Abstract
More intensive and/or frequent hemodialysis may provide clinical benefits to patients with end-stage renal disease; however, these dialysis treatments are more convenient to the patients if provided in their homes. Here we created a standardized model, based on a systematic review of available costing literature, to determine the economic viability of providing hemodialysis in the home that arrays costs and common approaches for assessing direct medical and nonmedical costs. Our model was based on data from Australia, Canada, and the United Kingdom. The first year start-up costs for all hemodialysis modalities were higher than in subsequent years with modeled costs for conventional home hemodialysis lower than in-center hemodialysis in subsequent years. Modeled costs for frequent home hemodialysis was higher than both in-center and conventional home hemodialysis in the United Kingdom, but lower than in-center hemodialysis and higher than conventional home hemodialysis in Australia and Canada in subsequent years. The higher costs of frequent compared to conventional home hemodialysis were because of higher consumable usage due to dialysis frequency. Thus, our findings reinforce the conclusions of previous studies showing that home-based conventional and more frequent hemodialysis may provide clinical benefit at reasonable costs.
Keywords: conventional in-center hemodialysis, costing study, end-stage renal disease, home hemodialysis, modeling, nocturnal home hemodialysis
There is a growing body of literature demonstrating that longer and/or more frequent hemodialysis provides substantially improved clinical, biochemical, and health outcomes benefits for end-stage renal disease patients over conventional hemodialysis (dialysis performed for 4 h per day, 3 times per week).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 The quality-of-life-related benefits of providing more intense hemodialysis to patients in their own home compared with in-center hemodialysis have been documented.1, 5, 7
Numerous studies have reported that conventional and more frequent home hemodialysis are less costly than conventional in-center hemodialysis.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 Existing published studies describing costs of home hemodialysis have been written from the payer, patient, and government perspectives. These economic studies were conducted in a variety of industrialized nations, with various assumptions, under an array of funding regimes. This diversity creates difficulty in generalizing the findings because of the unique characteristics and funding structures of individual programs. The lack of consistency within the published literature could help explain some of the substantial variability in the uptake of home hemodialysis.
The intent of this paper was to apply lessons from an analysis of original costing studies comparing in-center hemodialysis with home hemodialysis in developing a cost model from the payer's perspective. Owing to the heterogeneity of inputs used within the published analyses, the cost model is envisioned to provide payers and decision makers with a comprehensive, robust tool to accurately and transparently capture the cost of home hemodialysis services. This costing tool will aid in setting informed, evidence-based reimbursement policies to expand access to home hemodialysis in general and to more intensive home hemodialysis specifically.
RESULTS
Our comprehensive base case costing model is presented in Table 1, separated into perspectives from Australian, Canadian, and the UK health-care systems. Total costs for each modality were relatively consistent in year 1. From year 2 onward, conventional home hemodialysis is less expensive than in-center hemodialysis. Specifically, the model predicts that, over time and depending on location, conventional home dialysis would save payers between $7612 and $12,403 over the first year of conventional in-center hemodialysis. The model predicts that frequent home hemodialysis, with its increased costs of consumables and materials, would cost UK payers $4408 in subsequent years. However, frequent home hemodialysis would save Canadian payers $3411 and Australian payers $4036 in subsequent years compared with first year in-center hemodialysis costs.
Table 1. Cost model (all figures presented in 2010 US dollars).
