Abstract
Acute graft-versus-host disease (GvHD) is a major complication that prevents successful outcomes after allogeneic bone marrow transplantation (BMT), an effective therapy for hematological malignancies. Several studies demonstrate that donor T cells and host antigen-presenting cells along with several proinflammatory cytokines are required for the induction of GvHD and contribute to its severity. Increasing evidence demonstrates that human serum-derived αalpha-1- anti-trypsin (AAT) reduces production of proinflammatory cytokines, induces anti-inflammatory cytokines, and interferes with maturation of dendritic cells. Using well-characterized mouse models of BMT, we have studied the effects of AAT on GvHD severity. Administration of AAT early after BMT decreased mortality in three models of GvHD and reduced serum levels of proinflammatory cytokines in the allogeneic recipients compared with vehicle (albumin) treated animals. AAT treatment reduced the expansion of alloreactive T effector cells but enhanced the recovery of T regulatory T cells, (Tregs) thus altering the ratio of donor T effector to T regulatory cells in favor of reducing the pathological process. However, despite altering the ratio in vivo, AAT had no direct effects on either the donor T effector cells or T regulatory cells Tregs in vitro. In contrast, AAT suppressed LPS-induced in vitro secretion of proinflammatory cytokines such as TNF-α and IL-1β, enhanced the production of the anti-inflammatory cytokine IL-10, and impaired NF-κB translocation in the host dendritic cells. In light of its long history of safety in humans, these findings suggest that administration of AAT represents a novel unique and viable strategy to mitigate clinical GvHD.
Keywords: inflammation, interleukin
Allogeneic bone marrow transplantation (BMT) is a potentially curative therapy for many malignant and nonmalignant hematological diseases (1). The therapeutic potential of allogeneic BMT is offset by its major complication, graft-versus-host disease (GvHD) (1). The pathophysiology of GvHD involves donor T-cell interactions with host antigen-presenting cells (APC) and the subsequent production of proinflammatory cytokines, along with activation of alloreactive T effector cells (T effectors) that cause target organ damage (2–4). By contrast, donor-derived mature foxp3+ T regulatory cells (Tregs) can down-regulate alloreactivity (2, 3, 5–8). Several lines of evidence converge to implicate that the severity of both experimental and clinical GvHD is set by a network of proinflammatory cytokines that include IL-1β, tumor necrosis factor α (TNF-α), and IL-6 (2, 9, 10). Although experimental GvHD can be mitigated by blocking single cytokines (11, 12), such monotherapeutic strategies have failed to yield significant clinical benefits (13, 14). Therefore, agents that reduce multiple proinflammatory cytokines, as well as alter the ratio of donor T effectors to Tregs, may reduce the severity of GvHD.
Alpha-1-antitrypsin (AAT) is a circulating 52-kDa glycoprotein that is produced mainly by the liver (15). AAT is primarily known as a serine protease inhibitor and is encoded by the gene SERPINA1 (15). It is the most abundant, endogenous serine protease inhibitor in the circulation (15–18). AAT inhibits neutrophil elastase, and inherited deficiency in circulating AAT results in lung-tissue deterioration and liver disease (15). Serum AAT concentrations in healthy individuals range from 1.5 to 3.5 mg/mL and increase twofold during inflammation, coinciding with the role of AAT as an acute-phase protein (15). Several reports have suggested a negative association between AAT levels and the severity of several inflammatory diseases. For example, reduced levels or activity of AAT have been described in patients with HIV infection, diabetes mellitus, hepatitis C infection-induced chronic liver disease, and several types of vasculitis (19–21). Indeed, the addition of AAT to human peripheral blood mononuclear cells (PBMC) inhibits LPS-induced release of TNF-α and IL-1β but increases IL-1 receptor antagonist (IL-1Ra) and IL-10 production (22–25). AAT reduces in vitro IL-1β–mediated pancreatic islet toxicity, and AAT monotherapy prolongs islet allograft survival, promotes antigen-specific immune tolerance in mice (26, 27), and delays the development of diabetes in nonobese diabetic (NOD) mice (28, 29). AAT was shown to inhibit LPS-induced acute lung injury in experimental models (30, 31). Recently, AAT was shown to reduce the size of infarct and the severity of heart failure in a mouse model of acute myocardial ischemia-reperfusion injury (32).
Given the role of proinflammatory cytokines in the pathogenesis of acute GvHD (2) and the tissue-protective and anti-inflammatory properties of AAT (22–27), we sought to investigate the effects of AAT administration in several well-characterized murine models of allogeneic BMT. We hypothesized that administration of AAT early in the time course of allogeneic BMT, during the intensification of the proinflammatory cytokine cascade (2, 4), would suppress cytokine production and reduce the severity of systemic GvHD.
Consistent with this hypothesis, we found that monotherapy with clinical-grade human AAT (hAAT) reduced circulating proinflammatory cytokines, diminished GvHD severity, and prolonged animal survival after experimental allogeneic BMT.
Results
Administration of hAAT Decreases Severity and Mortality from GvHD.
To determine whether administration of hAAT affects the severity of GvHD and subsequent mortality from the condition, three models for GvHD were examined. In the first model, hAAT was administered in a dose-dependent manner in a MHC disparate B6 (H-2b) → B6D2F1 (H-2b/d) mouse model, and the severity of GvHD was examined (33). Lethally irradiated B6D2F1 mice received bone marrow (BM) and splenic T cells from syngeneic (B6D2F1) or allogeneic (B6) donors. BMT recipients were injected i.p. with 1, 2, or 4 mg per mouse of either hAAT or human albumin as control 2 d before transplantation and then every other day through day 13 after transplantation. This schedule was chosen to modulate the inflammatory cascade of acute GvHD, which is most severe before day 7 after transplantation (33–37). In addition, the dose of 2 mg per mouse was reportedly effective in allograft rejection and autoimmune models (26–28). The development of murine antibodies to hAAT in the present model is avoided by the profound immuno/myeloablation (34).
