Abstract
Objective
To determine among community patients with heart failure (HF), whether pulmonary artery systolic pressure (PASP) assessed by Doppler echocardiography was associated with death and improved risk prediction over established factors, using the integrated discrimination improvement (IDI) and net reclassification improvement (NRI).
Background
While several studies have focused on idiopathic pulmonary arterial hypertension, less is known about pulmonary hypertension among patients with HF, particularly on its prognostic value in the community.
Methods
Olmsted County residents with HF between 2003 and 2010 prospectively underwent assessment of ejection fraction (EF), diastolic function, and PASP by Doppler echocardiography.
Results
PASP was recorded in 1049 of 1153 patients (mean age 76±13, 51% women). Median PASP was 48 mmHg (25th-75th percentile, 37.0-58.0). There were 489 deaths after a follow-up of 2.7±1.9 years. There was a strong positive graded association between PASP and mortality. Increasing PASP was associated with an increased risk of death (HR 1.45, 95%CI 1.13-1.85 for tertile 2; HR 2.07, 95%CI 1.62-2.64 for tertile 3, versus tertile 1), independently of age, sex, comorbidities, EF and diastolic function. Adding PASP to models including these clinical characteristics resulted in an increase in the c-statistic from 0.704 to 0.742 (p=0.007), an IDI gain of 4.2% (p<0.001), and an NRI of 14.1% (p=0.002), indicating that PASP improved prediction of death over traditional prognostic factors. All results were similar for CV death.
Conclusion
Among community patients with HF, PASP strongly predicts death and provides incremental and clinically relevant prognostic information independently of known predictors of outcomes.
Keywords: heart failure, pulmonary hypertension, mortality, community
Introduction
Pulmonary hypertension (PH) is prevalent among patients with heart failure (HF), both among those with reduced and preserved left ventricular ejection fraction (EF)(1-3), with HF being one of the most common causes of PH (4,5). While there is substantial data on the rare idiopathic pulmonary arterial hypertension, less is known on the predictive value of pulmonary pressures in the more common PH associated with HF (4,6). Some studies indicate that PH portends a poor prognosis among patients with HF (1-3,7-11) but have notable limitations including retrospective design, limitation to prevalent cases and to subgroups of patients such as advanced HF, reduced or preserved EF. Further, chronic obstructive pulmonary disease (COPD) was often excluded, diastolic function was seldom assessed, and most studies included highly selected patients from echocardiographic or catheterization series or referrals to HF clinics. The only population-based study published thus far was limited to patients with preserved EF (3), therefore the prevalence, clinical characteristics and prognosis of PH among all community patients with HF remains uncertain. Further, publications focused chiefly on all cause death and information on the impact of PH on cardiovascular death is limited.
With the present population-based study, we addressed these gaps in knowledge by prospectively investigating all in and out-patients presenting with HF in Olmsted County. We aimed to examine the prevalence and prognostic role of PH estimated by pulmonary artery systolic pressure (PASP) from Doppler echocardiography, which is currently considered the best screening method for PH (6). We tested whether the associations were independent of other predictors such as EF and diastolic function, and investigated whether PASP improved risk prediction over established prognostic factors using the c-statistic, integrated discrimination improvement (IDI) and net reclassification improvement (NRI).
Methods
Study setting – The Rochester Epidemiology Project
This study was conducted in Olmsted County, Minnesota, and the methods are analogous to our previously published study of systolic and diastolic heart failure (12). Population-based epidemiological research is feasible in Olmsted County because it is relatively isolated from other urban centers and only a few providers (chiefly Mayo Clinic and Olmsted Medical Center) deliver nearly all health care to local residents. Each provider uses a medical record which captures information for all encounters and can be easily retrieved. These records are indexed through the Rochester Epidemiology Project, resulting in the linkage of medical records from all sources of care (13).
Identification of patients
As previously described, natural language processing of the unstructured text of the electronic medical record was used to prospectively identify patients presenting with clinical findings compatible with HF (12,14).
After real time case identification, subjects were immediately contacted to participate in the prospective study that included Doppler echocardiography and venous blood draw for the brain natriuretic peptide (BNP). If a clinical echocardiogram was not scheduled within one day of the enrollment, it was scheduled by the research team with experienced research sonographers performing the examinations under quality control similar to that in place for clinical examinations. Therefore, there was no difference between exams performed under clinical or research auspices. The complete records of potential cases were manually reviewed to verify the diagnosis of HF using both clinical and Framingham criteria (15) and to collect clinical data. The feasibility and reliability of the Framingham criteria to ascertain HF in Olmsted County have been previously published (16).
Participants provided written consent and the study was approved by the Mayo Clinic Institutional Review Board.
