Abstract
Background
Heart failure trials use a variety of measures of functional capacity and quality of life. Lack of formal assessments of the relationships between changes in multiple aspects of patient-reported health status and measures of functional capacity over time limit the ability to compare results across studies.
Methods
Using data from HF-ACTION (N = 2331), we used Pearson correlation coefficients and predicted change scores from linear mixed-effects modeling to demonstrate associations between changes in patient-reported health status measured with the EQ-5D visual analog scale (VAS) and the Kansas City Cardiomyopathy Questionnaire (KCCQ) and changes in peak VO2 and 6-minute walk distance at 3 and 12 months. We examined a 5-point change in KCCQ within individuals to provide a framework for interpreting changes in these measures.
Results
After adjustment for baseline characteristics, correlations between changes in the VAS and changes in peak VO2 and 6-minute walk distance ranged from 0.13 to 0.28, and correlations between changes in the KCCQ overall and subscale scores and changes in peak VO2 and 6-minute walk distance ranged from 0.18 to 0.34. A 5-point change in KCCQ was associated with a 2.50 ml/kg/min change in peak VO2 (95% confidence interval, 2.21–2.86) and a 112-meter change in 6-minute walk distance (95% confidence interval, 96–134).
Conclusions
Changes in patient-reported health status are not highly correlated with changes in functional capacity. Our findings generally support the current practice of considering a 5-point change in the KCCQ within individuals to be clinically meaningful.
Trial Registration
clinicaltrials.gov Identifier: NCT00047437
Introduction
Patient-reported outcome measures are important tools for evaluating health status in clinical trials and for monitoring patients in clinical care.1 Outcome measures that solicit patients’ perspectives of their disease offer unique information not captured by other commonly used metrics, such as clinical, functional, or physician-reported measures. Growing use of patient-reported outcome measures led the US Food and Drug Administration to call for more data supporting the construct validity of these instruments, to be supplemented with defined relationships among other outcome measures. However, relatively few studies have formally assessed the relationships between patient-reported outcome measures and other measures of disease severity in large samples of patients with heart failure, and none have comprehensively examined temporal changes in one measure compared with another.
Understanding of correlations between patients’ experiences of their disease and objective measures of disease severity over time is incomplete. For example, changes in B-type natriuretic peptide level after 6 weeks are not correlated with changes in the Kansas City Cardiomyopathy Questionnaire (KCCQ), a heart failure–specific patient-reported measure of health status.4 However, in a comparison between changes in KCCQ overall scores and cardiologists’ ratings of clinical change over a 6-week period on a 15-point global change scale, a mean change of 5 points on the KCCQ was considered clinically meaningful and was more strongly associated with assessments of clinical change than generic health status measures (eg, EQ-5D), B-type natriuretic peptide level, and 6-minute walk distance.5 Changes in the quality-of-life subscale of the KCCQ have been associated with changes in 6-minute walk distance,6 but no studies have assessed relationships with other KCCQ subscales or the overall scale. Likewise, no studies have assessed relationships between changes in the KCCQ and changes in peak VO2, the gold standard of functional measures. The KCCQ includes a subscale for physical limitations, which specifically targets patients’ reports of their ability to walk 1 block, climb stairs, and jog, among other activities, but no studies have examined how changes in this subscale correlate with changes in functional measures of physical ability.
It is important to understand how overall health status and its components are related to measures of functional capacity to inform study design and interpretation in clinical trials and observational studies that use these measures. With information about how these commonly used measures are related to each other over time, we can (1) add to the demonstrated validity and ability to detect change of the patient-reported health status measures and (2) develop a better framework for choosing study end points. Therefore, we used longitudinal data from a large multicenter study, Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION), to characterize relationships between changes in health status from the patient’s perspective and changes in functional capacity.
This study is unique in its ability to take advantage of linear mixed models to model trajectories of health status and functional measures over time; no previous studies correlating changes in outcome measures have had more than 2 longitudinal assessments of outcomes available. On the basis of cross-sectional correlations observed in HF-ACTION, we hypothesized that the KCCQ overall scale would have small correlations7 with peak VO2 and 6-minute walk distance (r < 0.30) and that the EQ-5D visual analog scale (VAS) would have negligible correlations with peak VO2 and 6-minute walk distance (r < 0.10). We expected that increased peak VO2 and longer 6-minute walk distance over time would be correlated with improved health status as assessed by the KCCQ overall scale and VAS. Finally, we expected the KCCQ physical limitations subscale to have stronger relationships with peak VO2 and 6-minute walk distance than the other KCCQ subscales or the overall score.
Methods
HF-ACTION was a multicenter, randomized controlled trial designed to test the long-term safety and efficacy of aerobic exercise training versus usual care in a large, multinational sample of patients with left ventricular dysfunction and heart failure.8 Enrollment criteria included left ventricular ejection fraction of 35% or less, New York Heart Association (NYHA) class II to IV heart failure, and ability and willingness to undergo exercise training. Patients were excluded if they were unable to exercise, were already exercising regularly, or had experienced a cardiovascular event in the preceding 6 weeks. The relevant institutional review boards of the participating centers and the coordinating center approved the protocol. This work was supported by grants from the National Heart, Lung, and Blood Institute. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Patient-Reported Health Status
The KCCQ is a disease-specific, 23-item, self-administered measure of health status for patients with heart failure with an overall summary score and subscales for physical limitations, symptoms, social limitations, self-efficacy, and quality of life.9 The KCCQ is scored from 0 to 100, with higher scores representing better health status. The KCCQ was self-administered at the baseline clinic visit, at 3-month intervals during clinic visits for the first 12 months, and annually thereafter for up to 4 years.
The VAS is a single-item “feeling thermometer” on which respondents rate their health state from 0 (worst imaginable) to 100 (best imaginable). It has been used previously to measure health status in patients with heart failure10–12 and was included in HF-ACTION as a generic measure of health status. The VAS was administered on the same schedule as the KCCQ.