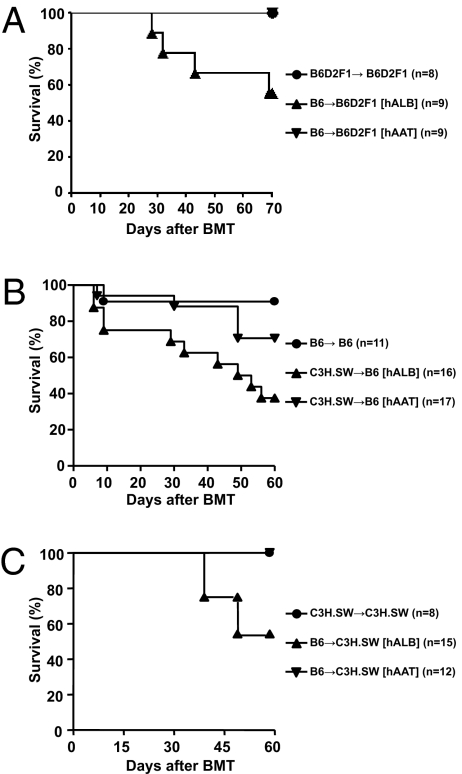
As shown in Fig. 1A, hAAT administration at 4 mg per mouse significantly reduced mortality from GvHD compared with allogeneic controls (100% survival vs. 50% survival at day 70; P < 0.02). hAAT administration at 1 mg per mouse had only modest effects on mortality, whereas at 2 mg per mouse hAAT appeared to have prolonged animal survival. Although mortality was reduced, the clinical scores remained higher for hAAT allo-recipients than for the group of syngeneic recipients: clinical GvHD severity scores on days 14, 21, and 28 in the hAAT allo-recipients were 2.1 ± 0.25, 3.2 ± 0.1, and 3.8 ± 0.3 whereas those of the syngeneic animals were 0.8 ± 0.3, 0.5 ± 0.2, and 0 (P < 0.05). All surviving mice displayed complete donor chimerism (97% ± 2%), as determined by fluorescence-activated cell sorter analysis, excluding both possibilities that graft failure had occurred and that the protection was the result of mixed chimerism (38). To demonstrate that reduction of GvHD by hAAT is strain independent, hAAT was also administered to experimental groups composed of C3H.SW (H-2b) → B6 (H-2b), a MHC-matched mouse model of acute GvHD that is driven by donor CD8+ T cells and is directed against minor histocompatibility antigens (39). Here, recipient B6 mice were conditioned and then transplanted with either syngeneic (B6) or allogeneic (C3H.SW) donor BM. As shown in Fig. 1B, administration of hAAT at 2 mg per mouse significantly improved survival compared with control allogeneic human albumin-treated mice (78% survival vs. 40% survival at day 60; P < 0.03).
Fig. 1.
AAT reduces mortality from GvHD. (A) B6D2F1 mice were irradiated with 1,000 centigray (cGy) of total-body irradiation on day −1 and transplanted with 5 × 106 T-cell–depleted BM cells and 2 × 106 CD90+T cells from either syngeneic F1 or allogeneic B6 donors. Each allo-recipient was injected i.p. with either 4 mg hAAT (n = 9) or human albumin (n = 9) for 6 d from day −2 to day +13. Data shown are combined from two similar experiments. Percentage survival after BMT is shown. For ▼ vs. ▲, P < 0.02. (B) B6 mice were given 1,000 cGy of total-body irradiation on day −1 and transplanted with 5 × 106 T-cell–depleted BM cells and 2 × 105 CD8+T cells from either syngeneic B6 or allogeneic C3H.SW donors. Each allo-recipient was injected i.p. with either 2 mg hAAT (n = 17) or human albumin (n = 16) for 6 d from day −2 to day +13 and was monitored for GvHD survival. Data shown are combined from three similar experiments. Percentage survival after BMT is shown. For ▼ vs. ▲, P = 0.029. (C) C3H.SW mice were irradiated as above and transplanted with 4 × 106 T-cell–depleted BM cells and 1 × 106 CD90+T cells from either syngeneic C3H.SW or allogeneic B6 donors. The allo-recipients were injected i.p. with either 2 mg hAAT (n = 12) or human albumin (n = 15) and were monitored for GvHD survival as above. Data shown are combined from two similar experiments. Percentage survival after BMT is shown. For ▼ vs. ▲, P = 0.019.
We next examined the effect of hAAT in MHC-matched minor disparate CD4+-dependent, but CD8+-driven, T-cell–mediated GvHD using the B6 (H-2b) → C3H.SW (H-2b) strain combination (6). As shown in Fig. 1C, administration of hAAT completely prevented mortality in allogeneic animals from GvHD compared with control human albumin-treated allogeneic animals (100% survival vs. 50% survival at day 40; P < 0.02). The reduction in GVHD was confirmed by histopathology of the skin at the end of the survival analyses on day +60 (1.8 ± 0.6 vs. 3.9 ± 0.4; P < 0.03).
AAT Alters Mature Donor T Effector-to-Treg Ratio After Allogeneic BMT.
The expansion of mature donor cytopathic effector T cells (Teffs) and Tregs was determined, and the ratio of Teffs to Tregs (Teffs:Tregs) was calculated in allogeneic transplants treated with hAAT. Mice (B6) were irradiated and transplanted with BM and CD8+ T cells from the allogeneic C3H.SW donors. Because expansion of alloreactive T cells peaks at 3–4 wk in this model, splenocytes from recipient animals were isolated at 4 wk and analyzed for donor Teff and Treg cell expansion by donor-specific congeneic markers.