Echocardiography-Doppler
In Olmsted County, all echocardiograms are performed and interpreted in the Mayo Clinic Echocardiographic Laboratory. M-mode, two-dimensional, Doppler, and Doppler tissue imaging were performed according to guidelines of the American Society of Echocardiography (17). Digital echocardiographic data containing a minimum of 3 consecutive beats (5 in atrial fibrillation) were acquired and transferred to a server for storage and archiving (ProSolv Echo Management System®). Left ventricular EF was measured by M-mode or two-dimensional echocardiography using the Quinones formula from the parasternal views (18), by the quantitative two-dimensional biplane volumetric Simpson method from four- and two-chamber views (17), and by the semiquantitative two-dimensional visual estimate method from multiple echocardiographic views. All methods have been previously validated (18). EF values were averaged when multiple measurements were performed. As recommended (19), preserved systolic function was defined as an EF greater than or equal to 50%. Left ventricular end-diastolic diameter, interventricular septal and posterior wall thickness were measured by M-mode or two-dimensional echo from the parasternal views at end-diastole as recommended by the American Society of Echocardiography, and they were used to calculate left ventricular mass which was indexed to body surface area (17).
As PASP is equal to right ventricular systolic pressure in the absence of pulmonary stenosis, PASP was estimated using Doppler echocardiography by calculating the right ventricular to right atrial pressure gradient during systole, approximated by the modified Bernoulli equation as 4v2, where v is the velocity of the tricuspid regurgitation jet in m/s. Right atrial pressure, estimated based on echocardiographic characteristics of the inferior vena cava and assigned a standardized value (20), was then added to the calculated gradient to give PASP (3). Although the agreement between echocardiographic estimates of PASP and invasively measured values on right heart catheterization is suboptimal (21), especially among patients with lung disease (22), these two methods are sufficiently correlated (20), to warrant the use of Doppler to screen for PH (21,23).
Diastolic function was assessed as previously published (12,24). It integrates Doppler measurements of the mitral inflow and Doppler tissue imaging of the mitral annulus (25), a sensitive and relatively load-independent measure of left ventricular relaxation (12,25,26). The algorithm relies on mitral inflow and Doppler tissue imaging, both methods that can be applied to large numbers of patients with high reproducibility (25). This approach enabled classifying diastolic function into 4 categories: normal, mild (impaired relaxation without evidence of increased filling pressures), moderate (impaired relaxation or pseudo-normal with moderate elevation of filling pressures), and severe (advanced reduction in compliance) (12,25). Diastolic function was categorized as indeterminate in the presence of missing data.
Patient characteristics
The characteristics at the time of HF diagnosis were collected from the medical records. Subjects were classified as outpatient cases if not hospitalized within 7 days of the outpatient diagnosis. Clinicians’ diagnoses were used to identify COPD, atrial fibrillation and flutter, hyperlipidemia, hypertension and current smoking. Diabetes mellitus was defined according to the American Diabetes Association criteria (27). The hemoglobin value closest to HF diagnosis (± 1 year) was used to define anemia (hemoglobin concentration <13.0 g/dL in men and <12.0 g/dL in women) (28). Body mass index (BMI) (kg/m2) was calculated using height (first available outpatient value) and weight (last outpatient value prior to HF diagnosis).
Myocardial infarction (MI) was defined by published criteria (29) and comorbidity was measured by the Charlson index, excluding COPD (30). Estimated glomerular filtration rate was calculated using the last serum creatinine value prior to the diagnosis of HF or the first value post HF diagnosis (+/− 1 year), with the Modification of Diet in Renal Disease Study (MDRD) equation (31).
Serum samples were stored at −70° C until laboratory testing was performed. BNP was measured by a 2-site immunoenzymatic sandwich assay on the DxI 800 automated immunoassay system (Beckman Instruments, Chaska, MN). Tests were performed in the Immunochemical Core Laboratory of Mayo Clinic, Rochester, Minn.
Follow-up for death relied on death certificates filed in Olmsted County, autopsy reports, obituary notices, and electronic files of death certificates obtained from the State of Minnesota Department of Vital and Health Statistics. Cardiovascular cause of death was based on codes from the 10th version of the International Classification of Diseases (ICD10) while relying on the American Heart Association categories for cardiovascular deaths (I00-I99) (32).
Statistical analysis
Data are presented as frequencies and percentages for categorical variables, mean (± standard deviation) for normally-distributed continuous variables, or median (25th, 75th percentile) for continuous variables with a skewed distribution. Trends in characteristics across groups were tested with Mantel-Haenszel chi-square for categorical variables and linear regression for continuous variables. Trends in skewed variables were analyzed after logarithmic transformation. PASP was categorized into tertiles and coded as one three-level variable.
Survival was analyzed with the Kaplan-Meier method according to PASP tertiles and was compared by the log-rank test. Cox proportional hazards regression was used to examine the association between PASP and all cause death and cardiovascular (CV) specific death, univariately and while controlling for baseline characteristics. Covariate data were missing in less than 1% of the patients. The proportional hazard assumption was tested using the Schoenfeld residuals and was found to be valid.
To determine whether PASP offered value in predicting 1-year mortality beyond traditional risk indicators, the incremental value of PASP was assessed using the c-statistic, IDI and NRI. It is recognized that reporting a significant association between a predictor and outcome is not sufficient to demonstrate value in risk prediction (33). It is suggested that the c-statistic not be the sole determinant of clinical utility and measures of risk discrimination (IDI) and reclassification (NRI) be used to evaluate the value of a risk indicator (33).
The IDI measures the change in the difference in the mean predicted probabilities of death between those dead and alive at 1 year after inclusion of PASP in the base model. Our base model included the covariates in our final Cox proportional hazards regression model (age, sex, incident HF status, comorbidity index, anemia, EF, diastolic function and COPD). PASP was log-transformed and modeled continuously.
