Fig. 3a).
Figure 3.
TNF suppresses TLR4 signaling and induces A20 expression. (a) Human primary macrophages were stimulated with LPS (10 ng/ml) or TNF (10 ng/ml) for 24 hr and challenged with 10 ng/ml LPS for the indicated times. I-κBα amounts and ERK, p38 and JNK phosphorylation were assessed by immunoblotting. (b, c) Human primary macrophages were stimulated with LPS or TNF for the indicated times. SOCS1, SOCS3, IRAK-M, SHIP1 and A20 expression was measured by (b) real-time PCR and (c) immunoblotting. Data are representative of four to twelve independent experiments. (d) Human macrophages transfected with control or A20-specific siRNA were treated with LPS or TNF for 24 hr and then challenged with 10 ng/ml LPS. IL-6 in culture supernatants was measured by ELISA. Percent tolerization of IL-6 was calculated by dividing IL-6 production of LPS- or TNF-pretreated cells by that of non-tolerized cells. Data are a summary of 3 independent experiments. P value was calculated by unpaired Student’s t-test. *, P = 0.038.
We next investigated whether TNF induced expression of signaling inhibitors SOCS1, SOCS3, IRAK-M and SHIP1 that have been implicated in LPS-induced tolerance4. Primary LPS stimulation rapidly induced increased expression of SOCS1 and SOCS3 (Fig. 3b) that preceded or coincided with the development of tolerance at the 6 hr time point (Fig. 1b). LPS increased expression of IRAK-M and SHIP1 with delayed kinetics (24 hr time point) (Fig. 3b and 3c); the magnitude of IRAK-M and SHIP1 induction appeared smaller in human macrophages relative to previous reports using murine macrophages. In contrast to LPS, TNF minimally induced SOCS1 and SOCS3 expression, but did induce a delayed increase in SHIP1 and IRAK-M (Fig. 3b and 3c). However, induction of SHIP1 and IRAK-M proteins by TNF was modest and was not observed in all donors, suggesting that SHIP1 and IRAK-M may not be sufficient to induce tolerance. Therefore, we analyzed expression of other known inhibitors of TLR signaling. Strikingly, TNF induced delayed but sustained expression of A20 mRNA (Fig. 3b) and substantially increased A20 protein expression at the 24 hr time point (Fig. 3c) that coincided with the development of TNF-induced tolerance (Fig. 1b). Robust and sustained TNF-induced A20 expression was consistently observed in > 10 donors and suggested a role for A20 in mediating TNF-induced tolerance. Indeed, RNAi-mediated knockdown of A20 expression reversed TNF-induced tolerization of IL-6 expression (Fig. 3d), indicating a role for A20 in mediating TNF-induced tolerance, which contrasts with the lack of an effect of A20 deficiency on LPS-induced tolerance35. Collectively, these results show that TNF-induced tolerance is associated with diminished TLR signaling, but is distinct from LPS-induced tolerance in terms of regulation of NF-κB signaling and the pattern of induction of signaling inhibitors, with a more prominent role for A20 in TNF-induced tolerance.
TNF-induced tolerance is mediated by GSK3
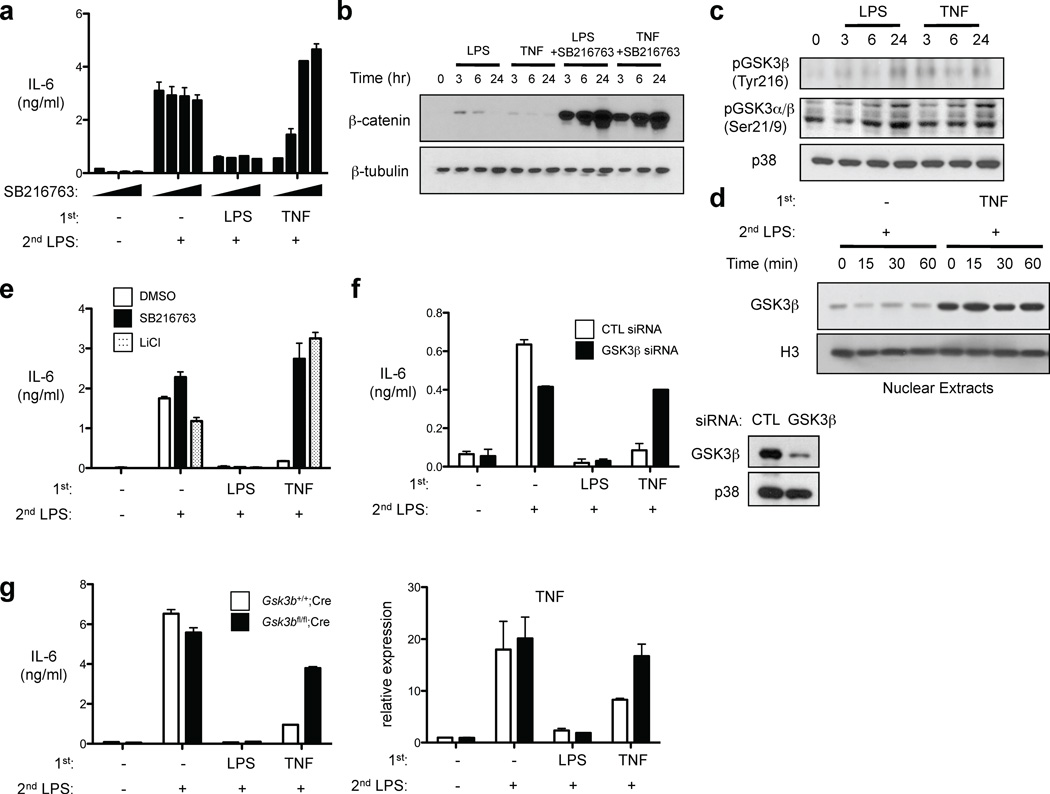
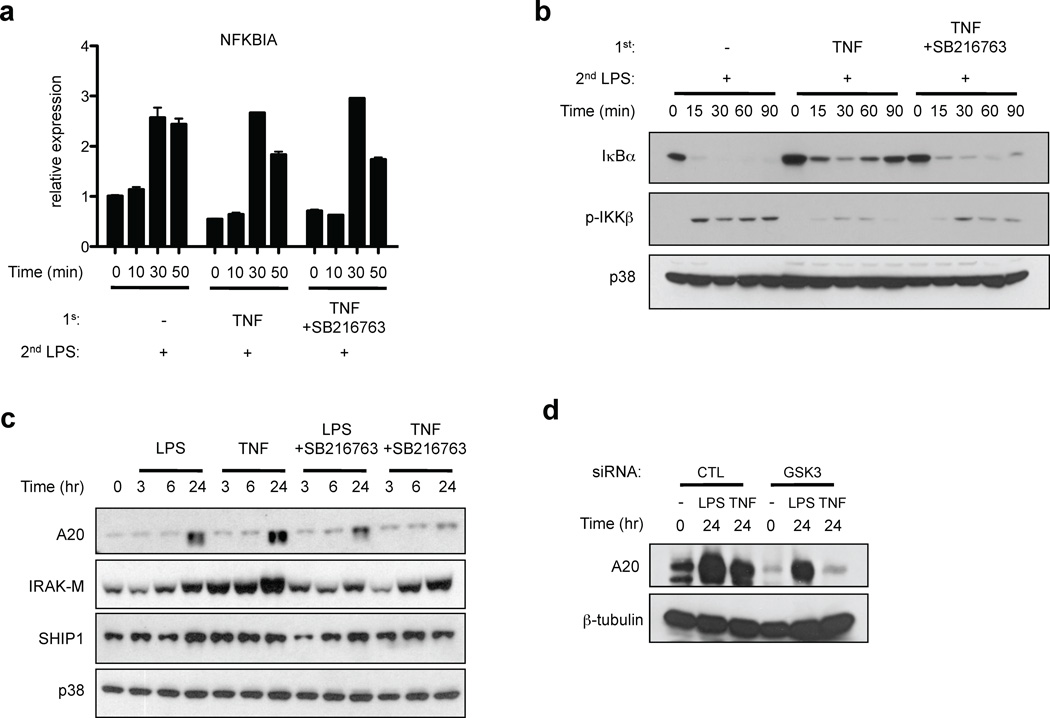
We wished to further investigate mechanisms by which TNF induces macrophage tolerance, and next tested the effects of a panel of inhibitors of pathways known to regulate macrophage cytokine production. Interestingly, TNF-induced tolerance was reversed in a dose-dependent manner by the GSK3 inhibitor SB216763, with essentially complete reversal of tolerance observed at higher concentrations of SB216763 (Fig. 4a). In contrast, LPS-induced tolerization of IL-6 was not at all affected by SB216763. GSK3 is constitutively active in most cell types and we analyzed its activity and regulation by TNF in macrophages. A standard approach to following GSK3 activity is to measure its