Abstract
The ability of human CMV (HCMV) to enter and establish a latent infection in myeloid cells is crucial for survival and transmission in the human population. Initial pathogen binding and entry triggers a number of antiviral responses, including the activation of proapoptotic cell death pathways, which must be countered during latency establishment. However, mechanisms responsible for a prosurvival state in myeloid cells upon latent HCMV infection remain completely undefined. We hypothesized that the cellular antiapoptotic machinery must be initially activated by HCMV to promote early survival events upon entry. Here we show that HCMV transiently protects nonpermissive myeloid cells from chemical and virus entry induced cell death by up-regulating a key myeloid cell survival gene, myeloid cell leukemia (MCL)-1 protein. The induction of MCL-1 expression was independent of viral gene expression but dependent on activation of the ERK–MAPK pathway by viral glycoprotein B. Inhibition of ERK-MAPK signaling, inhibition of HCMV fusion, antibody-mediated neutralization of glycoprotein B signaling or expression of a shRNA against MCL-1 all correlated with increased cell death in response to virus infection or chemical stimulation. Finally we show that activation of ERK–MAPK signaling impacts on long-term latency and reactivation in hematopoietic cells. Thus, HCMV primes myeloid cells for from the initial virus-cell encounter. Given the importance of ERK and MCL-1 for myeloid cell survival, the successful establishment of HCMV latency in myeloid progenitors begins at the point of virus entry.
Human CMV (HCMV) infection of healthy individuals is usually asymptomatic and results in the establishment of a lifelong latent infection (1). Although infection can be asymptomatic, primary infection, reinfection, or reactivation from latency in neonates, immunosuppressed transplant recipients, late-stage AIDS cases, and critically ill patients in intensive care can result in serious morbidity and mortality (2–4). In contrast to the broad cellular tropism of HCMV for lytic infection (reviewed in ref. 5), latent infection appears to be restricted to a subpopulation of hematopoietic CD34+ bone marrow progenitor cells that give rise to the cells of the myeloid lineage within peripheral blood (6). HCMV latency is operationally defined by the absence of lytic gene expression following infection and the ability of the virus to reenter the lytic life cycle (i.e., reactivation) at a later date when the appropriate cellular environment is encountered.
Like most pathogens, HCMV manipulates the host cell to create a cellular environment conducive for virus survival. However, HCMV binding and entry to the surface of the cell has profound effects on the cellular environment (7, 8), with some changes of no immediate apparent benefit to the virus, including a strong innate immune response characterized by the rapid induction of inflammatory cytokine and IFN-stimulated gene expression promoting a highly antiviral state (7–9). Virus binding is also to activate cellular pathways, including PI3K/Akt (10), MAP kinase ERK1/2 (11), and p38 (12), as well as signaling through TLR receptors (13), which results in significant reprogramming of cellular gene expression (8). Although it is not known what the precise effect many of these individual changes have on HCMV infection, it is clear that, globally, the virus can isolate the facets of signaling that benefit HCMV replication while inhibiting the aspects that are detrimental and that this reprogramming of the host cell response begins with viral entry and persists throughout lytic infection (7, 8, 14, 15).
The very early stages of virus binding and entry represent the first of many proapoptotic signals that HCMV triggers upon infection. During lytic infection, expression of an impressive armory of viral antiapoptotic functions (UL36-38; β2.7) (16, 17) has a profound contribution to the survival of infected cells. However, what mediates this during nonpermissive infections was less clear. Consequently, we hypothesized that HCMV targeted the host cellular machinery to elicit initial protection from apoptosis by using one of the pathways that it activates upon binding and entry. The role of myeloid progenitor cells in HCMV latency (6) led us to assess the role, if any, of myeloid cell leukemia (MCL)-1 protein, which plays an obligate role in myeloid cell survival (18). Originally identified from a myeloid leukemia cell line (19), MCL-1 is an inherently unstable (t1/2, 3 h) antiapoptotic member of the BCL-2 family (19) under complex regulation (20) in a cell type-specific manner. Indeed, during the course of our own studies, it was shown that sustained PI3K activation in infected monocytes resulted in prolonged MCL-1 protein expression promoting survival of short-lived monocytes in response to HCMV infection, with clear implications for virus persistence (21). As this was consistent with our own hypothesis, we chose to investigate this further in CD34+ cells—the natural site of HCMV latency.
In this study, we have assessed the impact of HCMV infection on the viability of infected nonpermissive myeloid cells immediately after infection, as progenitor myeloid cells represent a major site for the establishment of HCMV latency within the human host (6). In this report, we show that glycoprotein (g) B (gB), a protein essential for HCMV entry into cells (22), mediates protection of cells. We observe that activation of the ERK pathway, which correlates with transient up-regulation of MCL-1 upon virus binding and entry, is key to survival. We hypothesize that the ability of HCMV to initiate the establishment of latency in myeloid progenitors begins with transient induction of a favorable cellular environment upon binding and entry, which, at least in part, involves the induction of ERK–MAPK signaling and the downstream target MCL-1.
Results
HCMV Induces Transient MCL-1 Expression, Which Correlates with Protection from Cisplatin A-Induced Cell Death.
