Abstract
In many social animals, group-mates cooperate to defend their range against intrusion by neighboring groups. Because group size tends to be highly variable, such conflicts are often asymmetric. Although numerical superiority is assumed to provide a competitive advantage, small groups can generally defend their ranges, even when greatly outnumbered. The prevailing explanation for this puzzling phenomenon is that individuals in relatively large groups experience a greater temptation to flee from conflicts, in effect leveling the balance of power. Using playback experiments simulating territorial intrusions by wild capuchin monkey (Cebus capucinus) groups, we show that such a collective action problem does indeed undermine the competitive ability of large groups. Focal capuchins were more likely to run away from territorial intrusions when their group had a numeric advantage; each one-individual increase in relative group size raised the odds of flight by 25%. However, interaction location had a more important impact on individuals' reactions, creating a strong home-field advantage. After controlling for relative group size, the odds that a focal animal fled were 91% lower in experiments that occurred in the center compared with on the edge of its group's range, whereas the odds that it rushed toward the speaker were more than sixfold higher. These location-dependent patterns of defection and cooperation create a competitive advantage for residents over intruders across a wide range of relative group sizes, which may stabilize range boundaries and provide a general explanation for how groups of widely divergent sizes can coexist, even in the face of intense intergroup competition.
Keywords: intergroup aggression, territoriality, resource holding potential, Barro Colorado Island, Panama
In social animals ranging from ants (1) to humans (2), group-mates cooperate to defend shared resources and repel territorial intrusions by neighboring groups. A fundamental assumption of many models of social evolution is that large group size confers a competitive advantage in such contests (3, 4), thus providing an important benefit of group living. Empirical data, however, are equivocal. Competitive ability increases with group size in many species (1, 2, 5–7), but numerical superiority rarely ensures victory (8, 9), and a link between group size, group strength, and individual reproductive success has been demonstrated in only a handful of species (7, 10, 11). Though field observations have revealed that intergroup conflicts can lead to the displacement (8), dispossession (12, 13), and even the extinction (14–16) of weak social groups, these extreme outcomes are rare. Despite the fitness benefits that can be gained by annexing neighbors' ranges, large groups rarely usurp the territories of their smaller neighbors. Why can't (or why don't) large groups exploit their competitive advantage more effectively? Prevailing hypotheses propose that because territorial defense in group-living species is a collaborative act, it is subject to a classic collective action problem (17), which disproportionately affects the competitive ability of large social groups (18). Compared with their counterparts in small groups, members of large groups are predicted to face a greater temptation to hold back during intergroup conflicts and allow their group-mates to assume the risks associated with fighting. However, the dynamics of home-range ownership provide an alternate explanation. We propose that location-based payoff asymmetries play a key role in decreasing power asymmetries between unevenly matched groups, giving resident groups a competitive advantage over intruding groups, regardless of their size. Many animal species behave as if they value central portions of their home range more highly than peripheral areas (19), and thus the motivation of a resident individual to participate in an intergroup conflict may be substantially higher than that of a member of the intruding group. Such center/edge effects, although well known in territorial birds (20), have rarely been reported in social species (21–23), and the role that they play in mediating the balance of power between competing social groups remains unknown. Here, we use playback experiments simulating territorial intrusions by wild capuchin monkey (Cebus capucinus) groups to investigate how group-size asymmetries and resident/intruder status influence patterns of individual participation in intergroup conflicts, and determine the relative importance of these forces for shaping the balance of power among capuchin social groups.
Results
The subjects of this study were members of four wild white-faced capuchin social groups on Barro Colorado Island, Panama (9°9′ N, 79°51′ W). We previously demonstrated in this population that the outcome of aggressive encounters among six neighboring groups depended on both the relative size of the competing groups and the location of the interaction (8). Numerical superiority conferred a competitive advantage, but the effect was not uniform across space; small groups near the center of their own home range consistently defeated larger neighbors.