substrate β-catenin, which is rapidly degraded after GSK3-mediated phosphorylation and is stabilized and expressed when GSK3 is inactivated. Consistent with high basal GSK3 activity in human macrophages17,18, β-catenin protein was not detected at baseline (Fig. 4b) despite high expression of β-catenin mRNA (data not shown). As expected17,18, LPS transiently and weakly suppressed GSK3 activity, as assessed by transient expression of low levels of β-catenin (Fig. 4b); TNF stimulation elicited similar but lesser effects. In contrast, inhibition of GSK3 using SB216763 resulted in massive upregulation of β-catenin expression at all time points that were tested (Fig. 4b). These results confirm that GSK3 was active in human macrophages at the time point (24 hr post treatment) when TNF-induced tolerance was established. Consistent with these results, a 24 hr treatment with TNF did not increase levels of inactivating serine 9/21 phosphorylation of GSK3; instead, TNF modestly but consistently increased GSK3β tyrosine 216 phosphorylation, a posttranslational modification associated with GSK3 activation (Fig. 4c). In addition, TNF increased nuclear expression of GSK3β (Fig. 4d), thus facilitating GSK3β access to new substrates. These results indicate that GSK3β is regulated by TNF and active in TNF-tolerized macrophages, and thus we wished to more rigorously test the role of GSK3 in mediating TNF-induced tolerance. First, we found that LiCl, which inhibits GSK3 by a different mechanism than does SB216763, also potently reversed TNF-induced tolerance while having no apparent effect on LPS-induced tolerance (Fig. 4e). We confirmed and extended these results using genetic approaches. Downregulation of GSK3β by RNA interference restored the production of IL-6 in TNF-tolerized human primary macrophages (Fig. 4f). In addition, we used BMDMs from mice with a myeloid-specific deletion of Gsk3b36. TNF was not able to induce tolerance, as assessed by LPS-induced IL-6 production and Tnfa gene expression, in GSK3β-deficient BMDMs, whereas LPS-induced tolerance was intact (Fig. 4g). Collectively, these results provide compelling evidence that TNF-induced tolerance is mediated by GSK3. Dependence on GSK3 distinguishes TNF-induced tolerance mechanistically from LPS-induced tolerance that did not require GSK3.
Figure 4.
TNF-induced tolerance is mediated by GSK3. (a) Human macrophages treated with or without SB216763 (1–20 µM) were cultured with LPS or TNF for 24 hr and then challenged with 10 ng/ml LPS. Culture supernatants were harvested 24 hr later and IL-6 was measured by ELISA. (b, c) Human macrophages treated with or without SB216763 (10 µM) were stimulated with LPS or TNF for the indicated times. Whole cell extracts were analyzed by immunoblotting. (d) Human macrophages treated with or without SB216763 (10 µM) were stimulated with LPS or TNF for 24 hr and then challenged with 10 ng/ml LPS for the indicated times. Nuclear extracts were analyzed by immunoblotting. (e) Human macrophages treated with or without SB216763 (10 µM) or LiCl (20 mM) were cultured with LPS or TNF for 24 hr and then challenged with 10 ng/ml LPS and IL-6 was measured by ELISA. (f) Human macrophages transfected with control or GSK3β-specific short interfering RNA duplexes (siRNA) were treated with LPS or TNF for 24 hr and then challenged with 10 ng/ml LPS. IL-6 in culture supernatants was measured by ELISA. (g) BMDMs from mice lacking myeloid GSK3β or genetically matched controls were stimulated with LPS (100 ng/ml) or TNF (40 ng/ml) for 24 hr and then challenged with 10 ng/ml LPS for 1 hr (right panel) or 24 hr (left panel). TNF mRNA and IL-6 protein were assessed by real-time PCR and ELISA, respectively. Data are representative of at least three experiments.
