Abstract
Background
Current guidelines recommend using aspirin, clopidogrel, beta-blockers, statins, and angiotensin converting enzyme (ACE) inhibitors after acute myocardial infarction (AMI). Although there is evidence that patients often stop taking these medications prematurely, long-term data reflecting the actual reality of care are lacking. We studied prescription prevalence and treatment persistence of secondary prevention in patients who had an AMI by analyzing relevant claims data from a German sickness fund, the Techniker Krankenkasse (these data are not necessarily representative of the entire German population).
Methods
Insurees who were discharged from the hospital between 2001 and 2006 with AMI as their main discharge diagnosis were classified as users or non-users of each of the types of drug listed above on the basis of the prescriptions that they obtained in the first 90 days after they left the hospital. Treatment persistence was statistically assessed with survival analysis. Switches from one drug class to another were not examined.
Results
Of 30 028 AMI patients, 82% were initially prescribed a beta-blocker, 73% a statin, 69% an ACE inhibitor, 66% aspirin (without self-medication), and 61% clopidogrel. Five years after discharge, 10% of the patients for whom aspirin was initially prescribed were still taking it; the corresponding figures for the other drug classes were 17% for statins, 31% for ACE inhibitors, and 36% for beta-blockers. The greatest drop in treatment persistence occurred approximately one year after the AMI.
Conclusion
Treatment persistence with recommended medication after AMI is still in need of improvement. Patient education should start as soon as possible after infarction, because the greatest drops in medication use appear to occur within one year after AMI.
Cardiovascular diseases are the leading cause of death in Germany (1). In 2009, 356 462 people died from cardiovascular diseases; 56 226 of these deaths were due to myocardial infarction. Numerous randomized, controlled studies—and the meta-analyses based on them—can demonstrate that reinfarction risk and patient mortality after myocardial infarction can be considerably reduced by lifestyle changes and by using HMG-CoA reductase inhibitors (statins), angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, aspirin, and clopidogrel (e1– e7). Accordingly, recommendations for the long-term use of these drug classes in post myocardial infarction patients have been included in the clinical guidelines (2–6; current versions: e8–e13). Despite this strong supportive evidence, studies have revealed discrepancies between the recommended therapies and the actual health care provided (7– 12). This is due not only to medical undertreatment following the inpatient stay but also to the problem of high discontinuation rates (13, 14).
Until now, the health care situation in Germany has been only evaluated in regional studies with small patient cohorts, over a maximum time span of 12 months (7, 8, 11, 12). Our large-scale, nationwide study analyzed patients discharged from hospital between 2001 and 2006 with a discharge diagnosis of acute myocardial infarction (AMI). We aimed to determine whether their drug therapy was consistent with that recommended by the guidelines, and how many continued the initial therapy for the five years following their hospital discharge.
Methods
Study population
Individuals were included in the study if they had at least one inpatient stay from January 1, 2001, to September 30, 2006, with a main discharge diagnosis of AMI (ICD-10 I21 or ICD-9 410), and if they had been continuously covered by the sickness fund Techniker Krankenkasse (TK) for at least one year prior to, and 90 days following, the hospital stay. Patients were excluded from the study if:
no information was available for their insurance number (pseudonymized), age, or sex;
the main discharge diagnosis additionally contained an „A“ (signifying „exclusion of“), „V“ („suspected of“), or „Z“ („state afterward“);
the hospital stay was shorter than three days;
they had had an infarction in the year prior to, or in the 90 days following, the primary infarction.
Patients were also excluded if, within the 90 days following their hospital discharge, they could not be observed in an outpatient setting for at least one day due to additional hospital stays.
Data source and software analysis
The TK claims data from January 1, 2000, to February 28, 2007, were used as the data source. All analyses were performed with the software SAS (version 8.2). P values of <0.05 were considered to be statistically significant.
Prescription prevalence
Study participants were classified according to their prescriptions (for aspirin, clopidogrel, beta-blockers, ACE inhibitors, or statins) during the 90 days following discharge as users or non-users of the relevant drug classes. The active components of the drugs were classified according to the Anatomical Therapeutic Chemical (ATC) classification code (Table 1).
