Abstract
In November 2007, the container ship Cosco Busan released 54,000 gallons of bunker fuel oil into San Francisco Bay. The accident oiled shoreline near spawning habitats for the largest population of Pacific herring on the west coast of the continental United States. We assessed the health and viability of herring embryos from oiled and unoiled locations that were either deposited by natural spawning or incubated in subtidal cages. Three months after the spill, caged embryos at oiled sites showed sublethal cardiac toxicity, as expected from exposure to oil-derived polycyclic aromatic compounds (PACs). By contrast, embryos from the adjacent and shallower intertidal zone showed unexpectedly high rates of tissue necrosis and lethality unrelated to cardiotoxicity. No toxicity was observed in embryos from unoiled sites. Patterns of PACs at oiled sites were consistent with oil exposure against a background of urban sources, although tissue concentrations were lower than expected to cause lethality. Embryos sampled 2 y later from oiled sites showed modest sublethal cardiotoxicity but no elevated necrosis or mortality. Bunker oil contains the chemically uncharacterized remains of crude oil refinement, and one or more of these unidentified chemicals likely interacted with natural sunlight in the intertidal zone to kill herring embryos. This reveals an important discrepancy between the resolving power of current forensic analytical chemistry and biological responses of keystone ecological species in oiled habitats. Nevertheless, we successfully delineated the biological impacts of an oil spill in an urbanized coastal estuary with an overlapping backdrop of atmospheric, vessel, and land-based sources of PAC pollution.
Keywords: embryology, heart development, free radical, forensic chemistry, natural resource injury assessment
On November 7, 2007, the container ship Cosco Busan collided with the San Francisco–Oakland Bay Bridge, breaching two port fuel tanks and spilling approximately 54,000 gallons of bunker fuel into San Francisco Bay. This spill occurred in the spawning and rearing habitat for the largest coastal population of Pacific herring (Clupea pallasii) along the continental United States (1). Herring are a keystone species in the pelagic food web, and this population supports the last commercial finfish fishery in San Francisco Bay.
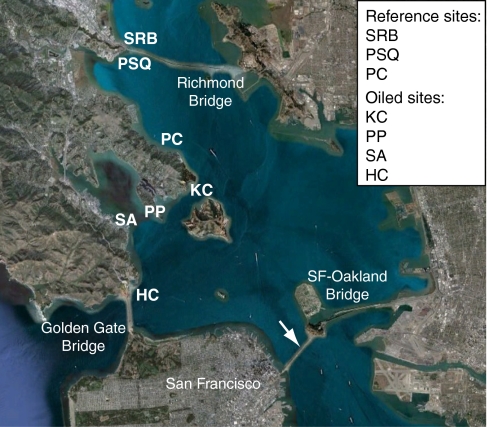
The Cosco Busan spill visibly oiled shorelines adjacent to North Central Bay areas where herring have historically spawned (November through March; Fig. 1). Although visibly oiled shorelines were cleaned, some extensively, only 52% of the oil was recovered from surface waters and land or lost to evaporation (2). The amount of hidden or subsurface oil that may have remained near herring spawning areas is unknown. The toxicity of crude oil to herring early life stages was extensively documented in the aftermath of the 1989 Exxon Valdez spill, which also occurred contemporaneously to Pacific herring spawning in Prince William Sound, Alaska (3–6). Therefore, the contamination of intertidal and shallow subtidal zones with Cosco Busan bunker oil posed a toxic threat to herring spawn and by extension, the productivity and abundance of the San Francisco Bay spawning population. Adult herring deposit adhesive, demersal eggs on shallow nearshore vegetation and other substrates. This proximity to oiled shorelines increases the risk of herring embryo exposure to water-soluble, toxic compounds that derive from oil as it weathers (i.e., shifts in chemical composition) over time.
Fig. 1.
Satellite view of study sites in central San Francisco Bay. Landmarks include the Golden Gate Bridge to the west and the Richmond Bridge to the north. Oiled sites include HC, SA, PP, and KC. Nonoiled reference sites include PC, PSQ, and SRB. Arrow indicates location of oil release.
Research on Alaska North Slope crude oil following the Exxon Valdez spill, together with studies during the past two decades that used other sources of crude oil and other teleost species, have yielded two central insights (7). First, teleost embryos exposed to trace concentrations of crude oil constituents dissolved in water exhibit a common syndrome of developmental abnormalities (8–17). Gross features include pericardial and yolk sac edema, small jaw, and body axis defects. Of these, edema appears to be the most sensitive indicator of oil exposure and toxicity (9, 11, 18). Second, studies in zebrafish and Pacific herring have shown that petroleum-induced edema is cardiogenic. Specifically, crude oil contains a directly cardiotoxic fraction attributable to the most abundant polycyclic aromatic compounds (PACs), the tricyclic fluorenes, dibenzothiophenes, and phenanthrenes (17–20). These compounds interfere with the physiological function of the embryonic heart, producing cardiac arrhythmia in herring embryos at tissue concentrations as low as 0.8 μmol/kg wet weight (19). Although embryos with edema do not survive as feeding larvae, externally normal embryos surviving Alaska North Slope crude oil exposure grew up to be adults with subtle changes in heart shape and reduced aerobic (i.e., swimming) capacity (21).