Functional Capacity
The measures of functional capacity in this study were peak VO2 and 6-minute walk distance. Patients performed a maximal exercise test with gas exchange measurements on a treadmill using the modified Naughton protocol.13 Patients who were unable to perform an exercise test on a treadmill underwent cycle ergometry testing using a 10 W/min ramp protocol. Patients also performed a 6-minute walk test.14 These measures were collected at baseline, 3 months, 12 months, and 24 months.
Patient Characteristics
Patient characteristics used as covariates included sociodemographic and clinical history characteristics collected at baseline.
Statistical Analysis
We describe baseline patient characteristics using percentages for categorical variables and medians and interquartile ranges or means and standard deviations for continuous variables. We used SAS version 9.1 for all analyses.
We used Pearson correlation coefficients to demonstrate associations between changes in patient-reported health status and changes in measures of functional capacity. We examined relationships graphically to determine whether this approach was appropriate. Because the use of observed change-from-baseline scores in this context may produce bias due to the influence of measurement error and missing follow-up data,15 we used full maximum likelihood estimation to model all available data (up to 4 years) from each patient and generate the best linear unbiased prediction (“BLUP”) of change from baseline to 3 months and baseline to 12 months from linear mixed effects models. Mixed models take all available longitudinal information into account to model trajectories over time and can result in less bias from measurement error and missing data compared to using observed change-from-baseline scores,16 assuming unobserved variables do not explain the probability of missingness over and above what is explained by observed variables.17 Examining raw change-from-baseline scores at 3 and 12 months in a study that collected data at many more time points would be equivalent to ignoring the intermediate data points and the information they supply about patients’ underlying trajectories across time (ie, ignoring 5 of 8 data points per participant). Furthermore, such an approach would not include participants who missed the 3- or 12-month assessments. Our approach decreases the influence of missing data on the conclusions by using as much of the data as possible to model underlying trajectories over time.
After examining the observed mean trajectories for each of the patient-reported and functional capacity end points, we found that the trajectories were not strictly linear, as the initial increase from baseline to 3 months was very steep. (This was the same period of the trial during which exercise training was supervised.) Thus, we modeled time using a piecewise linear model with a joint point (“jump”) at 3 months. A likelihood ratio test for model reduction confirmed the better fit of the piecewise model. The piecewise model was used to produce the best linear unbiased predictions of change. Each of the mixed models included a jump from baseline to 3 months and a linear increase after 3 months, as well as 28 baseline covariates,18 each of which had less than 1% missing data. All interaction terms between baseline covariates and the jump at 3 months, as well as between baseline covariates and time after 3 months were tested simultaneously using an omnibus likelihood-ratio test. When the omnibus test was significant, we tested the individual interactions separately. We included significant interaction pairs in the final models. For each model, all participants who had at least a baseline score on the outcome variable contributed data to that model. We compared the correlations between peak VO2 and 6-minute walk distance and the KCCQ physical limitations subscale with the correlations between functional capacity and the other KCCQ subscales and the overall score based on the Fisher z transformation adjusted for correlated correlation coefficients.19
To graphically display relationships between changes in health status and functional measures over time and to estimate minimally important changes within individuals, we used predicted changes at 12 months, as estimated from the mixed models. Estimating change requires converting a correlation into a regression parameter. Because the size of the regression parameter can depend on which variable is modeled as the outcome, we used ordinary least squares regression in which the KCCQ overall score was regressed on peak VO2 or 6-minute walk distance. This approach reflects the most common model of patient outcomes, the Wilson and Cleary model, which assumes that patient-reported health status is a function of the patient’s underlying physiological status.20 Other approaches are possible, such as ordinary least squares regression in which the functional end points are regressed on health status, or orthogonal regression, which treats functional end points and the KCCQ overall score symmetrically.21 However, without underlying conceptual frameworks for these models, their plausibility is not compelling. We used the bootstrap method with replacement to estimate confidence intervals, and we constructed the confidence intervals using the bias-corrected and accelerated method.22
Results
Table 1 shows the baseline characteristics of the study population. Baseline characteristics did not differ by treatment group.18 Table 2 shows the visit-level missing data through 12 months of follow-up, accounting for patients who died or withdrew from the study.
Table 1.
Baseline Characteristics of the Study Population (N = 2331)*
| Characteristic† | Patients |
|---|---|
| Treatment group, No. (%) | |
| Usual care | 1172 (50.3) |
| Exercise training | 1159 (49.7) |
| Age, median (IQR), y | 59.3 (51.1–68.0) |
| Female, No. (%) | 661 (28.4) |
| Diabetes mellitus, No. (%) | 748 (32.1) |
| Peripheral vascular disease, No. (%) | 157 (6.8) |
| Chronic obstructive pulmonary disease, No. (%) | 249 (10.8) |
| Previous myocardial infarction, No. (%) | 979 (42.0) |
| Previous revascularization, No. (%) | 903 (38.7) |
| Atrial fibrillation/atrial flutter, No. (%) | 488 (20.9) |
| Ischemic etiology of heart failure, No. (%) | 1197 (51.4) |
| Ejection fraction, median (IQR), % | 24.7 (20.2–30.1) |
| Implantable cardioverter-defibrillator, No. (%) | 938 (40.2) |
| Biventricular pacemaker, No. (%) | 419 (18.0) |
| Use of β-blocker, No. (%) | 2203 (94.5) |
| Use of angiotensin-converting enzyme inhibitor, No. (%) | 1736 (74.5) |
| Use of HMG-CoA reductase inhibitor, No. (%) | 1097 (47.1) |
| Presence of 2 diuretics, No. (%) | 136 (5.8) |
| Spironolactone or eplerenone, No. (%) | 1051 (45.1) |
| New York Heart Association classification, No. (%) | |
| II | 1477 (63.4) |
| III/IV | 854 (36.6) |
| Canadian Cardiovascular Association functional classification | |
| of angina, No. (%) | |
| I | 200 (8.6) |
| II | 136 (5.8) |
| III | 36 (1.6) |
| IV | 6 (0.3) |
| No angina | 1950 (83.8) |
| Systolic blood pressure, median (IQR) | 111 (100–126) |
| Diastolic blood pressure, median (IQR) | 70 (60–78) |
| Heart rate, median (IQR) | 70 (63–77) |
| Body mass index, median (IQR) | 29.9 (26.0–35.1) |
| Exercise duration, median (IQR), min | 9.6 (6.9–12.0) |
| Smoking status, No. (%) | |
| Never | 866 (37.3) |
| Current | 388 (16.7) |
| Past | 1066 (46.0) |
| No. of heart failure–related hospitalizations in the past 6 months, No. (%) | |
| 0 | 1701 (73.6) |
| 1 | 464 (20.1) |
| 2 | 94 (4.1) |
| 3+ | 52 (2.3) |
| Beck Depression Inventory II, median (IQR) | 8.0 (4.0–15.0) |
| Perceived Social Support Scale, median (IQR) | 6.0 (5.2–6.7) |
| 6-minute walk distance, mean (SD), m | 364.5 (104.7) |
| Peak VO2, mean (SD), mL/kg/min | 14.9 (4.7) |
| EQ-5D visual analog scale, mean (SD) | 65.5 (19.0) |
| KCCQ overall summary scale, mean (SD) | 66.2 (20.6) |
| KCCQ subscales, mean (SD) | |
| Physical limitations | 69.4 (21.9) |
| Symptoms | 73.1 (20.8) |
| Quality of life | 59.7 (24.7) |
| Social limitations | 62.4 (27.5) |
Abbreviations: IQR, interquartile range; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Percentages may not sum to 100 because of rounding.
