Abstract
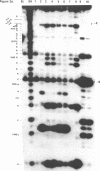
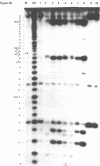
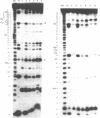
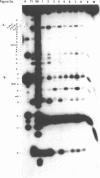
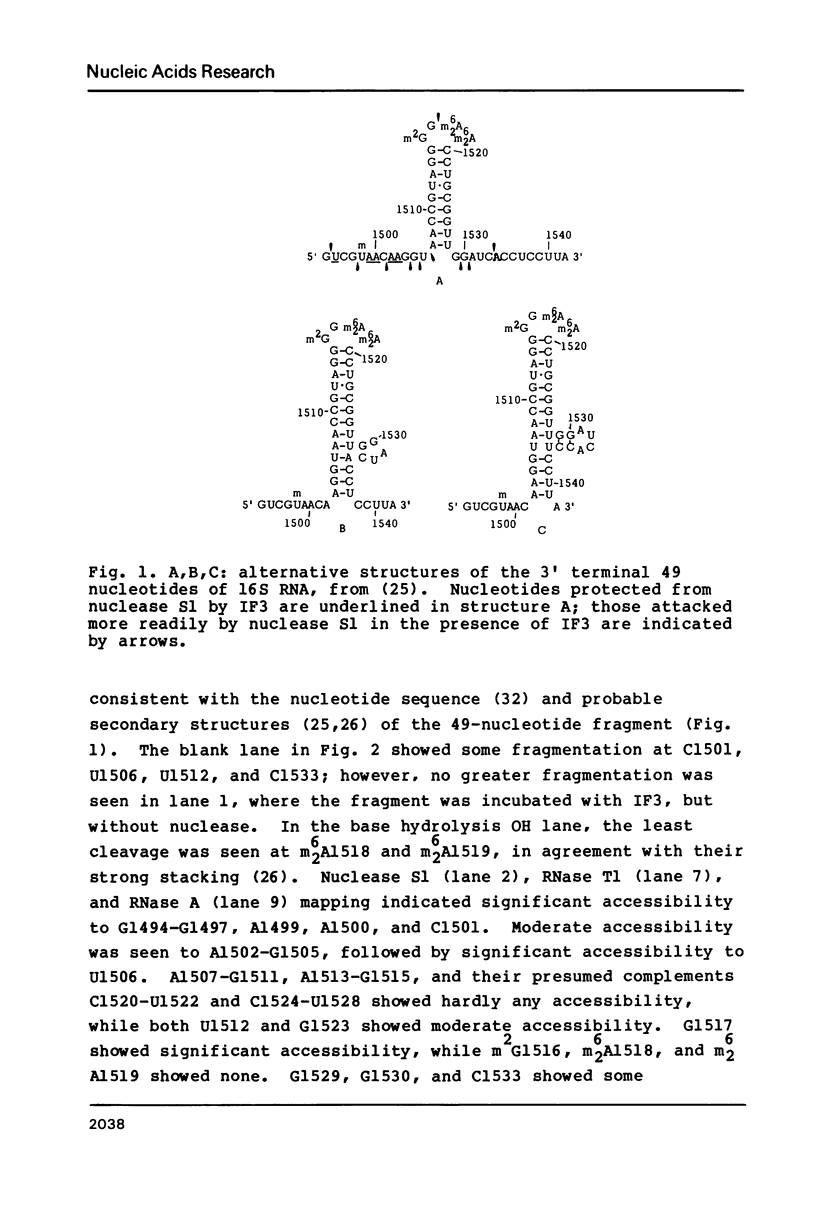
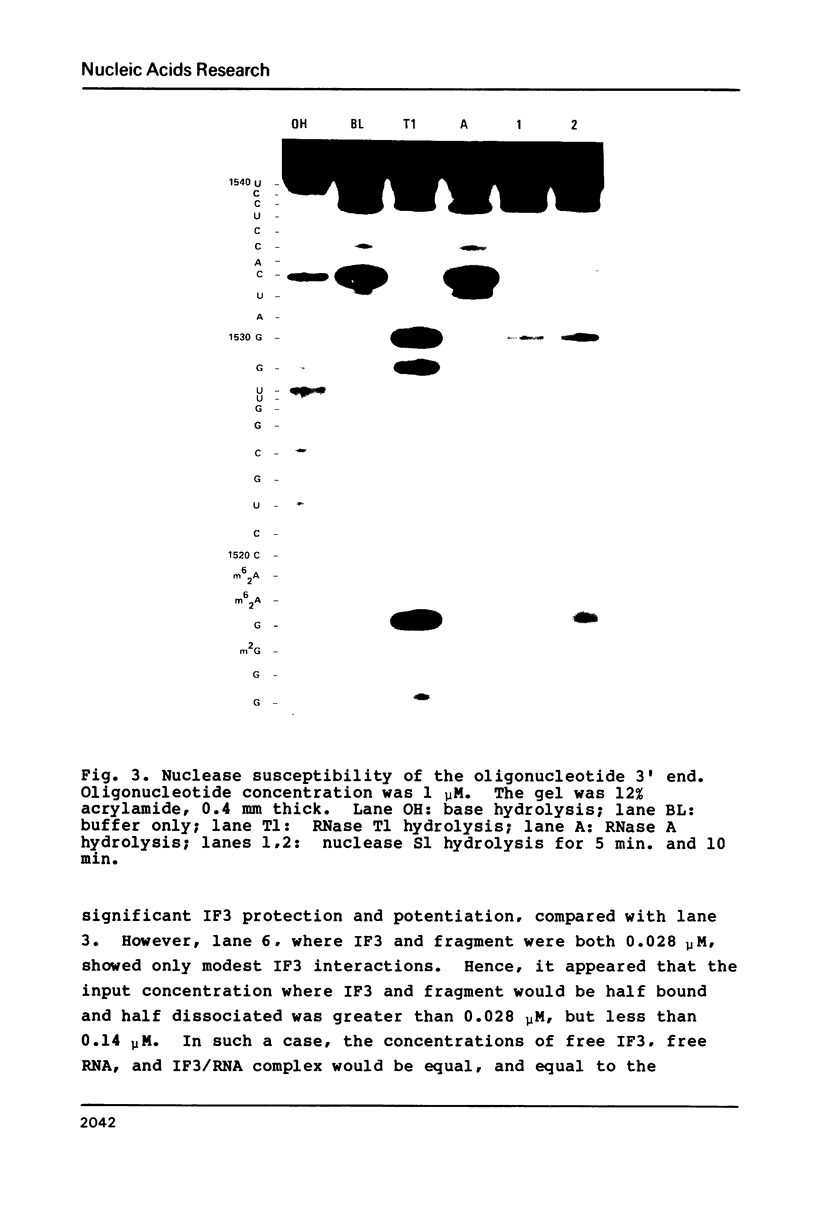
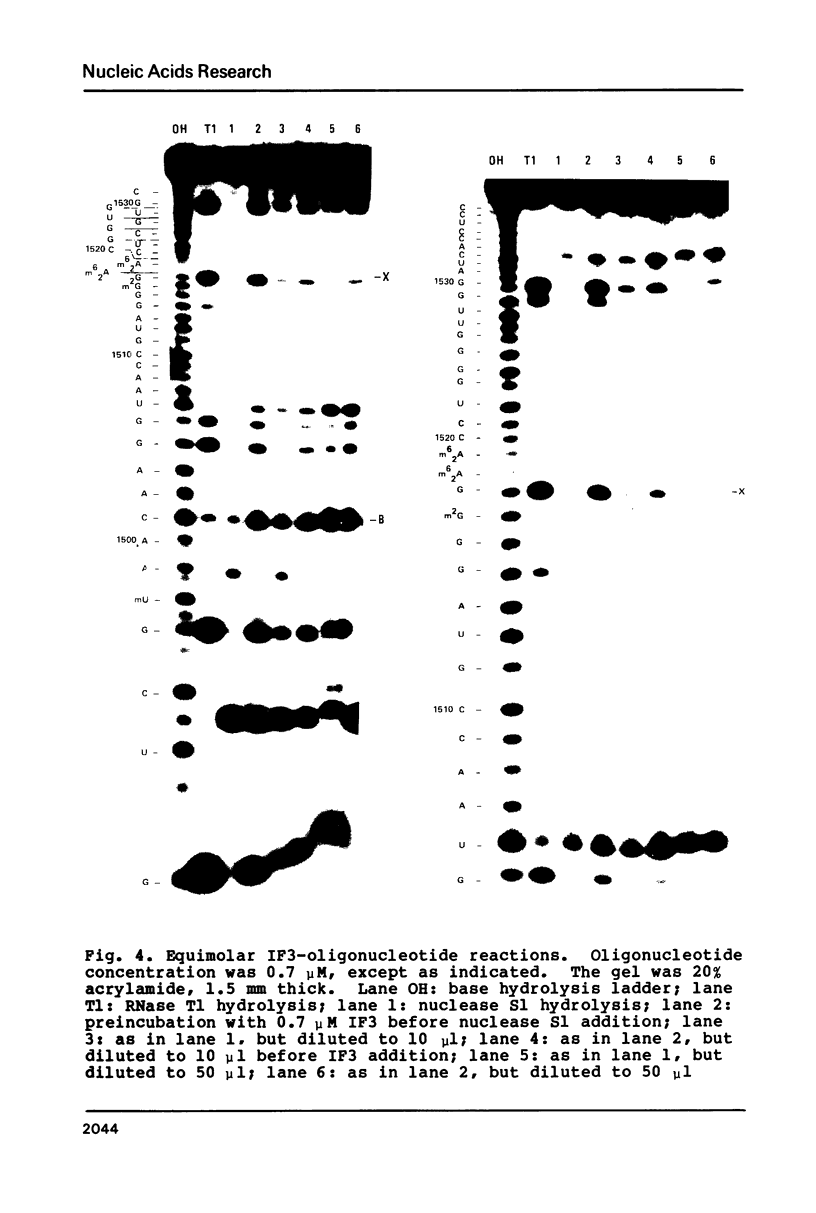
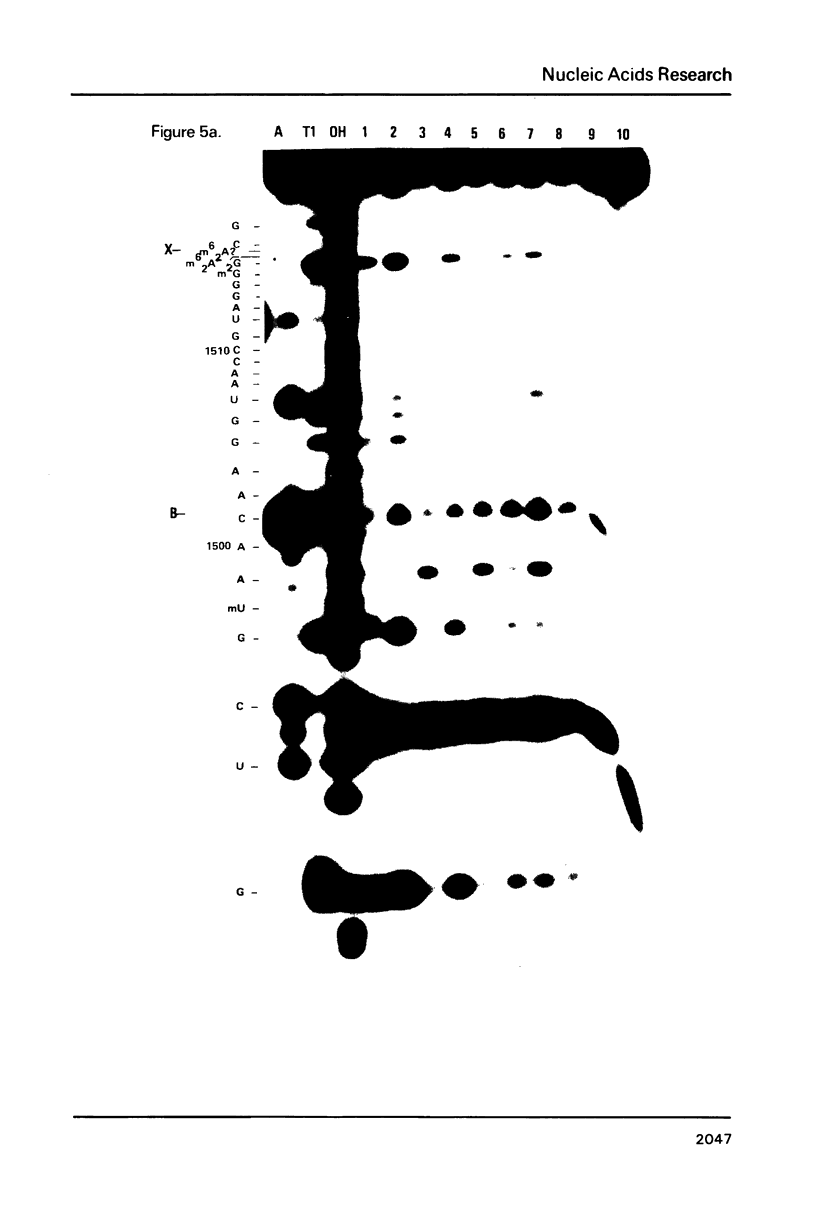
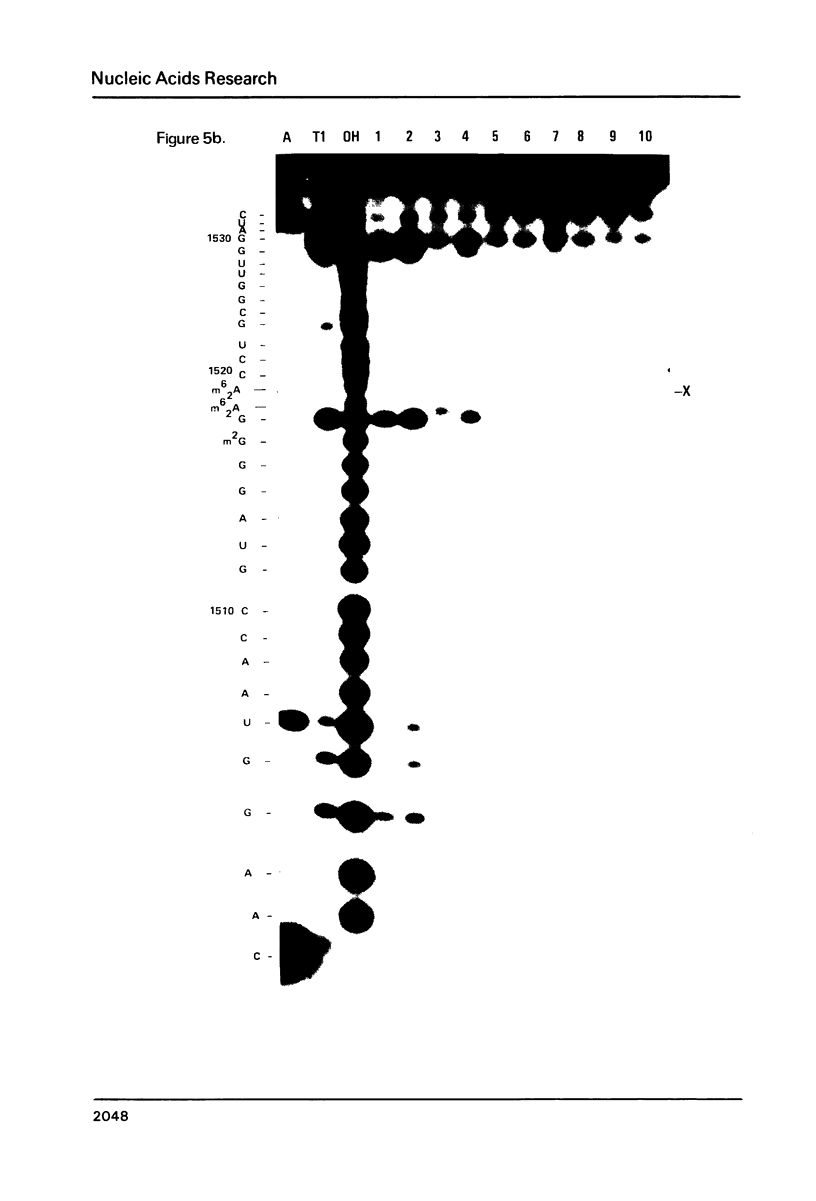
Escherichia coli translational initiation factor 3 (IF3) may be crosslinked to the 3' end of 16S RNA in 30S ribosomal subunits. In order to determine the sequence to which IF3 may bind in vivo, samples of 5'-32P labelled 3' terminal 49-nucleotide fragment of 16S RNA were incubated 5 min. at 37 degrees in 40 mM Tris-HOAc, pH 7.4, 100 mM NaCl, 1 mM Mg (OAc)2, 1 mM ZnSO4, with or without IF3, then reacted a further 5 min with nuclease S1, RNase T1, or RNase A. Base pairing between the 5' and 3' legs of the fragment occurs in the absence of IF3, but is disrupted by IF3 binding. IF3 appears to protect some residues near the 5' end of the fragment (U1495, A1499, A1500, A1502, and A1503) from nuclease S1, and potentiates S1 attack on others (G1494, G1497, C1501, G1504, G1505, U1506, G1517, G1529, G1530, and C1533). A series of equimolar reactions at increasing dilution imply an association constant range of 1.4-7.0 X 10(7) M-1.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baan R. A., Hilbers C. W., Van Charldorp R., Van Leerdam E., Van Knippenberg P. H., Bosch L. High-resolution proton magnetic resonance study of the secondary structure of the 3'-terminal 49-nucleotide fragment of 16S rRNA from Escherichia coli. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1028–1031. doi: 10.1073/pnas.74.3.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baan R. A., van Charldorp R., van Leerdam E., van Knippenberg P. H., Bosch L., de Rooij J. F., van Boom J. H. The 3'-terminus of 16 S ribosomal RNA of Escherichia coli. Isolation and purification of the terminal 49-nucleotide fragment at a milligram scale. FEBS Lett. 1976 Dec 1;71(2):351–355. doi: 10.1016/0014-5793(76)80968-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Gallinaro H., Lazar E., Jacob M. The conformation of chicken, rat and human U1A RNAs in solution. Nucleic Acids Res. 1981 Feb 25;9(4):841–858. doi: 10.1093/nar/9.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer D., Wittmann-Liebold B. The primary structure of the initiation factor IF-3 from Escherichia coli. FEBS Lett. 1977 Jul 15;79(2):269–275. doi: 10.1016/0014-5793(77)80801-6. [DOI] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman N. M., Noller H. F. Protection of specific sites in 16 S RNA from chemical modification by association of 30 S and 50 S ribosomes. J Mol Biol. 1977 Jan 5;109(1):131–149. doi: 10.1016/s0022-2836(77)80049-1. [DOI] [PubMed] [Google Scholar]
- Cooperman B. S., Expert-Bezançon A., Kahan L., Dondon J., Grunberg-Manago M. IF-3 crosslinking to Escherichia coli ribosomal 30 S subunits by three different light-dependent procedures: identification of 30 S proteins crosslinked to IF-3--utilization of a new two-stage crosslinking reagent, p-nitrobenzylmaleimide. Arch Biochem Biophys. 1981 May;208(2):554–562. doi: 10.1016/0003-9861(81)90544-0. [DOI] [PubMed] [Google Scholar]