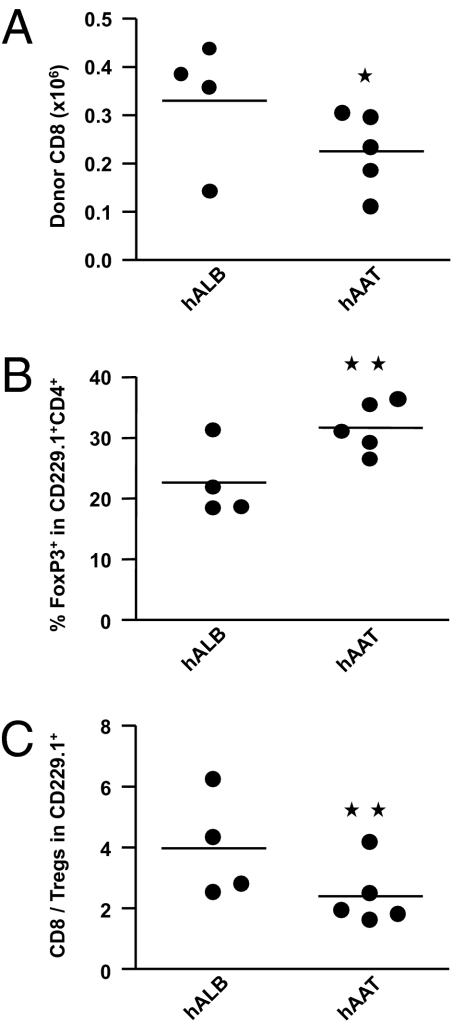
As shown in Fig. 2 A–C, administration of hAAT resulted in reduced expansion of splenic CD8+ donor Teffs. In contrast, the expansion of donor Tregs was increased, resulting in the reduction of the Teff:Treg ratio compared with the control animals.
Fig. 2.
hAAT reduces Teff expansion but enhances Treg expansion. B6 mice were irradiated and transplanted with either allogeneic C3H.SW donor. The recipient animals received either hAAT or albumin as above. Expansion of C3H.SW donor (CD229.1+) CD8+ and CD4+FoxP3+ T cells was analyzed using day 29 spleen cells. Absolute numbers of C3H.SW donor-derived (CD229.1+) (A) CD8+ and (B) CD4+ FoxP3+ T cells are shown. (C) The ratio of CD8+ to CD4+ FoxP3+ T-cell absolute numbers is shown. Each point represents one individual mouse (n = 4–5/group). *P < 0.05 for A and P < 0.04 for B and C.
To exclude the possibility of confounding effects of donor BM-derived Tregs in these analyses, and to analyze the kinetics of mature donor Teffs:Tregs, we used B6 GFP+ foxp3 knock-in mice as donors for Tregs (GFP+ and Ly5.1+) and mature Teffs (GFP− and Ly5.1+). In a similar manner, B6 Ly5.2 (CD45.1) used as the source of donor BM is responsible for CD45.2+ mature Teff (GFP−) or Tregs (GFP+). These cells were infused into the MHC-matched but minor disparate C3H.SW animals, and the expansion of the mature Teffs and Tregs was determined.
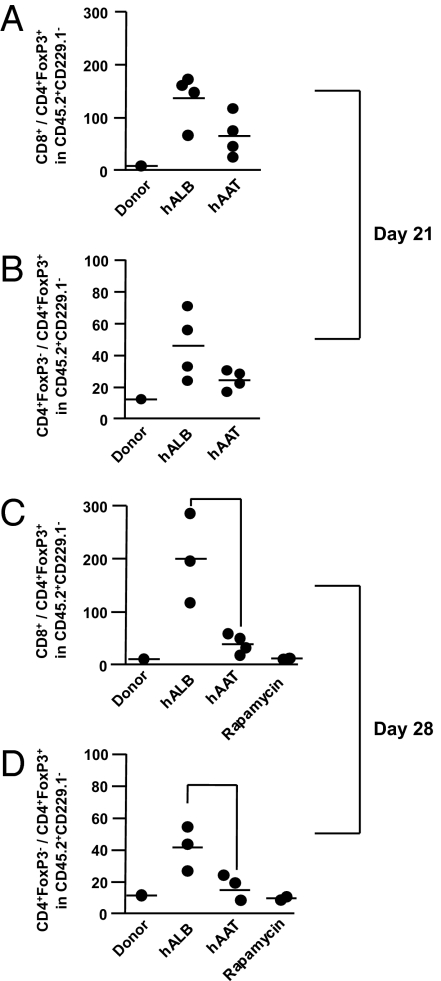
Because both CD4+ and CD8+ T cells are responsible for mortality in this model, we analyzed the ratio of both mature donor CD4+ Teffs and mature CD8+ Teffs to the mature donor GFP+ Tregs on day 21 and day 28 after BMT (6). As shown in Fig. 3 A–D, the ratio of Teffs:Tregs was significantly altered for both CD4+ and CD8+ Teffs on both day 21 and day 28. The effect of hAAT was compared with rapamycin, an immunosuppressive agent that has been shown to enhance Tregs in these models (40). As shown in Fig. 3 C and D, alteration in the Teffs:Tregs ratio by hAAT produced effects similar to those induced by rapamycin on day 28.
Fig. 3.
hAAT alters the ratio of mature donor Teff to Treg cells. C3H.SW mice were irradiated and transplanted with either allogeneic B6GFP+Foxp3 knock-in donor. The recipient animals received either hAAT or rapamycin or the control vehicle as above. Expansion of mature donor (CD45.2+CD45.1−CD229.1−GFP−) CD4 and CD8+ effectors and the mature donor (CD45.1−CD45.2+CD229.1−GFP+) Tregs was analyzed in the peripheral blood on day +21 and day +28 for the ratio of CD8+ to CD4+ FoxP3+ T-cell absolute numbers of mature donor-derived (CD45.2+CD45.1−CD229.1+). Each point represents one individual mouse (n = 2–5/group). (A) CD4+:Treg ratio on day +21, vehicle vs. hAAT; P = 0.037. (B) CD8+:Treg ratio on day +21, vehicle vs. hAAT, P = 0.022. (C) CD4+:Treg ratio on day +28, vehicle vs. hAAT; P = 0.0223. (D) CD8+:Treg ratio on day +28, vehicle vs. hAAT; P = 0.0127.