The number of missing values was 20 in history of chronic obstructive pulmonary disease, 1 in history of atrial fibrillation/atrial flutter, 3 in Canadian Cardiovascular Association functional classification of angina, 4 in systolic blood pressure, 5 in diastolic blood pressure, 5 in heart rate, 7 in body mass index, 22 in CPX duration, 11 in smoking status, 20 in number of heart failure-related hospitalization in the past 6 month, 9 in Beck Depression Inventory II, and 17 in Perceived Social Support Scale. Missing data for KCCQ, Peak VO2, EQ-5D VAS, and 6-minute walk distance are reported in Table 2. There was no missing data for other baseline variables.
Table 2.
Patients With Missing Data by Study Visit
| Expected No. of Patients | Patients With Missing KCCQ, No. (%)* | Patients With Missing EQ-5D VAS, No. (%)* | Patients With Missing Peak VO2, No. (%)* | Patients With Missing 6-Minute Walk Distance, No. (%)* | |
|---|---|---|---|---|---|
| Baseline | 2331 | 1 (< 0.1) | 57 (2.4) | 56 (2.4) | 51 (2.2) |
| 3 months | 2281 | 243 (10.7) | 280 (12.3) | 399 (17.5) | 434 (19.0) |
| 6 months | 2240 | 332 (14.8) | 374 (16.7) | — | — |
| 9 months | 2196 | 383 (17.4) | 423 (19.3) | — | — |
| 12 months | 2101 | 345 (16.4) | 379 (18.0) | 649 (30.9) | 646 (30.7) |
Abbreviation: KCCQ, Kansas City Cardiomyopathy Questionnaire; VAS, visual analog scale.
Percentages are calculated from the number of patients expected at each visit, accounting for patients who died or withdrew from the study.
Table 3 shows the Pearson correlation coefficients comparing changes in patient-reported health status and changes in the functional capacity measures at 3 and 12 months. After adjustment for baseline patient characteristics, there were small correlations between VAS scores and peak VO2 or 6-minute walk distance. After adjustment for baseline patient characteristics, there were small to moderate correlations between changes in the KCCQ overall score and changes in peak VO2 and 6-minute walk distance. All correlations were significantly different from zero. The correlations between the measures of functional capacity and the KCCQ physical limitations subscale were slightly higher than for the other KCCQ domains. Where these correlations were significantly different from the correlation with the physical limitations scale, they are noted in the table.
Table 3.
Correlations Between Changes in Health Status and Changes in Functional Capacity
| Health Status and Functional Capacity | 3 Months, r (95% CI)*† | 12 Months, r (95% CI)*† |
|---|---|---|
| KCCQ overall score | ||
| Peak VO2, mL/kg/min | 0.29 (0.21 to 0.39) | 0.31 (0.24 to 0.39) |
| 6-minute walk distance, m | 0.26 (0.20 to 0.34)c | 0.31 (0.26 to 0.38) c |
| KCCQ subscales | ||
| Physical limitation | ||
| Peak VO2, mL/kg/min | 0.28 (0.19 to 0.40) | 0.32 (0.25 to 0.41) |
| 6-minute walk distance, m | 0.31 (0.23 to 0.42) | 0.34 (0.28 to 0.43) |
| Symptoms | ||
| Peak VO2, mL/kg/min | 0.26 (0.18 to 0.35) | 0.27 (0.20 to 0.35)c |
| 6-minute walk distance, m | 0.25 (0.17 to 0.35)c | 0.29 (0.22 to 0.37) c |
| Quality of life | ||
| Peak VO2, mL/kg/min | 0.24 (0.15 to 0.34)c | 0.26 (0.18 to 0.34)c |
| 6-minute walk distance, m | 0.18 (0.11 to 0.25)c | 0.23 (0.17 to 0.30)c |
| Social limitation | ||
| Peak VO2, mL/kg/min | 0.28 (0.20 to 0.38) | 0.30 (0.23 to 0.38) |
| 6-minute walk distance, m | 0.22 (0.16 to 0.30)c | 0.29 (0.23 to 0.37)c |
| EQ-5D Visual Analog Scale | ||
| Peak VO2, mL/kg/min | 0.13 (0.02 to 0.25) | 0.16 (0.07 to 0.27) |
| 6-minute walk distance, m | 0.18 (0.07 to 0.31) | 0.28 (0.19, to 0.39) |
Abbreviations: CI, confidence interval; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Adjusted for all significant patient characteristics listed in Table 1 and their interactions.
Confidence intervals estimated using the bootstrap method with replacement and constructed using the bias-corrected and accelerated method.
Correlation coefficient significantly different from corresponding coefficient with KCCQ physical limitation subscale at P < .05.