NRI measures improvement in risk classification using event-specific reclassification tables. Logistic regression was used to determine the predicted probabilities for 1-year all-cause mortality for each patient using the base model. The probabilities were then ranked and categorized into tertiles (<13%, 13 to <23%, and ≥23%). Patients were reclassified according to the 1-year predicted probabilities of death after addition of PASP into the model. The NRI quantified the net improvement in risk reclassification (higher predicted probability of death in subjects dead at 1 year, lower predicted probability of death in subjects alive at 1 year). Similar methods were used to determine the NRI for 1-year CV specific mortality. The tertile cutpoints for predicted probability of CV death were: <6%, 6 to<12% and ≥12%.
All statistical tests were two-sided and a p-value of 0.05 was selected for the threshold of statistical significance. Analyses were performed using SAS® statistical software, version 9.2 (SAS Institute Inc., Cary, NC) and Splus® statistical software, version 8 (TIBCO Software, Inc., Palo Alto, CA).
Results
Patient identification and characteristics
Between September 4, 2003 and June 30, 2010, 8460 Olmsted County residents were identified from the electronic medical record as potential candidates for inclusion. After manual record review, 1938 individuals with active HF (both incident and prevalent) were approached for participation. Among these, 1300 consented (participation rate of 67.1%) and 1153 underwent echocardiography at a median (25th-75th percentile) of 1 (0-3) day within the diagnosis of HF. PASP could not be measured on 104 patients, resulting in a final study population of 1049.
The mean±SD age of study subjects was 76±13years, 1016 (96.9%) met Framingham criteria for HF and 51% were women. The burden of comorbidity was high in this population as 572 (54.5%) of the subjects presented with a modified Charlson comorbidity index of 3 or greater. Among the 1049 study subjects, 282 (27%) were diagnosed in the outpatient setting, while 767 (73%) were inpatients; 538 (51%) were incident HF cases, while the remaining 511 (49%) were prevalent cases.
Pulmonary artery systolic pressure
The median PASP was 48 mmHg (25th-75th percentile, 37.0-58.0). When the upper limit of pulmonary pressure was defined as 35 mmHg, only 21% of patients in this cohort had normal pulmonary pressures. Baseline characteristics were examined according to the tertiles of the distribution of PASP defined as < 41, 41-54 and > 54 mmHg (Table 1). Among patients with PASP < 41 mmHg, 64.4% had normal PASP (defined ≤ 35mmHg).
Table 1. Clinical Characteristics of Patients with HF in Olmsted County*.
| N missing |
Total (N=1049) |
Tertile 1 PASP<41 mmHg (N=343) |
Tertile 2 PASP=41- 54 mmHg (N=370) |
Tertile 3 PASP>54 mmHg (N=336 |
p value (trend) |
|
|---|---|---|---|---|---|---|
| Age, mean ±SD, y | -- | 75.6±13.3 | 73.4±13.4 | 76.2±13.0 | 77.1±13.3 | <0.001 |
| Men | -- | 517 (49.3) | 188 (54.8) | 172 (46.5) | 157 (46.7) | 0.035 |
| Cardiovascular risk factors | ||||||
| Hypertension | -- | 897 (85.5) | 294 (85.7) | 311 (84.1) | 292 (86.9) | 0.664 |
| Current smoker | -- | 97 (9.3) | 37 (10.8) | 32 (8.7) | 28 (8.3) | 0.269 |
| Diabetes mellitus | -- | 347 (33.1) | 113 (32.9) | 110 (29.7) | 124 (36.9) | 0.278 |
| Hyperlipidemia | -- | 783 (74.6) | 274 (79.9) | 269 (72.7) | 240 (71.4) | 0.011 |
| BMI, median (Q1- Q3) |
-- | 27.5 (24-32) | 28.4 (25-32) | 27.5 (24-31) | 26.5 (23-31) | 0.015 |
| Comorbidities | ||||||
| Prior MI | -- | 251 (23.9) | 83 (24.2) | 86 (23.2) | 82 (24.4) | 0.952 |
| COPD | -- | 293 (27.9) | 89 (26.0) | 110 (29.7) | 94 (28.0) | 0.552 |
| Estimated glomerular filtration rate, median (Q1-Q3), mL/min per 1.73 m2 |
203 | 52.2 (38-64) | 54.2 (44-67) | 52.1 (39-64) | 48.1 (34-59) | <0.001 |
| Anemia | 4 | 537 (51.4) | 146 (42.7) | 189 (51.4) | 202 (60.3) | <0.001 |
| Atrial fibrillation/flutter |
1 | 379 (36.2) | 98 (28.6) | 155 (42) | 126 (37.5) | 0.015 |
| Comorbidity index | -- | 0.096 | ||||
| 0 | 156 (14.9) | 58 (16.9) | 52 (14.1) | 46 (13.7) | ||
| 1-2 | 321 (30.6) | 106 (30.9) | 121 (32.7) | 94 (28.0) | ||
| ≥3 | 572 (54.5) | 179 (52.2) | 197 (53.2) | 196 (58.3) | ||
| HF characteristics and severity indices | ||||||
| NYHA class | -- | 0.001 | ||||
| I | 63 (6.0) | 29 (8.5) | 16 (4.3) | 18 (5.4) | ||
| II or III | 647 (61.7) | 222 (64.7) | 234 (63.2) | 191 (56.9) | ||
| IV | 339 (32.3) | 92 (26.8) | 120 (32.4) | 127 (37.8) | ||
| BNP, pg/mL, median (Q1-Q3) |
154 | 426 (209- 836) |
306 (133-566) | 417 (233- 736) |
675 (320-1170) | <0.001 |
Data are presented as N (%), unless otherwise specified.