Based on this previous work, we initiated a 3-y study beginning 3 mo after the Cosco Busan spill to assess PAC exposure, sublethal cardiac toxicity, developmental abnormalities, and hatching success in herring embryos in San Francisco Bay. We moored cages containing artificially fertilized embryos, together with passive water sampling devices for PACs (i.e., polyethylene membrane devices; PEMDs), at six sites. Four of these sites were visibly oiled immediately after the spill, whereas two “reference” sites were not oiled but contiguous with the same heavily urbanized shoreline (Table 1 and Fig. 1). We also collected naturally spawned embryos from five intertidal sites, four of which were adjacent to the caged embryos (Table 1 and Fig. 1). At the time of embryo incubations, there was no visible oiling at any of the sites, although small tar balls were occasionally found on shore. Embryos from all sites were transported to a laboratory for live imaging by using digital photo- and videomicroscopy and for incubation to hatching. We measured PACs and a suite of persistent organic pollutants (POPs) routinely found in urban environments, including polychlorinated biphenyls (PCBs) and organochlorine pesticides, in embryos in 2008 and 2010. Additionally, ovaries and whole bodies of prespawning adult herring entering San Francisco Bay in 2008 were analyzed for PACs and POPs to evaluate the potential for maternal transfer of contaminants.
Table 1.
Physical shoreline characteristics of sample sites and types of samples collected each year
| Subtidal sampling |
||||||
| Site | SCAT rating | Cleanup | Adjacent land use/maritime use | Incubated caged embryos* | PEMDs | Intertidal sampling* |
| KC | Oiled; heavy | Extensive wiping, removal of rock | Residential, undeveloped forest | 2008 | Yes | 2008, 2010 |
| HC | Oiled; moderate-light | Extensive wiping of rip-rap | Marina, major highway | 2008 | Yes | ND |
| SA | Oiled; very light-light | Some wiping | Marina, commercial, residential | 2008 | Yes | 2008, 2010 |
| PP | Oiled; light | Some wiping | Residential | 2008 | Yes | 2008, 2010 |
| SRB | No oil | NA | Commercial parking lot, major highway | 2008 | Yes | 2008 |
| PC | No oil | NA | Residential, public green space | Not sampled | No | 2009, 2010 |
| PSQ | No oil | NA | Commercial/industrial parking lots, major highway | 2008 | Yes | Not sampled |
NA, not applicable; ND, no spawn detected; SCAT = shoreline cleanup assessment team.
*All caged and naturally spawned embryos were assessed for sublethal exposure to PACs and POPs, except the 2009 samples.
Results
Cardiac Defects in Caged Embryos Incubated in Subtidal Zone at Oiled Sites.
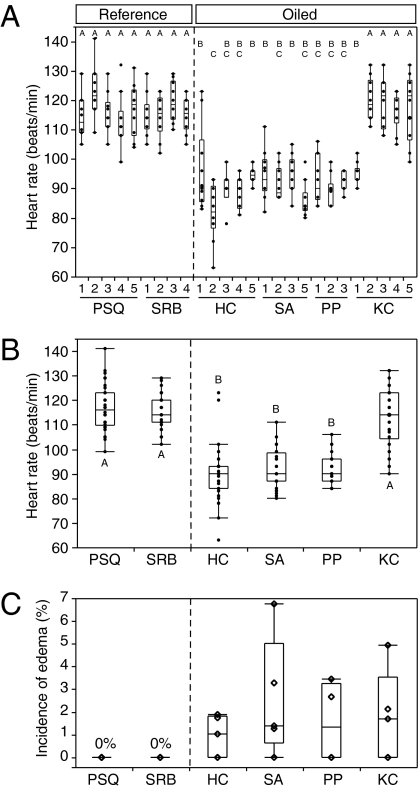
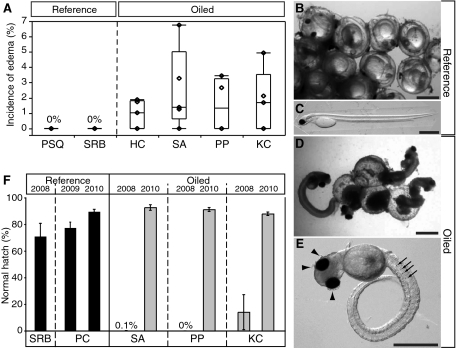
Sublethal cardiac effects were observed in caged embryos placed subtidally at oiled sites in 2008 (Fig. 2). Pronounced bradycardia was observed in embryos from 13 of 17 cages deployed at the four oiled sites, with significant variability among cages at a given site (Fig. 2 A and B). At three of the oiled sites, embryos from all cages measured showed a significant bradycardia in the range of 90 beats/min [Horseshoe Cove (HC), 90 ± 1; Sausalito (SA), 92 ± 1; and Peninsula Point (PP), 92 ± 1]. Only one of five cages at the Keil Cove (KC) oiled site showed significant bradycardia (cage 1, 95 ± 1 beats/min), and the overall mean (114 ± 2 beats/min) was statistically indistinguishable from that in reference sites. There was no significant variation among heart rates within the five cages from each of the two reference sites (Fig. 2A), and both sites had an overall mean heart rate of 116 ± 1 beats/min (Fig. 2B). Mean temperatures were indistinguishable among sites (SI Appendix, Table S1), indicating that differences in heart rate were not caused by incubation temperatures. Hatching success of caged embryos was high, with average normal hatch rates (i.e. percentage of hatched larvae with normal morphology) ranging from 79 ± 5% to 97 ± 2% at all sites, compared with 91 ± 1% in controls held back in the laboratory (SI Appendix, Fig. S2). With all types of morphological abnormalities pooled, all oiled sites, but also reference site Point San Quentin (PSQ), had hatch rates (normal larvae) significantly lower than those in reference site San Rafael Bay (SRB) and laboratory controls. However, pericardial edema, which is produced by more severe impairment of cardiac rhythm in developing fish embryos, was observed only at each oiled site in a small but significant percentage of larvae hatched from subtidal cages, ranging from 0.9% to 2.5% (Fig. 2C). In contrast, no edema was observed among 652 larvae hatched from subtidal cages at either reference site (PSQ, n = 308; SRB, n = 344).