- Czernilofsky A. P., Kurland C. G., Stöffler G. 30S ribosomal proteins associated with the 3'-terminus of 16S RNA. FEBS Lett. 1975 Oct 15;58(1):281–284. doi: 10.1016/0014-5793(75)80279-1. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Dahlberg J. E. Binding of ribosomal protein S1 of Escherichia coli to the 3' end of 16S rRNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2940–2944. doi: 10.1073/pnas.72.8.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaf F. K., Klaasen-Boor P. Purification and characterization of a complex between cloacin and its immunity protein isolated from Enterobacter cloacae (Clo DF13). Dissociation and reconstitution of the complex. Eur J Biochem. 1977 Feb 15;73(1):107–114. doi: 10.1111/j.1432-1033.1977.tb11296.x. [DOI] [PubMed] [Google Scholar]
- Draper D. E., Pratt C. W., von Hippel P. H. Escherichia coli ribosomal protein S1 has two polynucleotide binding sites. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4786–4790. doi: 10.1073/pnas.74.11.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper D. E., von Hippel P. H. Interaction of Escherichia coli ribosomal protein S1 with ribosomes. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1040–1044. doi: 10.1073/pnas.76.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimark R. L., Kahan L., Johnston K., Hershey J. W., Traut R. R. Cross-linking of initiation factor IF3 to proteins of the Escherichia coli 30 S ribosomal subunit. J Mol Biol. 1976 Aug 5;105(2):219–230. doi: 10.1016/0022-2836(76)90108-x. [DOI] [PubMed] [Google Scholar]