To investigate how individual behavioral strategies give rise to this pattern, we conducted 27 playback experiments on four of our original study groups, simulating territorial intrusions by groups of different sizes in different locations (Table S1). We tested whether the probability that a focal animal rushed toward the speaker (i.e., participated in group defense) was affected by its sex, the relative size of its group (the number of adults in the focal group minus the number of adults in the playback group), and the location of the simulated encounter (range center vs. edge). Previous studies of intergroup competition in capuchins have reported that although individual participation is highly variable, males tend to take a more active role than females (24, 25). Consistent with these results, we found that the odds of participating were 80% lower for females than males [generalized estimating equation (GEE) multiple logistic regression: X21 = 11.56, P = 0.0007; Table 1]. Nevertheless, after statistically controlling for sex, the odds that a focal animal participated were 683% higher if the playback occurred near the center vs. the edge of the range (X21 = 4.54, P = 0.033). This is strong evidence that both males and females were more motivated to confront intruders in the exclusively used, and thus high-value, center of their range, than in the shared areas along its border.
Table 1.
Individual participation in simulated territorial intrusions
| Variable | Parameter estimate | SE | χ2 | P |
| Intercept | −2.62 | 1.02 | 6.55 | 0.01 |
| Sex | ||||
| Female | −1.59 | 0.47 | 11.56 | 0.0007 |
| Male | — | — | — | — |
| Location | ||||
| Center | 2.06 | 0.97 | 4.54 | 0.033 |
| Edge | — | — | — | — |
| Relative group size | −0.17 | 0.14 | 1.59 | 0.206 |
| Relative group size by location | ||||
| Center | 0.3 | 0.16 | 3.53 | 0.061 |
| Edge | — | — | — | — |
Multiple logistic regression of the probability that the focal animal rushed toward the playback speaker vs. sex, location, relative group size, and the relative group size × location interaction term. Nonsignificant terms were removed. n = 54 focal samples from 27 playback experiments.
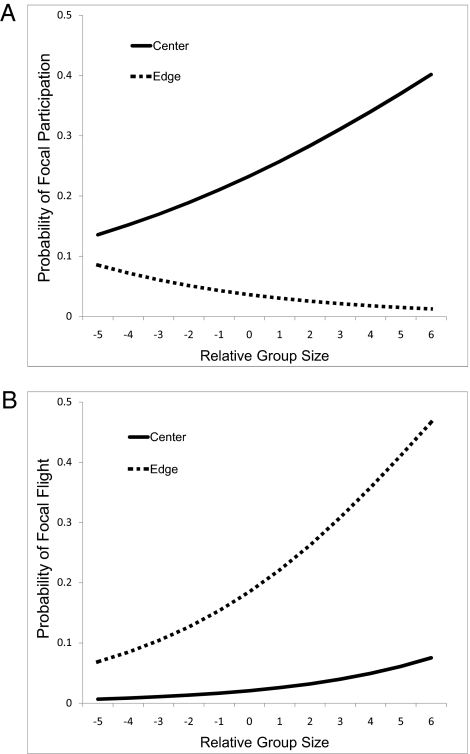
Location is not the only factor that can influence the payoffs associated with participating in intergroup conflicts; individuals are also expected to weigh the potential costs of engaging in territorial defense. If the probability of participation is independent of group size, then individuals should be more willing to participate in intergroup conflicts when they outnumber their opponents (26) and their risk of injury is accordingly low (27). Indeed, when faced with simulated incursions in the center of their range, focal animals tended to be more likely to move to confront the intruders when their group had a numeric advantage (X21 = 3.53, P = 0.06; Fig. 1A and Table 1). Large relative group size did not, however, increase the odds of focal participation in experiments conducted at the edge of the group's range (Table 1 and Fig. 1A), indicating that low costs are not always sufficient to ensure cooperation, especially if the benefits of winning are likely to be small.
Fig. 1.
Predicted values from a multiple logistic regression of the probability that the focal animal (A) rushed toward and (B) ran away from the playback speaker as a function of relative group size and location.