Table 1. ATC codes for medical substance identification.
| ATC code | Name |
| B01AC06, N02BA01 | Acetylsalicylic acid (aspirin) |
| B01AC04 | Clopidogrel |
| C07 | Beta blockers |
| C08 | Calcium channel blockers |
| C09A, C09B | ACE inhibitors |
| C10AA, C10BA, C10BX | HMG-CoA reductase inhibitors (statins) |
ACE, angiotensin converting enzyme;
ATC, Anatomical Therapuetic Chemical Classification
Treatment persistence
The treatment persistence group consisted of patients who had adhered to a continuous drug therapy during the observation period. A drug therapy was considered to be continuous when, starting from the 90 day observation period following the discharge date, it was recorded that the prescription for the assigned drug class had been refilled within a time frame of „prescription end date + 90 days“. If this was not the case, the therapy was considered to have been discontinued (discontinuation criterion).
Patients were observed until the discontinuation criterion had been fulfilled, their insurance coverage was ended, the end of observation period was reached (on December 31, 2006), or reinfarction occurred (in this case, the first such occurrence was considered; patients underwent censored observation). Treatment persistence during this time was determined for each drug group with survival time analyses, using the Kaplan-Meier method (15).
Episodes of drug coverage and dosage determination for the prescribed drug
The length of an episode of drug coverage, measured in days, was determined from the number of prescribed daily does contained in a package. The an episode of drug coverage was calculated from the dispensing date as follows:
End of the episode of drug coverage = date of dispense + length of the episode of drug coverage – one day.
To determine the end of an episode of drug coverage, it was essential to know the prescribed daily dose (PDD). Although the PDD was not in the claims data, it is possible to determine this from the copies of all filled prescriptions that are kept by the health insurance companies—as the dosages prescribed by the physicians are often visible on these, the PDD can sometimes be identified. For this, the first prescriptions that were written following the hospital discharge were selected for each patient and drug class. From each drug class, 2% of prescriptions were randomly selected, and the corresponding written prescription copies were anonymously reviewed to determine the dosage recommendations. The dosages recommended by the physicians were compared with:
the corresponding defined daily dose (DDD);
the dosages (lowest and highest) stated in the product characteristics summary;
an assumed daily dose of one tablet.
We also analyzed which of these three values came the closest to the PDD. This was then used to determine the effective range.
Results
Study population
From about 6.7 million individuals who were insured by TK, 30 028 had had an AMI from 2001 to 2006 and fulfilled the study criteria (Table 2). The average age was 63 ± 12 years (with a mean of 63 years), and 81% were men. The average age of the women in this group was higher than that of the men (68 ± 13 years vs. 62 ± 11 years). The incidence of reinfarction during the follow-up period was 4%.
Table 2. Baseline characteristics of the study population.
| Age group | Sex | n | % |
| <50 | Male | 3164 | 88.21 |
| Female | 423 | 11.79 | |
| Total | 3587 | 100 | |
| 50–64 | Male | 8343 | 86.72 |