BMI=body mass index; BNP= brain natriuretic peptide; COPD=chronic obstructive pulmonary disease; HF=heart failure; MI=myocardial infarction; NYHA= New York Heart Association; PASP=pulmonary artery systolic pressure; Q1=25th percentile; Q3=75th percentile.
Patients with higher PASP were more likely to be older, female, have anemia, atrial fibrillation, lower creatinine clearance and BMI, and a higher NYHA class and BNP level, while they were less likely have hyperlipidemia (Table 1). Increasing PASP was associated with larger left atrial volume index, higher E/e’, and worse diastolic function, however PASP was not associated with left ventricular mass index, EF or left ventricular end-diastolic diameter (Table 2).
Table 2. Echocardiographic characteristics of patients with HF in Olmsted County*.
| N missing |
Total (N=1049) |
Tertile 1 PASP<41 mmHg (N=343) |
Tertile 2 PASP=41-54 mmHg (N=370) |
Tertile 3 PASP>54 mmHg (N=336) |
p value (trend ) |
|
|---|---|---|---|---|---|---|
| LV mass index (g/m2) | 401 | 113 (91-137) | 112 (90-136) | 114 (92-135) | 115 (91-140) | 0.729 |
| LVEDD (mm) | 46 | 51 (46-57) | 50 (46-57) | 51.3 (46-57) | 51 (46-57) | 0.697 |
| LA volume index (ml/m2) |
586 | 47 (38-58) | 43 (34-54) | 48 (38-61) | 50.5 (41-59) | <0.001 |
| E/e’ ratio | 152 | 18 (13-25) | 15 (11-20) | 18 (13-25) | 20 (15-30) | <0.001 |
| EF, mean ±SD | 2 | 47.6 (16.5) | 48.1 (15.5) | 47.3 (17.1) | 47.6 (16.9) | 0.654 |
| Diastolic Function, N (%) |
<0.001 | |||||
| Normal | 72 (6.9) | 25 (7.3) | 29 (7.8) | 18 (5.4) | ||
| Mild | 49 (4.7) | 23 (6.7) | 16 (4.3) | 10 (3.0) | ||
| Moderate | 618 (58.9) | 223 (65.0) | 218(58.9) | 177 (52.7) | ||
| Severe | 183 (17.5) | 42 (12.2) | 64 (17.3) | 77 (22.9) | ||
| Indeterminate | 127 (12.1) | 30 (8.7) | 43 (11.6) | 54 (16.1) |
Data are presented as median (25th-75th quartile) unless otherwise specified.
EF=ejection fraction; LA= left atrial; LV= left ventricular; LVEDD=left ventricular end-diastolic diameter; other abbreviations as in Table 1.
Mortality
After a mean ± SD follow-up of 2.7±1.9 years, 489 patients died. There was a strong positive graded association between PASP and risk of all-cause death after HF (p<0.001, Figure 1). One-year mortality estimates for patients in tertile 1, 2 and 3 were 8%, 19%, and 28%, respectively. Compared to patients in the lowest PASP tertile, patients in the middle tertile had an unadjusted 72% increased risk of death (HR 1.72, 95% CI, 1.35-2.18; Figure 2), while those in the highest tertile had over a 2.5 times increased risk of death (HR 2.64, 95% CI, 2.09-3.33). Adjustment for age and sex and further adjustment for incident HF status, comorbidity index, anemia, EF, diastolic function and COPD attenuated these results, although in the final multivariable model, patients in the middle PASP tertile still had a 45% increased risk of death (HR 1.45, 95% CI, 1.13-1.85) while those in the highest tertile were over twice as likely to die compared to patients in the lowest tertile (HR 2.07, 95% CI, 1.62-2.64). The interaction between EF and PASP was not significant (p=0.357), nor was the interaction between diastolic function and PASP (p=0.589).
Figure 1. Overall survival by PASP tertiles among HF patients.
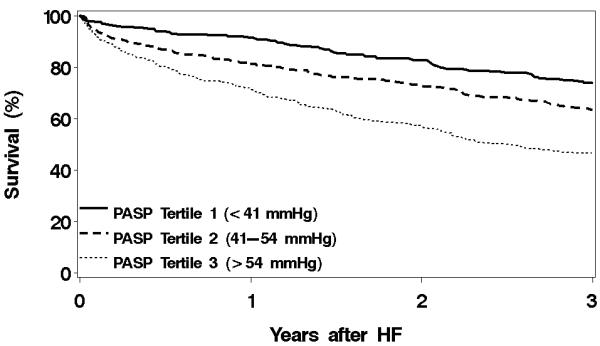
Overall survival by pulmonary artery systolic pressure (PASP) tertiles among heart failure (HF) patients. N=1049. p<0.001.