Individual participation in cooperative territorial defense is expected to be subject to a classic collective action problem (18, 28). Whenever possible, individuals should rely on the efforts of their group-mates rather than participating themselves, enjoying the benefits of defense without experiencing the costs of fighting. Our results show that groups are more susceptible to the collective action problem when they outnumber their opponent. Individuals were more likely to run away from the speaker when their group had a numeric advantage, regardless of location (X21 = 5.06, P = 0.03; Table 2). This pattern of cheating suggests that the risk of injury does not drive individuals to avoid territorial conflicts; instead, animals run away when doing so will have relatively little impact on the final outcome. Participation by a particular individual may be critical to the competitive success of a small group but irrelevant to that of a big group. The odds of defection also depended strongly on the location of the contested area. After controlling for relative group size, the odds that a focal animal fled were 91% greater at the edge than in the center of its group's range (GEE multiple logistic regression: X21 = 4.28, P = 0.04; Table 2 and Fig. 1B).
Table 2.
Individual flight from simulated territorial intrusions
| Variable | Parameter estimate | SE | χ2 | P |
| Intercept | −1.48 | 0.58 | 6.55 | 0.011 |
| Location | ||||
| Center | −2.3711 | 1.15 | 4.28 | 0.039 |
| Edge | — | — | — | — |
| Relative group size | 0.22 | 0.1 | 5.06 | 0.025 |
Multiple logistic regression of the probability that the focal animal ran away from the playback speaker vs. sex, location, relative group size, and the relative group size × location interaction term. Nonsignificant terms were removed. n = 54 focal samples from 27 playback experiments.
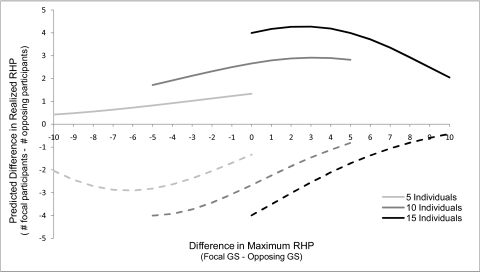
Variation in group members' motivation to participate in, or run away from, territorial conflicts should have a profound impact on the competitive ability of their social groups. A group's overall strength—its maximum resource holding potential (RHP) (29, 30)—may be relatively constant, but its competitive ability in any given fight, its realized RHP, is expected to fluctuate depending on patterns of individual participation and defection. When group members’ interests are perfectly aligned, the realized RHP of their group should be the same as its maximum RHP (i.e., all available members should fight). However, when conflicts of interest among group members arise, a group's realized RHP may be significantly lower than the maximum that could have been achieved if all group-mates had cooperated fully. Although we were unable to determine the total number of individuals that approached the simulated intruders in our experiments because dense foliage limited visibility, the odds that at least one member of the focal group arrived at the playback speaker (the strongest level of response to the playback we observed) increased almost 14-fold if the playback occurred in the center vs. on the edge of the group's range, regardless of the relative size of the groups (GEE multiple logistic regression, X21 = 6.97, P = 0.008; Table 3). Additionally, there was a statistically significant interaction between playback location and relative group size (Table 3); in the center of the focal group's range, each one-individual increase in relative group size increased the odds of group members arriving at the speaker by 25%. To better understand the relationship between the relative size of competing groups and their competitive ability, we used the parameters from our logistic regression analysis of individual participation to extrapolate the difference in a group's realized RHP as a function of relative group size and the location of the interaction (Fig. 2). Both this model and the group-level response to our playback experiments show that although groups that outnumbered their opponents were able to convert that numerical superiority to a competitive advantage when defending the center of their range against neighboring intruders, they failed to do so when they themselves encroached into the ranges of neighboring groups.
Table 3.
Group-level response to simulated territorial intrusions
| Variable | Parameter estimate | SE | χ2 | P |
| Intercept | −2.9 | 0.95 | 9.36 | 0.002 |
| Location | ||||
| Center | 2.68 | 1.02 | 6.97 | 0.008 |
| Edge | — | — | — | — |
| Relative group size | −0.26 | 0.16 | 2.56 | 0.111 |
| Relative group size by location | ||||
| Center | 0.49 | 0.03 | 4.37 | 0.036 |
| Edge | — | — | — | — |
Multiple logistic regression of participation (arrival at the playback speaker) by at least one member of the focal group vs. location, relative group size, and the relative group size × location interaction term. n = 27 playback experiments.
Fig. 2.