GSK3 promotes rapid termination of NF-κB signaling and A20 expression
To determine the mechanism by which GSK3 mediates TNF-induced tolerance, we first investigated the role of GSK3 in the suppression of TLR signaling in TNF-tolerized macrophages. Inhibition of GSK3 had no effect on the diminished levels of LPS-induced MAPK activation in TNF-tolerized macrophages (Fig. 5a). However, inhibition of GSK3 nearly completely abolished the rapid resynthesis of I-κBα that was observed after LPS stimulation of TNF-tolerized cells, thereby extending the duration of I-κBα protein downregulation and NF-κB signaling to be similar to control nontolerized macrophages (Fig. 5b, lanes 8–12). In contrast, I-κBα protein expression remained high in LPS-tolerized macrophages even when GSK3 was inhibited (Fig. 5c), consistent with the lack of reversal of LPS-induced tolerance by inhibition or ablation of GSK3 (Fig. 4). These results suggest that GSK3 is required for rapid termination of NF-κB pathway signaling by newly synthesized I-κBα in TNF-tolerized macrophages. I-κBα-mediated postactivation repression of NF-κB signaling is a well established inhibitory mechanism by which newly synthesized I-κBα not only traps NF-κB proteins in the cytoplasm, but also translocates to the nucleus and facilitates removal of NF-κB subunits from selected gene promoters and subsequent export to the cytoplasm34. Accordingly, we detected substantial nuclear accumulation of resynthesized I-κBα 90 minutes after LPS stimulation of TNF-tolerized macrophages, and this nuclear accumulation was abrogated when GSK3 was inhibited (Fig. 5d). In addition, ChIP analysis showed that LPS-induced recruitment of NF-κB p65 to the endogenous IL6 and TNF promoters was considerably diminished in TNF-induced tolerant cells (Fig. 5e). Consistent with increased IL6 expression (Fig. 4a), NF-κB p65 occupancy at the IL6 promoter was enhanced when GSK3 was inhibited (Fig. 5f). Collectively, the results suggest that GSK3 mediates TNF-induced tolerance at least in part by promoting rapid reaccumulation of newly synthesized I-κBα, thereby attenuating expression of NF-κB-dependent genes.
Figure 5.
GSK3 regulates kinetics of I-κBα expression and postinduction repression of NF-κB signaling in LPS-stimulated TNF-tolerized macrophages. (a – c) Human macrophages were pretreated for 24 hr with LPS or TNF with or without SB216763 (10 µM) and then challenged with 10 ng/ml LPS for the indicated times. IκBα amounts and ERK and p38 phosphorylation were assessed by immunoblotting of whole cell lysates. (d) Human macrophages were pretreated with LPS or TNF with or without SB216763 (10 µM) and then challenged with 10 ng/ml LPS for the indicated times. Leptomycin B (10 µM) was added together with LPS to inhibit CRM1/exportin1-mediated nuclear export. Cells were stained with anti-I-κBα (green) and DAPI (blue). (e, f) Human macrophages were pretreated for 24 hr with LPS or TNF with or without SB216763 (10 µM) and then stimulated with 10 ng/ml LPS for 1 hr or 3 hr, for analysis of TNF and IL6, respectively. NF-κB p65 recruitment to TNF and IL6 promoters was assessed by ChIP. Data are representative of three to eight independent experiments.
We next examined the mechanism by which GSK3 accelerated and increased I-κBα protein re-expression after LPS stimulation of TNF-tolerized macrophages. Transcription of the NFKBIA gene encoding I-κBα is rapidly and directly activated by NF-κB to engage rapid feedback inhibition after various activating stimuli7,37,38. Consistent with intact but transient activation of NF-κB signaling (Fig. 5b), NFKBIA mRNA was induced after LPS stimulation of TNF-tolerized macrophages (Fig. 6a); thus NFKBIA is a nontolerizable gene. NFKBIA mRNA induction by LPS did not substantially differ among naïve, tolerized and SB216763-treated tolerized cells (Fig. 6a), and thus the rapid re-accumulation of I-κBα protein after LPS stimulation of tolerized cells (Fig. 5b and 6b) could not be explained by changes in NFKBIA mRNA induction. These results suggested that I-κBα protein rapidly re-accumulated in LPS-stimulated cells because of diminished degradation, which is induced by IKK-mediated phosphorylation. This notion was supported by the finding that LPS-induced activation of IKKβ was diminished in TNF-tolerized cells (Fig. 6b), which provides an explanation for decreased I-κBα degradation. Interestingly, inhibition of GSK3 partially but consistently reversed the attenuation of IKKβ activation in tolerized cells, indicating that GSK3 regulates LPS-induced signaling upstream of IKKβ. As activation of IKKβ is regulated by A20, which we had implicated in TNF-induced tolerance (Fig. 3d), we tested the role of GSK3 in A20 expression. Interestingly, inhibition of GSK3 abrogated TNF-induced expression of A20 (Fig. 6c); multiple other chemical inhibitors had no effect on A20 expression, thereby showing specificity of regulation of delayed and sustained A20 expression by GSK3 signaling (Supplementary Fig. 4). Inhibition of GSK3 had modest and variable effects on IRAK-M and SHIP1 expression, and on LPS-induced expression of A20. This result further supports the idea that IRAK-M and SHIP1 may contribute to TNF-induced tolerance in a subset of donors, but are not the most important players in TNF-induced, GSK3-mediated tolerance. A role for GSK3 in TNF-induced sustained A20 expression was further supported by genetic evidence showing decreased A20 expression when GSK3 expression was knocked down using RNAi (Fig. 6d). These results indicate that GSK3 regulates LPS-induced NF-κB signaling in tolerized macrophages in part by mediating TNF-induced sustained accumulation of A20.
Figure 6.
TNF-induced A20 expression is mediated by GSK3. (a – c) Human macrophages were pretreated for 24 hr with LPS or TNF with or without SB216763 (10 µM) and then challenged with 10 ng/ml LPS for the indicated times. (a) I-κBα mRNA and (b, c) I-κBα, A20, IRAK-M and SHIP1 amounts and IKKβ phosphorylation were assessed by real-time PCR and immunoblotting, respectively. (d) Human macrophages transfected with control or GSK3-specific RNAi were treated with LPS or TNF for 24 hr. A20 expression was determined by immunoblotting. Data are representative of at least three independent experiments.