Figure 2. The association between PASP tertiles and all-cause mortality.
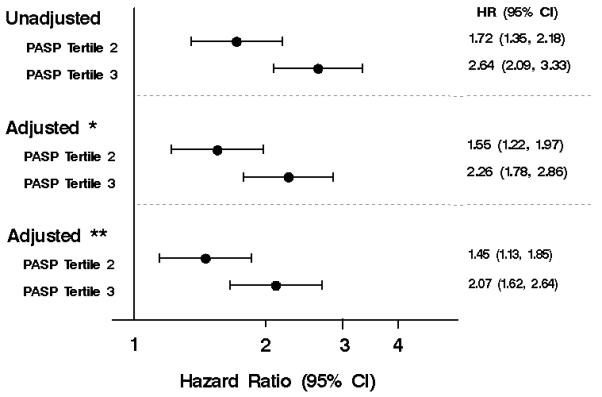
The unadjusted and adjusted hazard ratios for risk of all-cause mortality using Cox proportional hazards regression.
Adjusted*=adjusted for age and sex; Adjusted**=adjusted for age, sex incident HF status, comorbidity index, anemia, ejection fraction (EF), diastolic function and chronic obstructive pulmonary disease (COPD). Other abbreviations as in Figure 1.
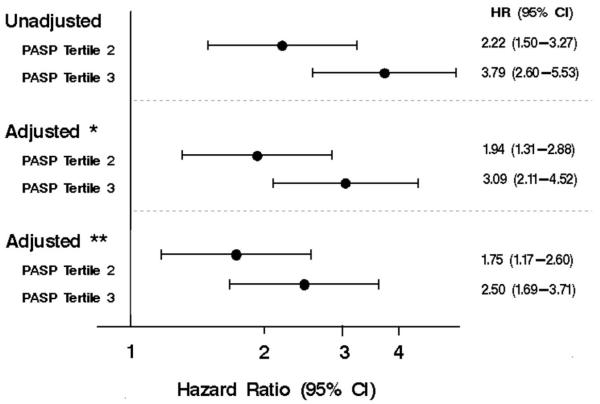
We could not obtain cause of death for 74 patients; thus they were excluded from CV specific death analyses. During follow-up, 218 patients died of CV death. There was a similar graded association between PASP and risk of CV specific death. (p<0.001, Figure 3). One-year mortality estimates for patients in tertile 1, 2 and 3 were 4%, 10% and 17%, respectively. Patients in the middle PASP tertile had an unadjusted 2-fold increased risk of CV death (HR 2.22, 95% CI, 1.50-3.27; Figure 4) compared to patients in the lowest tertile, while those in the highest tertile had nearly a 4-fold increased risk of CV death (HR 3.79, 95% CI, 2.60-5.53). After adjustment for the same covariates as in the all-cause death analysis, patients in the middle tertile still had an 75% increased risk of CV death (HR 1.75, 95% CI 1.17-2.60) while those in the highest tertile were 2.5 times as likely to die of CV death compared to patients in the lowest tertile (HR 2.50, 95% CI 1.69-3.71). There was not a significant interaction between EF and PASP (p=0.079) or diastolic function and PASP (p=0.201).
Figure 3. Survival from CV death by PASP tertiles among HF patients.
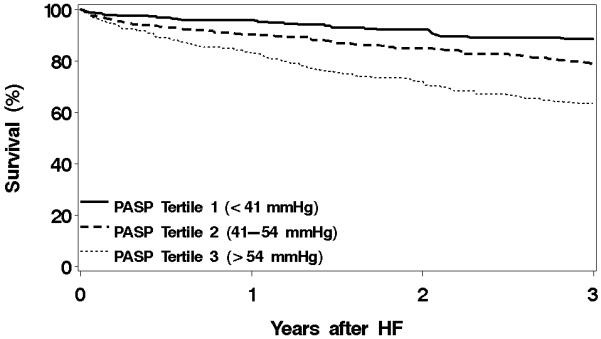
Survival from cardiovascular (CV) death by PASP tertiles among HF patients. N=975. p<0.001.
Other abbreviations as in Figure 1.
Figure 4. The association between PASP tertiles and CV mortality.
The unadjusted and adjusted hazard ratios for risk of CV mortality using Cox proportional hazards regression. Adjusted*=adjusted for age and sex; Adjusted**=adjusted for age, sex incident HF status, comorbidity index, anemia, EF, diastolic function and COPD. Other abbreviations as in Figures 1, 2 and 3.
Impact of PASP on Risk Prediction
The addition of PASP to a prognostic model including age, sex, incident HF status, comorbidity index, anemia, EF, diastolic function and COPD resulted in an increase in the c-statistic from 0.704 to 0.742 (p=0.007), an IDI gain of 4.2% (p<0.001), and an NRI of 14.1% (p=0.002) (Table 3), indicating that PASP offered additional value in predicting 1-year all-cause mortality over traditional prognostic factors. Similar results were found when predicting 1-year CV mortality. After the addition of PASP, the c-statistic increased from 0.720 to 0.765 (p=0.020), there was an IDI gain of 3.6% (p<0.001), and an NRI of 13.5% (p=0.025) (Table 4).