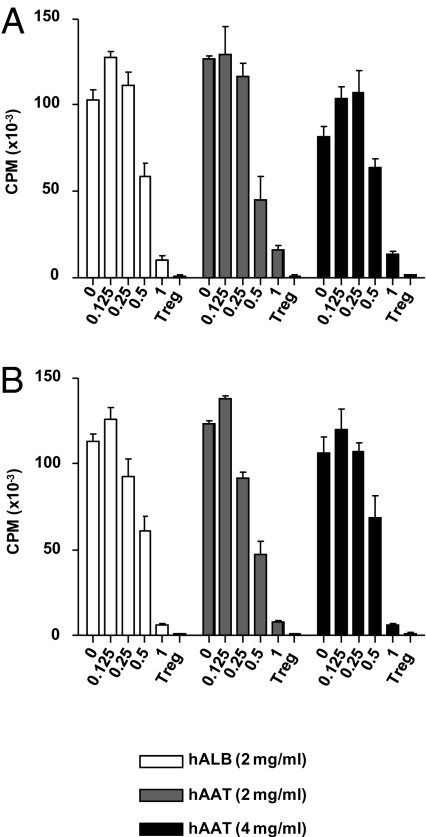
Given the alteration in the expansion and the ratio of the donor Teffs and Tregs, we next determined whether hAAT had direct effects on donor Teff responses in vitro. The proliferation of Treg-depleted donor T cells (BALB/c) to allogeneic (B6) BM-derived dendritic cells (DC) was equivalent regardless of whether the donor T cells were pretreated with hAAT or vehicle (Fig. 4A).
Fig. 4.
AAT does not affect T-cell responses in vitro. (A) BALB/c splenic CD4+25− T cells were treated overnight with hAAT or albumin at either 2 or 4 mg/mL and were then used as responder in an MLR with B6 BM DCs as stimulators. Freshly isolated BALB/c CD4+CD25+ Tregs were added at the indicated ratios to the above cultures, and T-cell proliferation was determined by 3H-thymidine incorporation at 96 h. Data are mean ± SEM of quadruplicate cultures. P = not significant, control vs. hAAT-treated CD4+ T cells. Data shown are from one of two similar experiments. (B) Treg treatment with hAAT. BALB/c splenic CD4+25+Tregs were treated overnight with hAAT or albumin at either 2 or 4 mg/mL and were then used as suppressors of freshly isolated BALB/c CD4+25− at the indicated ratios in an MLR with B6 BM DCs as stimulators. T-cell proliferation was determined by 3H-thymidine incorporation at 96 h. Data are mean ± SEM of quadruplicate cultures. P = NS at all of the ratios, control vs. hAAT-treated CD4+25+ Tregs. Data shown are from one of two similar experiments.
To determine the direct impact of hAAT on T cells without a confounding effect of hAAT on accessory cells, anti-CD3–induced T-cell activation was examined in the presence of hAAT. Consistent with previous reports (28), addition of AAT did not interfere with T-cell responses (Fig. 4). In addition, hAAT allowed uninterrupted T-cell lysis of host-type conA blast cells following priming [57% and 63% at 50:1 Effector:Target (E:T) ratio; P = not significant].
We next determined whether hAAT might directly enhance Treg functions, as determined by their ability to suppress T effector cells. CD4+CD25+ T cells from BALB/c animals were harvested, sorted, and pretreated with hAAT or vehicle. The cells were then used at varying ratios to analyze their ability to suppress BALB/c CD25− T effector cell proliferation in a mixed lymphoctye reaction (MLR). As shown in Fig. 4B, Tregs suppressed the proliferation of T effector cells in an equivalent manner regardless of their pretreatment with hAAT. These data demonstrate that in vitro hAAT does not have a direct effect on T cells and that the in vivo alterations in the ratios of donor T effector cells to donor Tregs is likley an indirect effect.
AAT Inhibits Proinflammatory Cytokine Release After Allogeneic BMT.
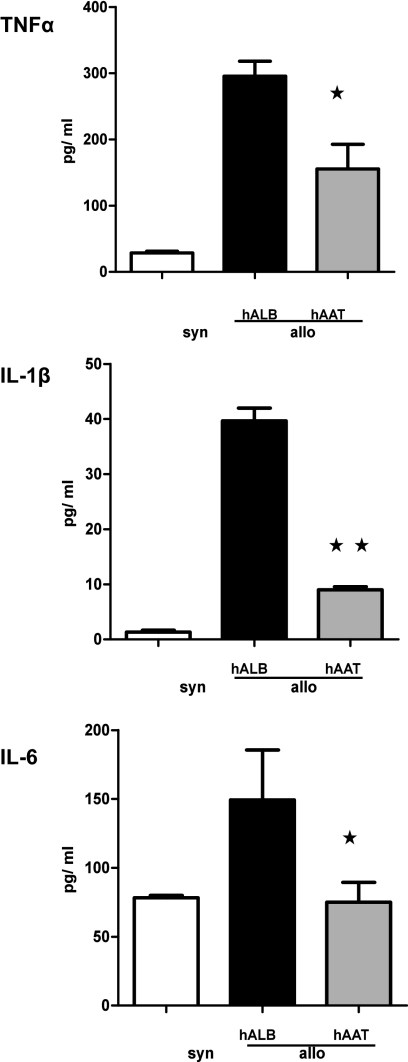
The presence of proinflammatory cytokines has been shown to enhance the expansion of Teffs while mitigating the responses of Tregs (41, 42). In particular, IL-1β, TNF-α, and IL-6 have been shown to play a critical role in determining the severity of GvHD (2, 9, 10). Therefore, we reasoned that the alteration of the Teffs:Tregs ratio and the protection from GvHD might be the consequence of the suppression of proinflammatory cytokine secretion by hAAT. Mice (B6D2F1) were irradiated and transplanted with BM and T cells and were injected with either hAAT or albumin as before. Serum samples were analyzed on day +7 from transplantation for TNF-α, IL-1β, and IL-6. Administration of hAAT significantly reduced serum levels of all three proinflammatory cytokines compared with control allo-recipients (Fig. 5).
Fig. 5.