Predicted difference in the number of active participants (realized RHP) as a function of size, relative to the opposing group, and the location of the territorial conflict. Values were extrapolated from experimental data on the approach/retreat response of individual animals, as described in the text. Solid lines represent conflicts occurring in the center, and dashed lines represent conflicts at the edge of the focal group's range. We assume that territorial conflicts in the center of the focal group's range occur on the edge of the opposing group's range, and vice versa. When defending the center of their range, focal groups are always predicted to have more active participants (greater realized RHP) than their opponents, even if they are greatly outnumbered. In contrast, capuchin groups, no matter how many members they have, are always predicted to have fewer active participants than their opponent in territorial conflicts that occur at the edge of their range.
Discussion
Despite the important role that collective action problems play in theoretical models of animal cooperation (18, 31), few studies have demonstrated context-dependent patterns of defection in wild animals (32). Our results demonstrate that collective action problems are real and indeed pose a significant challenge that social species must overcome. The patterns of individual participation and defection that we document create context-dependent variability in the realized RHP of capuchin social groups, with important implications for intergroup relationships. The tendency for individuals (and hence groups) to respond more vigorously to territorial challenges in the center of their range and to fall victim to a collective action problem near the borders creates a home-field advantage that stabilizes range boundaries and prevents large groups from successfully invading the ranges of their weaker neighbors (8). We demonstrate that fluctuations in the balance of power between competing groups arise from a set of behavioral responses to the costs and benefits of cooperative territorial defense, which are undoubtedly shared with other social species, and thus provide a general explanation for how groups of widely divergent sizes can coexist.
Our findings challenge a key assumption of models of social evolution (3, 4)—namely, that large group size provides an advantage in intergroup resource competition. Although our large groups often enjoyed increased competitive ability compared with smaller groups, their advantage was not unconditional; the balance of power between groups fluctuated in a location-dependent manner. A better understanding of the causes of these fluctuations, including the role that genetic relatedness plays in shaping patterns of individual cooperation and defection, is key to resolving a long-standing debate regarding the role of between-group competition in the evolution of social species (3, 33). Specifically, further research is required to determine if certain species or groups are better able to overcome the collective action problem posed by cooperative territorial defense and, if so, how? These questions are crucial for determining the importance of intergroup conflict as a selective pressure and, as intergroup conflict is hypothesized to have played a central role in human evolution (34–36) and contributed to our development of behavioral strategies that mitigate collective action problems (37, 38), will yield important insight into our own evolutionary history.
Materials and Methods
Animal Tracking and Home-Range Estimation.
One or two adults in each of the four focal groups were captured and fitted with radio collars (39), and their movements were tracked using an automated radio telemetry system (8). We used these data to calculate 50% and 95% fixed-kernel home ranges (40) for each study group using the program BIOTAS (Ecological Software Solutions, LLC), and to ensure that focal groups had not interacted with any of their neighbors on days we conducted playback experiments.
Playback Experiments.
To investigate the causes of variation in individual- and group-level responses to territorial intrusions, we broadcast vocalizations of neighboring capuchin social groups of different sizes within the range of each of our study groups. Playback experiments were conducted either in the center (defined as within the 50% kernel home range) or on the edge (defined as within 100 m of the 95% kernel home range boundary) of the focal group's range (Fig. S1). The location of the focal group was verified using a hand-held GPS unit (Garmin GPSMAP 60CSx; Garmin Ltd.) before the start of each experiment. Vocalizations were recorded using a Marantz PMD660 portable recorder (Marantz America, Inc.) and a Sennheiser ME66 shotgun microphone (Sennheiser Electronic Corp.). The auditory stimuli were meant to simulate the presence of another group and consisted of 1 min of group feeding noises, including food-associated calls and the sounds of falling fruits and moving monkeys, punctuated half-way through by screams associated with a within-group fight. Each stimulus consisted of calls from a single group. We attempted to standardize stimuli by ensuring that (i) group feeding noises included vocalizations from individuals of all age/sex classes, (ii) the screams we used were made by adults in the context of a within-group fight related to food, and (iii) the duration of the scream component was consistent across stimuli (∼5 s). Playback volume was adjusted to ensure that the sound pressure level of the screams was between 65 and 69 dB