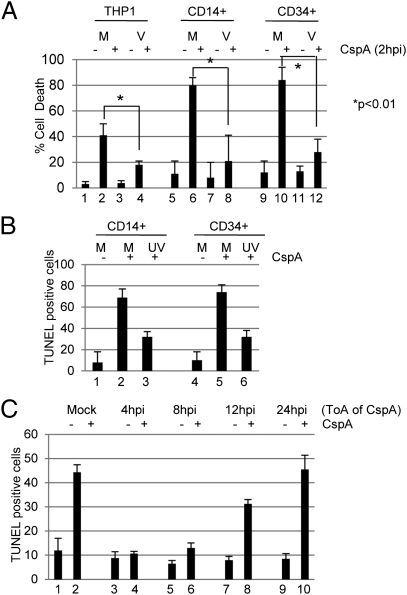
A hallmark of myeloid progenitor cells is differentiation-dependent permissiveness for lytic infection. The myelomonocytic cell line, THP1, exhibits the same differentiation-dependent permissiveness for lytic infection as primary cells (23) and thus can, potentially, be used to model aspects of nonpermissive infection in vitro (6). A major component of the innate cellular antiviral response is cell death induction upon infection. We hypothesized that HCMV could induce a prosurvival environment to combat this, and, indeed, HCMV infection provided significant protection from cell death triggered by the DNA alkylating drug cisplatin A (Fig. 1A), of which MCL-1 is a natural antagonist. Interestingly, protection was independent of viral gene expression, as UV-inactivated virus elicited the same protection in both CD14+ and CD34+ cell infections (Fig. 1B). Furthermore, this initial protection was transient, as latently infected CD34+ cells 24 h postinfection (hpi) were no longer resistant to cisplatin A-induced cell death (Fig. 1C).
Fig. 1.
HCMV transiently protects myeloid cells from cell death. (A) THP1 (bars 1–4), CD14+ (bars 5–8), or CD34+ (bars 9–12) cells mock- (M) or HCMV (V)-infected were incubated with DMSO (−) or 25 μM cisplatin (+) 2 hpi for 4 h and then assayed by trypan blue staining for cell viability 18 h later (n = 3). Significance was measured by using Student t test. (B) CD14+ (bars 1–4) or CD34+ (bars 5–8) cells mock- (M) or UV-HCMV (UV)-infected were incubated with DMSO (−) or 25 μM cisplatin (+) 2 hpi for 4 h and then assayed by TUNEL 24 h later to measure apoptosis. (C) CD34+ cells mock- or HCMV-infected and then incubated with 25 μM cisplatin A at 2 to 24 hpi were analyzed by TUNEL 24 h later to measure apoptosis.
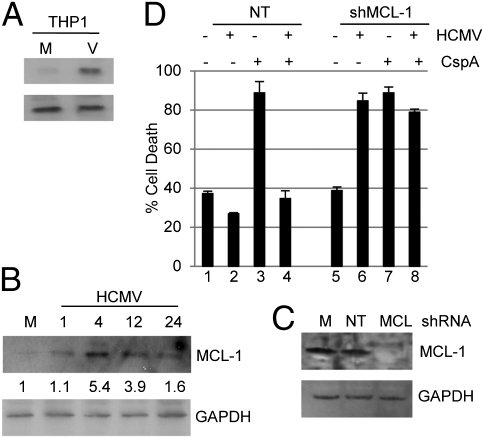
Thus, our initial observations were in agreement with a prior study that showed that the survival of CD14+ monocytes was promoted by prolonged overexpression of MCL-1 (21). Similarly, we observed elevated MCl-1 RNA and protein expression in CD34+ cells and THP1 cells at very early times after infection (Fig. 2 A and B and Fig. S1). However, in contrast, we also observed that, in CD34+ cells, unlike reported in monocytes (21), MCL-1 expression rapidly returned to approaching basal levels after initial binding events (Fig. 2B), correlating with the transient protection observed (Fig. 1C). Unfortunately, shRNA inhibition of MCL-1 expression was overtly deleterious to CD34+ cell viability, but, by using the THP1 cell line, we observed that inhibition HCMV-induced HCMV MCL-1 expression abrogated protection from cisplatin A-induced cell death (Fig. 2C). Of note, infection of MCL-1–KO cells with HCMV, even in the absence of a chemical cell death stimulus, triggered high levels of cell death (Fig. 2C, bar 6), suggesting that MCL-1 was required to counter death signals activated by infection.
Fig. 2.
HCMV up-regulation of MCL-1 correlates with protection from cell death. (A and B) MCL-1 and GAPDH protein expression was analyzed in mock- (M) and HCMV (V)-infected cells 4 hpi of THP1 cells (A) and 0–24 hpi were analyzed in CD34+ cells infected with HCMV (B). Densitometry shows the ratio change from mock. (C) Western blot for MCL-1 and GAPDH expression was performed on THP1 cells 24 h after transduction with mock (M) or a nontargeting (NT) or shMCL-1 expressing (MCL) lentiviral vector. (D) THP1 cells were first transduced with either nontargeting (bars 1–4) or shMCL-1–expressing (bars 5–8) lentiviral vectors for 24 h and then mock- or HCMV-infected. At 2 hpi, they were incubated with DMSO or cisplatin A for 4 h. Following rescue in fresh media, cell viability was assayed by trypan blue staining 18 h after cisplatin A treatment.
HCMV gB Is a Key Component of Initial Survival Response.