| Female | 1278 | 13.28 | |
| Total | 9621 | 100 | |
| 65–74 | Male | 5654 | 80.82 |
| Female | 1342 | 19.18 | |
| Total | 6996 | 100 | |
| ≥ 75 | Male | 2452 | 63.11 |
| Female | 1433 | 36.89 | |
| Total | 3885 | 100 | |
| Total | Male | 19913 | 81.42 |
| Female | 4476 | 18.58 | |
| Total | 24089 | 100 | |
| Time of infarction (year) | n | % | |
| 2001 | 3245 | 13.47 | |
| 2002 | 3461 | 14.37 | |
| 2003 | 3970 | 16.48 | |
| 2004 | 4455 | 18.49 | |
| 2005 | 4966 | 20.62 | |
| 2006 | 3992 | 16.57 | |
| Employment status | n | % | |
| Self-employed | 1559 | 6.47 | |
| Employee/worker | 10125 | 42.03 | |
| Pensioner/unemployed/welfare recipient/other | 12405 | 51.50 | |
| Cardiac medication in the year preceding infarction | n | % | |
| Acetylsalicylic acid (aspirin) | 4039 | 16.77 | |
| Beta-blocker | 7892 | 32.76 | |
| ACE inhibitor | 8687 | 36.06 | |
| Statin | 4594 | 19.07 | |
| Inpatient treatment | n | % | |
| Angiography | 15863 | 65.85 | |
| Bypass | 1099 | 4.56 | |
| Cardiac pacemaker | 211 | 0.88 | |
| PCI Total | 12593 | 52.28 | |
| PCI with stent | 10881 | 45.17 | |
| PCI with non-drug-eluting stent | 9601 | 39.86 | |
| PCI with drug-eluting stent | 1548 | 6.43 | |
PCI, percutaneous coronary intervention; ACE, angiotensin-converting enzyme
Prescription prevalence
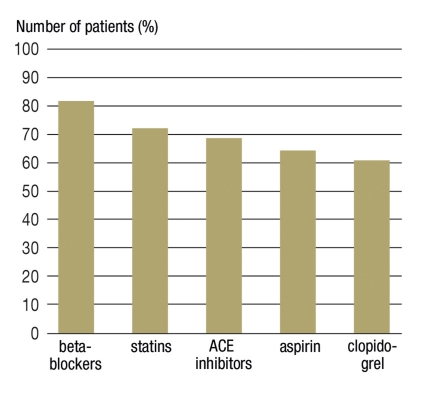
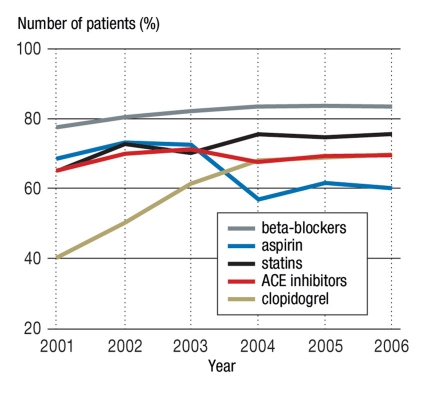
During the 90 days following the hospital discharge, 82% of patients received a beta-blocker, 73% a statin, 69% an ACE inhibitor, 66% aspirin, and 61% clopidogrel treatment (Figure 1); 3% of the patients did not receive any of these drug groups. Of those who received aspirin, 28% also received clopidogrel, a beta-blocker, an ACE inhibitor, and a statin. An increase in the 90-day prescription prevalence was observed from 2001 to 2006 for all drug classes except for aspirin (Figure 2).
Figure 1.
Number of patients with at least one prescription in the respective drug class (90 days following hospital discharge) n = 24 089; ACE, angiotensin converting enzyme
Figure 2.
Number of patients with at least one prescription for the respective drug class (90 days following hospital discharge) n = 24 089; ACE, angiotensin converting enzyme
Prescribed dosage
In total, 2152 prescription copies were analyzed to validate the dosage recommendations. The PDD was indicated on only 15% of these copies. The assumption that one tablet was taken daily came the closest to the PDD and was therefore used to determine the treatment persistence.
Treatment persistence
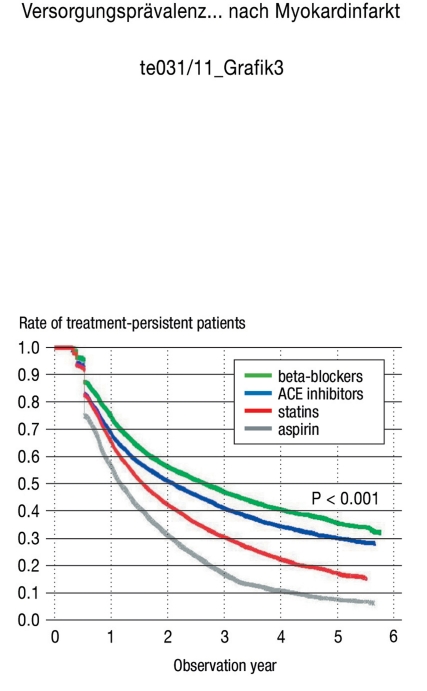
During the 5-year observation period, there was a noticeable decline in the continuation of the therapy. Specifically, only 10% of the aspirin users, 17% of the statin users, 31% of the ACE inhibitor users, and 36% of the beta-blocker users persisted with the therapy over 5 years. The largest decline was observed in the first year following the infarction (Figure 3).
Figure 3.
Treatment persistence over time
⊓beta-blocker = 19 818, ⊓ACE inhibitor = 16 672,
⊓statin = 17 602, ⊓aspirin = 15 906
Curve profile explanation: Note that, since the discontinuation criterion allowed a 90-day gap, determining the therapy discontinuation rate was methodologically restricted to 90 days after the observation start point. However, several censored observations (that influenced the survival chances) were present at that point, causing the curve profile to sharply drop at the first therapy discontinuation value.