- Hershey J. W., Yanov J., Johnston K., Fakunding J. L. Purification and characterization of protein synthesis initiation factors IF1, IF2, and IF3 from Escherichia coli. Arch Biochem Biophys. 1977 Aug;182(2):626–638. doi: 10.1016/0003-9861(77)90543-4. [DOI] [PubMed] [Google Scholar]
- Johnson B., Szekely M. Specific binding site of e. coli initiation factor 3 (IF3) at a 3'-terminal region of MS2 RNA. Nature. 1977 Jun 9;267(5611):550–552. doi: 10.1038/267550a0. [DOI] [PubMed] [Google Scholar]
- Keren-Zur M., Boublik M., Ofengand J. Localization of the decoding region on the 30S Escherichia coli ribosomal subunit by affinity immunoelectron microscopy. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1054–1058. doi: 10.1073/pnas.76.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughrea M., Dondon J., Grunberg-Manago M. The relationship between the 3'-end of 16 S RNA and the binding of initiation factor IF-3 to the 30 S subunit of E. coli. FEBS Lett. 1978 Jul 15;91(2):265–268. doi: 10.1016/0014-5793(78)81188-0. [DOI] [PubMed] [Google Scholar]
- Laughrea M., Moore P. B. On the relationship between the binding of ribosomal protein S1 to the 30 S subunit of Escherichia coli and 3' terminus of 16 S RNA. J Mol Biol. 1978 Jun 5;121(4):411–430. doi: 10.1016/0022-2836(78)90391-1. [DOI] [PubMed] [Google Scholar]
- Lestienne P., Dondon J., Plumbridge J. A., Howe J. G., Mayaux J. F., Springer M., Blanquet S., Hershey J. W., Grunberg-Manago M. Expression of the gene for Escherichia coli initiation factor IE-3 in vivo and in vitro. Eur J Biochem. 1982 Apr;123(3):483–488. doi: 10.1111/j.1432-1033.1982.tb06556.x. [DOI] [PubMed] [Google Scholar]