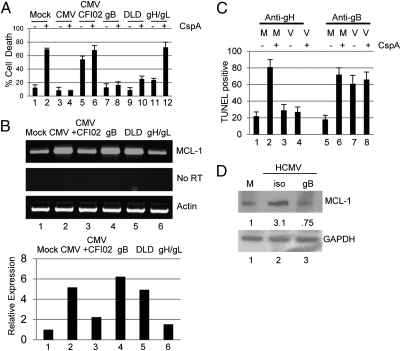
These data suggested an event associated with virus binding and entry was important for this initial protection from cell death. HCMV entry involves a number of virion–cell surface protein interactions involving predominantly gB and gH/gL complexes. Thus, we first asked whether either of these recombinant proteins elicited protection (Fig. 3A). A protective effect was observed with recombinant gB, but not gH/gL, which was recapitulated with just the DLD peptide. Similarly, CFI02-mediated inhibition of gB activity abrogated HCMV protection from cell death. Furthermore, protection correlated with elevated MCL-1 RNA expression (Fig. 3B). We next asked whether neutralization of gB function would abrogate protection from cell death. By using previously characterized anti-gB (ITC88) and anti-gH (MSL109) therapeutic antibodies, targeting gB-, but not gH-, containing complexes abrogated protection from cell death of CD34+ cells (Fig. 3C) and THP1 cells (Fig. S2A). Furthermore, blockade of gB binding alone promoted cell death—even in the absence of addition of cisplatin (bar 7). In contrast, incubation with MSL109 had no impact on virus-mediated protection from cisplatin A-induced cell death (bar 4). Finally, this correlated with an anti-gB antibody-induced impairment of HCMV-mediated up-regulation of MCL-1 expression upon infection of CD34+ cells (Fig. 3D).
Fig. 3.
HCMV-mediated protection from cell death is gB-dependent. (A) THP1 cells incubated with mock (bars 1 and 2), HCMV (bars 3 and 4), HCMV plus CFI02 (bars 5 and 6) or recombinant gB (bars 7 and 8), DLD (bars 9 and 10), or gH/gL (11 and 12) proteins were incubated with DMSO or cisplatin A 2 h after addition of ligand. After 18 h, cell death was assayed by trypan blue staining. (B) RT-PCR for MCL-1 and actin expression in THP1 cells incubated with mock, HCMV, HCMV plus CFI02, recombinant gB, DLD, or gH/gL protein for 2 h. (C) Media (M) or HCMV (V) preincubated with anti-gH (bars 1–4) or anti-gB (bars 5–8) antibodies was used to infect CD34+ cells which, at 4 hpi, were incubated with 0.1% DMSO (−) or cisplatin A (+) for 3 h, then rescued in fresh media. Cell death was measured by TUNEL staining 24 hpi. (D) Western blot analysis for MCL-1 protein expression in mock or HCMV-infected following earlier incubation with anti-gB or isotype control. Densitometry shows the ratio change from mock.
Transient Prolife Signals at Point of Entry Are ERK–MAPK-Dependent and Result in More Efficient Establishment of Latency and Reactivation.
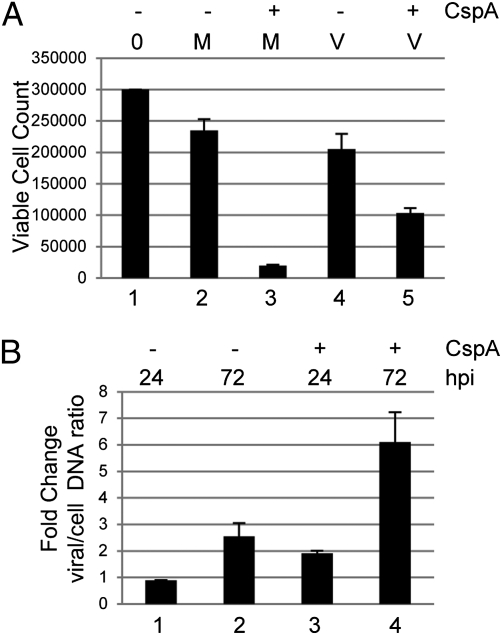
Thus far, these data suggested HCMV-mediated up-regulation of MCL-1 was one component of a transient survival response initiated at the point of infection to prevent cell death. We next asked whether this initial survival phenotype had any impact on the establishment of a long-term latent infection. To test this, we reasoned that the infection of myeloid cells at low multiplicity of infection (MOI) and transient treatment with cisplatin A should promote an enrichment of latently infected cells, as these would be the only cells to survive in a mixed population of infected and uninfected cells. To test this, we infected CD34+ cells at low MOI and treated with cisplatin A 3 hpi. We then “rescued” the treated cells in with fresh media and assayed for viable cell number and the carriage of viral genomes. Unsurprisingly, the transient addition of cisplatin A resulted in a significant decrease in the total number of viable primary cells over time, although to a lesser degree, in the infected population, presumably because of the survival of a small proportion of infected cells (Fig. 4A). Consistent with survival of infected cells, a PCR analysis of genome carriage suggested an enrichment of viral DNA (Fig. 4B), likely because the proportion of genome-positive cells in the remaining viable cells was increasing. As the prodeath trigger was delivered at 3 hpi, these data suggested that virus initiates a transient prosurvival response at very early times of infection, which can have downstream impact on the establishment of a long-term latent pool of infected CD34+ cells.
Fig. 4.