at a distance of 5 feet from the speaker, a criterion we selected based on measures of the sound pressure level of screams made in the field. A total of 12 stimuli were made using Raven Lite 1.0 software (41) and were broadcast from an iPod (Apple, Inc.) using a MiniVox Lite speaker (Anchor Audio, Inc.). Each stimulus was broadcast only once to each of the study groups, and experiments were not conducted if there had been an aggressive encounter between the focal group and any of their neighbors that day. To facilitate logistics, and make behavioral responses to the experiments easier to identify, we conducted playbacks only when the focal group was not traveling. During playback experiments, the speaker was placed ∼80 m from the focal individuals (measured using a Garmin GPSMAP 60CSx; Garmin Ltd.) in the direction of the home range of the group whose vocalizations were being broadcast. We selected two adults (usually one male and one female) as the focal individuals for each experiment. Focals were never part of the same subgroup (defined as within five body lengths of one another), and in most cases were in different trees. Females with dependent offspring were not selected as focal individuals because the presence of a vulnerable infant might reasonably be expected to influence whether a female chooses to participate in potentially dangerous intergroup interactions. Observers (one per focal individual) recorded the capuchins' reaction to the simulated territorial intrusion, including whether they approached or retreated from the speaker. A focal individual was scored as approaching if it left the tree it was in and moved at least 5 m toward the speaker at an angle of ≤45°. Similarly, to be categorized as retreating, a focal had to leave the crown of its tree and move at least 5 m away from the speaker at an angle of ≥135°. In these analyses, we consider only immediate responses to the experiment, meaning movements that were initiated during the playback. Focal individuals were followed for 10 min or until they rejoined their group or resumed habitual activities, such as foraging, feeding, or resting. Because of highly obstructed viewing conditions, we were unable to confidently determine the number or identity of all group members who approached the speaker. However, we did record the total number of group members who actually arrived at the playback speaker.
Data Analysis.
We conducted three multiple logistic regressions to examine the factors affecting the response to a simulated encounter with a neighboring social group. We used the GEE method (PROC GENMOD) in SAS 9.2 (SAS Institute) to control for multiple experimental treatments on the same combination of social groups (e.g., five playbacks of vocalization from FC group to BLT group). In all three regressions, the explanatory variables were (i) location (center/edge), (ii) relative group size (number of adults in the focal group minus the number of adults in the playback group), (iii) distance between focal group and the speaker, and (iv) location × relative group size interaction term. We did not have the statistical power to parcel out the effects of absolute and relative group size, because these variables were highly correlated. For regressions 1 and 2, we included sex of the focal animal as an additional explanatory variable. We followed a backward elimination procedure, removing variables with P > 0.05.
In the first regression (Table 1), we asked whether the focal individual rushed toward the speaker (y/n). Distance to the speaker was not statistically significant (P = 0.50) and was removed from the model. In the second regression, we modeled the probability that the focal individual ran away from the speaker. We sequentially removed sex (P = 0.42), location × relative group size (P = 0.89), and distance to the speaker (0.37) to generate the final model (Table 2). Finally, we used the third regression to examine the factors affecting the probability that at least one adult in the experimental group arrived at the location of the playback speaker. Again, distance to the speaker was not statistically significant (P = 0.87) and was removed, yielding the final model (Table 3).
All research described in this paper was approved by the Institutional Animal Care and Use Committee at the Smithsonian Tropical Research Institute (assurance no. 2008-03-12-08) and complied with the laws of the Republic of Panama.
Supplementary Material
Acknowledgments
Important logistical support was provided by members of the Automated Radio Telemetry System Initiative, particularly Daniel Obando, Alejandro Ortega, Roland Kays, and Martin Wikelski, and by the staff at the Smithsonian Tropical Research Institute field station on Barro Colorado Island. We thank Lucia Tórrez, Nena Robles, and Anyuri González for help conducting the experiments; Robert Lessnau, Rose Laughter, George Middleton, and Oldemar Valdes for assistance with animal capture; and Ben Hirsch, Martin Wikelski, Richard Wrangham, Daniel Rubenstein, Stefan Schnitzer, Eldredge Bermingham, two anonymous reviewers, and the editor of the article for comments on a previous draft of this manuscript. We thank the Autoridad Nacional del Ambiente and the government of the Republic of Panama for permission to conduct this research. Funding for this work was provided by the Smithsonian Tropical Research Institute, the Wenner-Gren Foundation for Anthropological Research, Princeton University, and the Max Planck Institute for Ornithology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115937109/-/DCSupplemental.
