Abstract
Both cardiac myocytes and cardiac stem cells (CSCs) express the receptor of growth hormone releasing hormone (GHRH), activation of which improves injury responses after myocardial infarction (MI). Here we show that a GHRH-agonist (GHRH-A; JI-38) reverses ventricular remodeling and enhances functional recovery in the setting of chronic MI. This response is mediated entirely by activation of GHRH receptor (GHRHR), as demonstrated by the use of a highly selective GHRH antagonist (MIA-602). One month after MI, animals were randomly assigned to receive: placebo, GHRH-A (JI-38), rat recombinant GH, MIA-602, or a combination of GHRH-A and MIA-602, for a 4-wk period. We assessed cardiac performance and hemodynamics by using echocardiography and micromanometry derived pressure-volume loops. Morphometric measurements were carried out to determine MI size and capillary density, and the expression of GHRHR was assessed by immunofluorescence and quantitative RT-PCR. GHRH-A markedly improved cardiac function as shown by echocardiographic and hemodynamic parameters. MI size was substantially reduced, whereas myocyte and nonmyocyte mitosis was markedly increased by GHRH-A. These effects occurred without increases in circulating levels of growth hormone and insulin-like growth factor I and were, at least partially, nullified by GHRH antagonism, confirming a receptor-mediated mechanism. GHRH-A stimulated CSCs proliferation ex vivo, in a manner offset by MIA-602. Collectively, our findings reveal the importance of the GHRH signaling pathway within the heart. Therapy with GHRH-A although initiated 1 mo after MI substantially improved cardiac performance and reduced infarct size, suggesting a regenerative process. Therefore, activation of GHRHR provides a unique therapeutic approach to reverse remodeling after MI.
Keywords: heart failure, cardiac stem cells, cardiac regeneration
Mortality from cardiovascular disease has decreased over time as therapeutic advances have become more elaborate. Despite this progress, no current treatment fully reverses the primary cause of impaired heart function, the loss of cardiomyocytes. Because the worldwide prevalence of heart failure (HF) continues to increase, any intervention that improves this condition would promise a beneficial clinical outcome and should be further explored (1, 2).
Expression of mRNA for growth hormone releasing hormone (GHRH) has been detected in extrapituitary tissues, including the heart (3, 4), consistent with widespread biologic signaling beyond the hypothalamic pituitary axis. Recently, it was shown that the heart harbors GHRH receptors (GHRHRs) that can be activated by GHRH (1–44) (5) and synthetic GHRH agonists (6), suggesting that the ischemic heart is also a target of GHRH signaling. However, our current knowledge on the role of GHRH signaling in the heart is still limited. Granata et al. (5) reported that GHRH (1–44) promoted survival of cardiomyocytes and protected rat hearts from ischemia-reperfusion injury. Subsequently,we demonstrated that a potent GHRH agonist (GHRH-A; JI-38) stimulated substantial cardiac repair after acute ischemic injury in a rodent model independently of growth hormone (GH) or insulin-like growth factor I (IGF-I) (6). JI-38 is a synthetic analog of human GHRH with high activity and greater metabolic stability due to incorporation of amino acid substitutions that increase the peptide's resistance to degradation (7).
Experimental and clinical studies aimed at developing either GH or IGF-I therapeutically have met so far with mixed results. In addition, compounds in this class can produce side effects such as fluid retention, hypertension, arrhythmias, risk of diabetes, and increased body weight. Accordingly, activation of the cardiovascular GHRH/GHRHR axis has the potential to exert beneficial effects that are based on direct receptor action and can eliminate the side effects of GH or IGF-I (8, 9).
Whereas our previous results demonstrated the efficacy of GHRH-A at preventing remodeling, it remains important to assess whether this effect could actually reverse remodeling of the chronically injured heart. Therefore, we tested the hypothesis that activation of GHRHR in the heart using a potent GHRH-A can reverse ventricular remodeling and improve cardiac performance after chronic cardiac injury. We used a selective GHRH antagonist (MIA-602) to inhibit or block these actions to test whether the effects of the agonist are fully receptor mediated.
Results
As illustrated in SI Appendix, Fig. S1, body weights (BW) were similar for all treatment groups at baseline. BW at week 8 was increased by treatment with rat recombinant GH (rrGH) (P < 0.01), GHRH-A, and GHRH (A+Ant) (P < 0.05) in comparison with placebo and MIA-602; however, heart weight (HW), HW/BW, and HW/tibia length (HW/TL) ratios did not change.