Induction of cell death in a mixed population of cells promotes enrichment of genome-positive cells. (A) CD34+ cells (3 × 105) mock- (M) or HCMV (V)-infected were further incubated with cisplatin (+) or DMSO (−) for 3 hpi. After a further 4 h, cells were plated in fresh media and cultured for 72 h. Viability was assayed by trypan blue. Error bars represent a triplicate counts taken from three independent experiments. (B) DNA was harvested from cultures at 3, 24, and 72 hpi and, following IE and GAPDH PCR, bands were analyzed by densitometry to quantify viral and cellular DNA content. The ratio was expressed as a fold increase from 3 h. Error bars represent a triplicate analysis of a representative experiment (n = 3).
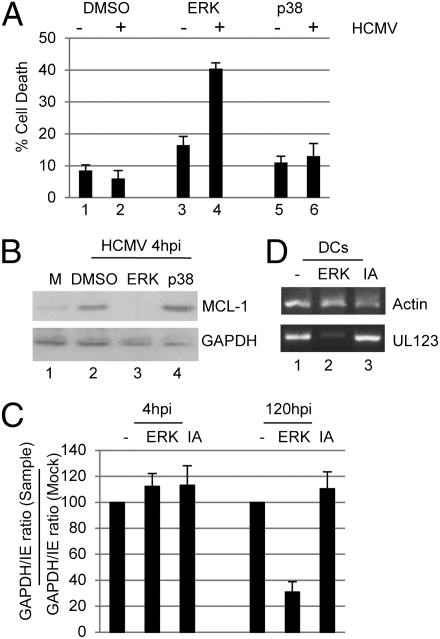
At the interface of virus entry and MCL-1 up-regulation is the activation of a number of cellular pathways. Transfection assays (THP1s) with an MCL-1 luciferase reporter construct identified virus-induced ERK–MAPK activation as a potential mechanism (Fig. S3A). To test this further in the context of latency establishment, we again turned to CD34+ cells. By using a reversible inhibitor of the ERK–MAPK pathway in CD34+ cells, the transient inhibition of ERK–MAPK signaling resulted in a substantial induction of cell death upon virus infection (Fig. 5A), which correlated with decreased MCL-1 expression (Fig. 5B, bar 4), supporting a role for ERK–MAPK signaling during the establishment of latency. These immediate effects manifest as a defect in the long-term carriage of HCMV genomes (Fig. 5C) and decreased reactivation upon differentiation to dendritic cells (Fig. 5D). As such transient activation of the ERK–MAPK pathway at the point of infection has long-term implications—if fewer cells establish a latent infection, it follows that fewer reactivation events will occur manifesting, in these analyses, as decreased IE RNA expression.
Fig. 5.
Inhibition of ERK–MAPK signaling blocks HCMV-induced MCL-1 up-regulation and cell survival. (A) CD34+ cells incubated with DMSO or reversible inhibitors of ERK or p38 inhibitor signaling for 1 h were mock- (−) or HCMV (+)-infected. At 3 hpi, cells were rescued in fresh media and then TUNEL-stained following a further 24 h culture. (B) Western blot analysis at 4 hpi of MCL-1 expression in CD34+ cells mock- (M) or HCMV-infected pretreated with DMSO, or inhibitors of ERK or p38 signaling. (C) PCR was performed on DNA isolated from infected CD34+ cells 4 and 120 hpi pretreated with ERK inhibitor or inactive analogue for 1 h, and the ratio of cellular to viral DNA expressed as a percentage of the mock control. (D) RT-PCR for UL123 and actin expression was performed on RNA isolated from mature dendritic cells derived from latently infected CD34+ cells transiently cultured in ERK inhibitor or inactive analogue (IA) at the time of infection.
Discussion
Apoptosis forms one part of a complex response of the human host to virus and pathogen infection. Sometimes referred to as part of the “intrinsic” immune response, programmed cell death clearly plays a role in the control of infection (24). As a consequence, pathogens have evolved multiple mechanisms to evade the host immune response and cellular defense mechanisms in place to counter them.
Virus infection triggers apoptosis at multiple levels. The first of these responses is often in response to virus binding and thus is initiated before any de novo virus gene expression and is observed with a number of viruses including HIV, HSV, and HCV (25–28). HCMV envelope gB and gH proteins have been shown to engage and activate TLR-2 on the surface of cells (13, 29). Given that signaling through TLR-2 can activate proapoptotic pathways (30, 31), including in response to HCMV (32) and HSV (33) infection, it is likely that, in latent infection, the virus must subvert this or other yet unidentified prodeath signals. Indeed, our observations that inhibition alone of elements important for the HCMV antiapoptotic response in myeloid cells (i.e., ERK–MAPK pathway, MCL-1 shRNA expression, and gB signaling) elevated cell death upon infection supported the activation of a prodeath response countered during nonpermissive HCMV infection.