ACE, angiotensin converting enzyme
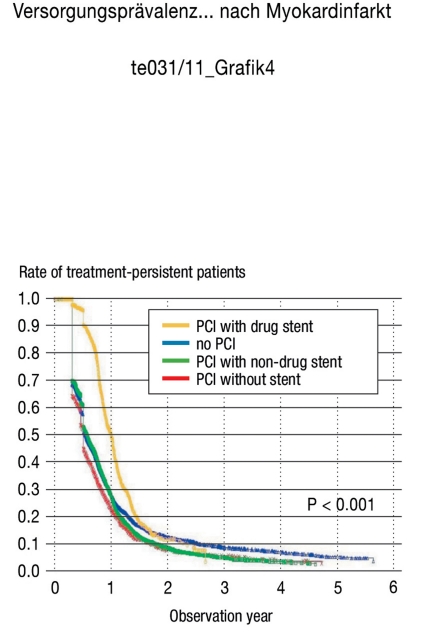
The changes in treatment persistence for the clopidogrel users were analyzed according to the intervention performed during the hospital stay (e.g., without a percutaneous coronary intervention [PCI], a PCI without a stent, a PCI with a non-drug-eluting stent implanted, or a PCI with a drug-eluting stent implanted) (Figure 4). The percentage of patients who persisted with therapy in the first three groups (e.g., without a PCI, PCI without a stent, or PCI with a non-drug-eluting stent) was between 65% and 70% after three months, between 45% and 55% after six months, and between 22% and 28% after one year. In contrast, treatment persistence for patients who had received a PCI implant with a drug-eluting stent was noticeably higher over time, with 90% persistence after six months, and 50% after one year.
Figure 4.
Treatment persistence over time (only clopidogrel)
⊓total = 14 747, ⊓PCI = 10 176,
⊓PCI with non-drug stent = 7 949, ⊓with drug stent = 1445
PCI = percutaneous coronary intervention, non-drug = non-drug-eluting, drug = drug-eluting
Curve profile explanation: Note that, since the discontinuation criterion allowed a 90-day gap, determining the therapy discontinuation rate was methodologically restricted to 90 days after the observation start point. However, several censure criteria (that influenced the survival chances) were present at that point, causing the curve profile to sharply drop at the first therapy discontinuation value.
Discussion
Despite the strong evidence supporting the use of aspirin, clopidogrel, beta-blockers, statins, and ACE inhibitors as secondary preventive medicine following AMI, recent studies have shown a discrepancy between the recommended therapy and that which is actually received. This study, based on nationwide claims data from the TK from January 1, 2000, to February 28, 2007, analyzed 30 028 AMI patients. During follow-up outpatient care, 82% of these patients received a beta-blocker, 73% a statin, 69% an ACE inhibitor, 66% aspirin, and 61% clopidogrel. Prescription prevalence increased for all drug classes except aspirin between 2001 and 2006, possibly due to better understanding and application of the existing guidelines.
The prescription prevalence rates observed here are higher than those found in other countries, such as the Netherlands (9) or Denmark (11), for all of the drug groups except aspirin. In contrast, another German study, based on a questionnaire given to the primary care physicians who continued outpatient treatment, reported almost equivalent or even slightly higher results (12). Specifically, the study found that, in the month following myocardial infarction in 4643 patients, 70% of them were prescribed aspirin and clopidogrel, 83% a beta-blocker, 71% an ACE inhibitor, and 84% a statin (note that this is higher than our value). These differences in values could be explained by the medical samples provided to the patients by the physicians, as these would not be documented in the claims data yet would be reported as a medication. Additionally, aspirin can be bought directly without a physician’s prescription and therefore without being documented in the insurance claims. Additionally, it should be noted that self-medication (over the counter) was removed from reimbursement through German statutory health insurance (GKV) in 2004, and although aspirin is still prescribed following myocardial infarction, this removal had a clear effect on the prescription prevalence (which dropped from 72% in 2003 to 57% in 2004). Whether the prescription decline observed in this study reflects an actual prescription decline remains unclear.