GH and IGF-I Levels.
The circulating levels of GH (SI Appendix, Fig. S2A) were similar in all groups, except the rrGH group. As expected, administration of rrGH substantially increased serum levels of GH (P < 0.0001 vs. all other groups). Surprisingly, IGF-I (SI Appendix, Fig. S2B) was increased in both rrGH and GHRH (A+Ant) groups (P < 0.0001 vs. placebo, GHRH-A, and MIA-602 groups) but without increases in GH level in the latter one.
Expression of GHRHR.
The expression of GHRHR in isolated cardiac myocytes assessed by immunostaining (SI Appendix, Fig. S3) was substantially increased in GHRH-A and rrGH groups [P < 0.05 vs. placebo, GHRH (A+Ant), and MIA-602]. In addition, RT-PCR (SI Appendix, Fig. S4) also revealed an overexpression of GHRHR in GHRH-A and rrGH groups (P < 0.05 vs. placebo).
Impact of GHRHR Activation on Ventricular Remodeling.
Baseline echocardiography documented similar parameters of LV dimension and function in all groups (Fig. 1 and SI Appendix, Table S1). Importantly, at the time of treatment (30 d after MI), cardiac function was markedly impaired relative to baseline, indicating the development of ventricular dysfunction after MI; all echocardiographic parameters revealed similar degrees of global LV functional deterioration and ventricular chamber enlargement in all groups at the time of treatment.
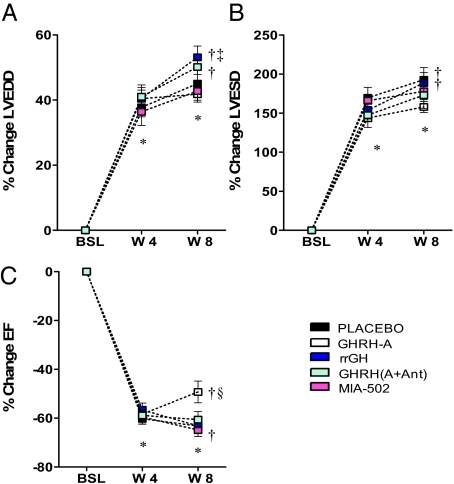
Fig. 1.
Impact of treatments on ventricular remodeling. Graphs correspond to changes over time in LV end-diastolic (LVEDD) (Upper Left), end-systolic (LVESD) diameters (Upper Right), and ejection fraction (EF) (Lower). All values represent mean ± SEM (n = 7–10), *P < 0.05 vs. baseline (BSL), same group; †P < 0.05 vs. wk 4 (W4), same group; ‡P < 0.05 vs. all other groups at week 8 (W8), except GHRH (A+Ant). §P < 0.05 vs. all other groups at wk 8.
Between the 4 and 8 wk evaluation, LVEDD (Fig. 1A) and LVESD (Fig. 1B) progressively increased in the placebo, rrGH, and GHRH (A+Ant) groups. This increase was prevented by GHRH-A (SI Appendix, Table S1). Moreover, the reduction in ejection fraction (EF) (Fig. 1C) due to MI was restored toward normal by ≈22% with the administration of GHRH-A [P < 0.05 vs. all other groups. Administration of MIA-602 blocked the favorable effects of GHRH-A on ventricular chamber size and EF.
Impact of GHRHR Activation on Cardiovascular Performance.
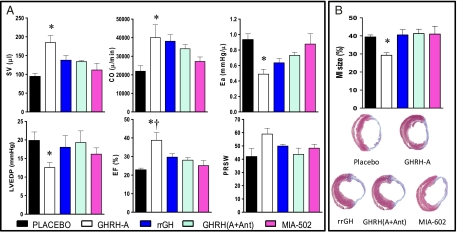
Fig. 2A and SI Appendix, Table S2 and Fig. S5 summarize analyses of hemodynamic parameters derived from pressure-volume loops in steady-state and loading conditions just before euthanasia. Load-dependent parameters of systolic function such as stroke volume (SV) and cardiac output (CO) were markedly increased by treatment with GHRH-A [P < 0.01 vs. placebo, GHRH (A+Ant), and MIA-602 groups]. The improvement in cardiac performance was, at least partially, due to significant reduction in ventricular afterload (Ea), P < 0.05 vs. placebo and MIA-602. In addition, LV end-diastolic pressure (LVEDP) was also reduced by therapy with GHRH-A (P < 0.05 vs. placebo). In agreement with our echocardiographic data, GHRH-A led to a sustained improvement of myocardial function, as determined by EF. Moreover, preload recruitable stroke work (PRSW) trended to be higher only in the GHRH-A group (P = 0.0547).