References
- 1.Hölldobler B. Foraging and spatiotemporal territories in the Honey Ant Myrmecocystus mimicus Wheeler (Hymenoptera: Formicidae) Behav Ecol Sociobiol. 1981;9:301–314. [Google Scholar]
- 2.Keeley LH. War Before Civilization. New York: Oxford Univ Press; 1996. [Google Scholar]
- 3.Wrangham RW. An ecological model of female-bonded primate groups. Behaviour. 1980;75:262–300. [Google Scholar]
- 4.Isbell LA. Contest and scramble competition: Patterns of female aggression and ranging behavior among primates. Behav Ecol. 1991;2(2):143–155. [Google Scholar]
- 5.Kruuk H, Macdonald D. Group territories of carnivores: Empires and enclaves. In: Sibly RM, Smith RH, editors. Behavioural Ecology: Ecological Consequences of Adaptive Behaviour. Oxford: Blackwell Scientific; 1984. pp. 521–536. [Google Scholar]
- 6.Cheney DL. Interactions and relations between groups. Primate Societies. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Chicago: Univ of Chicago Press; 1987. pp. 267–281. [Google Scholar]
- 7.Mosser A, Packer C. Group territoriality and the benefits of sociality in the African lion, Panthera leo. Anim Behav. 2009;78:359–370. [Google Scholar]
- 8.Crofoot MC, Gilby IC, Wikelski MC, Kays RW. Interaction location outweighs the competitive advantage of numerical superiority in Cebus capucinus intergroup contests. Proc Natl Acad Sci USA. 2008;105:577–581. doi: 10.1073/pnas.0707749105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radford AN, Du Plessis MA. Territorial vocal rallying in the green woodhoopoe: Factors affecting contest length and outcome. Anim Behav. 2004;68:803–810. [Google Scholar]
- 10.Robinson JG. Group-size in wedge-capped capuchin monkeys, Cebus olivaceus, and the reproductive success of males and females. Behav Ecol Sociobiol. 1988;23(3):187–197. [Google Scholar]
- 11.Williams J, Oehlert G, Carlis J, Pusey A. Why do male chimpanzees defend a group range? Anim Behav. 2004;68:523–532. [Google Scholar]
- 12.Mitani JC, Watts DP, Amsler SJ. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr Biol. 2010;20:R507–R508. doi: 10.1016/j.cub.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Jolly A, Pride E. Troop histories and range inertia of Lemur catta at Berenty, Madagascar: A 33-year perspective. Int J Primatol. 1999;20:359–373. [Google Scholar]
- 14.Nishida T, Hiraiwahasegawa M, Hasegawa T, Takahata Y. Group extinction and female transfer in wild chimpanzees in the Mahale National Park, Tanzania. Zeitschrift Fur Tierpsychologie-Journal of Comparative Ethology. 1985;67:284–301. [Google Scholar]
- 15.Sugiura H, Agetsuma N, Suzuki S. Troop extinction and female fusion in wild Japanese macaques in Yakushima. Int J Primatol. 2002;23(1):69–84. [Google Scholar]
- 16.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Cambridge, MA: Harvard Univ Press; 1986. [Google Scholar]
- 17.Sterck EHM, Watts DP, vanSchaik CP. The evolution of female social relationships in nonhuman primates. Behav Ecol Sociobiol. 1997;41:291–309. [Google Scholar]
- 18.Olson M. The Logic of Collective Action. Cambridge, MA: Harvard Univ Press; 1965. [Google Scholar]
- 19.Davies NB, Houston AI. Territory economics. In: Krebs JR, Davies NB, editors. Behavioural Ecology: An Evolutionary Approach. 2nd Ed. Oxford: Blackwell Scientific; 1984. pp. 148–169. [Google Scholar]
- 20.Giraldeau LA, Ydenberg R. The center-edge effect: The result of a war of attrition between territorial contestants? Auk. 1987;104:535–538. [Google Scholar]
- 21.Wich SA, Assink PR, Becher F, Sterck EHM. Playbacks of loud calls to wild Thomas langurs (primates; Presbytis thomasi): The effect of location. Behaviour. 2002;139(1):65–78. [Google Scholar]