|
Canada |
Australia |
United Kingdom |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Year 1 |
Subsequent years |
Year 1 |
Subsequent years |
Year 1 |
Subsequent years |
|||||||||||||
| ICHD | CHHD | FHHD | ICHD | CHHD | FHHD | ICHD | CHHD | FHHD | ICHD | CHHD | FHHD | ICHD | CHHD | FHHD | ICHD | CHHD | FHHD | |
| Total costs | 44,801 | 42,462 | 51,453 | 44,461 | 32,398 | 41,389 | 52,614 | 49,174 | 57,527 | 52,274 | 40,225 | 48,578 | 45,374 | 46,218 | 57,898 | 45,034 | 37,762 | 49,442 |
| Patient evaluation/recruitment, training costs | 6862a | 6862a | 1864a | 1864a | 6588a | 6588a | 2159a | 2159a | 5285a | 5285a | 1690a | 1690a | ||||||
| Home preparation | 2500b | 2500b | 1954c | 1954c | 2295d | 2295d | ||||||||||||
| Machine costs | 1429b | 7200b | 7200b | 1429b | 7200b | 7200b | 1429b | 7200b | 7200b | 1429b | 7200b | 7200b | 1259d | 4398d | 4398d | 1259d | 4398d | 4398d |
| Pump | 525e | 525e | 525e | 525e | 525e | 525e | ||||||||||||
| Consumables and peripheral costs | 5510f | 8047g | 16,014g | 5510f | 8047g | 16,014g | 9966c | 9966c | 20,367c | 9966c | 9966c | 20,367c | 12,290d | 12,290d | 24,580d | 12,290d | 12,290d | 24,580d |
| Total allied health-care costs | 12,324a | 1503a | 1503a | 12,324a | 1503a | 1503a | 17,467c | 4108c | 4108c | 17,467c | 4108c | 4108c | 10,510d | 4920d | 4920d | 10,510d | 4920d | 4920d |
| Medical equipment | 390b | 2340b | 2340b | 50b | 300b | 300b | 390b | 2340b | 2340b | 50b | 300b | 300b | 390b | 2340b | 2340b | 50b | 300b | 300b |
| Renal medication costs (total) | 7312h | 5335g | 6970g | 7312h | 5335g | 6970g | 10,020c | 10,020c | 10,020c | 10,020c | 10,020c | 10,020c | 4,870d | 4870d | 4870d | 4870d | 4870d | 4870d |
| Dialysis-related laboratory costs | 1071f | 1565g | 1173g | 1071f | 1565g | 1173g | 1071f | 1565g | 1173g | 1071f | 1565g | 1173g | 1071f | 1565g | 1173g | 1071f | 1565g | 1173g |
| Costs of in-center runs | 1672i | 1672i | 1672i | 1672i | 1672i | 1672i | 1672i | 1672i | 2761d | 2761d | 2761d | 2761d | ||||||
| Facility costs | 10,624f | 10,624f | 5948c | 5948c | 8405d | 8405d | ||||||||||||
| Dialysis water and electricity costs | 2155f | 3592f | 2155f | 3592f | 478c | 478c | 478c | 478c | 2155f | 3592f | 2155f | 3592f | ||||||
| Travel costs to and from dialysis | 1613j | 1613j | 1795k | 1795k | 2051d | 57 d | 57 d | 2051d | 57d | 57d | ||||||||
| Hospitalization costs | 4529l | 2757l | 1102g | 4529l | 2757l | 1102g | 4529l | 2757l | 1102g | 4529l | 2757l | 1102g | 4529l | 2757l | 1102g | 4529l | 2757l | 1102g |
Abbreviations: CHHD, conventional home hemodialysis; FHHD, frequent home hemodialysis; ICHD, in-center hemodialysis.
Data on hours from Komenda et al.35 Costs calculated using country-specific wages.
Estimate based on literature and author experience—amortized over 8 years or annual rental.
See Agar et al.2
See Mowatt et al.22
Pump cost assumptions based on data for Abbott Lifecare 5000 Pump Infusion System.
See McFarlane et al.26
See Kroeker et al.21
See Lee et al.15
Assumption of 11 in-center runs per year at $152 per run, which is the Medicare reimbursement rate for such a run.
Assumes Canadian reimbursement rate of $0.53 per km at 10 km one way for 3 visits per week for 1 year (Canadian Revenue Agency).
Assumes Australian reimbursement rate of $0.63 per km at 10 km one way for 3 visits per week for 1 year (Australian Taxation Office).