- Olson H. M., Glitz D. G. Ribosome structure: localization of 3' end of RNA in small subunit by immunoelectronmicroscopy. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3769–3773. doi: 10.1073/pnas.76.8.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldermans B., Van Buul C. P., Van Knippenberg P. H. Studies on the function of two adjacent N6,N6-dimethyladenosines near the 3' end of 16 S ribosomal RNA of Escherichia coli. II. The effect of the absence of the methyl groups on initiation of protein biosynthesis. J Biol Chem. 1979 Sep 25;254(18):9090–9093. [PubMed] [Google Scholar]
- Politz S. M., Glitz D. G. Ribosome structure: localization of N6,N6-dimethyladenosine by electron microscopy of a ribosome-antibody complex. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1468–1472. doi: 10.1073/pnas.74.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon C. L., Pawlik R. T., Gualerzi C. The topographical localization of IF3 on Escherichia coli 30 S ribosomal subunits as a clue to its way of functioning. FEBS Lett. 1982 Jan 25;137(2):163–167. doi: 10.1016/0014-5793(82)80339-6. [DOI] [PubMed] [Google Scholar]
- Pon C. L., Pawlik R. T., Gualerzi C. The topographical localization of IF3 on Escherichia coli 30 S ribosomal subunits as a clue to its way of functioning. FEBS Lett. 1982 Jan 25;137(2):163–167. doi: 10.1016/0014-5793(82)80339-6. [DOI] [PubMed] [Google Scholar]
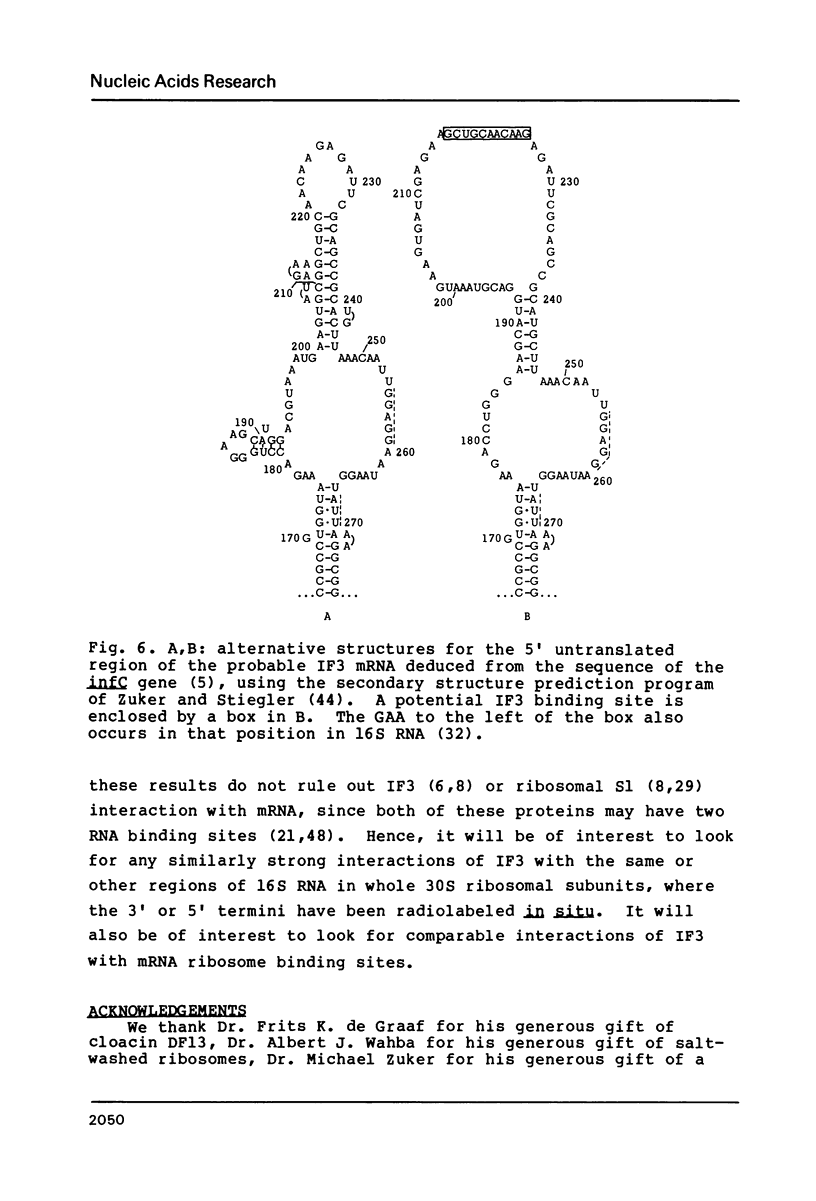