Fig. 2.
Hemodynamic parameters derived from pressure-volume loops (A) and infarct size (B). A shows stroke volume (SV), cardiac output (CO), arterial elastance (Ea), *P < 0.05 vs. placebo and MIA-602; LV end-diastolic pressure (LVEDP), *P < 0.05 vs. placebo (Student's t test); ejection fraction (EF), *P < 0.01 vs. placebo and MIA-602; †P < 0.05 vs. GHRH (A+Ant); preload recruitable stroke work (PRSW). All values represent mean ± SEM (n = 5–7). (B) MI size was significantly reduced by GHRH-A therapy, *P < 0.05 vs. all groups (n = 7–10). At the bottom, representative Masson's trichrome-stained sections of each group at midventricular level.
Impact on Scar Size, Capillary Density, and Cell Survival.
MI size was similar in all groups (Fig. 2B), except in rats treated with GHRH-A, which showed a significant scar reduction (P < 0.05 vs. all other groups). Capillary density (SI Appendix, Fig. S6) at the MI border zone was increased in all treated groups, but to a greater extent in the GHRH-A group (P < 0.0001 vs. placebo and MIA-602), whereas at the areas remote to MI, there were no differences. Apoptotic cells were detected by TUNEL assay (SI Appendix, Fig. S7). Overall, none of the treatments significantly reduced the expression of apoptotic cells at the chronic stage of MI.
Impact on Cellular Division, Proliferation, and Differentiation.
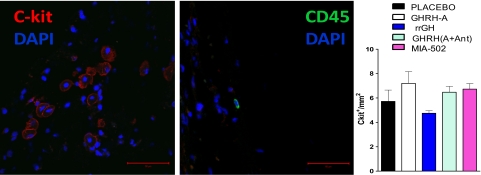
The number of cardiac stem cells (c-kitpos) was not different among groups but there was a trend for them to be higher after GHRH-A therapy and, as shown in Fig. 3, the majority of c-kitpos cells were not bone marrow derived; these cells were CD45neg and tryptaseneg and were clearly distinguishable from resident cardiac mast cells (c-kitpos CD45pos). Thus, similar to our previous study, the quantity of mast cells was minute.
Fig. 3.
Representative confocal micrograph image of c-kitpos (red), CD45pos (green), and nuclei (blue). (Scale bar: 50 μm.) (Right) Bar graph corresponds to expression of c-kitpos cells in the heart (n = 3–4).
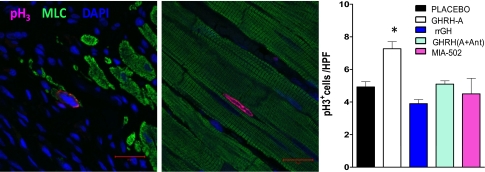
In addition, the presence of cellular mitosis was determined by the nuclear localization of phospho-histone H3 (pH3). Our results showed that the expression of pH3pos cells, including myocytes and nonmyocytes, was significantly higher in the rats treated with GHRH-A and rrGH at the infarct border zone (Fig. 4).
Fig. 4.
Immunostaining analysis of mitosis in heart tissues by phospho-histone H3 (pH3). Representative confocal micrograph images of pH3 (magenta), myosin light chain (MLC, green), and nuclei (DAPI, blue). (Scale bar: 20 μm.) Bar graph corresponds to expression of pH3pos cells at the border zone. Data represent mean ± SEM (n = 3), *P < 0.05 vs. placebo and rrGH groups.