See McFarlane et al.6
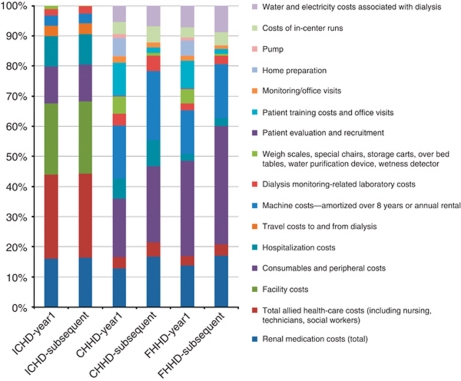
In-center hemodialysis costs are stable over time as the cost to provide hemodialysis in a center is conservatively assumed to stay relatively constant year after year. Renal medication, human resources, hospitalizations, consumables, and facility (overhead) costs are the primary cost drivers for in-center hemodialysis. Conventional home hemodialysis and frequent home hemodialysis costs in year 1 are greater than those in subsequent years, as costs of hemodialysis reduce in subsequent years after capital investments and training have been paid for. These conventional home and frequent hemodialysis costs are driven by renal medication, capital investment, patient training (primarily training nurse costs), machines, consumables (more for frequent home hemodialysis), home preparation, training, and hospitalization costs. To illustrate the impact of component costs, Figure 1 provides a summary of the cost drivers by modality for Canada.
Figure 1.
Component costs as a percentage of total costs, Canada. CHHD, conventional home hemodialysis; FHHD, frequent home hemodialysis; ICHD, in-center hemodialysis.
Sensitivity analysis
The sensitivity analysis demonstrated that changes to the major drivers of total cost, such as staffing costs and facility costs, can substantially impact the overall costs. For example, when evaluating the range of total costs while adjusting the total allied health-care costs to 25% of the original cost input, total costs for conventional home hemodialysis in Canada goes from being less than that for in-center hemodialysis to more than that in year 1. In comparison, when increasing the original cost input of total allied health-care costs by 25% in the United Kingdom in year 1, total costs for in-center hemodialysis move from being less than to more than total costs for conventional home hemodialysis. Across all component costs, there were four instances in year 1 (7.5%) and one instance in subsequent years (2.4%) where adjusting the component cost plus or minus 25% changed the total costs of a hemodialysis modality from being more than to less than another modality.
DISCUSSION
Derived from data in published cost studies, we have generated a transparent, logical costing model that includes both start-up and operating costs for conventional home hemodialysis and frequent home hemodialysis as compared with in-center hemodialysis. As anticipated, the results of our model are consistent with the findings of most costing studies to date, in that conventional home hemodialysis and frequent home hemodialysis are similar in cost to in-center hemodialysis in the first year (driven primarily by training costs) but can be less costly than in-center hemodialysis from the second year onward, depending on the frequency of dialysis.
The results of the model show that year 1 costs are largely a result of higher start-up costs, but lower human resource costs, for home hemodialysis submodalities as compared with in-center hemodialysis. In year 2 and beyond, conventional home hemodialysis is less expensive than in-center hemodialysis. Specifically, the model predicts that, over time and depending on location, conventional home dialysis would save payers between $7612 and $12,403 over the first year of conventional in-center hemodialysis. The model shows that frequent home hemodialysis, with its increased costs of consumables and materials, would cost UK payers $4,408 in subsequent years. However, frequent home hemodialysis would save Canadian payers $3411 and Australian payers $4036 in subsequent years compared with the first year in-center hemodialysis costs. Decreased staffing costs coupled with lower facility overhead and medication costs drove this cost differential. That is, there are initial training costs for home hemodialysis patients (and their family members). Once trained, there are minimal human resource costs associated with home hemodialysis modalities, whereas the human resource costs for in-center hemodialysis stay consistent over time as payers are responsible for the salaries of staff who provide dialysis.
A review of the published cost studies reveals that all three forms of home hemodialysis (conventional, nocturnal, and short daily home hemodialysis) may have economic advantages over in-center hemodialysis.5, 7, 14, 15, 16, 17, 18, 19 These studies also demonstrate the clinical advantages of more frequent intensive hemodialysis relative to less frequent hemodialysis while being cost neutral or cost saving to the payer.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
The most persuasive evidence (based upon study size, quality of study, and number of studies with consistent results) finds that costs for home hemodialysis are substantially lower than costs for in-center hemodialysis.1, 5, 7, 19, 26, 27 In addition, costs per quality-adjusted life year appear to be better for conventional home hemodialysis compared with in-center hemodialysis.28, 29, 30
These studies take numerous perspectives and include many different costing inputs. No study to date (to our knowledge) has attempted to summarize the findings of these studies to come to a comprehensive summary of the component cost inputs of conventional home hemodialysis or frequent home hemodialysis when compared with in-center hemodialysis.