- Sacerdot C., Fayat G., Dessen P., Springer M., Plumbridge J. A., Grunberg-Manago M., Blanquet S. Sequence of a 1.26-kb DNA fragment containing the structural gene for E.coli initiation factor IF3: presence of an AUU initiator codon. EMBO J. 1982;1(3):311–315. doi: 10.1002/j.1460-2075.1982.tb01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleich T., Wickstrom E., Twombly K., Schmidt B., Tyson R. W. Circular dichroism study of Escherichia coli initiation factor 3 binding to nucleic acids. Biochemistry. 1980 Sep 16;19(19):4486–4492. doi: 10.1021/bi00560a016. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobura J. E., Chowdhury M. R., Hawley D. A., Wahba A. J. Requirement of chain initiation factor 3 and ribosomal protein S1 in translation of synthetic and natural messenger RNA. Nucleic Acids Res. 1977 Jan;4(1):17–29. doi: 10.1093/nar/4.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Charldorp R., Heus H. A., Van Knippenberg P. H., Joordens J., De Bruin S. H., Hilbers C. W. Destabilization of secondary structure in 16S ribosomal RNA by dimethylation of two adjacent adenosines. Nucleic Acids Res. 1981 Sep 11;9(17):4413–4422. doi: 10.1093/nar/9.17.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Charldorp R., Van Knippenberg P. H. Sequence, modified nucleotides and secondary structure at the 3'-end of small ribosomal subunit RNA. Nucleic Acids Res. 1982 Feb 25;10(4):1149–1158. doi: 10.1093/nar/10.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Charldorp R., Verhoeven J. J., Van Knippenberg P. H., Haasnoot C. A., Hilbers C. W. A carbon-13 nuclear magnetic resonance study of the 3'-terminus of 16S ribosomal RNA of Escherichia coli specifically labeled with carbon-13 in the methylgroups of the m6(2)Am6(2)A sequence. Nucleic Acids Res. 1982 Jul 24;10(14):4237–4245. doi: 10.1093/nar/10.14.