TNF and GSK3 regulate chromatin accessibility
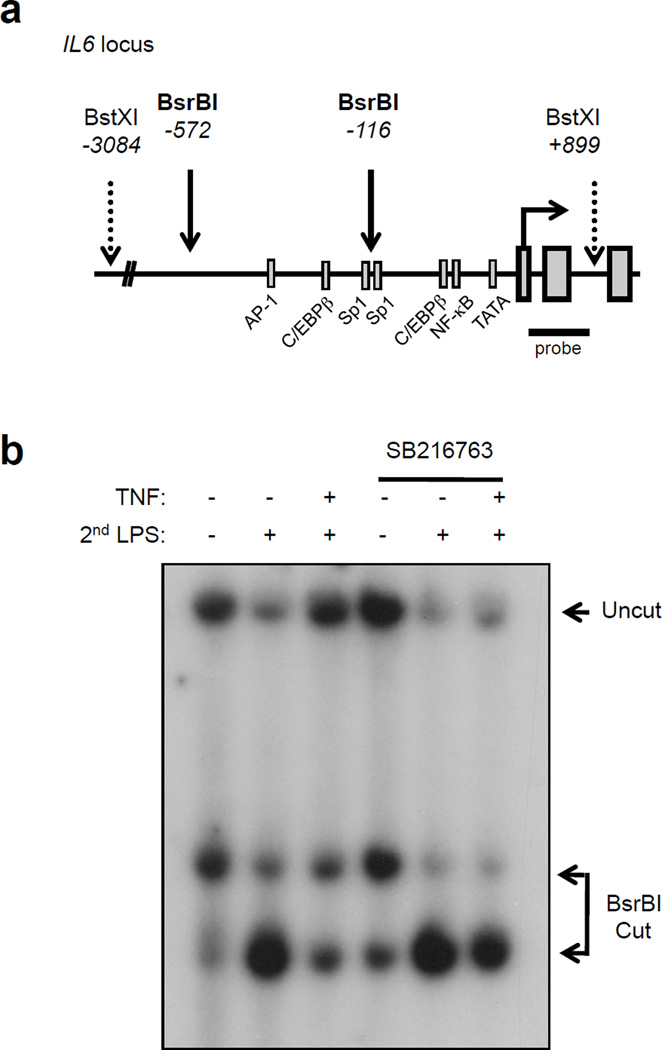
A hallmark of LPS-induced tolerance is acquisition of gene-specific chromatin modifications that suppress expression of tolerized genes. A potent mechanism of LPS-induced gene-specific tolerance is decreased chromatin accessibility at tolerized genes secondary to defective LPS-induced nucleosome remodeling, leading to a failure to overcome a nucleosome-imposed barrier to gene transcription5. We tested the effects of TNF pretreatment on chromatin accessibility at the IL6 locus in primary human macrophages using the restriction enzyme accessibility (REA) assay, a well-established method for measuring chromatin accessibility at endogenous gene loci7,39; increased chromatin accessibility is reflected by increased restriction enzyme cleavage. As expected, cleavage at BsrBI sites upstream of the IL6 transcription start site (Fig. 7a), as detected by induction of more rapidly migrating cleaved DNA fragments, was substantially increased by LPS stimulation of naïve cells (Fig. 7b, lanes 1 and 2). Strikingly, LPS-induced cleavage at BsrBI sites was attenuated in TNF-tolerized cells (Fig. 7b, lane 3). Thus, similar to LPS, TNF pretreatment suppressed LPS-induced nucleosome remodeling that is required for effective induction of IL6 expression40. Remarkably, inhibition of GSK3 in TNF-tolerized cells partially restored BsrBI accessibility (Fig. 7b), which correlated with restoration of IL6 gene expression (Fig. 4) and recruitment of NF-κB p65 to the IL6 locus (Fig. 5f). These results show that TNF regulates chromatin accessibility at an inflammatory gene locus and that GSK3 mediates TNF-induced tolerance in part by preventing increases in chromatin accessibility in response to secondary LPS challenge.
Figure 7.
TNF and GSK3 regulate chromatin accessibility at the IL6 promoter. (a) Schematic representation of the proximal IL6 promoter. (b) Human macrophages were pretreated for 24 hr with LPS or TNF with or without SB216763 (10 µM) and then challenged with 10 ng/ml LPS for 3 hr. Nuclei were digested with 50 U of BsrBI for 20 min at 37°C. Purified genomic DNA was analyzed by Southern blot. Data shown are representative of three independent experiments.
DISCUSSION
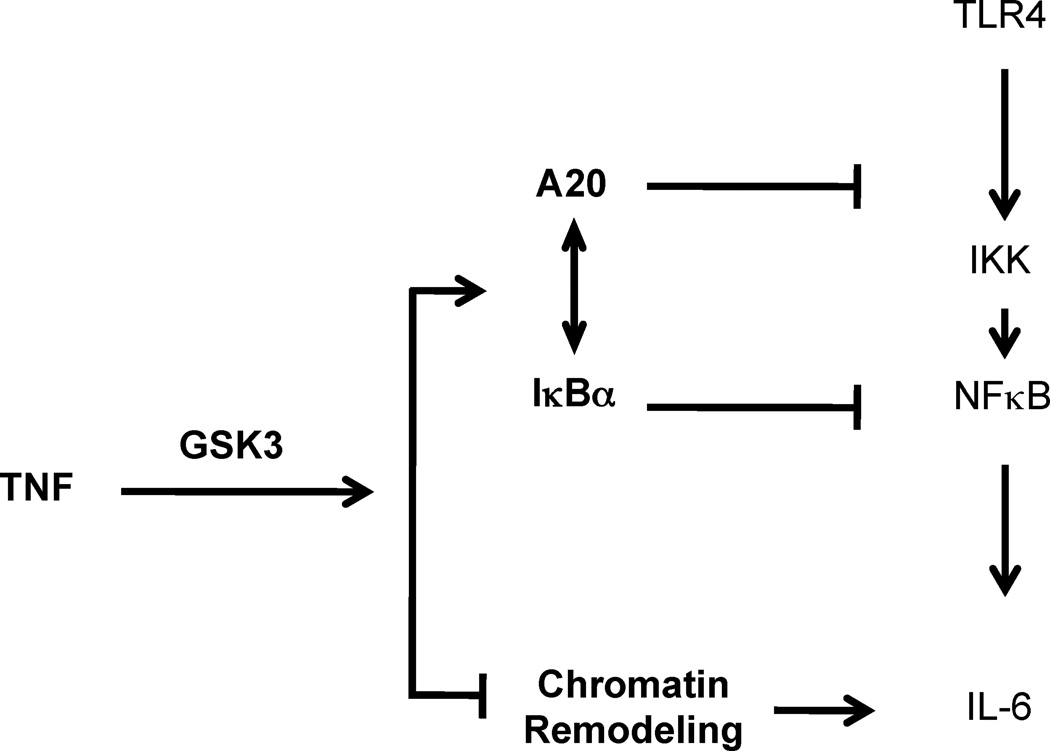
Induction of tolerance to endotoxin by endogenous cytokines has not been mechanistically investigated and signaling pathways and molecules that are important for inducing tolerization are not known. In this study we found that TNF induces tolerance in primary human and murine macrophages and confers protection from endotoxin toxicity and lethality in vivo. Hyporesponsiveness of macrophage inflammatory cytokine production to secondary LPS challenge was mediated by coordinate action of two inhibitory mechanisms – suppression of TLR-induced signaling and of chromatin remodeling. Both inhibitory mechanisms were dependant on GSK3, which suppressed chromatin accessibility and promoted rapid termination of TLR-induced NF-κB signaling by augmenting negative feedback mediated by A20 and I-κBα (Fig. 8). Thus, the mechanism of TNF-induced tolerance is partially distinct from TLR-induced tolerance, where NF-κB signaling is entirely blocked and GSK3 does not play an important role. Our findings identify an unexpected homeostatic function of TNF and reveal the important role of GSK3 in controlling signaling and chromatin remodeling pathways important for macrophage tolerance and thus regulation of cytokine production and inflammation.