Rates of home hemodialysis utilization vary greatly across the world. The number of patients on home hemodialysis has grown significantly over the past several years globally.31 There is considerable variation in reimbursement of hemodialysis expenses around the world. Canada and the United Kingdom have developed similar, although nonidentical, methods for reimbursing dialysis services. Canada's single-payer universal health-care system funds providers, hospitals, or provincial programs to provide all dialysis care. Care is often provided within a case rate payment: CDN $55,466 (US $45,094) per patient with end-stage renal disease.15 The UK's National Health Service funds dialysis care through a special commission on a countrywide basis through the use of health-related group payments for care. The health-related group for hemodialysis, LC01A, had an average payment per dialysis session of £153 for in-center hemodialysis and £83 for home hemodialysis in 2007, which translates to a yearly payment of £23,443 (US$37,393) and £18,270 (US$29,142) per year, respectively.32 The six Australian states are funded to provide dialysis through a mix of capitation and case payments.33 One state (Victoria) has added incentives of AUD$10,000/patient/year paid to renal services for each nocturnal home hemodialysis patient who is home installed, with out-of-pocket expenses for water, power, and cleaning reimbursed at AUD$1,200/year for each home hemodialysis patient.30
Given these varying payment systems for hemodialysis, payers may have difficulty synthesizing the published literature to determine what information is relevant to their context. Our costing model was developed to assist payers in understanding the component cost drivers of home versus in-center hemodialysis in more detail. Decision makers representing different localities or with costs not consistent with modeled data should adjust component costs accordingly if attempting to use the model to estimate costs in a specific local setting. This information can be used to inform coverage policy development, especially in reference to developing payment systems that encourage home hemodialysis options (conventional, nocturnal, and short daily home hemodialysis).
Our costing model has several limitations. As mentioned previously, our review of the existing costing literature yielded inconsistent evidence related to costs of conventional home, frequent home, and in-center hemodialysis between and within Australia, Canada, and the United Kingdom. Despite this, we feel that our model is transparent enough to allow for specific costs to be varied easily by decision makers in tailoring our analysis to almost any payer model. Because no one study captured all required cost inputs, the model used inputs from a variety of studies. As a result, heterogeneity exists within the methods used by the referenced cost studies. Our use of sensitivity analysis attempts to address this.
Costing data for home hemodialysis should be augmented by randomized control trial data. The National Institutes of Health (NIH)-sponsored Frequent Hemodialysis Network trial has recently published results on the clinical outcomes associated with more frequent in-center hemodialysis, although currently published data do not yet include a cost analysis.34, 35 In addition, cost-effectiveness analysis could be used to capture the clinical and cost advantages of home hemodialysis modalities (conventional, nocturnal, and short daily home hemodialysis) compared with in-center hemodialysis. Additional research and modeling in this area would help to systematically evaluate both clinical and cost variables in a variety of settings.
The costing model presented here uses a hybrid of existing costing data and modeled inputs for key cost drivers across three hemodialysis modalities in four countries with heterogeneous payer models. It can serve as a robust tool for payers and other decision makers to apply key cost drivers to make related decisions.
All home hemodialysis modalities have limitations and may not be the optimal solution for all patients. Improved access to dialysis delivery systems, with simpler operating procedures and portability, will allow more patients to undertake this modality. For those patients likely to receive kidney transplant within 1 year of dialysis, home hemodialysis likely does not represent an optimal approach, given the high start-up, home preparation, and training costs. However, the literature does reveal a large segment of dialysis patients for whom frequent home hemodialysis, via nocturnal home hemodialysis or short daily home hemodialysis, would be a beneficial approach. In many countries, utilization lags behind the evidence base.
Although the costs described within the model inform reimbursement decision making, they must be contextualized within the broader clinical literature. Reimbursement policy should consider these cost and clinical findings. Payers can take an active role in using reimbursement policy to encourage clinicians to use the most effective home hemodialysis approaches. This model provides a tool for payers to make a cost determination for their patient population; given the model's findings of two of the three locations incurring lower total costs after the first year of home hemodialysis, this could be an informative exercise for payers while potentially improving the quality of lives for patients.