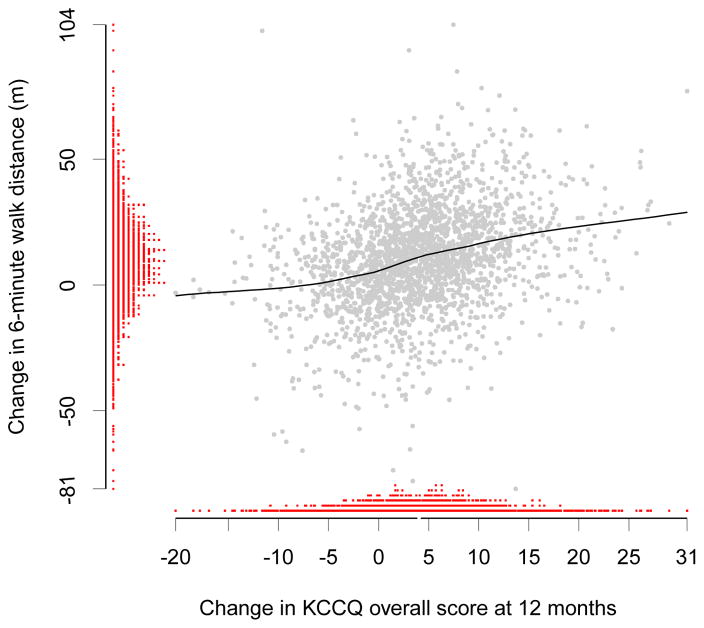
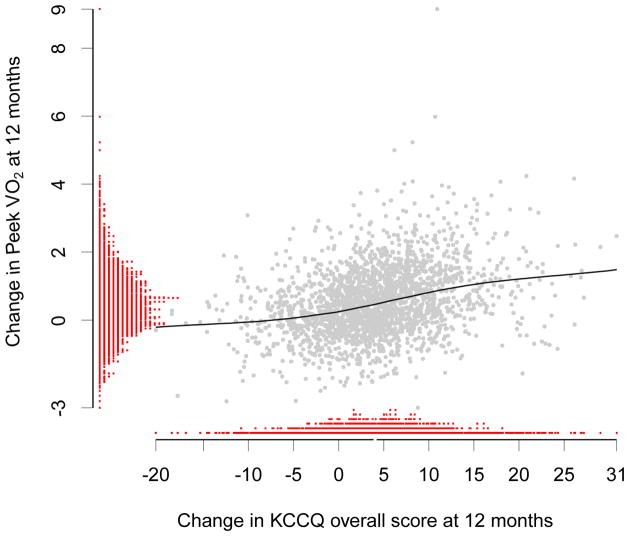
Figures 1 and 2 show the distributions of change in KCCQ overall score and changes in functional capacity measures at 12 months. They also display the LOESS curves and underlying scatter plots of the relationships between KCCQ and functional capacity. These relationships appear consistent with the magnitudes of the reported correlation coefficients.
Figure 1.
Distributions of and Relationship Between Changes in KCCQ and Changes in Peak VO2 at 12 Months
Abbreviation: KCCQ, Kansas City Cardiomyopathy Questionnaire.
*LOESS fit curve between the predicted change in KCCQ overall score and change in peak VO2 at 12 months.
Figure 2.
Distributions of and Relationship Between Changes in KCCQ and Changes in 6-Minute Walk Distance at 12 Months
Abbreviation: KCCQ, Kansas City Cardiomyopathy Questionnaire.
*LOESS fit curve between the predicted change in KCCQ overall score and change in 6-minute walk distance at 12 months.
Table 4 shows estimates of minimally important change. A 5-point change in KCCQ overall score was associated with a 2.50-ml/kg/min change in peak VO2 (95% CI, 2.21–2.86) and a 111.69-meter change in 6-minute walk distance (95% CI, 95.91–133.67).
Table 4.
Estimation of Minimally Important Change in KCCQ Overall Score at 12 Months
| Functional Capacity Variable | Parameter Estimate* | Change Corresponding to 5-Point Change in KCCQ (95% CI) |
|---|---|---|
| Peak VO2 | 2.00 | 2.50 (2.21–2.86) |
| 6-minute walk distance | 0.04 | 111.69 (95.91–133.67) |
Abbreviation: CI, confidence interval; KCCQ, Kansas City Cardiomyopathy Questionnaire.
From ordinary least squares regression model in which KCCQ overall score was regressed on the functional capacity variable, adjusted for all patient characteristics and their significant interactions.
Discussion
To our knowledge, ours is the first study to use longitudinal data to characterize relationships between changes in multiple aspects of patient-reported health status and changes in 2 commonly used functional measures of disease severity. The study population in HF-ACTION was large, relatively diverse, and balanced with respect to heart failure etiology, and the patients received evidence-based, guideline-supported therapy. We used a conservative approach to model these relationships and adjusted for 28 important potential confounders, such as age, depression, and functional class, as well as their interactions with time. As expected, increasing functional capacity was correlated with improved patient-reported health status. Changes in the KCCQ physical limitations subscale, which was designed to quantify patients’ exertional capacity, had the largest correlations with changes in functional measures of exercise capacity, consistent with previous cross-sectional analyses,23 though the differences between the physical limitations subscale and other domains were not always statistically significant.
In a cross-sectional analysis of group differences in patient-reported health status and functional capacity in HF-ACTION, there were small correlations between the KCCQ overall score and peak VO2 (r = 0.22) and 6-minute walk distance (r = 0.28) and negligible correlations between the VAS and peak VO2 (r = 0.09) and 6-minute walk distance (r = 0.11).24 Our findings examining changes over time show slightly higher correlations, though all correlations were still modest. Our findings support the clinical observation that patients with the same level of functional capacity can report different daily functioning, symptoms, and quality of life. The findings are also consistent with findings in other diseases,20 supporting the idea that patient-reported outcome measures like the KCCQ provide additional information to profiles of patient health. The KCCQ offers unique information in that it quantifies, from patients’ perspectives, the severity of heart failure symptoms and their impact on physical and social function and quality of life. This information is highly relevant to patients and should be used in conjunction with other objective outcome measures (eg, as co-primary end points in clinical trials).