Injection of hAAT inhibits in vivo proinflammatory cytokine production after BMT. B6D2F1 mice were exposed to 1,000 cGy of total-body irradiation and transplanted with 5 × 106 T-cell–depleted BM cells and 2 × 106 T cells from either allogeneic (B6) or syngeneic (B6D2F1) donors. Each F1 recipient of the allogeneic cells was injected i.p. with 4 mg hAAT or human albumin on days −2, +1, and +4. Sera from the recipient animals (n = 4–5/group) were obtained on day 7 after BMT and analyzed for TNF-α, IL-1β, and IL-6. Albumin-treated allogeneic controls (solid bars) vs. hAAT allogeneic recipients (open and gray bars) for TNF-α. *P < 0.04, IL-1β; Allo, **P < 0.03; IL-6, *P < 0.04.
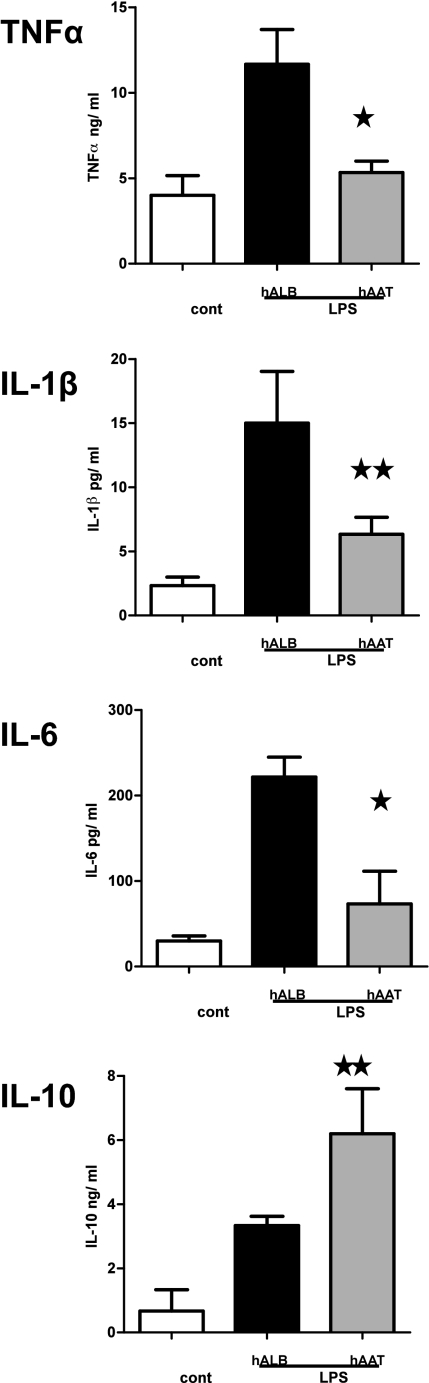
Given the lack of direct effect of hAAT monotherapy on donor T cells, we next reasoned that the reduction in proinflammatory cytokines and GvHD severity might be the consequence of an effect of hAAT on host APC. The effect of hAAT on dendritic cells was thus examined. BM-derived DC from B6 mice were incubated overnight with hAAT before stimulation with 100 ng/mL LPS for 8 h. The secretion of proinflammatory cytokines from AAT-treated DCs was significantly reduced (Fig. 6). In contrast, the secretion of an anti-inflammatory cytokine, IL-10, was significantly enhanced by hAAT compared with albumin-treated controls. The changes in IL-6 and IL-10 were also observed under similar conditions in host F4/80+ macrophages (Fig. S1).
Fig. 6.
AAT suppresses proinflammatory cytokine secretion by BM DCs. BM DCs were obtained from C57BL/6 animals as described in Materials and Methods. BM DCs were preincubated overnight with 4 mg/mL concentration of hAAT or hALB and were then stimulated for 8 h with LPS (100 ng/mL). Cytokine TNF-α, IL-1β, IL-6, and IL-10 levels in the supernatants were measured by ELISA. *P < 0.02; **P < 0.03.
AAT Inhibits LPS-Induced NF-κB Translocation in DCs.
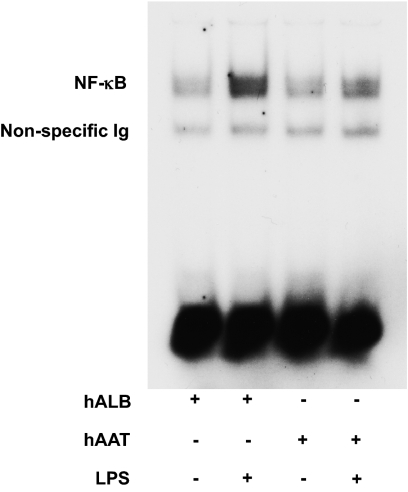
To examine a possible mechanism for the reduction of LPS-induced proinflammatory cytokine secretion by hAAT in BM DCs, NF-κB translocation into the nucleus was determined. AAT or human albumin were added to BM-derived DCs and then stimulated with LPS. NF-κB translocation was analyzed by electrophoretic mobility shift assay (EMSA) in the nuclear fraction of cell lysates. Treatment with hAAT significantly reduced LPS-induced translocation of NF-κB into the nucleus compared with control DCs (Fig. 7). However, consistent with previous observations (43), the levels of expression of MHC class II CD80 and CD86 were high following in vitro generation of DCs. Addition of AAT to these phenotypically mature DCs did not cause them to revert back into immature state as determined by expression of the above costimulatory molecules (Fig. S2). However, despite the lack of effect on phenotype, AAT attenuated the functional responses of DCs, the secretion of proinflammatory cytokines, and NF-κB translocation.
Fig. 7.
hAAT reduces LPS-induced nuclear translocation of NF-κB. BM DCs were obtained from C57BL/6 animals and were preincubated overnight with 4 mg/mL of hAAT or hALB and then stimulated for 6 h with LPS or hALB (100 ng/mL). NF-κB translocation was evaluated by the gel-shift EMSA assay. This figure is representative of one of two similar experiments.