Fig. 2.
Evidence of cardiac dysfunction in embryos and larvae incubated in subtidal cages at oiled sites in 2008. Box-and-whisker plots encompass the distribution of individual data points. (A) Mean heart rate (n = 30) by cage. One cage at SRB was lost at retrieval, one cage at PP was stripped of embryos before retrieval, and video from one cage each at SA and PP were of insufficient quality to provide heart rate data. (B) Mean heart rate by site. Mean heart rates from individual cages were pooled for a site mean. ANOVA indicated 34% of the total variance was caused by the cage, and showed significant effects of site (P < 0.001) and oiled state (P < 0.001). Letters A–C indicate statistically similar datasets identified by post-hoc means comparison with Tukey-Kramer HSD test (α = 0.05). Overall, oiled sites were statistically different from reference sites (Student t test, α = 0.05). (C) Incidence of pericardial edema in larvae hatched in the laboratory after incubation to 7 d postfertilization in subtidal cages. Edema tended to be observed more frequently at oiled sites (Wilcoxon rank-sum test, P = 0.06).
Necrotic Late Embryos in Natural Spawn Samples from Intertidal Zone at Oiled Sites.
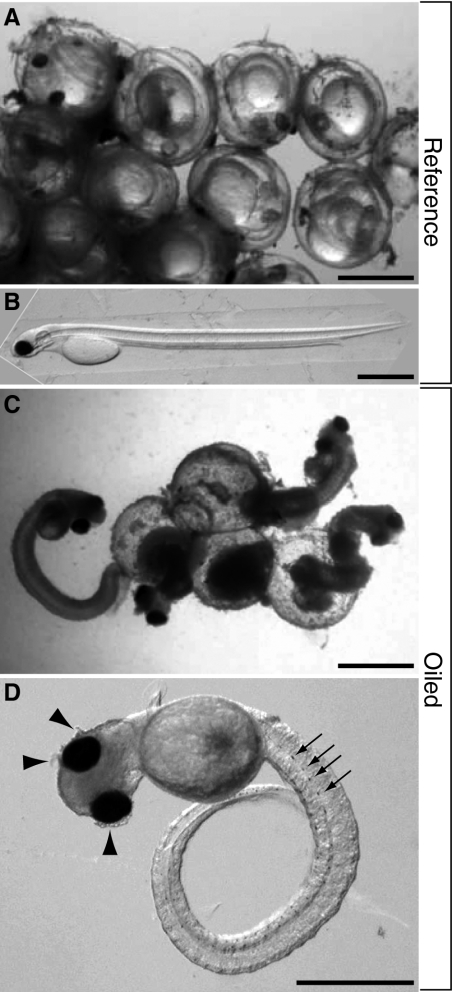
Abundant natural spawn occurred on macroalgae in the lower intertidal zone at four of the six sites where caged embryos were deployed in adjacent subtidal zones in 2008, comprising three oiled and one reference site (Table 1 and SI Appendix, Table S2 and Fig. S3). High rates of lethality and morphological abnormalities occurred in natural spawn from all three oiled sites. At the hatching stage, healthy herring embryos are normally translucent and colorless except for a darkly pigmented eye and a row of individual melanophores ventrally along the gut. This normal condition was observed in all embryos from each subtransect from reference site SRB (Fig. 3A). Upon dechorionation, SRB embryos uncurled (Fig. 3B) and were motile. Despite having developed fully pigmented eyes and ventral melanophores (indicating normal development approaching the hatching stage), the majority of embryos in samples from oiled sites were dead on examination in the laboratory, as indicated by absence of heart beats and motor activity (Movie S1), opacity of tissues such as the brain, spinal cord, and axial muscle, and disintegration of epidermal tissue (Fig. 3C). Because it was impossible to gain etiological insight from these necrotic embryos, we focused our image analysis on a small subset of living embryos that exhibited obviously beating hearts. Most of the living embryos from oiled sites exhibited opaque tissues, failure to straighten the body axis, and immotility (Fig. 3D and Table 2). In many cases, there was also loss of epidermal integrity (Fig. 3D, arrowheads). We consistently observed cardiac dysfunction at all three oiled sites in 2008 (i.e., arrhythmia, silent ventricle, severe bradycardia, minimal overall contractility, and loss of heart beat; Table 2), defects that were entirely absent in embryos from SRB.