HCMV has been shown to infect a number of different cell types, which suggests that the HCMV receptor is ubiquitously expressed or, alternatively, HCMV can use and engage multiple entry receptors on the surface of cells. Although the specific identity of HCMV entry receptor(s) remains to be definitively defined (34–36), the current model proposes that, upon initial docking to receptor(s), virion fusion and internalization are mediated by interactions with cellular integrins (37, 38) via a DLD within gB (37). In contrast with lytic infection, latent infection is restricted to a subpopulation of CD34+ hematopoietic cells in the bone marrow population (6), particularly the myeloid lineage. It is plausible that such selectivity could be driven by a latency-specific receptor. Alternatively, signaling pathways activated by HCMV infection of myeloid cells may promote an environment conducive for the establishment of latency. Given the pivotal roles that ERK and MCL-1 play in the function and survival of early hematopoietic myeloid progenitors (18), it is an attractive hypothesis that latency establishment in myeloid cells is initiated, in part, by the successful navigation of cell death in early myeloid progenitors. Whether long-term survival also involves prolonged ERK–MAPK activity is unknown; however, the up-regulation of a number of antiapoptotic functions has been described in experimental models of long-term HCMV latency (39). One model would propose that entry-mediated events provide a transient protection from the cell death, and then the “baton” is passed onto viral gene products expressed during long-term latency to maintain HCMV genomes. Clearly, if the virus cannot inhibit these initial prodeath events occurring upon entry, the efficiency of latency establishment is, logically, going to be severely impaired.
The data presented here argue a role for viral gB signaling and ERK activation during cell survival at the initial stages of CD34+ latent infection—a protection that correlates with MCL-1 expression. Furthermore, given that a major target of cisplatin A-induced cell death is MCL-1, MCL-1 plays an obligate role in hematopoietic cell survival in addition to the direct role MCL-1 plays in infected THP1 survival. Our data suggest that MCL-1 is an attractive target for the establishment of latency. Interestingly, HCMV has also been shown to activate the PI3K pathway in both permissive (10) and nonpermissive infection (40), which suggested that the protective effects of HCMV we observed could be mediated via this pathway also. Although we cannot preclude a role for the activation of these pathways in aspects of cell survival, in our hands, it was the ERK pathway that appeared important for initial survival of latently infected CD34+ cells in response to infection or the further stress of cisplatin A. Interestingly, the pathogenesis of chronic myeloid leukemia has also been associated with the activation of MCL-1 expression in an ERK-, but not PI3K-, dependent manner consistent with ERK-induced activation of MCL-1 being important for early myeloid cell survival (41). Indeed, given the increasing role for ERK–MAPK pathway activity in myeloid cell survival and function (42), the manipulation of this pathway provides obvious benefit to HCMV, and future studies will seek to evaluate the breadth of further responses to ERK–MAPK activation and, particularly, their implications for the establishment of latency. Reasons for the differences with a previous study (21) could be multiple. Although EGF-R can activate both PI3K and ERK–MAPK signaling, its role during entry remains contentious (35, 36), and, crucially, EGFR is not expressed on hematopoietic CD34+ cells (43). Furthermore, our studies were performed with clinical isolates, not laboratory strains, which in themselves appear to have phenotypic differences during latent infection. Finally, it is highly feasible that, upon infection of progenitor myeloid cells such as CD34+ cells and THP1 cells, the virus uses a concert of different pathways upon entry. This, in our opinion, is the most attractive hypothesis to explain differences between the two data sets, and illustrates how HCMV could use multiple pathways to manipulate common targets in different cell types. We also note that Chan et al. observed that transcription of MCL-1 was only slightly elevated at 24 hpi (21). Given that the half-life of MCL-1 protein is 3 h, the prolonged expression of MCL-1 seen in their study (21) may also suggest that other mechanisms, apart from the entry-mediated effects on which we focused, were functioning to stabilize the MCL-1 protein in their monocyte infections, which would be an interesting avenue for further study.
One of the key factors for the success of herpes viruses is the ability to establish lifelong latent infections in the host; in contrast with lytic infection, we are only just beginning to understand the mechanisms viruses use to subvert normal host responses during latent infection. The health threat from latent and persistent infections can manifest over many years and can result in severe disease and death under certain conditions. It is hoped, therefore, that an understanding of the mechanisms governing latency and persistence will not only help us in our attempts to control the threat from latent viruses but also provide further insight into the fundamental cellular pathways that these viruses target for survival, which begins at the point of virus binding and entry.
Materials and Methods
Virus, Cell Lines, Culture, and Reagents.
The clinical isolates VR1814 and TB40/e was purified from infected human retinal pigment epithelial cells by using sorbitol gradients as previously described (44). Equivalent results were obtained with either virus strain. For UV inactivation, HCMV was irradiated for 4 min (on ice) in a Stratagene cross-linker (10 × 105 μJ). Inactivation was confirmed by staining infected fibroblasts for IE gene expression by immunofluorescence. THP1 cells were maintained in RPMI 1640 medium supplemented with 10% FBS, penicillin (100 U/mL), streptomycin (100 μg/mL), and 2 mM L-glutamine. Primary CD14+ monocytes were isolated from the venous blood of anonymous donors who had given informed consent under the local rules governing the Novartis Blood Research Enrolment Program. Primary CD34+ hematopoietic cells were isolated from apheresis blood packs harvested from granulocyte colony-stimulating factor mobilized patients who had subsequently died. Typically, 50 mL of venous blood or apheresis product was diluted 1:1 in PBS solution, separated on a Ficoll gradient, purified by using a CD14+ or CD34+ cell direct isolation kit (Miltenyi Biotec) and magnetic-activated cell sorting, and then cultured in X-vivo 15 supplemented with 2 mM L-glutamine.