Discussion
The induction of acute GvHD is a consequence of donor T-cell responses to host alloantigens and the dysregulation of proinflammatory cytokine cascades (2, 4, 9, 10). Inflammatory cytokines play a key role in the pathogenesis of GvHD (2, 9, 10). In the current study, monotherapy with hAAT reduced several proinflammatory cytokines and GvHD mortality in multiple models.
The effect of AAT in regulating inflammatory processes is increasingly appreciated (44) in several experimental models (26–29, 45, 46). The concentrations and doses used in the current study are derived and further extended from these reports, according to which 2 mg hAAT per mouse is sufficient to allow islet allograft acceptance (a dose that is comparable to that used routinely in humans who are deficient in AAT) (27), and 0.5 mg/mL hAAT protects various cell types in vitro from multiple injuries. Thus, we explored in vivo doses of 1, 2, and 4 mg per mouse and also tested in vitro concentrations up to 4 mg/mL. The present data demonstrate that exogenous administration of hAAT after allogeneic BMT suppresses proinflammatory cytokines; alters the ratio of T effector cells to T regulatory cells; and, more importantly, reduces GvHD severity and related mortality.
In examining the cellular targets that can mediate such profound changes in animal model outcomes, we found that the presence of AAT did not directly alter the activity of T effector or T regulatory cells to host antigens in vitro, suggesting that the in vivo effects on donor T-cell subset expansion is likely an indirect effect, perhaps as a consequence of reduced inflammation. AAT appears to promote an environment that facilitates expansion of T regulatory cells and IL-10 (29, 30) while mitigating the expansion of donor T effectors by suppressing the proinflammatory milieu early after allogeneic BMT. Both rapamycin and AAT increase the ratio of Tregs after allogeneic BMT. Recent data have shown that rapamycin can directly modulate Tregs and that its effects can be further augmented by IL-2 (40, 47). In contrast to rapamycin, the mechanism of AAT in enhancing the ratio of Tregs might be more indirect, perhaps a consequence of increased secretion of IL-10, mitigation of the proinflammatory milieu (such as IL-6 that has been shown to affect the generation and stability of Tregs), and lack of impact of AAT on IL-2 secretion (27, 28, 48).
The anti-inflammatory effects of exogenous AAT observed in our study are consistent with several recent reports of this activity in solid organ allograft and autoimmunity models (29–32). Monotherapy with AAT was reported to reduce cytokine-mediated islet damage and promote tolerance toward islet cell allografts (29, 30). Similarly, treatment with AAT dampened inflammation without direct inhibition of T-cell activation and restored euglycemia and immune tolerance to β-cells in NOD mice with recent-onset type 1 diabetes (31, 46). Our data are also consistent with recent observations that AAT inhibits IL-32 and results in attenuation of GVHD (49). However, in contrast to those observations, in the present study, the allo-responses were not completely abrogated and tolerance was not induced after allo-BMT, as demonstrated by the persistence of some GvHD despite the reduction in mortality. Our observations nonetheless confirm the in vivo anti-inflammatory effects of AAT in clinically relevant models of GvHD.
We further extend the observations by demonstrating that the beneficial effects of hAAT are, at least in part, because of direct modulation of DC responses. The suppression of proinflammatory cytokine secretion responses of DCs by AAT is consistent with other studies (26, 30). Furthermore, similar suppression of proinflammatory cytokines from human PBMCs has been observed, supporting the applicability of these observations to the human context (25–28, 50, 51). The role of AAT in suppressing inflammation in humans is also supported by the observation that Staphylococcus epidermis-stimulated blood collected from AAT-deficient individuals demonstrated significantly greater IL-8, IL-6, and IL-1Ra production compared with blood from healthy donors (26). Our data show that AAT directly reduced LPS-stimulated NF-κB nuclear translocation and indicate that this might be a crucial mechanism by which AAT reduces DC responses. These data are consistent with other observations in other cell types that demonstrate inhibition of NF-κB activation by AAT (52, 53).
The role of AAT in the context of clinical acute GvHD has not been well explored. A negative correlation between fecal AAT concentrations and serum albumin levels in patients with severe gastrointestinal GvHD has been reported (54).The monocyte-macrophage contribution to serum levels of AAT after BMT has also been reported (18). It is possible that the levels of AAT might be elevated in acute GVHD similarly to the elevated levels of other acute-phase reactant proteins, such as CRP or ferritin (55). The increased production of AAT following many inflammatory conditions may be a protective regulatory response to any aggressive systemic inflammation, and, as such, greater-than-normal concentrations might be needed to further dampen the ongoing inflammation. Although these issues are yet to be resolved, our data demonstrate a potent role for AAT in reducing the early proinflammatory responses contributing to GvHD without having a direct effect on donor T cells. Because AAT replacement has already been safely used in several patients with little toxicity and is FDA approved, AAT may be examined in human allogeneic BMT recipients as an adjunct in the prevention or treatment of GvHD.
Materials and Methods
Human Alpha-1-Antitrypsin and Albumin.
hAAT (Aralast) and human albumin were obtained from Baxter. Rapamycin and LPS were purchased from LC Laboratories and Sigma, respectively.
Mice.
Female C57BL/6 (B6, H-2b, CD45.2) and C3H.SW (H-2b, CD45.2+) mice were purchased from the Jackson Laboratories. B6-Ly5.2 (H-2b, CD45.1+) and B6D2F1 (B6, H-2b/d, CD45.2+) mice were purchased from the Charles River Laboratories. GFP-FoxP3 knock-in mice were provided by Alexander Rudensky (University of Washington, Seattle, WA) and then bred at the University of Michigan animal facility.
Bone Marrow Transplantation.
Bone marrow transplantation was performed as described in detail in SI Materials and Methods. Briefly, recipient mice were irradiated (137Cs source) with 10 Gy total-body irradiation and injected with either syngeneic or allogeneic T cells along with T-cell–depleted bone marrow cells. Animal studies were approved by the University of Michigan Committee on the Use and Care of Animals.