Table 3. Reclassification of participants by 1-year all-cause mortality using model with PASP.
| Model with Established Risk Factors and PASP | |||
|---|---|---|---|
| Model with Established Risk Factors* |
<13% Risk | 13% to < 23% Risk | ≥23% Risk |
| Participants dead at 1 year (n=190) | |||
| <13% Risk | 14 | 8 | 3 |
| 13% to 23% Risk | 8 | 22 | 25 |
| ≥23% Risk | 5 | 14 | 91 |
| Participants alive at 1 year (n=781) | |||
| <13% Risk | 247 | 36 | 3 |
| 13% to 23% Risk | 103 | 121 | 47 |
| ≥23% Risk | 10 | 46 | 168 |
Table 4. Reclassification of participants by 1-year CV mortality using model with PASP.
| Model with Established Risk Factors and PASP | |||
|---|---|---|---|
| Model with Established Risk Factors* |
<6% Risk | 6% to <12% Risk | ≥12% Risk |
| Participants dead at 1 year (n=90) | |||
| <6% Risk | 6 | 0 | 2 |
| 6% to 13% Risk | 7 | 9 | 12 |
| ≥13% Risk | 1 | 4 | 49 |
| Participants alive at 1 year (n=781) | |||
| <6% Risk | 256 | 34 | 2 |
| 6% to 13% Risk | 104 | 94 | 52 |
| ≥13% Risk | 10 | 62 | 167 |
Sensitivity Analyses
Several ancillary analyses were conducted to assess the robustness of our results.
The distribution of PASP was similar when restricted to incident cases of HF, patients meeting Framingham criteria or to patients presenting in the outpatient setting.
Analyses in the subset of patients without COPD (n=756, 72%) yielded similar results for all-cause and CV death, as did analyses in the subset of patients without atrial fibrillation (n=669, 64%). Furthermore, results were similar when analyses were restricted to the subset of patients with an EF less than 45% (n=424, 40%).
Adjustment for BMI, hypertension, hyperlipidemia, atrial fibrillation, NYHA class, smoking status and estimated glomerular filtration rate yielded similar results, as did adjustment for BNP. Removing diastolic function from the final model and adjusting for E/e’ ratio yielded similar results, as did further adjustment for BNP and left atrial volume.
Survival in patients without measurable PASP (9%) was not significantly different from those with measurable PASP (log-rank p=0.997).
Discussion
The present data indicate that pulmonary pressures can be readily assessed by Doppler echocardiography among patients with HF in the community and that pulmonary hypertension is overwhelmingly present in this setting. PASP was a strong and independent predictor of all cause death and CV death independently of other known predictors, including diastolic function measures and BNP. Further, PASP improved risk prediction over established risk factors as assessed using novel risk prediction methodology.
Increased pulmonary pressures in HF
While PASP increases with age (34) and patients with HF are mostly elderly, PASP is higher in HF than in the general population (34). The upper limit of normal for PASP is commonly defined as 35 mmHg, while varying with age and BMI (35). Published studies used varying definitions of PH, which compromises our ability to compare even informally across studies and with our data (2,8,36,37). However, among studies which used similar definitions (3,9), the prevalence of PH ranged from 46-83%. The corresponding populations were, however, heterogeneous and selected populations (Table 5). In the present cohort, when PH was defined as PASP greater than 35 mmHg, PH was prevalent in the vast majority of subjects (79%).
Table 5. Selected studies reporting on PASP in the literature 2000-2010.
| Author | N | Year | Design | HF definition |
EF | Percent with measured PASP |
|---|---|---|---|---|---|---|
| Damy (9) | 1380 | 2001- 2008 |
Consecutive referrals to HF clinic |
Clinical | 26% preserved EF (>45%) 74% reduced EF |
26% of patients with LVSD 40% without LVSD |
| Adhyapak (36) |
147 | 2004- 2007 |
Consecutive HF patients with echocardiography series |
Framingham criteria |
Mean EF=39% |
100% |
| Khush (37) | 171 | 2000- 2003 |
Sub-study of ESCAPE trial |
Clinical | Only EF≤30% |
100% |
| Kjaergaard (7) |
1022 | 2001- 2004 |
Sub-study of patients screened for trial (ECHOS) |
Clinical | 24% preserved EF (>50%) 71% reduced EF |
38% |
| Grigioni (8) |
196 | 1996- 2003 |
Echocardiographic series |
Clinical | Mean EF=27% |
100% |
| Ghio (2) | 377 | 1992- 1998 |
Consecutive patients referred for HF management |
Clinical | only EF<35% |
100% |
| Lam (3) | 244 | 2003- 2005 |
Community patients with HF |
Framingham criteria |
only EF≥50% |
83% |
| Shalaby (11) |
270 | 2004- 2005 |
Echocardiographic series of patients with HF undergoing CRT |
Clinical | Not measured |
79% |
In addition, EF was similar across the tertiles of PASP and the effect of PH on outcome was similar both in patients with reduced and preserved EF. Thus PH was not associated with the severity of systolic dysfunction. Similar to previous reports (38), in the present study PH was associated with LA volume index, diastolic function grade, and left ventricular filling pressures (E/e’), confirming that patients included had type II PH according to WHO classification, thus secondary to left heart disease and due chiefly to elevation in left sided pressures. Sensitivity analyses by excluding patients with COPD showed the results were unchanged and confirm these findings.