We observed a considerable decrease in the treatment persistence in the years following infarction. Thus, only 10% of the aspirin users, 17% of the statin users, 31% of the ACE inhibitor users, and 36% of the beta-blocker users continuously persisted with their therapy over five years. Moreover, we clearly show here that the first year following infarction is the period in which the patient decides whether or not to discontinue taking their medication (at least on a regular basis), or in which the physicians decide to refrain from writing further prescriptions. This observation is consistent with the results of other studies (9, 13, 14). Nonetheless, a methodically-similar study carried out in the Netherlands showed a considerably higher persistence rate (the proportion of patients who persisted with therapy after five years was 82% for statin users, 58% for ACE inhibitors, and 55% for beta-blocker users), although the initial prescription prevalence was lower (9). This could be explained by the differences in the study populations and the resulting different initial risks. For example, the Dutch study excluded all patients who were relocated after the acute event to other hospitals or rehabilitation facilities. This could therefore mean that the patient cohort had an overall tendency towards a lower disease severity, and that it perhaps also had fewer co-existing diseases that lead to non-tolerance and therefore discontinuation of a medication.
With respect to the guidelines at the time of the study (2– 6; current versions: e8–e13), our study showed that the treatment persistence for the time-limited use of clopidogrel could be influenced by the type of inpatient intervention, as would be expected. Thus, while there was no clear difference between patients who had no PCI, PCI without a stent, or PCI with a non-drug-eluting stent, 90% of the patients who had undergone PCI with a drug-eluting stent implantation persisted with their therapy after 6 months, 50% after 12 months, and 20% after 18 months. According to the guidelines (2– 6; e8–e12), patients should be treated with a combination of aspirin and clopidogrel for between 1 to 12 months, depending on the type of infarction (non-ST elevation myocardial infarction [NSTEMI] or ST elevation myocardial infarction [STEMI]) and the type of implanted stent (drug-eluting or non-drug-eluting).
As the ICD codes have only recently (since 2005) allowed a distinction between STEMI and NSTEMI, it is difficult to determine whether the high persistence rate observed for patients who had no PCI, PCI without a stent, or PCI with a non-drug-eluting stent is appropriate, especially since the current guidelines now recommend a one-year administration of clopidogrel with aspirin for STEMI/NSTEMI patients after a stent implantation (e8, e10, 16).
A high proportion of insured individuals still received clopidogrel six months after having a drug-eluting stent implanted, which can be seen as a positive trend. The fact that 20% of patients still persisted with therapy after 18 months should not be interpreted as excessive, since it has recently become clear that discontinuing clopidogrel therapy increases the problem of late stent thrombosis with drug-eluting stents (17). The uncertainty about how long it is necessary to administer clopidogrel following implantation of this newer generation of stents prompted the American professional medical associations to recommend a longer administration period of at least one year (16, 18).
Advantages and limitations of this study
One advantage of this study was that it was based on data obtained about the prescriptions that had actually been dispensed at pharmacies. It should be emphasized that what the patient receives upon hospital discharge is only a medication recommendation. Studies based on such discharge medication recommendations have a tendency to overestimate the quality of the primary care, since the therapy decisions in the ambulatory sector often differ from those in the inpatient sectors, for various reasons (19, 20). A further advantage of this work is the long follow-up period of up to six years. Until now, only one study is available in the literature in which the follow-up was longer than two years (9). Additionally, since our study was performed completely independently of any research-oriented secondary data, there was no risk of recall bias.
One limitation of this study is that the study population is not necessarily representative of the general population in Germany. Thus, on average, the socio-economic factors differ from people insured with the statutory GKV insurance and those who are privately insured. However, people insured with TK have an overall higher socio-economic status than those insured through other GKV sickness funds, which might have positively affected their knowledge and attitudes about using medicine.
Several further limitations arise from using the claims data from the statutory GKV health insurance as the information source. For instance, the claims data do not contain any information regarding the medication that a patient received without a prescription, such as self-medication or samples from the physician, which can lead to underestimating the prescription prevalence for certain drug classes (especially for aspirin). Additionally, the claims data do not contain the prescriptions written by physicians that were not dispensed. Likewise, the claims document the medication received by the patient from the pharmacy, but not whether this medication was actually taken, which can also lead to overestimating the drug persistence.
Another potential limitation of our study is that we did not examine if the patients had switched from one drug class to another, or if they had interrupted and then later resumed a therapy. We also did not analyze the use of angiotensin II blockers as an alternative to ACE inhibitors, which could have led to underestimating both the prescription prevalence of agents that affect the renin-angiotensin system and the relevant treatment persistence. Therefore, this study only describes a part of the reality of secondary prevention care. It also remains unclear whether the decisions to discontinue therapies came from the physicians or the patients.