Bone Marrow Dendritic Cell Generation.
DCs were generated from BM in the presence of 20 ng/mL recombinant murine GM-CSF (Peprotech) for 7 d and isolated using CD11c microbeads (Miltenyi).
Flow Cytometric Analysis.
Flow cytometric analysis was performed using FITC, phycoerythrin (PE), PerCP-Cy5.5, or APC-conjugated monoclonal antibodies to mouse CD4 (clone RM4-4), CD229.1 (30C7), CD8a (53-6.7), CD25 (PC61.5), CD45.1 (A20), and CD45.2 (eBioscience) and analyzed on a FACSVantage SE (Becton Dickinson) or C6 cytometer (Accuri Cytometers) (see SI Materials and Methods for details).
EMSA.
CD11C+ DCs from B6 mice were treated with human albumin (hALB) or hAAT for 4 h and then treated with LPS or diluent for another 3 h after which EMSA was performed (see SI Materials and Methods for details).
ELISA.
ELISAs for TNF-α, IL-1β, IL-6, and IL-10 (BD Pharmingen) were performed according to the manufacturer's protocol.
In Vitro Suppression Assay.
CD4+CD25− and CD4+CD25+ T cells were isolated, and suppression assay was performed as described (6, 10, 34, 36).
Statistical Analysis.
Survival curves were plotted and compared by log-rank analysis; P < 0.05 was considered statistically significant. A paired t test was used to evaluate significant differences between groups in in vitro experiments.
Supplementary Material
Acknowledgments
This work is supported by National Institutes of Health Grants AI-075284, HL-090775, and CA-143379 (to P.R.) and AI 15614 (to C.A.D.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117665109/-/DCSupplemental.
References
- 1.Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007;357:1472–1475. doi: 10.1056/NEJMp078166. [DOI] [PubMed] [Google Scholar]
- 2.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 3.Paczesny S, Hanauer D, Sun Y, Reddy P. New perspectives on the biology of acute GVHD. Bone Marrow Transplant. 2010;45(1):1–11. doi: 10.1038/bmt.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antin JH, Ferrara JL. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80:2964–2968. [PubMed] [Google Scholar]
- 5.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: Take a billion or so and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tawara I, et al. A crucial role for host APCs in the induction of donor CD4+CD25+ regulatory T cell-mediated suppression of experimental graft-versus-host disease. J Immunol. 2010;185:3866–3872. doi: 10.4049/jimmunol.1001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193:1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teshima T, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–581. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 10.Tawara I, et al. Interleukin-6 modulates graft-versus-host responses after experimental allogeneic bone marrow transplantation. Clin Cancer Res. 2011;17(1):77–88. doi: 10.1158/1078-0432.CCR-10-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abhyankar S, Gilliland DG, Ferrara JL. Interleukin-1 is a critical effector molecule during cytokine dysregulation in graft versus host disease to minor histocompatibility antigens. Transplantation. 1993;56:1518–1523. doi: 10.1097/00007890-199312000-00045. [DOI] [PubMed] [Google Scholar]
- 12.Korngold R, Marini JC, de Baca ME, Murphy GF, Giles-Komar J. Role of tumor necrosis factor-alpha in graft-versus-host disease and graft-versus-leukemia responses. Biol Blood Marrow Transplant. 2003;9:292–303. doi: 10.1016/s1083-8791(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 13.Antin JH, et al. Interleukin-1 blockade does not prevent acute graft-versus-host disease: Results of a randomized, double-blind, placebo-controlled trial of interleukin-1 receptor antagonist in allogeneic bone marrow transplantation. Blood. 2002;100:3479–3482. doi: 10.1182/blood-2002-03-0985. [DOI] [PubMed] [Google Scholar]
- 14.Alousi AM, et al. Etanercept, mycophenolate, denileukin or pentostatin plus corticosteroids for acute graft vs. host disease: A randomized phase II trial from the BMT CTN. Blood. 2009;114:511–517. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med. 2009;360:2749–2757. doi: 10.1056/NEJMcp0900449. [DOI] [PubMed] [Google Scholar]
- 16.Aldonyte R, Jansson L, Ljungberg O, Larsson S, Janciauskiene S. Polymerized alpha-antitrypsin is present on lung vascular endothelium. New insights into the biological significance of alpha-antitrypsin polymerization. Histopathology. 2004;45:587–592. doi: 10.1111/j.1365-2559.2004.02021.x. [DOI] [PubMed] [Google Scholar]
- 17.Carrell RW. alpha 1-Antitrypsin: Molecular pathology, leukocytes, and tissue damage. J Clin Invest. 1986;78:1427–1431. doi: 10.1172/JCI112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krivit W, Miller J, Nowicki M, Freier E. Contribution of monocyte-macrophage system to serum alpha 1-antitrypsin. J Lab Clin Med. 1988;112:437–442. [PubMed] [Google Scholar]
- 19.Shapiro L, Pott GB, Ralston AH. Alpha-1-antitrypsin inhibits human immunodeficiency virus type 1. FASEB J. 2001;15(1):115–122. doi: 10.1096/fj.00-0311com. [DOI] [PubMed] [Google Scholar]
- 20.Kok KF, et al. Heterozygous alpha-I antitrypsin deficiency as a co-factor in the development of chronic liver disease: A review. Neth J Med. 2007;65(1):160–166. [PubMed] [Google Scholar]
- 21.Hashemi M, Naderi M, Rashidi H, Ghavami S. Impaired activity of serum alpha-1-antitrypsin in diabetes mellitus. Diabetes Res Clin Pract. 2007;75:246–248. doi: 10.1016/j.diabres.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Janciauskiene S, et al. Inhibition of lipopolysaccharide-mediated human monocyte activation, in vitro, by alpha1-antitrypsin. Biochem Biophys Res Commun. 2004;321:592–600. doi: 10.1016/j.bbrc.2004.06.123. [DOI] [PubMed] [Google Scholar]
- 23.Pott GB, Chan ED, Dinarello CA, Shapiro L. Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J Leukoc Biol. 2009;85:886–895. doi: 10.1189/jlb.0208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janciauskiene SM, Nita IM, Stevens T. Alpha1-antitrypsin, old dog, new tricks. Alpha1-antitrypsin exerts in vitro anti-inflammatory activity in human monocytes by elevating cAMP. J Biol Chem. 2007;282:8573–8582. doi: 10.1074/jbc.M607976200. [DOI] [PubMed] [Google Scholar]