PH in HF has been postulated to be the result of the combination of the passive effect of elevated left ventricular end diastolic pressure backward on the pulmonary venous circulation and an active vasoreactive process of vasoconstriction and pulmonary arterial remodeling (3,4). Pathology studies have shown remodeling changes in the elastic fibers of the pulmonary arterial wall, intimal fibrosis, and medial hypertrophy of pulmonary muscular arteries, with changes that are similar to or greater than those seen in idiopathic pulmonary arterial hypertension (39).
In the present study, PASP remained a strong predictor of death and CV death even after adjusting for diastolic function or E/e’. The statistical independence between diastolic function and PASP supports the hypothesis that, beyond the effect of post-capillary increased venous congestion, there is a superimposed active pre-capillary component of PH and that this plays a role in prognosis. Therefore, PH appears to be not only a marker of worse HF, but may have itself a direct deleterious effect.
Pulmonary pressures and outcomes
While there is substantial data on pulmonary pressures in idiopathic pulmonary arterial hypertension which is less common (5,6), much less is known about the more common PH in HF (6). Indeed, Table 5 summarizes selected published studies, which consist chiefly of convenience samples of patients referred to outpatient or inpatient clinics (2,9), to diagnostic testing such as echocardiography (8,11,36) or were subanalyses of clinical trials (7,37). These studies are thus subject to various degrees of selection bias, which hinders the inference that can be drawn from them. In addition, most published studies were limited to patients with severe HF (8,37) or to patients with reduced (2,8,37) or preserved EF (3) and often did not include the whole spectrum of HF. Indeed, the only community study on PH in HF, conducted by our group, was limited to subjects with preserved EF (3).
The present data address the aforementioned gaps in knowledge, reflect the comprehensive experience of a community and pertain to the complete spectrum of HF including in and out-patients, systolic and diastolic HF as well as incident and prevalent cases. In this setting of high clinical relevance, PH is a strong independent predictor of outcomes as there is a graded association between the severity of PH and death in a large community cohort, irrespective of whether the patient was admitted to the hospital or evaluated as an outpatient, in primary or in subspecialty care. The novel risk prediction methodology used herein provides information on the value of PH for risk discrimination and reclassification, two measures of the practical value of a risk indicator (33). As PASP is readily available from echocardiographic studies, which are recommended by professional guidelines for the evaluation of heart failure (40), it can be obtained with no additional burden to the patient and no additional cost to provide important incremental prognostic information.
Limitations and strengths-Clinical implications
Potential limitations should be acknowledged to facilitate the interpretation of the results. We did not account for right ventricular size and function as this information was not universally available. The inferior vena cava size and collapse were used to estimate right atrial pressure in a semiquantitative method, and right atrial pressure was added to the transtricuspid gradient (41). The reproducibility and reliability of Doppler in measuring PASP is lower than right heart catheterization (23); however this study could have not been performed using invasive methods. We recognize that right heart catheterization is the standard to accurately diagnose PH and determine its severity as well as impact on RV function. However Doppler estimates of PASP have been shown to have adequate correlation with invasive measures (42), therefore Doppler echocardiography is now considered the reference screening method to detect PH (6).
PASP could not be measured in 9% of the patients, which is much lower than what is usually reported (7,9). However, survival in patients without measurable PASP was not significantly different from those with measurable PASP.
Strengths of this study include the community-based approach, which enhances its external validity, and the novel case finding method, which enables rapid identification of all cases of HF (12). We captured the complete spectrum of HF by including incident and prevalent systolic and diastolic HF cases indentified during both in- and outpatient visits. Further, we relied on rigorous validated Doppler echocardiography techniques applied promptly after HF diagnosis.
In aggregate, these data support the concept that PH may be a central determinant in the outcome of HF and may, therefore, represent a potential therapeutic target. Further studies are warranted to test this hypothesis.
Conclusion
In a large community-based prospective cohort of subjects with HF, pulmonary pressures can be readily estimated by Doppler echocardiography. Pulmonary hypertension is a strong and independent predictor of mortality among patients with HF and provides incremental and clinically relevant prognostic information independently of known predictors of outcomes.
Acknowledgments
We thank Ellen Koepsell, RN, Mayo Clinic and Kay Traverse, RN, Mayo Clinic for their assistance with subject enrollment and data collection, and Kristie Shorter, Mayo Clinic for secretarial assistance. We thank the study sonographers: Jo-Ellen Ehrsam, RDCS, Mayo Clinic, Tammy Green, RDCS, Mayo Clinic, Mary E. Hagen, RN, RDCS, Mayo Clinic.
Supported by grants from the National Institutes of Health (R01 HL59205, R01 HL72435) and the Rochester Epidemiology Project from the National Institute on Aging (R01 AG034676).
Abbreviations List
- PH
pulmonary hypertension
- HF
heart failure
- EF
ejection fraction
- COPD
chronic obstructive pulmonary disease
- PASP
pulmonary artery systolic pressure
- IDI
integrated discrimination improvement
- NRI
net reclassification improvement
- BNP
brain natriuretic peptide
- BMI
body mass index
- MI
myocardial infarction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
These individuals were not compensated for their contributions.