MATERIALS AND METHODS
Three hemodialysis modalities were selected for cost modeling based on the number and quality of cost studies available. These are as follows: in-center hemodialysis, conventional home hemodialysis, and more frequent home hemodialysis. Frequent home hemodialysis is inclusive of nocturnal home hemodialysis (dialysis performed for 6–10 h per night for up to 7 nights per week) and short daily home hemodialysis (dialysis performed for 2 to 3 h per day for up to 7 days per week), which is based on the assumption that short daily home hemodialysis costs are similar to nocturnal home hemodialysis costs, although the authors recognize that small differences in resource utilization between the two modalities may exist. Short daily home hemodialysis costs were not specifically included in the model because of a lack of published, comprehensive cost data specific to that submodality available at the time of this analysis.
The current costing studies take different perspectives and were conducted under a variety of funding regimes. To provide a holistic view of costs across multiple countries, we constructed a standardized cost model from the payer/funder perspective using inputs from published costing studies. The purpose of the model is to provide payers and decision makers with a comprehensive, robust tool to accurately and transparently capture the cost of home hemodialysis services. This costing tool will aid in setting informed, evidence-based reimbursement policies to expand access to home hemodialysis in general and to more intensive home hemodialysis specifically.
The cost model is a transparent spreadsheet that summarizes component costs for in-center hemodialysis, conventional home hemodialysis, and frequent home hemodialysis within each of the three countries (Australia, Canada, and the United Kingdom). The three countries were selected for two reasons: first, over half of the available published cost data are related to them; and second, they represent distinct reimbursement systems that are informative to other similar country reimbursement systems. The United States was excluded from this analysis because of the unique funding system associated with Medicare's end-stage renal disease program and the limited US cost data specific to the different hemodialysis modalities.
The model was constructed from a payer's perspective and therefore includes component costs that are routinely reimbursed by payers (summarized in Table 2). Direct medical and well-documented direct nonmedical costs associated with dialysis (e.g., transportation to and from dialysis facilities) have been included. Indirect nonmedical costs (e.g., lost time from work and unpaid assistance from family members) are not included. Costs are considered in terms of start-up costs that would only be experienced in the first year of use, and then ongoing maintenance costs experienced in year 2 and beyond. As such, variables are segmented by one-time costs directly related to hemodialysis (e.g., dialysis training costs) and medical costs (e.g., consumable material costs, medications, professional reimbursement, and costs of emergency in-center hemodialysis runs) experienced in the first year of dialysis. Once these items were established, we added further line items that would be considered ‘standard' in a comprehensively reimbursed home hemodialysis program (e.g., wetness detectors, scales, nursing, and allied health costs for clinic visits).
Table 2. Characteristics of dialysis payment in each country studied based on specific cost variables (online).
| Canada41 | Australia42 | United Kingdom32 | ||
|---|---|---|---|---|
| Health system | Socialized | Socialized | ||
| Payment mechanism for dialysis | Case rate | Case rate | Case rate with payment by results program | |
| Direct costs | ||||
| Patient evaluation/recruitment, training costs | Within | Within or standard | Within | |
| Home preparation | NC | Min (via patient grant) | NC | |
| Machine costs—amortized over 8–10 years or annual rental | Standard | Within | Standard | |
| Pump | Standard | Within | Standard | |
| Consumables and peripheral costs | Standard | Within | Within | |
| Total allied health-care costs (including nursing, technicians, social workers) | Standard | Within | Standard | |
| Weigh scales, special chairs, storage carts, over bed tables, water purification device, wetness detector | Standard | Within | Standard | |
| Renal medication costs (total) | Standard | Within | Standard | |
| Dialysis monitoring-related laboratory costs | Standard | Within | Standard | |
| Hospitalization costs | Out of program | Out of program | Out of program | |
| Costs of in-center runs | Standard | Within | Standard | |
| Dialysis-related home utility costs | NC | Standard (from patient block grant) | Min | |
| Facility costs | Within | Within | Within | |
| Utility costs (water and electricity) | NC | Min (via patient grant) | Min | |
| Indirect cost variables | ||||
| Travel costs to and from dialysis | NC | Standard (from patient block grant) | Min | |
| Lost productivity | NC | NC | Min |
Abbreviations: Min, payer provides minimal benefit, potential out-of-pocket payment from patient; NC, not covered; Out of program, cost born by payer outside renal replacement therapy program; Standard, payer covers routine costs; Within, cost not separately reimbursed, but captured within global payment rate.