Figure 8.
Model for regulation of TNF-induced tolerance by GSK3. TNF induces endotoxin tolerance by two complementary GSK3-dependent inhibitory mechanisms. 1. Pretreatment with TNF attenuates proximal TLR4-induced signaling by GSK3-mediated increases in A20 and I-κBα expression. 2. TNF pretreatment suppresses chromatin remodeling in a GSK3-mediated manner. These two inhibitory mechanisms can work in series to fine tune the amplitude and specificity of TLR4-induced gene expression.
TNF is a potent inflammatory cytokine that is rapidly induced after initial exposure of cells to microbial products such as LPS, and plays a key role in mediating cell activation and the host response to microbial challenge. Indeed, high TNF production after initial LPS stimulation plays a key role in LPS-induced toxicity and lethality41. Thus, on balance TNF is generally regarded as an activating, inflammatory and potentially toxic cytokine. However, increasing evidence suggests a dual role for TNF, which can promote or suppress inflammation depending on timing and context8–13,42,43. For example, TNF is involved in promoting early inflammation but suppressing the later phases of autoimmunity in EAE44, and TNF blockade in human patients can induce exacerbations of multiple sclerosis. Mechanisms that underlie the immunoregulatory functions of TNF are not well understood. Our results reveal that in conjunction with initiating an initial burst of macrophage activation, TNF begins to engage feedback inhibitory mechanisms leading to macrophage tolerance that will limit subsequent inflammatory cytokine production and thereby TNF also serves a protective and homeostatic function in the earliest phases of innate responses. As TLR ligands induce tolerance independently of TNF4, TNF-induced tolerance will be more important for attenuating activation of cells that are not directly exposed to microbial ligands during the initial stages of infection. Thus, TNF-induced tolerization of macrophages surrounding a focus of infection would limit potentially toxic cytokine production if infection spreads, while selective gene regulation would preserve aspects of the host response, such as phagocytosis and production of anti-microbial peptides. Our in vivo results also support the notion that an early burst of systemic TNF production, despite potential for toxicity, also provides protection by preventing excessive cytokine responses in the event of subsequent dissemination of infection and systemic challenge with TLR ligands. TNF-induced tolerance may also contribute to homeostatic regulation of sterile inflammation in conditions where a tolerance-like state has been observed after tissue damage, such as surgery, ischemia/reperfusion and acute coronary syndrome30–32, and to attempts to limit the magnitude of chronic inflammation during autoimmune diseases. TNF-induced feedback inhibitory mechanisms are clearly insufficient to adequately restrain chronic inflammation in autoimmune diseases such as rheumatoid arthritis, possibly because the efficacy of these mechanisms is compromised by other cytokines such as IFN-γ33, or by hypomorphic allelic variants in genes important for tolerance, for example variants in TNFAIP3 (encodes A20) that have been linked with several autoimmune and inflammatory diseases45,46. Overall our findings highlight the importance of the homeostatic functions of TNF.
One interesting aspect of tolerance induced by both TLRs and TNF is coordinate suppression of TLR-induced signaling and of chromatin modifications that are required for downstream inflammatory gene induction. It is likely that these two inhibitory mechanisms cooperate to fine tune the amplitude and pattern of gene expression in restimulated tolerized cells. TNF-induced suppression of chromatin remodeling provides to our knowledge the first example of cytokine-induced epigenetic chromatin modification that confers transcriptional memory in innate immune cells. Upstream of chromatin remodeling, TNF-mediated tolerization altered the kinetics of activation of NF-κB, the most important signal required for inflammatory cytokine induction47. Two key negative regulators of NF-κB activation are A20, which inhibits signaling upstream of IKKs, and I-κBα, whose resynthesis terminates NF-κB signaling. Previous work has suggested that the key determinants of the amplitude and kinetics of NF-κB activation are cellular A20 concentrations present at the time of cell stimulation, and the kinetics of I-κBα protein re-expression after stimulation37. Interestingly, TNF tolerization affected both of these determinants of NF-κB activity. TNF gradually increased cellular A20 concentrations, which may explain the slow development of TNF-induced tolerance in human macrophages. TNF also accelerated I-κBα resynthesis after TLR4 stimulation, with attendant rapid termination of NF-κB signaling. Differential kinetics of NF-κB signaling lead to distinct patterns of gene activation47,48, and thus early termination of NF-κB signaling contributes to the altered pattern of gene expression observed in LPS-stimulated TNF-tolerized cells. For example, the transient burst of TLR-induced NF-κB signaling observed in TNF-tolerized cells can contribute to induction of nontolerized genes, such as the early response gene NFKBIA that is rapidly activated by NF-κB with minimal additional activation requirements7,38. In contrast, activation of secondary response genes requires later phases of NF-κB activity, after chromatin remodeling has occurred40,47, and thus rapid termination of NF-κB signaling in TNF-tolerized cells contributes to diminished induction of secondary response cytokine genes such as IL6 on LPS challenge. In contrast to TNF-tolerized macrophages, NF-κB signaling is completely abrogated in TLR-tolerized cells, with minimal degradation of I-κBα, likely secondary to a strong block in proximal signaling. Thus, the pattern of TLR-induced gene expression and the functional phenotype of TNF-tolerized macrophages are likely partially distinct from TLR-tolerized macrophages and will be further investigated in future work.