Previous studies of outpatients assessed at baseline and 6 weeks set the minimally important change for the KCCQ at 5 points.5 This determination was made by expert consensus and validated against physician-rated change using a 15-point scale. Thus, another goal of our study was to understand how a 5-point change translates into changes in commonly used measures of functional capacity over a longer period of time. Given the small correlations between the measures, the uncertainty in mapping patient-reported outcome scores onto measures of functional capacity must be recognized. Certainty will be greatest where the correlation between the self-reported health status outcome and the functional capacity outcome is large. Ironically, in such a situation, the utility of assessing both types of measures will be substantially lower because it will not provide much additional information. Based on work by Wilson and Cleary,20 which posits that functional capacity determines self-reported health status, our data suggest that a 5-point change in the KCCQ is clinically relevant, inasmuch as a 5-point change in the KCCQ corresponds to changes in measures of functional capacity that can be considered clinically meaningful. For example, the metabolic equivalent has traditionally been valued at 3.5 mL/kg/min, though recent research in patients with coronary heart disease suggests an even lower baseline (range, 2.47–2.84 ml/kg/min),25 similar to the change in peak VO2 that we found to be associated with a 5-point change in KCCQ (ie, 2.5 mL/kg/min). Furthermore, the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) demonstrated that resynchronization therapy—which improves the mortality rate among patients with NYHA class III and IV heart failure symptoms26—was associated with a 1.1 mL/kg/min change in peak VO2.27 Likewise, 112-meter change in 6-minute walk distance is greater than the values considered meaningful in previous studies, such as 20% change6 or ± 25 to 45 meters.
Limitations
Our study has some limitations. First, because the data are from a clinical trial, men and younger patients may be overrepresented compared with the typical heart failure population. Second, at the time of enrollment, patients were medically stable with respect to heart failure symptoms and were willing to perform exercise on a regular basis. Furthermore, only 23 patients in NYHA class IV enrolled in the trial, so the generalizability of our findings to patients with severe heart failure symptoms is unclear. Third, we used mixed models to estimate change scores, which assume that unobserved variables do not explain the probability of missingness over and above what is explained by observed variables. Given the large number of observed variables included in the models, we believe this was an appropriate approach and that the benefits of using mixed models for this application.
Conclusions
Changes in patient-reported health status, as measured by the VAS and the KCCQ and its subscales, are not highly correlated with changes in functional capacity, including peak VO2 and 6-minute walk distance. According to the predominant model of patient outcomes,20 our findings do not alter the recommendation of considering a 5-point change in the KCCQ within individuals to be clinically meaningful.5 This information will help to inform study design and interpretation in heart failure trials and observational studies that use the VAS or KCCQ.
Acknowledgments
Funding/Support: HF-ACTION was funded by grants 5U01HL063747, 5U01HL066461, 5U01HL068973, 5U01HL066501, 5U01HL066482, 5U01HL064250, 5U01HL066494, 5U01HL064257, 5U01HL066497, 5U01HL068980, 5U01HL064265, 5U01HL066491, and 5U01HL064264 from the National Heart, Lung, and Blood Institute and grants R37AG018915 and P60AG010484 from the National Institute on Aging.
Appendix. HF-ACTION Investigators
Executive Committee: Christopher M. O’Connor, MD, Kerry L. Lee, PhD, Stephen J. Ellis, PhD, David S. Rendall, BA, PA-C (Duke University, Durham, North Carolina); David J. Whellan, MD (Thomas Jefferson University, Philadelphia, Pennsylvania); Ileana L. Piña, MD (Case Western Reserve University, Cleveland, Ohio); Steven J. Keteyian, PhD (Henry Ford Hospital, Detroit, Michigan); Lawton S. Cooper, MD, MPH, Robin Boineau, MD, Lawrence J. Fine, MD, DrPH, Jerome L. Fleg, MD, Eric S. Leifer, PhD (National Heart, Lung, and Blood Institute, Bethesda, Maryland); Virginia Erickson, RN, PhD (University of California, Los Angeles); Jonathan G. Howlett, MD (Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia, Canada); Nancy Houston Miller, RN, BSN (Stanford University, Stanford, California); Debra Isaac, MD (Foothills Hospital, Calgary, Alberta, Canada); Robert McKelvie, MD (Hamilton Health Sciences, Hamilton, Ontario, Canada); Faiez Zannad, MD, PhD (Université Henri Poincaré, Nancy, France).
Coordinating Center (Duke Clinical Research Institute, Duke University, Durham, North Carolina): Sharon Boozer, BA, Patty Connolly, Anthony Doll, BA, Stephen J. Ellis, PhD, Camille Frazier, MD, MHS, Deb Mark, Michelle McClanahan-Crowder, AA, Marcia Meyer, RN, BSN, Brenda S. Mickley, BS, David S. Rendall, BA, PA-C, Molly Rich, Hoss Rostami, BSMSE, Sharon Settles, MS, Kathy Spinella, BBA, Jessica Staib, PhD, Omar Thompson, BA.
Steering Committee (U01 Grant Sites): William Abraham, MD, Danuta Biniakiewicz, PhD, Joann Homan, RN (Ohio State University, Columbus); Vera Bittner, MD, MSPH, Meredith Fitz-Gerald, RN, BSN (University of Alabama, Birmingham); Gregory Ewald, MD, Heidi Craddock, RN, Jean Flanagan, RN, MSN (Washington University, St Louis, Missouri); Gregg Fonarow, MD, Virginia Erickson, RN, PhD (University of California, Los Angeles); Wilson Colucci, MD, Nancy Z. Lim, RN, Elena Tokareva, PhD (Boston Medical Center, Boston, Massachusetts); Rami Alharethi, MD, Ray Hershberger, MD, Deirdre Nauman (Oregon Health and Science University, Portland); Steven J. Keteyian, PhD, Matthew Saval, MS (Henry Ford Hospital, Detroit, Michigan); Dalane W. Kitzman, MD, Brittney L. Fray, MS, Brian Moore, MS (Wake Forest University, Winston-Salem, North Carolina); Ileana L. Piña, MD, Marianne Vest, MA, BSN (Case Western Reserve University, Cleveland, Ohio); Andrew Smith, MD, Gail Snell, RN, BSN, CCRC (Emory University, Atlanta, Georgia); Eugene Wolfel, MD, Mona Cantu, RN, NP (University of Colorado Hospital, Aurora).