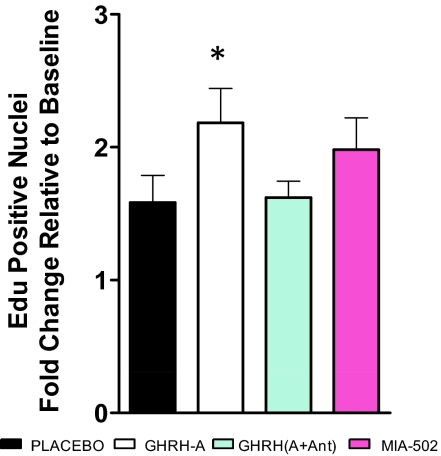
Next, we determined the impact of GHRH-A activity on cardiac stem cells (CSCs) division in vitro by incorporation of the thymidine analog EdU during S phase of the cell cycle. Our data (Fig. 5) showed an increase in the proliferation of CSCs after pretreatment with GHRH-A (P < 0.05 vs. placebo, Student's t test), whereas other treatments did not show a difference. Importantly, we also confirmed by fluorescence-activated cell sorting (FACS) analysis that CSCs express the GHRH-R (SI Appendix, Fig. S8).
Fig. 5.
Impact of GHRH-A activity in cell division of cardiac stem cells (CSCs). Values represent fold change (mean ± SEM) of incorporation of the thymidine analog EdU for 16 h relative to baseline. GHRH-A treatment significantly increased the rate of DNA synthesis relative to vehicle (DMSO), *P < 0.05, Student's t test (n = 5–8).
As illustrated in SI Appendix, Fig. S9, the expression of the transcription factor GATA-4 at the border zone was increased by therapy with GHRH-A or rrGH [P < 0.05 vs. placebo, MIA-602, and GHRH (A+Ant) groups].
Effects of Treatments at the Molecular Level.
Gene expression analysis after treatments revealed increased levels of several genes (SI Appendix, Fig. S4). Real-time PCR values of the relative expression of mRNA for anti- and proapoptotic genes (Bcl2 and Bax, respectively) were significantly increased in all treated groups in comparison with the placebo group. Stromal derived factor 1 (SDF-1) was up-regulated only in GHRH-A and GHRH (A+Ant) groups, whereas stem cell factor was overexpressed in GHRH-A, MIA-602, and GHRH (A+Ant) groups. Therapy with GHRH-A and rrGH also increased the expression of vascular endothelial factor A (VEGF-A) mRNA. Interestingly, mRNA for IGF-I was only up-regulated in the combination group. Cyclin A2 expression was highly increased by GHRH-A therapy and, importantly, down-regulated by MIA-602 treatment.
mRNA for GHRH was up-regulated in all treated groups, but not in the rrGH-treated rats. However, mRNA for GHRHR showed overexpression in the groups treated with GHRH-A, rrGH, and GHRH (A+Ant) but not with MIA-602.
Discussion
The results of this study indicate that the administration of GHRH-A in a chronic stage of myocardial injury can reverse ventricular remodeling and improve cardiac performance. Concurrently, infarct size was significantly reduced and, more importantly, none of these effects were accompanied by increases in circulating levels of GH or IGF-I, and were, for the most part, abrogated by the GHRH-antagonist (MIA-602). Our in vivo and in vitro findings confirm that the effects observed are receptor mediated, and importantly suggest that the effects result from a multifactorial mechanism involving cell-cycle reentry, angiogenesis, and likely cardiac stem cell activation. Together these data support the possibility of a unique therapeutic principle for chronic ischemic HF.
To determine the cardioprotective mechanisms of GHRH-A, capillary density, rate of apoptosis, cell cycle activity (mitosis), and expression of cardiac c-kitpos cells and genes related to these pathways were studied (6, 10, 11). Our previous study in acute MI (6) showed that beneficial functional effect of GHRH-A therapy were mediated mainly through reduction of fibrosis, apoptosis, and recruitment of c-kitpos cells—effects that were associated with myocardial regeneration. In the current study, we documented a similar reduction in scar size and improvement in EF. At a cellular level, we observed a trend in c-kitpos cell increase in vivo, but documented that GHRH-A stimulated augmentation of CSCs proliferation ex vivo. Importantly, cell cycle reentry of myocytes was also augmented by GHRH-A in vivo, suggestive of an increase in the pool of transient-amplifying cells originating from CSCs (11). The direct stimulation of CSCs by GHRH-A is further supported by documentation of the GHRHR on these stem cells by FACS.