Inhibition of the MAPK pathways was performed by using the MAPK inhibitor set II (Calbiochem). This kit contained MEK/ERK inhibitors PD98059 (10 μM), U0126 (1–10 μM), and p38 inhibitor SB203580 (5–25 μM). Furthermore, the inactive analogue UO124 (1–10 μM) was also used (Calbiochem). All inhibitors were added directly to the culture media with 0.1% DMSO used as the solvent control 1 h before virus infection.
To perform neutralization experiments, HCMV was preincubated with either anti-gB (ITC88) or anti-gH (MSL-109) for 1 h before infection of cells. ITC88 was originally isolated from hybridomas established from the lymphocytes of a healthy HCMV carrier and recognizes an epitope between amino acids 67 and 86 of gB (45). MSL109 was isolated from the B cells of a patient who survived an HCMV infection (46) and was subsequently shown to target an epitope of gH (47).
Experimental Infection of Myeloid Cells.
THP1 cells were cultured in serum-free RPMI medium 3 h before infection. CD14+ and CD34+ cells were infected and maintained in serum-free X-vivo 15 media (BioWhittaker) throughout culture. One hour after infection, suspension cells were pelleted (300 g) and media were replaced. For adherent CD14+, the media was replaced directly. For analysis of reactivation, CD34+ cells were differentiated as described previously (48).
Nucleic Acid Isolation, Reverse Transcription, PCR, and Western Blot.
Ten micrograms of DNase I-treated RNA isolated using RNAeasy spin columns was reverse-transcribed by using an ImpromII RT kit (Promega). DNA was harvested from infected cells by using the sodium perchlorate method.
Gene specific primers were then used to amplify target sequences by PCR using PCR 2× MasterMix (Promega) with the following cycling conditions: 95 °C (5 min), then 20 to 30 cycles of 94 °C (40s), 55 °C (40 s), and 72 °C (60 s), and then a final extension at 72 °C for 10 min. Primers were as follows: MCL-1, 5′-TGC AGG TGT GTG CTG GAG TAG and 5′-GCT CTT GGC CAC TTG CTT TTC’; GAPDH, 5′-GAG TCA ACG GAT TTG GTC GT and 5′-TTG ATT TTG GAG GGA TTC TCG; Actin, 5′-GCT CCG GCA TGT GCA and 5′-AGG ATC TTC ATG AGG TAG T; IE72, 5′-CAT CCA CAT CTC CCG CTT AT and 5′-CAC GAC GTT CCT GCA GAC TAT G.
For Western blot, 105 cells were lysed in Laemmli buffer and subject to SDS/PAGE electrophoresis. Following transfer, blots were incubated with anti-MCL-1 (1:500; Cell Signaling) or anti-GAPDH (1:2,000; Abcam) for 1 h followed by detection with the appropriate HRP-conjugated secondary antibody. Specific bands were visualized by ECL detection (Amersham).
Densitometry was performed on Western blots or ethidium bromide gels using ImageJ software (National Institutes of Health). Typically, samples were normalized to GAPDH (mock in WB) or actin (DNA-PCR) for comparative analysis.
Cell Death Assays.
Induction of cell death was achieved by using the chemotherapeutic drug cisplatin A (49). Cisplatin A (25μM; Sigma-Aldrich) or 0.1% DMSO (mock) control was added for 4 h to trigger the apoptotic pathway. As the effects of cisplatin A-induced cell death are not evident until at least 12 to 24 h after treatment (50), cell viability, measured by using trypan blue staining, was performed 18 h after administration of cisplatin A. Number of dead (i.e., blue) cells was expressed as a percentage of total cells as a measure of cell viability. Alternatively, cells were stained with a TUNEL detection kit (Roche) as described by the manufacturer and analyzed for specific apoptotic cell death by immunofluorescent microscopy.
Lentiviral Vector for Delivery of shRNA to MCL-1.
An MCL-1 shRNA expressing vector was generated using the pLKO.1 vector system as previously described (51). Essentially, an shRNA insert with EcoRI and NcoI sticky ends was generated by using the oligos 5′-CCGG-GGGACTGGCTAGTTAAACAAAG-CTCGAG-CTTTGTTTAACTAGCCAGTCCC-TTTTTG and 5′-AATTCAAAAA-GGGACTGGCTAGTTAAACAAAG-CTCGAG-CTTTGTTTAACTAGCCAGTCCC and inserted into pLKO.1. A nontargeting shRNA was used a control (shRNA sequence, GTGGACTCTTGAAAGTACTAT) and has been previously verified (52). Lentiviral particles were produced as previously described (51). For transduction, 106 THP1 cells were pelleted and suspended in 100 μL of media supplemented with 10% polyethylene glycol and then incubated with 107 particles of nontargeting or MCL-1–expressing lentiviral vectors for 3 h. Cells were then rescued and cultured for 24 h in RPMI-10 at approximately 2.5 × 105 cells/mL. Transduced cells were then infected with HCMV (MOI of 5) and cultured up to 48 hpi in RPMI-10 medium following treatment with DMSO or cisplatin A for 2 h.
Supplementary Material
Acknowledgments
The authors acknowledge Nathan Brown and Ashley Shea for technical assistance, Darlys Schott (Dana-Farber Cancer Institute) and Carole Lachance (Novartis Institutes for Biomedical Research) for coordinating the collection of apheresis and venous blood products, and Dale Porter and Jerry Donovan for the shMCL-1 and shRNA control expressing lentiviral vectors. This research was funded by Novartis Institutes for Biomedical Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114966108/-/DCSupplemental.