The block in TLR signaling in TLR-tolerized macrophages has been extensively characterized and is mediated in part by newly expressed signaling inhibitors such as IRAK-M. De novo gene expression in response to initial tolerizing LPS stimulation is also required for induction of suppressive chromatin modifications at tolerized gene loci5. However, the identity of gene products required for chromatin modification is not known, and the signaling pathways activated by initial TLR stimulation that lead to tolerance are not known. Our findings provide the first insights into pathways required for tolerance by identifying a key role for GSK3 in mediating TNF-induced tolerance. We have linked GSK3 to regulation of basal A20 expression, rapid resynthesis of I-κBα, and suppression of chromatin remodeling at the IL6 locus. This discovery provides insights into pathways that mediate endotoxin tolerance and opens avenues towards identification of GSK3-dependent downstream genes and effector molecules that suppress chromatin remodeling at inflammatory cytokine gene loci.
In naïve macrophages, GSK3 is constitutively active and, depending on context, can augment proinflammatory cytokine production after acute stimulation, at least in part by suppressing IL-10 production and increasing NF-κB activity by a mechanism that involves increased interaction with the coactivator CBP17. Acute stimulation of macrophages with TLR ligands transiently increases GSK3 serine 9/21 phosphorylation and thereby inactivates GSK3 as part of a feedback inhibition mechanism that is suppressed by IFN-γ17,18. In contrast, in tolerized cells that have been exposed to TNF for longer periods, there was a switch in GSK3 function, such that GSK3 mediated the suppression of inflammatory cytokine expression that is associated with macrophage tolerance. One mechanism by which this switch in GSK3 function was achieved is increased GSK3-mediated expression of A20 and I-κBα, which themselves are encoded by NF-κB-activated genes. Thus, during tolerization GSK3 still supports expression of NF-κB target genes, but a shift towards high expression of genes involved in feedback inhibition of NF-κB results in attenuation of the classical inflammatory NF-κB-mediated response. Such shifts in the balance between activation vs. feedback inhibition of NF-κB signaling depending on prior environmental cues, such as prolonged TNF treatment, helps explain the context-dependent and often paradoxical effects of GSK3 on NF-κB signaling that have been previously reported19,25–29. Previous work has connected the GSK3β isoform with NF-κB16, and our results using GSK3β RNAi and gene deletion support an important role for GSK3β in tolerance. Interestingly, in contrast to regulation of GSK3β serine phosphorylation in acutely stimulated macrophages17,18, longer tolerizing treatment with TNF increased GSK3β tyrosine phosphorylation, thereby increasing its activity. In addition, TNF promoted nuclear localization of GSK3β, where GSK3β gains access to new substrates. GSK3-mediated phosphorylation of its substrates requires previous priming phosphorylation by a different kinase16, and TNF induces sustained signaling and phosphorylation of various cellular proteins in macrophages49. Thus, TNF likely alters cellular responses to GSK3 signaling at least in part by changing substrate availability and inducing priming phosphorylation of new substrates. Overall, our findings support a model whereby longer term TNF exposure couples GSK3 signaling to suppression of inflammatory cytokine production by promoting feedback inhibition of NF-κB and suppressing chromatin remodeling.
The early phase of TNF-induced signaling and gene induction has been well characterized and linked to acute inflammatory responses. Work from our lab and others characterizing later phases of TNF responses in macrophages suggests a more complex role for TNF, with induction of IFN-STAT1 responses and differentiation into multinucleated cells49. In this study, we have extended this more nuanced understanding of the role of TNF in macrophages by demonstrating TNF-mediated induction of a potent feedback mechanism that suppresses inflammatory cytokine production. Feedback inhibition via tolerization of macrophages restrains the magnitude of acute inflammatory responses, as shown by our in vitro and in vivo findings, and may contribute to the protective role of TNF in autoimmune diseases such as multiple sclerosis and lupus14,15. However, TNF is a major driver of inflammation in other autoimmune diseases such as rheumatoid arthritis and inflammatory bowel disease, indicating that in these settings TNF-mediated feedback inhibition is insufficient to resolve inflammation. This may occur because the homeostatic functions of TNF are compromised by counter-regulatory signaling pathways such as IFN-γ-STAT133,50, or by hypomorphic TNFAIP3 alleles associated with these diseases45,46. In this context, our findings provide insights that can be exploited therapeutically to boost the suppressive and homeostatic functions of TNF by targeting GSK3 and downstream pathways and molecules with predominantly homeostatic functions. Selective modulation of these pathways can potentially regulate macrophage function to limit inflammatory cytokine production while maintaining host defense in infectious and inflammatory settings.
METHODS
Cell culture and mice
CD14+ monocytes were purified from fresh peripheral blood mononuclear cells (PBMCs) with anti-CD14 magnetic beads (Miltenyi Biotec) as described18 and were cultured in RPMI 1640 medium with 10% FBS (Hyclone) and 10 ng/ml of M-CSF (Peprotech). All mice were maintained in specific pathogen-free conditions in the Animal Facility of the Hospital for Special Surgery. Mice were used at 6–10 weeks of age. C57BL/6J and Tnfrsf1a−/−Tnfrsf1b−/− mice were from the Jackson Laboratory. Gsk3bflox/flox mice were previously described35. We generated mice with myeloid-specific deletion of GSK3β by crossing Gsk3bflox/flox mice with mice harboring a lysozyme M-driven Cre transgene on the C57/BL6 background (Jackson). Mouse BMDMs were obtained as described18 and were maintained in DMEM supplemented with 20% FBS and mouse M-CSF (10 ng/ml; Peprotech). The experiments using human cells and mice were approved by, respectively, the Hospital for Special Surgery Institutional Review Board and Institutional Animal Care and Use Committee.
Reagents
Recombinant human and mouse TNF were from Peprotech (endotoxin levels were below limit of detection, this comes from the product sheet < 0.1 pg/ µg). LPS, SB216763, LiCL and Leptomycin B were purchased from Sigma, Pam3Cys was purchased from EMC Microcollections. Antibodies to IκBα (4812), p-ERK (4377), p-p38 (4631), p-JNK (9251), A20 (4625), IRAK-M (4369), GSK3β (9332), p-GSKα/β (Ser21/9, 9331) were from Cell Signaling. Antibodies specific for SHIP1 (sc-8425) and p38α (sc-535) were from Santa Cruz Biotechnology. Antibody specific for p-GSK3β (Tyr216, 612312) was from BD Pharmingen.