The model also provides a sum of all component costs per hemodialysis modality for year 1 and for subsequent years to derive a realistic cost picture that differentially accounts for start-up costs from ongoing costs experienced in subsequent years. It is recognized that the majority of start-up costs would be experienced in the first several months of a patient's dialysis care, but for the purposes of this model annual costs were used.
Published costing studies and government-sponsored report data were used to derive annual best cost estimates for each component cost on a dialysis modality-specific basis. Where available, country-specific data were utilized. When country-specific data were not available, we used published data from the Canadian setting. These data were collected from the Manitoba Renal Program at Seven Oaks General Hospital in Winnipeg, Canada, where one of the authors practices.36 Where data conflict existed (e.g., varying prices of dialysis machines), data inputs were selected from published studies prioritized by study, country perspective, and quality of the study. (Modifications were made based on the literature to reflect differences between the Canadian, Australian, and UK programs in which extrapolations were used.) Published hospitalization cost data were not differentiated by year of dialysis in the literature. We conservatively used an estimate for ‘year 1' costs for both ‘year 1' costs and ‘subsequent years' costs to keep a proxy for hospitalization costs in the model without influencing any one modality's results.
For each cost variable, costs were inflated to 2010 dollars using country-specific consumer price index and wage index from the year in the articles. To standardize costs across countries when country-specific data were not available, costs were then converted to US dollars using appropriate currency exchange rates.37, 38, 39, 40 (Although the use of purchasing power parity was considered, the authors ultimately chose this methodology as the costs are not intended to be compared across countries and the literature suggests that the CPI-based approach is an appropriate methodology for this type of analysis.)
Sensitivity analyses were conducted on all variables using a standard range of plus or minus 25%. The standard range was chosen because the source studies did not universally supply published sensitivity ranges. Each variable was initially analyzed to consider its impact on the base case cost analysis. Cost input values were then each varied plus or minus 25% to measure their impact on overall costs to determine the percentage of time that, across the plus or minus 25% range, the total costs of the hemodialysis modalities changed in relative rank order.
Acknowledgments
We acknowledge David R Walker, PhD, Paul M Just, PharmD, and Janeen DuChane, PhD, for their work in the literature review and methodology analysis of this study. This research was funded through an unrestricted grant by Baxter Healthcare Corporation, McGaw Park, IL.
All the authors declared no competing interests.
References
- Agar JWM. Nocturnal hemodialysis in Australia and New Zealand. Nephrology. 2005;10:222–230. doi: 10.1111/j.1440-1797.2005.00409.x. [DOI] [PubMed] [Google Scholar]
- Agar JWM, Knight RJ, Simmonds R, et al. Nocturnal haemodialysis: an Australian cost comparison with conventional satellite haemodialysis. Nephrology. 2005;10:557–570. doi: 10.1111/j.1440-1797.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- Chan CT, Harvey PJ, Picton P, et al. Short-term blood pressure, noradrenergic, and vascular effects of nocturnal home hemodialysis. Hypertension. 2003;42:925–931. doi: 10.1161/01.HYP.0000097605.35343.64. [DOI] [PubMed] [Google Scholar]