References
- 1.Ho M. Epidemiology of cytomegalovirus infections. Rev Infect Dis. 1990;12(suppl 7):S701–S710. doi: 10.1093/clinids/12.supplement_7.s701. [DOI] [PubMed] [Google Scholar]
- 2.Limaye AP, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legendre C, Pascual M. Improving outcomes for solid-organ transplant recipients at risk from cytomegalovirus infection: Late-onset disease and indirect consequences. Clin Infect Dis. 2008;46:732–740. doi: 10.1086/527397. [DOI] [PubMed] [Google Scholar]
- 4.Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: Disease mechanisms and prospects for intervention. Clin Microbiol Rev. 2009;22:99–126. doi: 10.1128/CMR.00023-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinzger C, Digel M, Jahn G. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol. 2008;325:63–83. doi: 10.1007/978-3-540-77349-8_4. [DOI] [PubMed] [Google Scholar]
- 6.Sinclair J, Sissons P. Latency and reactivation of human cytomegalovirus. J Gen Virol. 2006;87:1763–1779. doi: 10.1099/vir.0.81891-0. [DOI] [PubMed] [Google Scholar]
- 7.Zhu H, Cong JP, Mamtora G, Gingeras T, Shenk T. Cellular gene expression altered by human cytomegalovirus: Global monitoring with oligonucleotide arrays. Proc Natl Acad Sci USA. 1998;95:14470–14475. doi: 10.1073/pnas.95.24.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmen KA, et al. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. Proc Natl Acad Sci USA. 2001;98:7140–7145. doi: 10.1073/pnas.121177598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehme KW, Compton T. Innate sensing of viruses by toll-like receptors. J Virol. 2004;78:7867–7873. doi: 10.1128/JVI.78.15.7867-7873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson RA, Wang X, Ma XL, Huong SM, Huang ES. Human cytomegalovirus up-regulates the phosphatidylinositol 3-kinase (PI3-K) pathway: inhibition of PI3-K activity inhibits viral replication and virus-induced signaling. J Virol. 2001;75:6022–6032. doi: 10.1128/JVI.75.13.6022-6032.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle KA, Pietropaolo RL, Compton T. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol Cell Biol. 1999;19:3607–3613. doi: 10.1128/mcb.19.5.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson RA, Huong SM, Huang ES. Activation of the mitogen-activated protein kinase p38 by human cytomegalovirus infection through two distinct pathways: A novel mechanism for activation of p38. J Virol. 2000;74:1158–1167. doi: 10.1128/jvi.74.3.1158-1167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compton T, et al. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77:4588–4596. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortunato EA, McElroy AK, Sanchez I, Spector DH. Exploitation of cellular signaling and regulatory pathways by human cytomegalovirus. Trends Microbiol. 2000;8:111–119. doi: 10.1016/s0966-842x(00)01699-1. [DOI] [PubMed] [Google Scholar]
- 15.Alwine JC. Modulation of host cell stress responses by human cytomegalovirus. Curr Top Microbiol Immunol. 2008;325:263–279. doi: 10.1007/978-3-540-77349-8_15. [DOI] [PubMed] [Google Scholar]
- 16.McCormick AL. Control of apoptosis by human cytomegalovirus. Curr Top Microbiol Immunol. 2008;325:281–295. doi: 10.1007/978-3-540-77349-8_16. [DOI] [PubMed] [Google Scholar]
- 17.Reeves MB, Davies AA, McSharry BP, Wilkinson GW, Sinclair JH. Complex I binding by a virally encoded RNA regulates mitochondria-induced cell death. Science. 2007;316:1345–1348. doi: 10.1126/science.1142984. [DOI] [PubMed] [Google Scholar]
- 18.Opferman JT, et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 19.Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci USA. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warr MR, Shore GC. Unique biology of Mcl-1: Therapeutic opportunities in cancer. Curr Mol Med. 2008;8:138–147. doi: 10.2174/156652408783769580. [DOI] [PubMed] [Google Scholar]
- 21.Chan G, et al. PI3K-dependent upregulation of Mcl-1 by human cytomegalovirus is mediated by epidermal growth factor receptor and inhibits apoptosis in short-lived monocytes. J Immunol. 2010;184:3213–3222. doi: 10.4049/jimmunol.0903025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isaacson MK, Juckem LK, Compton T. Virus entry and innate immune activation. Curr Top Microbiol Immunol. 2008;325:85–100. doi: 10.1007/978-3-540-77349-8_5. [DOI] [PubMed] [Google Scholar]
- 23.Weinshenker BG, Wilton S, Rice GP. Phorbol ester-induced differentiation permits productive human cytomegalovirus infection in a monocytic cell line. J Immunol. 1988;140:1625–1631. [PubMed] [Google Scholar]
- 24.Everett H, McFadden G. Apoptosis: An innate immune response to virus infection. Trends Microbiol. 1999;7:160–165. doi: 10.1016/s0966-842x(99)01487-0. [DOI] [PubMed] [Google Scholar]
- 25.Meyaard L, et al. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 26.Ameisen JC, Capron A. Cell dysfunction and depletion in AIDS: The programmed cell death hypothesis. Immunol Today. 1991;12:102–105. doi: 10.1016/0167-5699(91)90092-8. [DOI] [PubMed] [Google Scholar]