The origin of cardiac c-kit cells could represent either circulating cells likely originating in bone (12, 13) or cardiac stem cells (11, 14). Our costaining of these cells with CD45 was able to differentiate them from hematopoietic origin, and, also most likely cardiac mast cells (15). Thus, the present trend toward an increase in c-kit cells in vivo, accompanied by an increase in pH3 cells, and the ex vivo results demonstrating that GHRH-A augments CSCs proliferation, all support the notion that GHRH-A's stimulate cardiac stem cell mediated tissue repair.
Our data also showed an increased expression of the transcription factor GATA-4 in response to GHRH-A, which is known to play important roles in regulating cell differentiation, proliferation, organ morphogenesis and, noted more recently, in regulation of apoptosis (16). Data by Rysa et al. (17) suggested that GATA-4 is an antiapoptotic factor required for adaptive responses and a key regulator of hypertrophy and hypertrophy-associated genes in the heart. Moreover, the reversal of reduced GATA-4 activity prevented adverse postinfarction remodeling through myocardial angiogenesis, antiapoptosis, and stem cell recruitment (17, 18).
We measured the expression level of various genes to gain further insights into the molecular underpinnings of our findings. Importantly, supporting the finding that GHRH-A stimulates cellular regeneration, we found increased levels of cyclin A2 mRNA in these hearts, and that this effect was blocked in the group receiving MIA-602. Previous work by Cheng et al. (19) demonstrated that cyclin A2 induces cardiac regeneration after MI and prevents HF through induction of a side population of cells with enhanced proliferative capacity.
We also tested the possibility that increased release of soluble factors (paracrine effects) contributed to the improvement in functional cardiac performance in the heart (20–23). This proposition is compatible with the results of Gnecchi et al. (24), which have implicated growth factors, such as VEGF-A, IGF-I, and basic fibroblast growth factor, in post-MI cardiac repair. In support of this paracrine hypothesis, our results revealed that the mRNA level for VEGF-A is significantly up-regulated in both GHRH-A and rrGH-treated rats relative to the placebo group. Higher expression of VEGF-A has also been associated with better collateral circulation development after ischemia (25, 26) and, consequently, better clinical outcome. More recently, Tang et al. (27) reported that activation of VEGF/SDF-1 signaling promotes myocardial repair at least in part through cardiac stem cell mobilization. SDF-1 is well known as a homing factor for a variety of stem cell populations and, therefore, for improving cardiac function post-MI (28, 29). This finding is also consistent with our results, which show up-regulation of SDF-1 in the GHRH-A and combination-treated groups.
Surprisingly, mRNA for IGF-I was only increased in the combination group, whereas IGF-I levels in the serum were increased in both rrGH and combination-treated rats. We postulated that the discrepancies observed in the circulating levels of GH and IGF-I in the combination group can be explained by the fact that stimuli other than GH, such as prolactin (PRL), platelet-derived growth factor, or nutritional status, can also lead to an increase in IGF-I synthesis (30, 31). Dardenne et al. (32) reported that cells expressing GH receptor also express receptors for PRL. Because the effect of administration of GHRH antagonist was not completely abolished in the combination group, one could speculate that the interval (5 min) between the administration of GHRH antagonist (MIA-602) and agonist (GHRH-A) presumably was not adequate to block or attenuate the effects of GHRH-A (JI-38); however, in fact, GH level was not increased in this group.
The impact of GHRH-A's on the GH/IGF-I axis warrants mention. Physiologically, spikes in GH secretion result from pulsatile release of GHRH. We administered s.c. a GHRH-agonist analog twice daily for 4 wk, and elevations in circulating GH and IGF-I levels did not occur. However, the agonist would be expected to produce a GH spike starting 15–30 min after injection and lasting for 30–60 min (i.e., 60–90 min after injection), a measurement at 12–24 h would not detect this elevation.
The current study is limited because the pharmacokinetics of the agonist are not fully characterized. Our results clearly indicate that the half-life of JI-38 is adequate to stimulate the reported myocardial processes. Indeed, as our results indicate, the doses delivered failed to activate the endocrine GH–IGF-I axis. Additional work will be required to clarify the pharmacodynamics, pharmacokinetics, metabolism, and breakdown products of JI-38.