Fig. 3.
Representative images of intact and dechorionated naturally spawned embryos from oiled and nonoiled intertidal sites sampled in 2008. Images were made by using oblique coherent contrast illumination on a Nikon SMZ1000 stereomicroscope, allowing high relief for colorless, transparent specimens. (A) An undissected cluster of embryos in the chorions from reference site SRB with normal translucency. Eyes are darkly pigmented and the trunk and tail are coiled around the brightly transparent yolk. (B) Representative embryo from SRB uncoiled after dechorionation, with anterior to the left and dorsal at top. Longitudinal features evident with oblique coherent contrast illumination from dorsal to ventral are the transparent neural tube, notochord, and gut. (C) An undissected cluster of nonviable embryos from oiled site PP showing pigmented eyes, opacity indicative of deteriorating tissues, and spontaneously ruptured chorions. (D) Representative late-stage embryo from PP with cardiac activity after dechorionation. Arrows indicate ventral pigment cells, arrowheads indicate loss of epidermal integrity.
Table 2.
Incidence of abnormalities in viable embryos in natural spawn collected from oiled and nonoiled sites in 2008
| Site | Body axis defect | Tissue opacity | Edema | Arrhythmia |
| SA (oiled) | 60 ± 11 | 41 ± 10 | 33 ± 7 | 48 ± 7 |
| PP (oiled) | 98 ± 2 | 96 ± 2 | 11 ± 4 | 91 ± 11 |
| KC (oiled) | 90 ± 5 | 86 ± 7 | 11 ± 2 | 70 ± 6 |
| SRB (nonoiled) | 0 | 0 | 1.0 ± 0.6 | 0 |
Table shows mean percent and SEM from eight samples (≥20 embryos per sample) at each site.
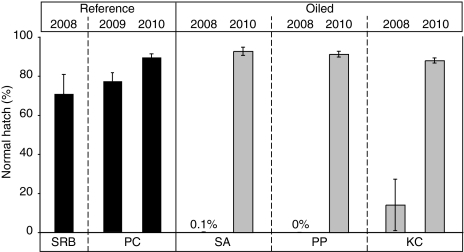
In 2008, essentially no live larvae hatched from the natural spawn we collected from oiled sites SA and PP, and samples from oiled site KC had a normal hatch rate of only 14 ± 13% (Fig. 4). In contrast, at reference site SRB, hatching began within 1 day following collection, and 71 ± 10% of embryos hatched to produce morphologically normal larvae (Fig. 4). Herring did not spawn in 2009 at any of the sites that had been oiled in 2008, but they did spawn in 2009 at a third nonoiled reference location [Paradise Cove (PC); sampled in January]. The natural spawn from PC in 2009 occurred at a higher elevation in the intertidal zone than the naturally spawned sites sampled in 2008, and were distributed on rocks as well as macroalgae. A relatively high proportion of 2009 embryos from this site failed to gastrulate (28 ± 7%); nevertheless, viable embryos from PC produced a normal hatch rate of 77 ± 4% (Fig. 4).
Fig. 4.
Hatching rates of larvae from natural spawn samples. Values represent mean ± SEM normal hatch rates, calculated as percent morphologically normal larvae hatched from total eggs in eight transect subsamples per site. Grand totals of eggs assessed for each site were 535 (SRB, 2008), 820 (PC, 2009), 683 (PC, 2010), 968 (SA, 2008), 385 (SA, 2010), 549 (PP, 2008), 919 (PP, 2010), 469 (KC, 2008), and 726 (KC, 2010).
In 2010, herring once again spawned at several locations where natural spawn was collected in 2008. The overlap was exact at two oiled sites (PP, KC) and partial at a third oiled site (SA). (To distinguish areas of overlap between 2008 and 2010, the SA site was subsequently divided into an inner-marina SA1 subsite and outer SA2 subsite; Table 3 and SI Appendix, Fig. S1A). Herring also spawned again at the PC reference site, at an intertidal depth equivalent to the samples collected in 2008. Larvae at all three oiled sites had normal cardiac and spontaneous motor activity (Movie S1). Furthermore, hatching rates were similar among the oiled and reference sites (Fig. 4), and these were similar to the relatively high hatching rates for nonoiled reference sites sampled in previous years (SRB in 2008 and PC in 2009). However, we observed a significantly higher incidence of pericardial edema in larvae from oiled KC site (3.5 ± 0.7%) relative to the other sites [SA, 1.6 ± 0.3%; PP, 1.0 ± 0.3%; and PC, 1.2 ± 0.3%; ANOVA, P < 0.003, Tukey–Kramer honestly significant differences (HSD) test].
Table 3.