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duin J., Kurland C. G., Dondon J., Grunberg-Mangago M., Branlant C., Ebel J. P. New aspects of the IF3-ribosome interaction. FEBS Lett. 1976 Feb 15;62(2):111–114. doi: 10.1016/0014-5793(76)80030-0. [DOI] [PubMed] [Google Scholar]
- Wahba A. J., Chae Y. B., Iwasaki K., Mazumder R., Miller M. J., Sabol S., Sillero M. A. Initiation of protein synthesis in Escherichia coli. I. Purification and properties of the initiation factors. Cold Spring Harb Symp Quant Biol. 1969;34:285–290. doi: 10.1101/sqb.1969.034.01.034. [DOI] [PubMed] [Google Scholar]
- Weiel J., Hershey J. W. Fluorescence polarization studies of the interaction of Escherichia coli protein synthesis initiation factor 3 with 30S ribosomal subunits. Biochemistry. 1981 Sep 29;20(20):5859–5865. doi: 10.1021/bi00523a032. [DOI] [PubMed] [Google Scholar]
- Wickstrom E. Escherichia coli initiation factor IF3 binding to AUG and AUG-containing single strands and hairpin loops, and nonspecific binding to polymers. Biochim Biophys Acta. 1974 Apr 27;349(1):125–130. doi: 10.1016/0005-2787(74)90015-x. [DOI] [PubMed] [Google Scholar]
- Wickstrom E. Physical parameters of Escherichia coli translational initiation factor 3 binding to poly(A). FEBS Lett. 1981 Jun 1;128(1):154–156. doi: 10.1016/0014-5793(81)81103-9. [DOI] [PubMed] [Google Scholar]
- Wickstrom E., Tinoco I., Jr The stability of RNA hairpin loops containing A-U-G: An-U-G-Um. Biopolymers. 1974 Nov;13(11):2367–2383. doi: 10.1002/bip.1974.360131116. [DOI] [PubMed] [Google Scholar]