Collectively, our findings demonstrate that GHRH-A therapy, when initiated 1 mo after MI once ventricular remodeling has already occurred, substantially improves the degree of cardiac dysfunction and reduces infarct size, suggesting that the regenerative process is still potentially activated at this late stage, and these benefits are independent on the GH or IGF-I. Together our findings support the possibility that the use of potent GHRH agonists could have therapeutic benefits in patients with acute MI, chronic ischemic heart disease, and possibly a broader array of diseases of heart muscle.
Materials and Methods
Animal Model.
All experiments involving rats were carried out in accordance with protocols reviewed and approved by the University of Miami Animal Care and Use Committee in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication no. 85–23, revised 1996).
MI was induced by permanent ligation of the left coronary artery in female 6-mo-old Fisher-344 rats as described (33). Animals were randomly assigned to receive placebo (DMSO + propylene glycol), GHRH-agonist (GHRH-A [JI-38]; 50 μg/kg), GHRH-antagonist (MIA-602; 50 μg/kg), combination of GHRH-A plus GHRH-antagonist [GHRH (A+Ant), same dose as for each treatment alone] or rat recombinant GH (rrGH; 0.5 mg/kg) starting 4 wk after MI. All treatment was given s.c. twice daily for 4 wk.
Drugs.
rrGH was supplied by Dr. A. F. Parlow from National Hormone and Pituitary Program (University of California-Harbor, Torrance, CA) and GHRH-A (JI-38) and GHRH-antagonist (MIA-602) were synthesized in the laboratories of A.V.S. (7, 9, 34). For additional information, see SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Wayne Balkan for reviewing the manuscript and J. B. Fernandez for technical assistance. This work was supported by National Institutes of Health R01 Grants HL107110, AG025017, HL084275, HL65455, HL094848; and by National Heart, Lung, and Blood Institute Grant U54-HL081028 (all to J.M.H.). The studies in the A.V.S. laboratory were supported by the Medical Research Service of the Department of Veterans Affairs, the South Florida Veterans Affairs Foundation for Research and Education, and the Division of Hematology/Oncology, Department of Pathology and Medicine, Miller School of Medicine, University of Miami.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119203109/-/DCSupplemental.
References
- 1.Kanashiro-Takeuchi RM, Schulman IH, Hare JM. Pharmacologic and genetic strategies to enhance cell therapy for cardiac regeneration. J Mol Cell Cardiol. 2011;51:619–625. doi: 10.1016/j.yjmcc.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang H, Kloner RA. Improving regenerating potential of the heart after myocardial infarction: Factor-based approach. Life Sci. 2010;86:461–472. doi: 10.1016/j.lfs.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Lin-Su K, Wajnrajch MP. Growth Hormone Releasing Hormone (GHRH) and the GHRH Receptor. Rev Endocr Metab Disord. 2002;3:313–323. doi: 10.1023/a:1020949507265. [DOI] [PubMed] [Google Scholar]
- 4.Matsubara S, Sato M, Mizobuchi M, Niimi M, Takahara J. Differential gene expression of growth hormone (GH)-releasing hormone (GRH) and GRH receptor in various rat tissues. Endocrinology. 1995;136:4147–4150. doi: 10.1210/endo.136.9.7649123. [DOI] [PubMed] [Google Scholar]
- 5.Granata R, et al. Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia-reperfusion injury in rat heart. Cardiovasc Res. 2009;83:303–312. doi: 10.1093/cvr/cvp090. [DOI] [PubMed] [Google Scholar]
- 6.Kanashiro-Takeuchi RM, et al. Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction. Proc Natl Acad Sci USA. 2010;107:2604–2609. doi: 10.1073/pnas.0914138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izdebski J, et al. Synthesis and biological evaluation of superactive agonists of growth hormone-releasing hormone. Proc Natl Acad Sci USA. 1995;92:4872–4876. doi: 10.1073/pnas.92.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aimaretti G, et al. GHRH and GH secretagogues: Clinical perspectives and safety. Pediatr Endocrinol Rev. 2004;2(Suppl 1):86–92. [PubMed] [Google Scholar]
- 9.Kiaris H, Schally AV, Kalofoutis A. Extrapituitary effects of the growth hormone-releasing hormone. Vitam Horm. 2005;70:1–24. doi: 10.1016/S0083-6729(05)70001-7. [DOI] [PubMed] [Google Scholar]
- 10.Schuleri KH, et al. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am J Physiol Heart Circ Physiol. 2008;294:H2002–H2011. doi: 10.1152/ajpheart.00762.2007. [DOI] [PubMed] [Google Scholar]