Steering Committee (Non-U01 Grant Sites): Kirkwood Adams, MD, Jana Glotzer, RN, MSN, ACNP, Valerie Johnson, RN, Kate Schumacher (University of North Carolina Hospitals, Chapel Hill); Gordon Blackburn, PhD, Carrie Geither, RN, Susan Moore, RN, BSN (Cleveland Clinic Foundation, Cleveland, Ohio); A. Bleakley Chandler Jr, MD, Shanda Browning Vaughn, RN, Paula J. Easler, RN, CCRC, Debbie Williams (University Hospital, Augusta, Georgia); Julius M. Gardin, MD, Kelly Dimick, RN, Sharon K. Sklar, LPN, Sherri Teller, RN, CCRP (St John Hospital and Medical Center, Detroit, Michigan); Jalal K. Ghali, MD, Karen Hale-Stenson, RN (Louisiana State University Health Sciences Center, Shreveport); Mihai Gheorghiade, MD, Theresa Strzelczyk, APN, CNS (Northwestern University Medical Center, Chicago, Illinois); Maryl R. Johnson, MD, Cassondra Vander Ark, RN, MS, CCRC (University of Wisconsin, Madison); Lee R. Goldberg, MD, MPH, Andrew Kao, MD, Jennifer Dekerlegand, MPT, PhD (Hospital of the University of Pennsylvania, Philadelphia); William E. Kraus, MD, Johanna Johnson, MS, Brian D. Duscha, MS (Duke University Medical Center, Durham, North Carolina); Mandeep R. Mehra, MD, Hector Ventura, MD, Bobbett Harris, RN (Ochsner Clinic Foundation, New Orleans, Louisiana); Monica Colvin-Adams, MD, Kathy Duderstadt, BSN, Karen Meyer, RN, BSN, Melissa Steger, RN, CCRC (University of Minnesota Medical Center, Fairview); Barry Cabuay, MD, Ron M. Oren, MD, Page Scovel, RN, BSN, CCRC (University of Iowa Hospitals and Clinic, Iowa City); Andrew Kao, MD, Tracey Stevens, MD, Karen Haffey, RN, BSN, CCRC, Christy Mandacina, Ann Stewart, RN, BSN (Mid America Heart Institute, Kansas City, Missouri); Ann M. Swank, PhD, CSCS, John Manire, MS (University of Louisville, Kentucky); Paul D. Thompson, MD, Ludmila Cosio-Lima, PhD, Marie Lagasse, MS (Hartford Hospital, Hartford, Connecticut); Tehmina Naz, MD, Lynne Wagoner, MD, Susan K. Roll, RN, BSN (University of Cincinnati, Cincinnati, Ohio); Frank G. Yanowitz, MD, Johnny Walker, Adam Mueller (LDS Hospital, Salt Lake City, Utah); Peter McCullough, MD, Cathy Coleman, RN, BSN, CCRC, Kimberly A. Dorrell, Tamika Washington (William Beaumont Hospital, Royal Oak, Michigan); Eileen Handberg, PhD, James A. Hill, MD, Jacqueline Bakos, RN, Alice Boyette, Pamela Smith, Cynthia Williams, RN, BSN, MS (University of Florida, Gainesville); Dalynn Badenhop, PhD, Susan Schroeder; Kelly Walter (Medical University of Ohio, Toledo); Peter Kokkinos, PhD, Elisse Collins, Lauren Korsak, MS (VA Medical Center, Washington, DC); Eric Eichhorn, MD, Allison Leonard, RN, BSN, Tina Worley, RN, BSN (Medical City Dallas Hospital, Dallas, Texas); Gerald Fletcher, MD, Phil Peasley, RN, Pam Oldano, RN (Mayo Clinic, Jacksonville, Florida); John Kostis, MD, Nora M. Cosgrove, RN, BS (University of Medicine and Dentistry of New Jersey, New Brunswick); Udho Thadani, MD, Lisa Rogan, RN, BSN, Michelle Thresher, RN, BSN, John Turner, RN (University of Oklahoma Health Sciences Center, Oklahoma City); Denise Barnard, MD, Denise Herman, MD, Annette Contasti, RN, Marcy Sagerian, RN (University of California, San Diego Medical Center); Elizabeth Ofili, MD, Anekwe Onwuanyi, MD, Sunday Nkemdiche, MD (Morehouse School of Medicine, Atlanta, Georgia); Howard Eisen, MD, James Fitzpatrick, MD, Joyce Wald, DO, Jennie Wong, RN, CCRP (Temple University Hospital, Philadelphia, Pennsylvania); Myrvin Ellestad, MD, Leslie Kern, RN, PhD (Long Beach Memorial Medical Center, Long Beach, California); Ezra A. Amsterdam, MD, Mary J. Burns, RN (University of California-Davis Medical Center, Sacramento); Savitri Fedson, MD, Ravi K. Garg, MD, Peggy Bennett, RN, Linda Bond, RN, MSN (University of Chicago Hospitals, Chicago, Illinois); Leway Chen, MD, MPH, Janice Schrack, RN, BSN (Strong Memorial Hospital, Rochester, New York); Douglas Pearce, MD, Linda Bond, MSN, RN, Greg Palevo, Margarett Serfass, RN (Saint Thomas Hospital, Nashville, Tennessee); Daniel Forman, MD, Maria M. Lopez, Yemi Talabi-Oates, April Williams (Brigham and Women’s Hospital and Boston VA Medical Center, Boston, Massachusetts); David Truitte, MD, Cindy Baumann, RN, CCRN (Lynchburg General Hospital, Lynchburg, Virginia); Jenny Adams, PhD, Anne Lawrence, RN (Baylor Hamilton Heart and Vascular Hospital, Dallas, Texas); Dennis McNamara, MD, Emily Gruendler, RN, BSN, Virginia Schneider (University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania); Steven Hutchins, MD, Alyce Hartwick, RN (Heart Clinic Arkansas, Little Rock); Paul