Whether long-term treatment persistence outside of randomized controlled trials lead to a significant reduction of recurring infarction and death has yet to be clarified and requires further research. Studies investigating this should consult data sources in addition to the claims data because, in our opinion, the validity of the information given in the claims data about the end of the insurance period (which could be due to death, for example) needs to be questioned. This type of analysis was unfortunately not possible within the frame of this study.
Conclusion
This investigation revealed that, while drug treatment of patients after an AMI clearly improved during our period of observation, there is scope for further improvement to treatment persistence. One way to improve treatment persistence would be to offer educational measures for both the patients and their family members, with the involvement of their primary care physicians. This training should take place immediately following the infarction, since this study demonstrates that the decision for or against a therapy is taken within the first year after the hospital discharge.
Key Messages.
Guidelines for secondary prevention after myocardial infarction recommend a continuous use of aspirin, clopidogrel, beta-blockers, statins, and ACE inhibitors; however, long-term studies analyzing the reality of the care received are lacking.
We analyzed data from the sickness fund Techniker Krankenkasse for 30 028 patients treated in the hospital for acute myocardial infarction (AMI) between 2001 and 2006; following hospital discharge, 82% were initially prescribed a beta-blocker, 73% a statin, 69% an ACE inhibitor, 66% aspirin (excluding self-medication), and 61% clopidogrel.
Only a small percentage of these initial users continued their therapy with the same drug class over 5 years (with 10% for aspirin, 17% for statins, 31% for ACE inhibitors, and 36% for beta-blockers).
Although we observed an improvement during the observation period in the patients’ prescription care after AMI, the medication persistence remains to be optimized.
Patient education, with the involvement of the primary care physicians, should begin immediately after infarction, since the decision to continue or discontinue a drug therapy is usually made in the first year after hospital discharge.
Acknowledgments
Translated from the original German by Veronica A. Raker, PhD.
Footnotes
Conflict of interest statement
Dr. P. H. Mangiapane received lecture fees from pharmaceutical chambers and associations as well as from the Berlin-Chemie company. Her travel expenses incurred for analyzing the claims data were reimbursed by the Techniker Krankenkasse (B-HH).
Prof. Busse received consulting fees from the BKK Bundesverband, the GlaxoSmithKline company, and the VfA. Congress fees as well as travel and accommodation expenses, were reimbursed by Eucomed (Association of Medical Technology Manufacturers). He received lecturer fees from different sickness funds (including TK and BKK) as well as from the AstraZeneca, Merck, Wyeth, and GlaxoSmithKline companies.
References
- 1.Statistisches Bundesamt. Todesursachenstatistik 2008. Wiesbaden: Statistisches Bundesamt; 2009. Fachserie 12, Reihe 4. [Google Scholar]
- 2.Bertrand ME, Simoons ML, Fox KA, Wallentin LC, Hamm CW, McFadden E, et al. Management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2002;23:1809–1840. doi: 10.1053/euhj.2002.3385. [DOI] [PubMed] [Google Scholar]
- 3.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. ACC/AHA guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction - 2002. Circulation. 2002;106:1893–1900. doi: 10.1161/01.cir.0000037106.76139.53. [DOI] [PubMed] [Google Scholar]
- 4.Van de Werf F, Ardissino D, Betriu A, Cokkinos DV, Falk E, Fox KA, et al. Management of acute myocardial infarction in patients presenting with ST-segment elevation. The task force on the management of acute myocardial infarction of the European Society of Cardiology. Eur Heart J. 2003;24:28–66. doi: 10.1016/s0195-668x(02)00618-8. [DOI] [PubMed] [Google Scholar]
- 5.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction - executive summary. Circulation. 2004;110:588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 6.Smith SC, Jr, Blair SN, Bonow RO, Brass LM, Cerqueira MD, Dracup K, Fuster V, et al. AHA/ACC Scientific Statement: AHA/ACC guidelines for preventing heart attack and death in patients with atherosclerotic cardiovascular disease: 2001 update: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 2001;104:1577–1579. doi: 10.1161/hc3801.097475. [DOI] [PubMed] [Google Scholar]
- 7.Böger GI, Hoopmann M, Busse R, Budinger M, Welte T, Böger RH. Drug therapy of coronary heart disease—are therapeutic guidelines being paid attention to? Z Kardiol. 2003;92:466–475. doi: 10.1007/s00392-003-0942-3. [DOI] [PubMed] [Google Scholar]