- 11.Hatzistergos KE, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogawa M, et al. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med. 1991;174:63–71. doi: 10.1084/jem.174.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edling CE, Hallberg B. c-Kit—a hematopoietic cell essential receptor tyrosine kinase. Int J Biochem Cell Biol. 2007;39:1995–1998. doi: 10.1016/j.biocel.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: A paradigm shift in human myocardial biology. Circ Res. 2011;109:941–961. doi: 10.1161/CIRCRESAHA.111.243154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Sperr WR, et al. The human cardiac mast cell: Localization, isolation, phenotype, and functional characterization. Blood. 1994;84:3876–3884. [PubMed] [Google Scholar]
- 16.Suzuki YJ, Evans T. Regulation of cardiac myocyte apoptosis by the GATA-4 transcription factor. Life Sci. 2004;74:1829–1838. doi: 10.1016/j.lfs.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Rysa J, et al. GATA-4 is an angiogenic survival factor of the infarcted heart. Circ Heart Fail. 2010;3:440–450. doi: 10.1161/CIRCHEARTFAILURE.109.889642. [DOI] [PubMed] [Google Scholar]
- 18.Heineke J, et al. Cardiomyocyte GATA4 functions as a stress-responsive regulator of angiogenesis in the murine heart. J Clin Invest. 2007;117:3198–3210. doi: 10.1172/JCI32573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng RK, et al. Cyclin A2 induces cardiac regeneration after myocardial infarction and prevents heart failure. Circ Res. 2007;100:1741–1748. doi: 10.1161/CIRCRESAHA.107.153544. [DOI] [PubMed] [Google Scholar]
- 20.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stastna M, Abraham MR, Van Eyk JE. Cardiac stem/progenitor cells, secreted proteins, and proteomics. FEBS Lett. 2009;583:1800–1807. doi: 10.1016/j.febslet.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stastna M, Chimenti I, Marbán E, Van Eyk JE. Identification and functionality of proteomes secreted by rat cardiac stem cells and neonatal cardiomyocytes. Proteomics. 2010;10:245–253. doi: 10.1002/pmic.200900515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chimenti I, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gnecchi M, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 25.Banai S, et al. Upregulation of vascular endothelial growth factor expression induced by myocardial ischaemia: Implications for coronary angiogenesis. Cardiovasc Res. 1994;28:1176–1179. doi: 10.1093/cvr/28.8.1176. [DOI] [PubMed] [Google Scholar]
- 26.Waltenberger J, Kranz A, Beyer M. Neovascularization in the human heart is associated with expression of VEGF-A and its receptors Flt-1 (VEGFR-1) and KDR (VEGFR-2). Results from cardiomyopexy in ischemic cardiomyopathy. Angiogenesis. 1999;3:345–351. doi: 10.1023/a:1026585900398. [DOI] [PubMed] [Google Scholar]
- 27.Tang JM, et al. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res. 2011;91:402–411. doi: 10.1093/cvr/cvr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxena A, et al. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224–2231. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segers VF, et al. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 30.Teale JD, Marks V. The measurement of insulin-like growth factor I: Clinical applications and significance. Ann Clin Biochem. 1986;23:413–424. doi: 10.1177/000456328602300406. [DOI] [PubMed] [Google Scholar]
- 31.Clemmons DR. Modifying IGF1 activity: An approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov. 2007;6:821–833. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- 32.Dardenne M, Mello-Coelho V, Gagnerault MC, Postel-Vinay MC. Growth hormone receptors and immunocompetent cells. Ann N Y Acad Sci. 1998;840:510–517. doi: 10.1111/j.1749-6632.1998.tb09589.x. [DOI] [PubMed] [Google Scholar]
- 33.Kanashiro-Takeuchi RM, Heidecker B, Lamirault G, Dharamsi JW, Hare JM. Sex-specific impact of aldosterone receptor antagonism on ventricular remodeling and gene expression after myocardial infarction. Clin Transl Sci. 2009;2:134–142. doi: 10.1111/j.1752-8062.2009.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schally AV, Varga JL, Engel JB. Antagonists of growth-hormone-releasing hormone: An emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab. 2008;4:33–43. doi: 10.1038/ncpendmet0677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.