Campbell, MD, Michele Esposito (Northeast Medical Center, Concord, North Carolina); Carol Buchter, MD, Rebecca A. Letterer, RN, BSN (University of Washington Medical Center, Seattle); Robert Taylor, MD, Cheri Wells, RN, MSN (University of New Mexico Health Sciences Center, Albuquerque); Bruce Johnson, PhD, Beth Kaping, RN, Susan Leathes, RN (Mayo Clinic, Rochester, Minnesota); Joseph O’Bryan, MD, Lynn Langley, RN (Southwest Florida Heart Group, Fort Myers); Edward T. Hastings, MD, Cassandra Clancy, RN, CCRC (St Luke’s Medical Center, Milwaukee, Wisconsin); Neil Agruss, MD, Christine Lawless, MD, Robin Fortman, MS, APN/CNP, CCRC (Central DuPage Hospital, Winfield, Illinois); Timothy R. McConnell, PhD, Deb Wantz, MSN, RN, CCNS, CCRC (Geisinger Medical Center, Danville, Pennsylvania); Mary N. Walsh, MD, Regina Margiotti, CMS, CCRC (The Care Group, Indianapolis, Indiana); Stuart Russell, MD, Elizabeth Heck, RN, BSN (Johns Hopkins Hospital, Baltimore, Maryland); Justine Lachmann, MD, Diane Lippman, RN, Jeannette McLaughlin, RN (St Francis Hospital, Roslyn, New York); Joel Landzberg, MD, Susan Mathus, RN, BSN (Hackensack University Medical Center, Hackensack, New Jersey); David W. Cullinane, MD, Wyatt Voyles, MD, Dione Lenz, RN, Scott Kaczkowski, BS, CCRC (Medical Center of the Rockies Foundation, Loveland, Colorado); Jack L. David, MD, Eve Gillespie, MD, PhD, Pat Keane-Richmond, RN, CCRC (Glacier View Cardiology, Kalispell, Montana); Steven K. Krueger, MD, Lori Heiss, RN, BS (Bryan LGH Heart Institute, Lincoln, Nebraska); Stephen Gottlieb, MD, Nancy Greenberg, RN, BSN, MS (University of Maryland School of Medicine, Baltimore); Neil Gordon, MD, Emily Parks, BS, Melanie Willoughby, RN, BSN, CCRN (St Joseph’s/Candler Hospital, Savannah, Georgia); Marvin W. Kronenberg, MD, Jennie Glenn, RN, Carol Madison, RN (Vanderbilt University Medical Center, Nashville, Tennessee); Malcolm Arnold, MD, Julie K. Smith, RN (London Health Sciences Centre, London, Ontario, Canada); Eduardo Azevedo, MD, Glen Drobot, MD, Estrellita Estrella-Holder, RN, BN, MSA, CCNC (St Boniface General Hospital, Winnipeg, Manitoba, Canada); Jonathan Howlett, MD, Darlene Cooley-Warnell, Sheila Yarn, RN (Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia, Canada); Debra Isaac, MD, Jane Grant, RN, Kim Lyzun (Foothills Hospital, Calgary, Alberta, Canada); Marie-Helene LeBlanc, MD, Rachel Vienneau, RN, BSc (Hôpital Laval, Sainte-Foy, Quebec, Canada); Robert S. McKelvie, MD, Linda Beare, Jill Hancock, LRN (Hamilton Health Sciences, Hamilton, Ontario, Canada); Gordon Moe, MD, Delores Golob, RN, BA (St Michael’s Hospital, Toronto, Ontario, Canada); Kenneth Melvin, MD, Anne Cymet, RN, Judith Renton, RN (Toronto General Hospital, Toronto, Ontario, Canada); Anil Nigam, MD, Julie LaLonge (Montreal Heart Institute, Montreal, Quebec, Canada); Karim Djaballah, MD (Hôpital Brabois, Vandoeuvre-lès-Nancy, France); Patrick Aebehard, MD (Centre Cardiologique du Nord, Saint-Denis, France); Marie Christine Iliou, MD (Hôpital Broussais, Paris, France); Remi Sabatier, MD, Annette Belin, MD (Centre Hospitalier Universitaire de Caen, France); Alain Cohen-Solal, MD (Hôpital Beaujon, Clichy, France); Luc Hittinger, MD (Hôpital Henri Mondor, Créteil, France).
Data and Safety Monitoring Board: Bertram Pitt, MD (chair), (University of Michigan, Ann Arbor); Philip A. Ades, MD (University of Vermont, South Burlington); Lotfy L. Basta, MD (San Francisco, California); Victor Froelicher, MD (Stanford University and Palo Alto VA Medical Center, Palo Alto, California); Mary Elizabeth Hamel, MD (Beth Israel Deaconess Medical Center, Boston, Massachusetts); Barry M. Massie, MD (San Francisco VA Hospital, San Francisco, California); Lemuel Moyé, MD, PhD (University of Texas Health Science Center, Houston); Lynda H. Powell, PhD (Rush University, Chicago, Illinois).
Cardiopulmonary Exercise Core Lab: William E. Kraus, MD (director); Johanna Johnson, MS, Lucy Piner, MS (Duke University, Durham, North Carolina); Daniel Bensimhon, MD (codirector) (LeBauer Cardiovascular Research Foundation, Greensboro, North Carolina); Stuart Russell, MD (Johns Hopkins University, Baltimore, Maryland).
Echo Core Lab: Julius M. Gardin, MD (director); Renee L. Bess, BS, RDCS, RVT, Gerald I. Cohen, MD (St John Hospital and Medical Center, Detroit, Michigan).
Heart Rate Training Core Lab: Steven J. Keteyian, PhD (director); Jonathan K. Ehrman, PhD (associate director); Clinton A. Brawner, MS (coordinator) (Henry Ford Hospital, Detroit, Michigan).