- 27.Galvan V, Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc Natl Acad Sci USA. 1998;95:3931–3936. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng L, et al. Hepatitis C virus infection induces apoptosis through a Bax-triggered, mitochondrion-mediated, caspase 3-dependent pathway. J Virol. 2008;82:10375–10385. doi: 10.1128/JVI.00395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boehme KW, Guerrero M, Compton T. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J Immunol. 2006;177:7094–7102. doi: 10.4049/jimmunol.177.10.7094. [DOI] [PubMed] [Google Scholar]
- 30.Tang SC, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabroe I, Dower SK, Whyte MK. The role of Toll-like receptors in the regulation of neutrophil migration, activation, and apoptosis. Clin Infect Dis. 2005;41(suppl 7):S421–S426. doi: 10.1086/431992. [DOI] [PubMed] [Google Scholar]
- 32.Chan G, Guilbert LJ. Ultraviolet-inactivated human cytomegalovirus induces placental syncytiotrophoblast apoptosis in a Toll-like receptor-2 and tumour necrosis factor-alpha dependent manner. J Pathol. 2006;210:111–120. doi: 10.1002/path.2025. [DOI] [PubMed] [Google Scholar]
- 33.Aravalli RN, Hu S, Lokensgard JR. Toll-like receptor 2 signaling is a mediator of apoptosis in herpes simplex virus-infected microglia. J Neuroinflammation. 2007;4:11. doi: 10.1186/1742-2094-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soroceanu L, Akhavan A, Cobbs CS. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature. 2008;455:391–395. doi: 10.1038/nature07209. [DOI] [PubMed] [Google Scholar]
- 35.Isaacson MK, Feire AL, Compton T. Epidermal growth factor receptor is not required for human cytomegalovirus entry or signaling. J Virol. 2007;81:6241–6247. doi: 10.1128/JVI.00169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Huong SM, Chiu ML, Raab-Traub N, Huang ES. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature. 2003;424:456–461. doi: 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]
- 37.Feire AL, Koss H, Compton T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci USA. 2004;101:15470–15475. doi: 10.1073/pnas.0406821101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Huang DY, Huong SM, Huang ES. Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat Med. 2005;11:515–521. doi: 10.1038/nm1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slobedman B, et al. Impact of human cytomegalovirus latent infection on myeloid progenitor cell gene expression. J Virol. 2004;78:4054–4062. doi: 10.1128/JVI.78.8.4054-4062.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith MS, Bentz GL, Smith PM, Bivins ER, Yurochko AD. HCMV activates PI(3)K in monocytes and promotes monocyte motility and transendothelial migration in a PI(3)K-dependent manner. J Leukoc Biol. 2004;76:65–76. doi: 10.1189/jlb.1203621. [DOI] [PubMed] [Google Scholar]
- 41.Aichberger KJ, et al. Identification of mcl-1 as a BCR/ABL-dependent target in chronic myeloid leukemia (CML): Evidence for cooperative antileukemic effects of imatinib and mcl-1 antisense oligonucleotides. Blood. 2005;105:3303–3311. doi: 10.1182/blood-2004-02-0749. [DOI] [PubMed] [Google Scholar]
- 42.Goldfarb AN. ERK expands its empire. Leuk Res. 2005;29:1235–1236. doi: 10.1016/j.leukres.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Spyridonidis A, et al. Purging of mammary carcinoma cells during ex vivo culture of CD34+ hematopoietic progenitor cells with recombinant immunotoxins. Blood. 1998;91:1820–1827. [PubMed] [Google Scholar]
- 44.Compton T. Analysis of cytomegalovirus ligands, receptors and the entry pathway. In: Sinclair J, editor. Methods in Molecular Medicine: Cytomegalovirus Protocols. Totowa, NJ: Humana Press; 2000. pp. 53–65. [DOI] [PubMed] [Google Scholar]
- 45.Ohlin M, Sundqvist VA, Mach M, Wahren B, Borrebaeck CA. Fine specificity of the human immune response to the major neutralization epitopes expressed on cytomegalovirus gp58/116 (gB), as determined with human monoclonal antibodies. J Virol. 1993;67:703–710. doi: 10.1128/jvi.67.2.703-710.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drobyski WR, et al. Phase I study of safety and pharmacokinetics of a human anticytomegalovirus monoclonal antibody in allogeneic bone marrow transplant recipients. Transplantation. 1991;51:1190–1196. doi: 10.1097/00007890-199106000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Britt WJ, Mach M. Human cytomegalovirus glycoproteins. Intervirology. 1996;39:401–412. doi: 10.1159/000150510. [DOI] [PubMed] [Google Scholar]
- 48.Reeves MB, MacAry PA, Lehner PJ, Sissons JG, Sinclair JH. Latency, chromatin remodeling, and reactivation of human cytomegalovirus in the dendritic cells of healthy carriers. Proc Natl Acad Sci USA. 2005;102:4140–4145. doi: 10.1073/pnas.0408994102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberg B, Vancamp L, Krigas T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature. 1965;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- 50.Barry MA, Behnke CA, Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990;40:2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- 51.Moffat J, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 52.Wiederschain D, et al. Single-vector inducible lentiviral RNAi system for oncology target validation. Cell Cycle. 2009;8:498–504. doi: 10.4161/cc.8.3.7701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.