- Lacson E, Diaz-Buxo JA. Daily and nocturnal hemodialysis: how do they stack up. Am J Kidney Dis. 2001;38:225–239. doi: 10.1053/ajkd.2001.26079. [DOI] [PubMed] [Google Scholar]
- Lindsay RM, and the Daily/Nocturnal Dialysis Study Group The London, Ontario, Daily/Nocturnal Hemodialysis Study. Semin Dial. 2004;17:85–91. doi: 10.1111/j.0894-0959.2004.17202.x. [DOI] [PubMed] [Google Scholar]
- McFarlane PA, Pierratos A, Redelmeier DA. Cost savings of home nocturnal versus conventional in-center hemodialysis. Kidney Int. 2002;62:2216–2222. doi: 10.1046/j.1523-1755.2002.00678.x. [DOI] [PubMed] [Google Scholar]
- Mohr PE, Neumann PJ, Franco SJ, et al. The case for daily dialysis: its impact on costs and quality of life. Am J Kidney Dis. 2001;37:777–789. doi: 10.1016/s0272-6386(01)80127-x. [DOI] [PubMed] [Google Scholar]
- Shurraw S, Zimmerman D. Vascular access complications in daily dialysis: a systematic review of the literature. Minerva Urol Nefrol. 2005;57:151–163. [PubMed] [Google Scholar]
- Traeger J, Galland R, Delawari E, et al. Six years' experience with short daily hemodialysis: do the early improvements persist in the mid and long term. Hemodial Int. 2004;8:151–158. doi: 10.1111/j.1492-7535.2004.01089.x. [DOI] [PubMed] [Google Scholar]
- Williams AW, O'Sullivan DA, McCarthy JT. Slow nocturnal and short daily hemodialysis: a comparison. Semin Dial. 1999;12:431–439. [Google Scholar]
- Culleton BF, Walsh M, Klarenbach SW, et al. Effect of frequent nocturnal hemodialysis versus conventional hemodialysis on left ventricular mass and quality of life. JAMA. 2007;298:1291–1299. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- Johansen KL, Zhang R, Huang Y, et al. Survival and hospitalization among patients using nocturnal and short daily compared to conventional hemodialysis: a USRDS study. Kidney Int. 2009;76:1040–1048. doi: 10.1038/ki.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M, Culleton B, Tonelli M, et al. A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health-related quality of life. Kidney Int. 2005;67:1500–1508. doi: 10.1111/j.1523-1755.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- De Wit GA, Ramsteijn PG, de Charro FT. Economic evaluation of end stage renal disease treatment. Health Policy. 1998;44:215–232. doi: 10.1016/s0168-8510(98)00017-7. [DOI] [PubMed] [Google Scholar]
- Lee H, Manns B, Taub K, et al. Cost analysis of ongoing care of patients with end-stage renal disease: the impact of dialysis modality and dialysis access. Am J Kidney Dis. 2002;40:611–622. doi: 10.1053/ajkd.2002.34924. [DOI] [PubMed] [Google Scholar]
- Maiorca R, Sandrini S, Cancarini GC, et al. Integration of peritoneal dialysis and transplantation programs. Perit Dial Int. 1997;17 (Suppl 2:S170–S174. [PubMed] [Google Scholar]
- Moore R, Marriott N. Cost and price in the NHS: the importance of monetary value in the decision-making framework--the case of purchasing renal replacement therapy. Health Serv Manage Res. 1999;12:1–14. doi: 10.1177/095148489901200101. [DOI] [PubMed] [Google Scholar]
- National Institute for Clinical Excellence Final appraisal determination: the clinical and cost effectiveness of home compared with hospital haemodialysis for patients with end-stage renal failureAvailable at http://guidance.nice.org.uk/TA48/Guidance/pdf/English .