Summary of selected PAC concentrations (ng/g wet weight) measured in naturally spawned and caged embryos collected in 2008 and 2010
| Matrix/y (n) | Site | Mean ∑PACs* | Mean FLA | Mean Alkyl-DBTs† | Frequency C2/C3-DBT, %‡ |
| Cage/2008 (4) | SRB (reference) | 17 ± 3 | 0.8 ± 0.3 | Below LLOQ | 0 |
| Spawn/2008 (8) | SRB (reference) | 21 ± 2 | 1.1 ± 0.2 | 0.05 ± 0.03 | 0 |
| Cage/2008 (5) | PSQ (reference) | 23 ± 2 | 1.0 ± 0.2 | 0.09 ± 0.06 | 0 |
| Spawn/2010 (8) | PC (reference) | 28 ± 3 | 0.5 ± 0.1 | 0.05 ± 0.05 | 13 |
| Cage/2008 (4) | HC (oiled) | 52 ± 10 | 3.7 ± 0.9 | 0.48 ± 0.23 | 50 |
| Cage/2008 (5) | SA (oiled) | 48 ± 6 | 2.7 ± 0.5 | 0.51 ± 0.13 | 80 |
| Spawn/2008 (5) | SA1 (oiled) | 81 ± 40 | 2.9 ± 0.7 | 0.49 ± 0.29 | 20 |
| Spawn/2008 (3) | SA2 (oiled) | 18 ± 3 | 0.6 ± 0.1 | Below LLOQ | 0 |
| Spawn/2010 (3) | SA2 (oiled) | 27 ± 1 | 1.4 ± 0.1 | Below LLOQ | 0 |
| Cage/2008 (4) | PP (oiled) | 21 ± 1 | 0.8 ± 0.1 | Below LLOQ | 0 |
| Spawn/2008 (8) | PP (oiled) | 19 ± 5 | 0.8 ± 0.1 | 0.12 ± 0.08 | 25 |
| Spawn/2010 (8) | PP (oiled) | 23 ± 1 | 0.6 ± 0.1 | Below LLOQ | 0 |
| Cage/2008 (5) | KC (oiled) | 24 ± 3 | 0.7 ± 0.1 | 0.21 ± 0.10 | 40 |
| Spawn/2008 (8) | KC (oiled) | 45 ± 18 | 3.8 ± 2.2 | 0.28 ± 0.09 | 75 |
| Spawn/2010 (8) | KC (oiled) | 34 ± 9 | 1.8 ± 1.0 | 0.48 ± 0.16 | 100 |
DBT, dibenzothiophene; FLA, fluoranthene.
*Mean and SEM values from given n.
†Lower limit of quantification (LLOQ) values for individual analytes ranged from < 0.15 to < 0.46; samples with values lower than the LLOQ were treated as 0 in means.
‡Frequency of samples with both C2- and C3-DBT detected.
Analyses of PACs and POPs in Prespawn Adults and Embryos.
Measurements of PACs and POPs in prespawn adult herring entering San Francisco Bay in February 2008 suggest that maternal transfer did not contribute significant levels of these compounds to embryos (SI Appendix, Table S3). Summed PACs (∑PACs) were very low (near the limit of detection), with wet weight concentrations ranging from 8.6–13 ng/g in ovaries and only one quarter the concentration (23–52 ng/g) of maternal whole bodies. Dichlorodiphenyltrichloroethanes (DDTs) and PCBs were the most abundant classes of POPs (2.8 ± 0.3 ng/g and 1.6 ± 0.4 ng/g, wet weight, respectively) detected in whole bodies; levels of DDTs were higher and PCBs lower than in prespawn adult herring from Puget Sound, Washington (22) (SI Appendix, Table S3). Other classes of POPs (e.g., chlordanes, polybrominated diphenyl ethers, hexachlorocyclohexanes) were also detected in whole bodies of herring but were below quantification limits in ovaries. Consistent with adult ovary data, PCB levels in natural spawn were low, near detection limits (SI Appendix, Table S3).
In caged and naturally spawned embryos, comparison of individual classes of PACs representing petrogenic or pyrogenic sources revealed significant differences among sites (Table 3), despite generally low mean ∑PAC concentrations that were in many cases statistically indistinguishable from maternal ovary levels according to standard parametric tests (complete data provided in Dataset S1). Sulfur-containing dibenzothiophenes are petrogenic PACs and often used for characterizing sources of oil in the environment (23–26). Although Cosco Busan bunker oil is low in sulfur compared with other fuels, with a C2-dibenzothiophene/C2-phenanthrene ratio of 0.26, the alkyl-dibenzothiophenes are nevertheless one of this bunker oil's most abundant identified PAC classes. Total alkyl-dibenzothiophene levels tended to be higher in natural spawn embryos from each oiled site and in caged embryos from three of four oiled sites than from nonoiled sites in 2008 (Wilcoxon rank-sum test, P = 0.08). C2- and C3-dibenzothiophene together were also detected more frequently in caged and naturally spawned embryos from oiled sites in 2008. In 2010, alkyl-dibenzothiophenes in natural spawn were not detected at oiled site PP but remained significantly elevated at oiled site KC (ANOVA, P = 0.03; Tukey–Kramer HSD, α = 0.05). The pyrogenic PAC fluoranthene appeared to be more uniformly distributed. Slightly lower fluoranthene levels at reference sites were not statistically significant (P = 0.28, Wilcoxon rank-sum test), and there was only a weak correlation between mean fluoranthene and total alkyl-dibenzothiophene concentrations (r2 = 0.6).