- 8.Frilling B, Schiele R, Gitt AK, Zahn R, Schneider S, Glunz HG, et al. Too little aspirin for secondary prevention after acute myocardial infarction in patients at high risk for cardiovascular events: Results from the MITRA study. Am Heart J. 2004;148:306–311. doi: 10.1016/j.ahj.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 9.van der Elst ME, Bouvy ML, de Blaey CJ, de Boer A. Preventive drug use in patients with a history of nonfatal myocardial infarction during 12-year follow-up in The Netherlands: a retrospective analysis. Clin Ther. 2005;27:1806–1814. doi: 10.1016/j.clinthera.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff B, Silber S, Richartz BM, Pieper L, Klotsche J, Wittchen HU. Inadequate medical treatment of patients with coronary artery disease by primary care physicians in Germany. Clin Res Cardiol. 2006;95:405–412. doi: 10.1007/s00392-006-0399-2. [DOI] [PubMed] [Google Scholar]
- 11.Gasse C, Jacobsen J, Larsen AC, Schmidt EB, Johannesen NL, Videbaek J, et al. Secondary medical prevention among Danish patients hospitalised with either peripheral arterial disease or myocardial infarction. Eur J Vasc Endovasc Surg. 2007;35:51–58. doi: 10.1016/j.ejvs.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Zeymer U. Secondary prevention in outpatients with coronary artery disease. Adherence with recommendations within 4 weeks after hospital discharge. Dtsch Med Wochenschr. 2007;132:2367–2370. doi: 10.1055/s-2007-991659. [DOI] [PubMed] [Google Scholar]
- 13.Akincigil A, Bowblis JR, Levin C, Jan S, Patel M, Crystal S. Longterm adherence to evidence based secondary prevention therapies after acute myocardial Infarction. J Gen Intern Med. 2007;45:S56–S65. doi: 10.1007/s11606-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blackburn DF, Dobson RT, Blackburn JL, Wilson TW, Stang MR, Semchuk WM. Adherence to statins, beta-blockers and angiotensin-converting enzyme inhibitors following a first cardiovascular event: a retrospective cohort study. Can J Cardiol. 2005;21:485–488. [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–481. [Google Scholar]
- 16.Kushner FG, Hand M, Smith SC, Jr, King SB III, Anderson JL, Antman EM, et al. 2009 Focused Updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction. Circulation. 2009;120:2271–2306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 17.Siegmund-Schultze N, Meyer R. Absetzen von Clopidogrel erhöht Infarktrisiko. Dtsch Arztbl. 2008;105(13) [Google Scholar]
- 18.Grines CL, Bonow RO, Casey DE, Jr, Gardner TJ, Lockhart PB, Moliterno DJ, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents. Circulation. 2007;115:813–818. doi: 10.1161/CIRCULATIONAHA.106.180944. [DOI] [PubMed] [Google Scholar]
- 19.Busse R, Hoopmann M, Mildenstein M, Welte T, Budinger M, Bode-Böger S, et al. Projekt DII 1. Hannover: Norddeutscher Forschungsverbund Public Health; 1998. Kommunikationsprozess und Therapieentscheidungen an der Schnittstelle von stationärer und ambulanter Versorgung im West-/Ostvergleich. [Google Scholar]
- 20.Cochrane RA, Mandal AR, Ledger-Scott M, Walker R. Changes in drug treatment after discharge from hospital in geriatric patients. BMJ. 1992;305:694–696. doi: 10.1136/bmj.305.6855.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- e2.Kober L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med. 1995;333:1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- e3.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- e4.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- e5.Freemantle N, Cleland J, Young P, Mason J, Harrison J. Beta blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318:1730–1737. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e6.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- e7.Antithrombotic Trialists’ Colaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e8.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction. Circulation. 2007;116:e148–e304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- e9.Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez-Aviles F, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–1660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- e10.Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, et al. 2007 Focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial Infarction. Circulation. 2008;117:296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- e11.Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the task force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–2945. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- e12.Programm für Nationale VersorgungsLeitlinien. Nationale VersorgungsLeitlinie Chronische KHK. http://www.khk.versorgungsleitlinien.de/ 2010.
- e13.Smith SC, Jr, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113:2363–2672. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]