- Peeters P, Rublee D, Just PM, et al. Analysis and interpretation of cost data in dialysis: review of Western European literature. Health Policy. 2000;54:209–227. doi: 10.1016/s0168-8510(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Haycox A, Duggan AK. Optimising the future development of dialysis services in the UK. Br J Med Econ. 1996;10:27–35. [Google Scholar]
- Kroeker A, Clark W, Heidenheim P, et al. An operating cost comparison between conventional and home quotidian hemodialysis. Am J Kidney Dis. 2003;42:S49–S55. doi: 10.1016/s0272-6386(03)00538-9. [DOI] [PubMed] [Google Scholar]
- Mowatt G, Vale L, Perez J, et al. Systematic review of the effectiveness and cost-effectiveness of home versus hospital or satellite unit hemodialysis for people with end stage renal failure NHS R&D HTA Programme – NICE 2004http://www.nice.org.uk/nicemedia/live/11471/32447/32447.pdf . [DOI] [PubMed]
- Roderick P, Nicholson T, Armitage A, et al. An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales. Health Technol Assess. 2005;9:1–178. doi: 10.3310/hta9240. [DOI] [PubMed] [Google Scholar]
- Lockridge RS, McKinney JK. Is HCFA's reimbursement policy controlling quality of care for end-stage renal disease patients. ASAIO J. 2001;47:466–468. doi: 10.1097/00002480-200109000-00012. [DOI] [PubMed] [Google Scholar]
- Lockridge RS, Anderson HK, Coffey LT, et al. Nightly home hemodialysis in Lynchburg, Virginia: economic and logistic considerations. Semin Dial. 1999;12:440–447. [Google Scholar]
- McFarlane PA, Bayoumi AM, Pierratos A, et al. The quality of life and cost utility of home nocturnal and conventional in-center hemodialysis. Kidney Int. 2003;64:1004–1011. doi: 10.1046/j.1523-1755.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- McPhatter LL, Lockridge RS.Nutritional advantages of nightly home hemodialysis Nephrol News Issues 2002163134–36. [PubMed] [Google Scholar]
- MacLeod A, Grant A, Donaldson C, et al. Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews. Health Technol Assess. 1998;2:1–166. [PubMed] [Google Scholar]
- Winkelmayer WC, Weinstein MC, Mittleman MA, et al. Health economic evaluations: the special case of end-stage renal disease treatment. Med Decis Making. 2002;22:417–430. doi: 10.1177/027298902236927. [DOI] [PubMed] [Google Scholar]
- Croxson BE, Ashton T. A cost effectiveness analysis of the treatment of end stage renal failure. N Z Med J. 1990;103:171–174. [PubMed] [Google Scholar]
- MacGregor MS, Agar JW, Blagg CR. Home haemodialysis-international trends and variation. Nephrol Dial Transplant. 2006;21:1934–1945. doi: 10.1093/ndt/gfl093. [DOI] [PubMed] [Google Scholar]
- Newton C, Strain H.Payment by results for kidney dialysis project group UK Department of HealthAccessed online on 05 February 2010 at http://www.dh.gov.uk/publications .
- Healthcare Management Advisors for the Department of Human Services Renal dialysis costing and funding review 2006. Accessed online at http://docs.health.vic.gov.au/docs/doc/Renal-Dialysis-Costing-and-Funding-Review-Report-(draft)---December-2006-(consultants-report) .
- Suri RS, Garg AX, Chertow GM, et al. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int. 2007;71:349–359. doi: 10.1038/sj.ki.5002032. [DOI] [PubMed] [Google Scholar]
- Komenda P, Copland M, Makwana J, et al. The cost of starting and maintaining a large home hemodialysis program. Kidney Int. 2010;77:1039–1045. doi: 10.1038/ki.2010.37. [DOI] [PubMed] [Google Scholar]
- Wordsworth S, Ludbrook A. Comparing costing results in across country economic evaluations: the use of technology specific purchasing power parities. Health Econ. 2005;14:93–99. doi: 10.1002/hec.913. [DOI] [PubMed] [Google Scholar]
- Shi L, Hodges M, Drummond M, et al. Good research practices for measuring drug costs in cost-effectiveness analyses: an international perspective. Value Health. 2010;13:28–33. doi: 10.1111/j.1524-4733.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- Consumer Price Index, health and personal care, by province (Canada) Statistics CanadaAccessed via the internet on 3 March 2010 at http://www40.statcan.gc.ca/l01/cst01/econ161a-eng.htm .
- Consumer Price Index, Australia, Dec 2009 Australian Bureau of StatisticsAccessed via the internet on 3 March 2010 at http://www.abs.gov.au/ausstats/abs@.nsf/mf/6401.0 .
- Inflation, United Kingdom Office for National StatisticsAccessed via the internet on 3 March 2010 at http://www.ons.gov.uk/ons/rel/cpi/consumer-price-indices/december-2010/index.html .
- Diaz-Buxo JA, Schlaeper C, VanValkenburgh D. Evolution of home hemodialysis monitoring systems. Hemodial Int. 2003;7:353–355. doi: 10.1046/j.1492-7535.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- Harris A. The organization and funding of treatment of end-stage renal disease in Australia. Int J Health Care Finance Econ. 2007;7:113–132. doi: 10.1007/s10754-007-9018-7. [DOI] [PubMed] [Google Scholar]