Adherence Core Lab: Nancy Houston Miller, RN, BSN (codirector) (Stanford University, Stanford, California); James A. Blumenthal, PhD (codirector); Krista Barbour, PhD (Duke University, Durham, North Carolina); Tanya M. Spruill, PhD (Columbia University, New York, New York); Bess Marcus, PhD (Brown Medical School and Miriam Hospital, Providence, Rhode Island); Jim Raczynski, MD (University of Arkansas for Medical Sciences, Little Rock).
Biomarker and DNA Core Lab: Kirkwood Adams, MD (University of North Carolina, Chapel Hill); Mark Donahue, MD, Mike Felker, MD (Duke University, Durham, North Carolina).
Economics and Quality of Life Group: Kevin A. Schulman, MD, Kevin P. Weinfurt, PhD, Kathryn E. Flynn, PhD, Shelby Reed, PhD, Ann Burnette, BS, Linda Davidson-Ray, MA, Joëlle Y. Friedman, MPA, Yanhong Li, MS, Li Lin, MS, Betsy O’Neal, BA (Duke University, Durham, North Carolina).
Clinical End Point Committee: Michael Zile, MD (chair), Ralph H. Johnson (VA Medical Center and Medical University of South Carolina, Charleston); Daniel R. Bensimhon, MD (LeBauer Cardiovascular Research Foundation, Greensboro, North Carolina); Vera Bittner, MD, MSPH (University of Alabama, Birmingham); Robin Boineau, MD (National Heart, Lung, and Blood Institute, Bethesda, Maryland); Mark E. Dunlap, MD (Case Western Reserve University, Cleveland, Ohio); William E. Kraus, MD, Christopher M. O’Connor, MD (Duke University, Durham, North Carolina); Gordon Moe, MSC, MD, FRCP(C) (St Michael’s Hospital, Toronto, Ontario, Canada); John Wertheimer, MD (Thomas Jefferson University and Drexel University Medical Residents at Abington Memorial Hospital, Philadelphia, Pennsylvania); David J. Whellan, MD (Jefferson Medical College, Philadelphia, Pennsylvania).
Footnotes
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Additional Contributions: Stephen J. Ellis, PhD, Duke University, suggested consideration of alternative modeling approaches to estimate minimally important change. Damon M. Seils, MA, Duke University, assisted with manuscript preparation. Dr Ellis and Mr Seils did not receive compensation for their assistance apart from their employment at the institution where the study was conducted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spertus JA. Evolving applications for patient-centered health status measures. Circulation. 2008;118:2103–10. doi: 10.1161/CIRCULATIONAHA.107.747568. [DOI] [PubMed] [Google Scholar]
- 2.Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2006 doi: 10.1186/1477-7525-4-79. (Accessed at http://www.fda.gov/CDER/GUIDANCE/5460dft.pdf.) [DOI] [PMC free article] [PubMed]
- 3.Patrick DL, Burke LB, Powers JH, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10 (Suppl 2):S125–37. doi: 10.1111/j.1524-4733.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 4.Luther SA, McCullough PA, Havranek EP, et al. The relationship between B-type natriuretic peptide and health status in patients with heart failure. J Card Fail. 2005;11:414–21. doi: 10.1016/j.cardfail.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–15. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Masoudi FA, Rumsfeld JS, Havranek EP, et al. Age, functional capacity, and health-related quality of life in patients with heart failure. JCard Fail. 2004;10:368–73. doi: 10.1016/j.cardfail.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, New Jersey: Lawrence Erlbaum Associates, Publishers; 1988. [Google Scholar]
- 8.Whellan DJ, O’Connor CM, Lee KL, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–11. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–55. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 10.Schweikert B, Hahmann H, Leidl R. Validation of the EuroQol questionnaire in cardiac rehabilitation. Heart. 2006;92:62–7. doi: 10.1136/hrt.2004.052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eurich DT, Johnson JA, Reid KJ, Spertus JA. Assessing responsiveness of generic and specific health related quality of life measures in heart failure. Health Qual Life Outcomes. 2006;4:89. doi: 10.1186/1477-7525-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konstam MA, Neaton JD, Poole-Wilson PA, et al. Comparison of losartan and captopril on heart failure-related outcomes and symptoms from the losartan heart failure survival study (ELITE II) Am Heart J. 2005;150:123–31. doi: 10.1016/j.ahj.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 13.Wilson JR. Exercise and the failing heart. Cardiol Clin. 1987;5:171–81. [PubMed] [Google Scholar]
- 14.Bittner V, Weiner DH, Yusuf S, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators JAMA. 1993;270:1702–7. [PubMed] [Google Scholar]
- 15.Bryk AS, Raudenbush SW. Hierarchical Linear Models. Newbury Park: 1992. [Google Scholar]
- 16.Gibbons RD, Hedeker D, Elkin I, et al. Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Application to the NIMH treatment of Depression Collaborative Research Program dataset. Arch Gen Psychiatry. 1993;50:739–50. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- 17.Schafer JL. Analysis of Incomplete Multivariable Data. Chapman & Hall/CRC; 1997. [Google Scholar]
- 18.Flynn KE, Pina IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–9. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng XL, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychological Bulletin. 1992;111:172–5. [Google Scholar]
- 20.Wilson IIB, Cleary PPD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
- 21.Isobe T, Feigelson ED, Akritas MG, Babu GJ. Linear Regressions for Astronominy. Astrophysical Journal. 1990;364:104–13. [Google Scholar]
- 22.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 23.Myers J, Zaheer N, Quaglietti S, Madhavan R, Froelicher V, Heidenreich P. Association of functional and health status measures in heart failure. JCard Fail. 2006;12:439–45. doi: 10.1016/j.cardfail.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Flynn KE, Lin L, Ellis SJ, et al. Outcomes, health policy, and managed care: relationships between patient-reported outcome measures and clinical measures in outpatients with heart failure. Am Heart J. 2009;158:S64–71. doi: 10.1016/j.ahj.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savage PD, Toth MJ, Ades PA. A re-examination of the metabolic equivalent concept in individuals with coronary heart disease. J Cardiopulm Rehabil Prev. 2007;27:143–8. doi: 10.1097/01.HCR.0000270693.16882.d9. [DOI] [PubMed] [Google Scholar]
- 26.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 27.Young JB, Abraham WT, Smith AL, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685–94. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 28.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]