- 25.Churg A, et al. Alpha-1-antitrypsin and a broad spectrum metalloprotease inhibitor, RS113456, have similar acute anti-inflammatory effects. Lab Invest. 2001;81:1119–1131. doi: 10.1038/labinvest.3780324. [DOI] [PubMed] [Google Scholar]
- 26.Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci USA. 2005;102:12153–12158. doi: 10.1073/pnas.0505579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis EC, et al. alpha1-Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc Natl Acad Sci USA. 2008;105:16236–16241. doi: 10.1073/pnas.0807627105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koulmanda M, et al. Curative and beta cell regenerative effects of alpha1-antitrypsin treatment in autoimmune diabetic NOD mice. Proc Natl Acad Sci USA. 2008;105:16242–16247. doi: 10.1073/pnas.0808031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang B, et al. Alpha1-antitrypsin protects beta-cells from apoptosis. Diabetes. 2007;56:1316–1323. doi: 10.2337/db06-1273. [DOI] [PubMed] [Google Scholar]
- 30.Huang H, et al. Alpha1-antitrypsin inhibits angiogenesis and tumor growth. Int J Cancer. 2004;112:1042–1048. doi: 10.1002/ijc.20494. [DOI] [PubMed] [Google Scholar]
- 31.Jie Z, et al. Protective effects of alpha 1-antitrypsin on acute lung injury in rabbits induced by endotoxin. Chin Med J (Engl) 2003;116:1678–1682. [PubMed] [Google Scholar]
- 32.Toldo S, et al. Alpha-1 antitrypsin inhibits caspase-1 and protects from acute myocardial ischemia-reperfusion injury. J Mol Cell Cardiol. 2011;51:244–251. doi: 10.1016/j.yjmcc.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Reddy P, Negrin R, Hill GR. Mouse models of bone marrow transplantation. Biol Blood Marrow Transplant. 2008;14(1) Suppl 1:129–135. doi: 10.1016/j.bbmt.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill GR, et al. Total body irradiation and acute graft-versus-host disease: The role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 35.Reddy P, et al. Interleukin-18 regulates acute graft-versus-host disease by enhancing Fas-mediated donor T cell apoptosis. J Exp Med. 2001;194:1433–1440. doi: 10.1084/jem.194.10.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy P, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc Natl Acad Sci USA. 2004;101:3921–3926. doi: 10.1073/pnas.0400380101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill GR, et al. Differential roles of IL-1 and TNF-alpha on graft-versus-host disease and graft versus leukemia. J Clin Invest. 1999;104:459–467. doi: 10.1172/JCI6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001;14:417–424. doi: 10.1016/s1074-7613(01)00122-4. [DOI] [PubMed] [Google Scholar]
- 39.Shlomchik WD, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 40.Zeiser R, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111:453–462. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strom TB. Saving islets from allograft rejection. Proc Natl Acad Sci USA. 2005;102:12651–12652. doi: 10.1073/pnas.0506079102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 43.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: Endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 44.Arora PK, Miller HC, Aronson LD. alpha1-Antitrypsin is an effector of immunological stasis. Nature. 1978;274:589–590. doi: 10.1038/274589a0. [DOI] [PubMed] [Google Scholar]
- 45.Münch J, et al. Discovery and optimization of a natural HIV-1 entry inhibitor targeting the gp41 fusion peptide. Cell. 2007;129:263–275. doi: 10.1016/j.cell.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 46.Kalis M, Kumar R, Janciauskiene S, Salehi A, Cilio CM. α 1-antitrypsin enhances insulin secretion and prevents cytokine-mediated apoptosis in pancreatic β-cells. Islets. 2010;2(3):185–189. doi: 10.4161/isl.2.3.11654. [DOI] [PubMed] [Google Scholar]
- 47.Shin HJ, et al. Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4+CD25+Foxp3+ regulatory T cells. Blood. 2011;118:2342–2350. doi: 10.1182/blood-2010-10-313684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009;21:281–285. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marcondes AM, et al. Inhibition of IL-32 activation by α-1 antitrypsin suppresses alloreactivity and increases survival in an allogeneic murine marrow transplantation model. Blood. 2011;118(18):5031–5039. doi: 10.1182/blood-2011-07-365247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subramaniyam D, et al. Cholesterol rich lipid raft microdomains are gateway for acute phase protein, SERPINA1. Int J Biochem Cell Biol. 2010;42:1562–1570. doi: 10.1016/j.biocel.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 51.Subramaniyam D, et al. Effects of alpha 1-antitrypsin on endotoxin-induced lung inflammation in vivo. Inflamm Res. 2010;59:571–578. doi: 10.1007/s00011-010-0164-x. [DOI] [PubMed] [Google Scholar]
- 52.Aldonyte R, Jansson L, Janciauskiene S. Concentration-dependent effects of native and polymerised alpha1-antitrypsin on primary human monocytes, in vitro. BMC Cell Biol. 2004;5(5):11. doi: 10.1186/1471-2121-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou X, Shapiro L, Fellingham G, Willardson BM, Burton GF. HIV replication in CD4+ T lymphocytes in the presence and absence of follicular dendritic cells: Inhibition of replication mediated by α-1-antitrypsin through altered IκBα ubiquitination. J Immunol. 2011;186:3148–3155. doi: 10.4049/jimmunol.1001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papadopoulou A, Lloyd DR, Williams MD, Darbyshire PJ, Booth IW. Gastrointestinal and nutritional sequelae of bone marrow transplantation. Arch Dis Child. 1996;75:208–213. doi: 10.1136/adc.75.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paczesny S, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113:273–278. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.