References
- 1.Abramson SV, Burke JF, Kelly JJ, Jr., et al. Pulmonary hypertension predicts mortality and morbidity in patients with dilated cardiomyopathy. Ann Intern Med. 1992;116:888–95. doi: 10.7326/0003-4819-116-11-888. [DOI] [PubMed] [Google Scholar]
- 2.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–8. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 3.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–26. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guglin M, Khan H. Pulmonary hypertension in heart failure. J Card Fail. 2010;16:461–74. doi: 10.1016/j.cardfail.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Hoeper MM, Barbera JA, Channick RN, et al. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol. 2009;54:S85–96. doi: 10.1016/j.jacc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 7.Kjaergaard J, Akkan D, Iversen KK, et al. Prognostic importance of pulmonary hypertension in patients with heart failure. Am J Cardiol. 2007;99:1146–50. doi: 10.1016/j.amjcard.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 8.Grigioni F, Potena L, Galie N, et al. Prognostic implications of serial assessments of pulmonary hypertension in severe chronic heart failure. J Heart Lung Transplant. 2006;25:1241–6. doi: 10.1016/j.healun.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Damy T, Goode KM, Kallvikbacka-Bennett A, et al. Determinants and prognostic value of pulmonary arterial pressure in patients with chronic heart failure. Eur Heart J. 2010;31:2280–90. doi: 10.1093/eurheartj/ehq245. [DOI] [PubMed] [Google Scholar]
- 10.Butler J, Stankewicz MA, Wu J, et al. Pre-transplant reversible pulmonary hypertension predicts higher risk for mortality after cardiac transplantation. J Heart Lung Transplant. 2005;24:170–7. doi: 10.1016/j.healun.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 11.Shalaby A, Voigt A, El-Saed A, Saba S. Usefulness of pulmonary artery pressure by echocardiography to predict outcome in patients receiving cardiac resynchronization therapy heart failure. Am J Cardiol. 2008;101:238–41. doi: 10.1016/j.amjcard.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 12.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ., 3rd. History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.Pakhomov SV, Buntrock J, Chute CG. Prospective recruitment of patients with congestive heart failure using an ad-hoc binary classifier. J Biomed Inform. 2005;38:145–53. doi: 10.1016/j.jbi.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. Journal of the American College of Cardiology. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 16.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Quinones MA, Waggoner AD, Reduto LA, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–53. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 19.Yturralde RF, Gaasch WH. Diagnostic criteria for diastolic heart failure. Prog Cardiovasc Dis. 2005;47:314–9. doi: 10.1016/j.pcad.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Ommen SR, Nishimura RA, Hurrell DG, Klarich KW. Assessment of right atrial pressure with 2-dimensional and Doppler echocardiography: a simultaneous catheterization and echocardiographic study. Mayo Clin Proc. 2000;75:24–9. doi: 10.4065/75.1.24. [DOI] [PubMed] [Google Scholar]
- 21.Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988–93. doi: 10.1378/chest.10-1269. [DOI] [PubMed] [Google Scholar]
- 22.Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167:735–40. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- 23.McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 24.Redfield MM, Jacobsen SJ, Burnett JC, Jr., Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 25.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 26.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 27.American Diabetes Association clinical practice recommendations 1997. Diabetes Care. 1997;20(Suppl 1):S1–70. [PubMed] [Google Scholar]
- 28.Izaks GJ, Westendorp RG, Knook DL. The definition of anemia in older persons. JAMA. 1999;281:1714–7. doi: 10.1001/jama.281.18.1714. [DOI] [PubMed] [Google Scholar]
- 29.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–33. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 32.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 33.Pencina MJ, D’Agostino RB, Sr., D’Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 34.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119:2663–70. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104:2797–802. doi: 10.1161/hc4801.100076. [DOI] [PubMed] [Google Scholar]
- 36.Adhyapak SM. Effect of right ventricular function and pulmonary pressures on heart failure prognosis. Prev Cardiol. 2010;13:72–7. doi: 10.1111/j.1751-7141.2009.00053.x. [DOI] [PubMed] [Google Scholar]
- 37.Khush KK, Tasissa G, Butler J, McGlothlin D, De Marco T. Effect of pulmonary hypertension on clinical outcomes in advanced heart failure: analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) database. Am Heart J. 2009;157:1026–34. doi: 10.1016/j.ahj.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Neuman Y, Kotliroff A, Bental T, Siegel RJ, David D, Lishner M. Pulmonary artery pressure and diastolic dysfunction in normal left ventricular systolic function. Int J Cardiol. 2008;127:174–8. doi: 10.1016/j.ijcard.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Delgado JF, Conde E, Sanchez V, et al. Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. Eur J Heart Fail. 2005;7:1011–6. doi: 10.1016/j.ejheart.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 40.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 41.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of EchocardiographyEndorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Capomolla S, Febo O, Guazzotti G, et al. Invasive and non-invasive determinants of pulmonary hypertension in patients with chronic heart failure. J Heart Lung Transplant. 2000;19:426–38. doi: 10.1016/s1053-2498(00)00084-x. [DOI] [PubMed] [Google Scholar]