Analysis of protein and mRNA
ELISA, immunoblotting and real-time quantitative PCR were performed as previously described18. Briefly, ELISAs were performed with paired antibody sets, as recommended by the manufacturer (BD Pharmingen). Cytoplasmic, nuclear, or whole cell extracts were prepared as previously described33 and fractionated on 7.5% to 10% polyacrylamide gels by SDS-PAGE, transferred to polyvinylide fluoride membranes (Millipore), incubated with specific antibodies, and enhanced chemiluminescence was used for detection (Amersham). β-tubulin was assessed by immunoblotting to examine cytoplasmic contamination of nuclear lysates. Total RNA was extracted with the RNeasy Mini Kit (Qiagen) and reverse-transcribed using the First Strand cDNA Synthesis kit (Fermertas). Quantitative real-time PCR was performed in triplicate using iQ SYBR Green Supermix and an iCycler iQ thermal cycler (Biorad). Relative expression was normalized for levels of GAPDH. For immunofluorescence microscopy, human macrophages were plated on poly-D-lysine-coated coverslips (BD Biosciences Discovery Labware). Cells were stimulated with TNF or LPS, then were fixed with 4% formaldehyde for 15 min at room temperature and were stained with mouse antibody to IκBα (4814, Cell Signaling Tech.), followed by Alexa Fluor 488-conjugated donkey anti-mouse antibodies (Molecular Probes, Invitrogen). Coverslips were mounted with Vectashield mounting medium (Vector Laboratories) and were examined by epifluorescence microscopy with a Zeiss Axiophot microscope.
RNA interference
Prevalidated GSK3 or A20-specific short interfering RNAs (siRNAs) and non-targeting control siRNAs were purchased from Dharmacon. siRNAs were transfected into freshly isolated primary human monocytes with the Amaxa Nucleofector device set to program Y-001 using the Human Monocyte Nucleofector kit. Cells were washed once 30 min after transfection and cultured for an additional 2 d (A20) or 3 d (GSK3) prior to TNF stimulation.
ChIP assay
ChIP was performed using the ChIP Assay Kit (Millipore) according to the manufacturer’s instructions. 20 × 106 primary human macrophages per condition were fixed with 1% formaldehyde and incubated at 37°C for 5 min. Cells were lysed and chromatin was sheared by liquid phase sonication of samples in Eppendorf tubes that were immersed in ice-cold water (8 × 20 s with output set at level 5 using a Misonix sonicator 3000). Diluted chromatin preparations were incubated with antibody overnight and then incubated with protein A-agarose beads (Santa Cruz Biotechnology) for 1 hr at 4°C. Immunoprecipitated DNA was analyzed by quantitative real-time PCR and normalized relative to 28S rRNA-encoding gene segments that reflect amounts of non-specific background DNA precipitation in each reaction. Anti-NF-κB p65 (7970) was from Abcam, control rabbit IgG (2345) was from Santa Cruz Biotechnology. We used the following primers to amplify the Human IL6 and TNF promoters: IL6, 5’-GCAGAATGAGCCTCAGACATC-3’ and 5’-ACCCTCACCCTCCAACAAAG-3’; TNF, 5’-GGGAGTGTGAGGGGTATCCT-3’ and 5’-GCACCTTCTGTCTCGGTTTC-3’.
Restriction enzyme accessibility assay
Restriction enzyme accessibility assays were performed as described33. Briefly, nuclei were isolated and resuspended in the recommended New England Biolabs buffer containing 50 U of the BsrBI restriction enzyme for 20 min at 37°C. Digested genomic DNA was purified using the DNeasy Blood and Tissue Kit (Qiagen) according to the manufacturer’s instructions. Equal amounts of purified DNA were digested to completion with 50 U BstXI overnight at 37°C, precipitated, and analyzed by Southern blot using a radiolabeled DNA probe specific for the IL6 gene (+51 to +614).
Statistic analysis
Graphpad Prism 5.0 for Mac was used for statistical analysis; specific statistical tests performed are described in the figure legends.
Supplementary Material
ACKNOWLEDGMENTS
We thank G.D. Kalliolias and A. Yarilina for helpful discussions and J. Woodgett (University of Toronto) for providing Gsk3b floxed mice. This work was supported by grants from the NIH (to L.B.I.).