Distinct Patterns of PAC Inputs Evident from Analysis of PEMD Passive Samplers.
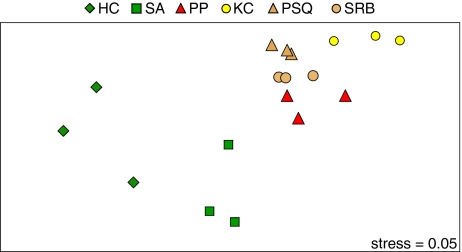
A comparison of PAC patterns in PEMDs deployed alongside the caged embryos indicated distinct PAC chemical fingerprints among the six subtidal sites (Fig. 5) that seemed to reflect their nearby upland development. Moreover, as expected for an urbanized estuary, all sites had mixed inputs of petrogenic and pyrogenic PACs (SI Appendix, Table S4). Nonmetric multidimensional scaling (nMDS) and an analysis of similarity (ANOSIM) revealed that PAC patterns were highly significantly different among sites (P = 0.001, R = 0.69; Fig. 5). A pairwise ANOSIM test showed that sites HC and SA, which have marinas and were oiled, exhibited similar PAC patterns (R = 0.33) and were both dissimilar to all other sites (R = 0.85–1.0). Similarly, reference sites PSQ and SRB, which are on opposite sides of a major highway (Richmond Bridge; Fig. 1), were indistinguishable from each other (R = 0.26) and were isolated from all other sites (R = 0.85–1.0). Oiled sites PP and KC, which are residential or minimally developed, were isolated from each other and all other sites (R = 0.85–1.0). Diagnostic PAC ratios also showed differences among sites (SI Appendix, Table S4). The ratio of sum alkyl-phenanthrenes to phenanthrene (MP/P) is widely used to distinguish pyrogenic from petrogenic sources (27), with the latter having MP/P ratios greater than 2. Conversely, the ratio of fluoranthene plus pyrene to sum C2- through C4-phenanthrene increases with increasing pyrogenic composition (26). The MP/P ratio was greater than 2 for only the most heavily oiled site, KC (MP/P ratio, 2.6), whereas the ratios of fluoranthene plus pyrene to C2- through C4-phenanthrene clustered into four groups that matched the nMDS analysis. This analysis is consistent with heterogeneous background PAC inputs among the six sites that are associated with the adjacent upland development and land use, as well as an elevation of petrogenic signal above the background level at the heavily oiled site KC.
Fig. 5.
Comparison of PAC patterns in PEMDs deployed at six sites indicates distinct PAC sources. nMDS was used to represent a large number of PAC compounds in low-dimensional (i.e., 2D) space. nMDS analysis was carried out using Primer version 6.0 as described in SI Appendix. Axes surround a unitless space within which samples are placed according to the degree of similarity in the relative abundance of five influential PAC compounds (preselected from 34 compounds using the Primer BEST routine). Similarity in PAC patterns determines the distance between points in the space: samples with similar patterns are placed close together and dissimilar patterns are further apart. The observed patterns are statistically different from a random configuration of points (stress, 0.05). PSQ and SRB are reference sites; HC, SA, PP, and KC are oiled sites.
Discussion
Unlike the Exxon Valdez oil spill that occurred in Prince William Sound, Alaska, the Cosco Busan spill occurred within a highly urbanized estuary with multiple inputs of petroleum hydrocarbons. This posed the difficult challenge of assessing the ecological impacts of the spill against the background of pollution in San Francisco Bay. Based on years of oil toxicity research since the Exxon Valdez spill, we anticipated that lingering oil toxicity, if any, would be evident as a small increase in the detection of sublethal cardiac effects (e.g., arrhythmia, edema) in herring embryos incubated near oiled shorelines. Although significant increases in bradycardia and pericardial edema were observed in caged embryos from oiled sites relative to nonoiled locations, natural spawn from oiled intertidal zones revealed an unexpectedly severe (i.e., lethal) form of developmental toxicity.
The high rate of natural spawn mortality at oiled sites in 2008 does not appear attributable to natural causes or anthropogenic causes unrelated to the spill. The spawning layers were not dense enough (i.e., fewer than four layers at all sites) to cause the density-dependent hypoxia that occurs when eggs are deposited in layers of greater than eight eggs thick (28–33). Euryhaline Pacific herring embryos develop normally at salinities of 8% to 28% (34, 35), and even suboptimal salinities would not be expected to cause acute mortality late in development. Natural spawn did not show evidence of accelerated development, as would be expected following exposures to high, potentially lethal temperatures (34, 36). Coating of herring eggs with fine sediments does not produce the late developmental mortality we observed here (37–41). Two sewage spills occurred during the 2008 spawning season; on January 13 in Richardson Bay, near oiled sites SA and PP, and on February 14 offshore of San Quentin Prison, near reference site PSQ. However, the available evidence indicates that sewage (i.e., concentrated sludge) is not acutely lethal to herring embryos (42). Finally, background PCB and DDT levels in ovaries and embryos from San Francisco Bay are not expected to cause the observed mortalities, as the levels were much lower than that associated with reduced hatching success in Baltic herring (43).