- 22.Whitehead JM. Vocally mediated reciprocity between neighboring groups of mantled howler monkeys Alouatta palliata palliata. Anim Behav. 1987;35:1615–1627. [Google Scholar]
- 23.Furrer RD, Kyabulima S, Willems EP, Cant MA, Manser MB. Location and group size influence decisions in simulated intergroup encounters in banded mongooses. Behav Ecol. 2011;22:493–500. [Google Scholar]
- 24.Crofoot MC. Mating and feeding competition in white-faced capuchins (Cebus capucinus): The importance of short- and long-term strategies. Behaviour. 2007;144:1473–1495. [Google Scholar]
- 25.Perry S. Intergroup encounters in wild white-faced capuchins (Cebus capucinus) Int J Primatol. 1996;17:309–330. [Google Scholar]
- 26.Kitchen DM, Beehner JC. Factors affecting individual participation in group-level aggression among non-human primates. Behaviour. 2007;144:1551–1581. [Google Scholar]
- 27.Manson JH, Wrangham RW. Intergroup aggression in chimpanzees and humans. Curr Anthropol. 1991;32:369–390. [Google Scholar]
- 28.Nunn CL, Deaner RO. Patterns of participation and free riding in territorial conflicts among ringtailed lemurs (Lemur catta) Behav Ecol Sociobiol. 2004;57(1):50–61. [Google Scholar]
- 29.Maynard Smith J, Parker GA. The logic of asymmetric contests. Anim Behav. 1976;24(1):159–175. [Google Scholar]
- 30.Parker GA. Assessment strategy and the evolution of fighting behaviour. J Theor Biol. 1974;47:223–243. doi: 10.1016/0022-5193(74)90111-8. [DOI] [PubMed] [Google Scholar]
- 31.Nunn CL, Lewis RJ. Cooperation and collective action in animal behaviour. In: Noe R, van Hooff JARAM, Hammerstein P, editors. Economics in nature. Cambridge: Cambridge Univ Press; 2001. pp. 42–66. [Google Scholar]
- 32.Heinsohn R, Packer C. Complex cooperative strategies in group-territorial African lions. Science. 1995;269:1260–1262. doi: 10.1126/science.7652573. [DOI] [PubMed] [Google Scholar]
- 33.van Schaik CP. Why are diurnal primates living in groups? Behaviour. 1983;87(1/2):120–143. [Google Scholar]
- 34.Avilés L. Solving the freeloaders paradox: Genetic associations and frequency-dependent selection in the evolution of cooperation among nonrelatives. Proc Natl Acad Sci USA. 2002;99:14268–14273. doi: 10.1073/pnas.212408299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowles S. Did warfare among ancestral hunter-gatherers affect the evolution of human social behaviors? Science. 2009;324:1293–1298. doi: 10.1126/science.1168112. [DOI] [PubMed] [Google Scholar]
- 36.Boyd R, Gintis H, Bowles S, Richerson PJ. The evolution of altruistic punishment. Proc Natl Acad Sci USA. 2003;100:3531–3535. doi: 10.1073/pnas.0630443100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saaksvuori L, Mappes T, Puurtinen M. Costly punishment prevails in intergroup conflict. Proc Royal Soc B Biol Sci. 2011;278:3428–3436. doi: 10.1098/rspb.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burton-Chellew MN, Ross-Gillespie A, West SA. Cooperation in humans: Competition between groups and proximate emotions. Evol Hum Behav. 2010;31(2):104–108. [Google Scholar]
- 39.Crofoot MC, et al. Field anesthesia and health assessment of free ranging white-faced capuchin monkeys (Cebus capucinus) in Panama. Int J Primatol. 2009;30(1):125–141. [Google Scholar]
- 40.Worton BJ. Kernel methods for estimating the utilization distribution in home-range studies. Ecology. 1989;70(1):164–168. [Google Scholar]
- 41.Charif RA, Ponirakis DW, Krein TP. Raven Lite 1.0. Ithaca, NY: Cornell Laboratory of Ornithology; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.