- Wickstrom E., Tyson R. W., Newton G., Obert R., Williams E. E. Stoichiometry of homopolynucleotide binding to Escherichia coli translational initiation factor 3. Arch Biochem Biophys. 1980 Mar;200(1):296–300. doi: 10.1016/0003-9861(80)90357-4. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Magrum L. J., Gupta R., Siegel R. B., Stahl D. A., Kop J., Crawford N., Brosius J., Gutell R., Hogan J. J. Secondary structure model for bacterial 16S ribosomal RNA: phylogenetic, enzymatic and chemical evidence. Nucleic Acids Res. 1980 May 24;8(10):2275–2293. doi: 10.1093/nar/8.10.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R. C., Steitz J. A., Moore P. B., Crothers D. M. The 3' terminus of 16S rRNA: secondary structure and interaction with ribosomal protein S1. Nucleic Acids Res. 1979 Dec 20;7(8):2399–2418. doi: 10.1093/nar/7.8.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieb C., Glotz C., Brimacombe R. Secondary structure comparisons between small subunit ribosomal RNA molecules from six different species. Nucleic Acids Res. 1981 Aug 11;9(15):3621–3640. doi: 10.1093/nar/9.15.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dieijen G., van Knippenberg P. H., van Duin J. The specific role of ribosomal protein S1 in the recognition of native phage RNA. Eur J Biochem. 1976 May 1;64(2):511–518. doi: 10.1111/j.1432-1033.1976.tb10330.x. [DOI] [PubMed] [Google Scholar]
- van Duin J., Kurland C. G., Dondon J., Grunberg-Manago M. Near neighbors of IF3 bound to 30S ribosomal subunits. FEBS Lett. 1975 Nov 15;59(2):287–290. doi: 10.1016/0014-5793(75)80394-2. [DOI] [PubMed] [Google Scholar]