Footnotes
AUTHOR CONTRIBUTIONS
S.P. designed and performed experiments and wrote the manuscript; K.-H. P.-M., J.C. and X.H. contributed to the signaling, REA and in vivo experiments, respectively; L.B.I. designed and supervised the research and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol Rev. 2009;227:248–263. doi: 10.1111/j.1600-065X.2008.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liew FY, Xu D, Brint EK, O'Neill LAJ. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 4.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 6.Foster SL, Medzhitov R. Gene-specific control of the TLR-induced inflammatory response. Clin Immunol. 2009;130:7–15. doi: 10.1016/j.clim.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smale R. Selective transcription in response to an inflammatory stimulus. Cell. 2010;140:833–844. doi: 10.1016/j.cell.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 9.Hodge-Dufour J, et al. Inhibition of interferon gamma induced interleukin 12 production: a potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc Natl Acad Sci USA. 1998;95:13806–13811. doi: 10.1073/pnas.95.23.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams-Skipp C, et al. Unmasking of a protective tumor necrosis factor receptor I-mediated signal in the collagen-induced arthritis model. Arthritis Rheum. 2009;60:408–418. doi: 10.1002/art.24260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blüml S, et al. Antiinflammatory effects of tumor necrosis factor on hematopoietic cells in a murine model of erosive arthritis. Arthritis Rheum. 2010;62:1608–1619. doi: 10.1002/art.27399. [DOI] [PubMed] [Google Scholar]
- 12.Zakharova M, Ziegler HK. Paradoxical anti-inflammatory actions of TNF-alpha: inhibition of IL-12 and IL-23 via TNF receptor 1 in macrophages and dendritic cells. J Immunol. 2005;175:5024–5033. doi: 10.4049/jimmunol.175.8.5024. [DOI] [PubMed] [Google Scholar]
- 13.Marino MW, et al. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kollias G, Douni E, Kassiotis G, Kontoyiannis D. On the role of tumor necrosis factor and receptors in models of multiorgan failure, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Immunol Rev. 1999;169:175–194. doi: 10.1111/j.1600-065x.1999.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 15.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu X, et al. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity. 2006;24:563–574. doi: 10.1016/j.immuni.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3) Trends Immunol. 2010;31:24–31. doi: 10.1016/j.it.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinbrecher KA, Wilson W, Cogswell PC, Baldwin AS. Glycogen synthase kinase 3beta functions to specify gene-specific, NF-kappaB-dependent transcription. Mol Cell Biol. 2005;25:8444–8455. doi: 10.1128/MCB.25.19.8444-8455.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buss H, et al. Phosphorylation of serine 468 by GSK-3beta negatively regulates basal p65 NF-kappaB activity. J Biol Chem. 2004;279:49571–49574. doi: 10.1074/jbc.C400442200. [DOI] [PubMed] [Google Scholar]
- 22.Saijo K, et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takada Y, et al. Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkappaBalpha kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J Biol Chem. 2004;279:39541–39554. doi: 10.1074/jbc.M403449200. [DOI] [PubMed] [Google Scholar]
- 24.Hoeflich KP, et al. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 25.Rodionova E, et al. GSK-3 mediates differentiation and activation of proinflammatory dendritic cells. Blood. 2007;109:1584–1592. doi: 10.1182/blood-2006-06-028951. [DOI] [PubMed] [Google Scholar]
- 26.Shen F, et al. IL-17 receptor signaling inhibits C/EBPbeta by sequential phosphorylation of the regulatory 2 domain. Sci Signal. 2009;2:ra8. doi: 10.1126/scisignal.2000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen E, Fan J, Peng T. Glycogen synthase kinase-3beta suppresses tumor necrosis factor-alpha expression in cardiomyocytes during lipopolysaccharide stimulation. J Cell Biochem. 2008;104:329–338. doi: 10.1002/jcb.21629. [DOI] [PubMed] [Google Scholar]
- 28.Vines A, et al. Novel anti-inflammatory role for glycogen synthase kinase-3beta in the inhibition of tumor necrosis factor-alpha- and interleukin-1beta-induced inflammatory gene expression. J Biol Chem. 2006;281:16985–16990. doi: 10.1074/jbc.M602446200. [DOI] [PubMed] [Google Scholar]
- 29.Jafferany M. Lithium and skin: dermatologic manifestations of lithium therapy. Int J Dermatol. 2008;47:1101–1111. doi: 10.1111/j.1365-4632.2008.03873.x. [DOI] [PubMed] [Google Scholar]
- 30.del Fresno C, et al. Inflammatory responses associated with acute coronary syndrome up-regulate IRAK-M and induce endotoxin tolerance in circulating monocytes. J Endotoxin Res. 2007;13:39–52. doi: 10.1177/0968051907078623. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki T, et al. Surgical stress induces endotoxin hyporesponsiveness and an early decrease of monocyte mCD14 and HLA-DR expression during surgery. Anesth Analg. 2001;92:1322–1326. doi: 10.1097/00000539-200105000-00046. [DOI] [PubMed] [Google Scholar]
- 32.Langdale LA, Kajikawa O, Frevert C, Liggitt HD. Sustained tolerance to lipopolysaccharide after liver ischemia-reperfusion injury. Shock. 2003;19:553–558. doi: 10.1097/01.shk.0000055238.25446.64. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Ivashkiv LB. IFN-{gamma} abrogates endotoxin tolerance by facilitating Toll-like receptor-induced chromatin remodeling. Proc Natl Acad Sci USA. 2010;107:19438–19443. doi: 10.1073/pnas.1007816107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Boone DL, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 36.Patel S, et al. Tissue-specific role of glycogen synthase kinase 3beta in glucose homeostasis and insulin action. Mol Cell Biol. 2008;28:6314–6328. doi: 10.1128/MCB.00763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Werner SL, et al. Encoding NF-kappaB temporal control in response to TNF: distinct roles for the negative regulators IkappaBalpha and A20. Genes Dev. 2008;22:2093–2101. doi: 10.1101/gad.1680708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 39.Weinmann AS, et al. Nucleosome remodeling at the IL-12 p40 promoter is a TLR-dependent, Rel-independent event. Nat Immunol. 2001;2:51–57. doi: 10.1038/83168. [DOI] [PubMed] [Google Scholar]
- 40.Ramirez-Carrozzi VR, et al. Selective and antagonistic functions of SWI/SNF and Mi-2beta nucleosome remodeling complexes during an inflammatory response. Genes Dev. 2006;20:282–296. doi: 10.1101/gad.1383206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 42.Christen U, et al. A dual role for TNF-alpha in type 1 diabetes: islet-specific expression abrogates the ongoing autoimmune process when induced late but not early during pathogenesis. J Immunol. 2001;166:7023–7032. doi: 10.4049/jimmunol.166.12.7023. [DOI] [PubMed] [Google Scholar]
- 43.Hassett S, Moynagh P, Reen D. TNF-alpha is a mediator of the anti-inflammatory response in a human neonatal model of the non-septic shock syndrome. Pediatr Surg Int. 2006;22:24–30. doi: 10.1007/s00383-005-1574-7. [DOI] [PubMed] [Google Scholar]
- 44.Kassiotis G, Kollias G. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J Exp Med. 2001;193:427–434. doi: 10.1084/jem.193.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stahl EA, et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet. 2010;42:508–514. doi: 10.1038/ng.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plenge RM, et al. Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nat Genet. 2007;39:1477–1482. doi: 10.1038/ng.2007.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Natoli G, Saccani S, Bosisio D, Marazzi I. Interactions of NF-kappaB with chromatin: the art of being at the right place at the right time. Nat Immunol. 2005;6:439–445. doi: 10.1038/ni1196. [DOI] [PubMed] [Google Scholar]
- 48.Werner SL, Barken D, Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science. 2005;309:1857–1861. doi: 10.1126/science.1113319. [DOI] [PubMed] [Google Scholar]
- 49.Yarilina A, et al. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat Immunol. 2008;9:378–387. doi: 10.1038/ni1576. [DOI] [PubMed] [Google Scholar]
- 50.Ivashkiv LB, Hu X. The JAK/STAT pathway in rheumatoid arthritis: pathogenic or protective? Arthritis Rheum. 2003;48:2092–2096. doi: 10.1002/art.11095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.