Our chemical analyses of PACs in embryos and PEMDs support the conclusion that embryos from oiled sites were exposed to oil, particularly at KC, even though a PAC “fingerprint” of Cosco Busan oil was not discernable against the background of urban PAC inputs. In particular, the trends of higher levels and increased frequency of alkyl-dibenzothiophene detections in embryos at oiled sites are consistent with lingering oil exposure at those sites that were visibly oiled several months before spawning. The PEMD data indicated unique patterns of PAC inputs across sites, consistent with the diversity of proximal land use patterns and vessel activities. However, embryonic phenotype did not follow this pattern of site-specific chemical variation. In contrast, the effects observed in natural spawn from each of the three oiled sites (SA, PP, and KC) were indistinguishable, and pericardial edema was observed only in larvae that were incubated subtidally at oiled sites. The only common feature linking these sites was a shoreline presence of visible oil in the weeks following the spill. The absence of these effects at reference sites and the marked recovery at oiled sites by 2010 indicate that background urban inputs of contaminants (e.g., via stormwater) were not likely causal. The most parsimonious explanation for the 2008 herring embryo mortality in San Francisco Bay is exposure to Cosco Busan oil.
PAC levels in the tissues of embryos collected from oiled intertidal sites in 2008 were below levels that would be expected to cause acute lethality based on laboratory studies (11, 19, 44). This, together with the dramatic difference in survival between intertidal spawn and embryos in nearby subtidal cages, implicates natural sunlight as a contributing factor in the observed embryolarval toxicity. Sequential exposures to crude oil and sunlight in the laboratory are acutely lethal to herring larvae at tissue PAC concentrations that are only sublethal without sunlight exposure (45). This presumably occurs via activation of PACs or other oil components by UV radiation (i.e., “phototoxicity”), thereby generating reactive oxygen species that cause membrane damage (46). Other studies in aquatic invertebrates and fish demonstrated that some bunker oils, as well as other petroleum sources that are low in conventionally measured PACs, have higher phototoxicity than predicted from the measured PACs (47, 48). Recent work in zebrafish has further shown that bunker oils have a much higher phototoxic potential than crude oil and cause an acutely lethal cellular necrosis when embryos are exposed sequentially to oil and sunlight (49). Finally, a 2009 laboratory study to investigate the potential role of sunlight in the observed 2008 natural spawn mortality showed that Cosco Busan bunker oil exhibited a phototoxic activity that (i) produced late embryonic mortality in herring embryos characterized by a loss of tissue integrity similar to that observed in the 2008 field-collected samples, (ii) was resistant to weathering, and (iii) was unexplained by conventionally measured tissue PAC concentrations, thus suggesting a causal role for one or more unmeasured compounds (50). Because modern bunker fuels contain the concentrated residuum of the crude oil refining process, they have much higher relative levels of many compounds, including the uncharacterized polar compounds that make up an “unresolved complex mixture” (51). The simplest explanation for our collective findings is that an uncharacterized and slowly weathering component of Cosco Busan bunker oil accumulated in the naturally spawned herring embryos, and then interacted with sunlight during low tides to produce lethal phototoxicity. Embryos in nearby cages, submerged beneath approximately 1 m or more of highly turbid San Francisco Bay water, exhibited canonical oil toxicity (i.e., bradycardia and pericardial edema) with no indication of a sunlight interaction.
Research following the Exxon Valdez oil spill established a new paradigm for oil toxicity to fish at early life stages (7), which demonstrated the central role PACs play in causing characteristic developmental deformities. Our ecological assessments of the Cosco Busan spill have extended and reinforced this paradigm by (i) highlighting the difference in effects on fish from exposure to oil of differing composition (i.e., crude vs. bunker), (ii) illustrating the potential significance of photo-enhanced toxicity and its interaction with local conditions, (iii) providing motivation to examine toxicity of a broader suite of petroleum product com-ponents, and (iv) emphasizing the exceptional vulnerability of fish early life stages to spilled oil. Our research showed strong evidence for a previously undescribed lethal toxicity in Pacific herring embryos associated with exposure to bunker oil, which may have been exacerbated by exposure to sunlight. Although bunker oil spills are typically smaller in volume than crude oil spills, they may have disproportionate impacts in ecologically sensitive, sunlit habitats.
Materials and Methods
Study Sites.
Sites were selected based on their likely proximity to oiled substrates as determined by Shoreline Cleanup Assessment Team surveys and other observations of oiling. Because the sites (Fig. 1 and Table 1) differed in the degree of oiling and cleanup, the actual amounts of residual oiling (including subsurface oil) at each location at the time of the assessment are unknown. Reference, nonoiled sites were chosen to be representative of an urban estuary and most closely match the habitat, temperature, and salinity conditions at corresponding sites within the spill zone. Satellite images indicating natural spawn subtransects and caged embryo/PEMD placements are shown in SI Appendix, Fig. S1.
General descriptions of natural spawn sample substrates are provided in SI Appendix, Table S2. The predominant substrates were brown and red bladed algae such as Fucus, Cryptopleura, and Chondracanthus, filamentous red algae (e.g., Gracilaria, Microcladia, or Odonthalia), and some green algae (Ulva). The highest-density spawn was at reference site SRB, where samples were almost exclusively on Fucus. Fucus also predominated at SA, but macroalgae were more variably mixed at PP and KC, with the former predominated by filamentous red forms. Typical samples are shown in SI Appendix, Fig. S2. The spawn at SRB was medium in density, approaching four layers of embryos. Spawning at the three oiled sites was less dense, ranging from very light “salt-and-pepper” density to light density with contiguous patches of a single layer.
Collection of Tissues from Adult Herring for Analysis of Maternal Contaminant Exposure.
Ovaries and whole bodies were collected from prespawning adult females to determine levels of POPs and PACs in ovaries and whole bodies to evaluate potential maternal transfer. Ovaries from the same 10 fish were placed into individual precleaned 240-mL glass jars (I-Chem), and the carcasses placed in individual zip-lock bags after recording fork length and weight. All tissue samples were frozen until analyzed. Bile was processed and analyzed for FACs, and ovaries and whole bodies for PACs and POPs, as described in the subsequent sections.
Collection of Herring Gametes and Preparation of Embryos.
Ripe prespawn herring were captured by hook-and-line, cast net, or midwater trawl, immediately sexed, and transferred to clean zip-lock bags and stored in a clean cooler on ice. Herring gonads were dissected and eggs fertilized as described elsewhere (35). Test fertilizations were conducted with eggs from individual females, and those with high fertilization success (≥90%) were pooled for mass fertilizations. Three separate catches of fish were used among the sites. Mean (± SEM) female weights for sites were as follows: HC and PP, 82.2 ± 13.6 g (n = 7); SA, KC, and SRB, 70.3 ± 8.4 g (n = 12); and PSQ, 78.7 ± 4.1 g (n = 20). Milt was pooled from five males for each experiment. Gametes were stored at 4 °C for a maximum of 72 h to allow a successive series of daily cage deployments. Eggs were kept from clumping before fertilization with polyvinyl alcohol (35), and were distributed onto 13 × 54-cm nylon mesh sheets for mounting in cages (15-cm3 lidded aluminum baskets; Fisher Scientific). The top and bottom of each cage was lined with the same nylon mesh, and each sheet with adhering fertilized eggs was then folded to line the sides of the cages, with the corners secured in place with cable ties. Eggs faced the interior of the cage, which was effectively lined entirely with nylon mesh to prevent the entrance of small egg-eating predators. Before transport for deployment, cages were placed in large zip-lock bags containing seawater diluted to 16 psu with Millipore-filtered water and held in clean 20-L plastic buckets. Cages were transported to sites on ice in coolers, and deployed as follows: HC, February 10, 2008; PP, February 11; SA, February 12; KC, February 13; SRB, February 14; and PSQ, February 18.
Deployment of Embryo Cages, PEMDs, and Data Loggers.
Anchor-buoy units for embryo cages and PEMDs consisted of a braided polypropylene line attached to a pair of concrete blocks joined by cable ties for a combined weight of 27 kg. Each line had two floats, a 20-cm inflatable surface marker, and a smaller crab-pot buoy mounted at a position to float on the surface at low tide. The latter served to maintain a vertical line no matter the tide level. Cages and PEMDs were attached with cable ties below the smaller float at a point 18 to 30 cm above the concrete blocks. Anchor-buoy units were installed along an isobath at −0.9 to −1.7 m, 1 to 2 d before embryo cage deployment, to allow any disturbed bottom sediments to clear.
The vessel used for cage and PEMD deployment was powered with a four-stroke engine. The vessel was fueled in advance of all operations and was cleaned after fueling and before operations to avoid contamination. Mooring locations were approached downwind, and all work related to deployment/retrieval of cages/PEMDs was from the bow or center. A designated boat operator handled all mechanical systems on board, and separate personnel carried out deployments. Upon arrival at a site, the bagged cages were lowered to a free diver in the water, followed by subsurface removal of the zip-lock bag and attachment to the mooring line by using two heavy-duty cable ties interlaced into the strands of the mooring line. PEMDs were handled similarly, with removal of aluminum foil wrap below the surface. When these had been secured, global positioning system coordinates and exact depth of water were documented. After a 7-d period of incubation at the field sites, retrieval of cages and PEMDs essentially reversed this process, with bagging below the surface before being handed onto the vessel. Sealed bags with cages were placed in a cooler for transfer to the laboratory.
Salinity and temperature data were collected from one anchor-buoy unit per site by using a SeaStar DST CTD recorder (Star-Oddi) attached to an embryo cage in a mesh bag. The recorders were activated for testing with salinities and temperature confirmed by using a refractometer and handheld thermometer 12 to 24 h before deployment, and programmed to collect temperature and conductivity data at 30-min intervals. Data were downloaded from recorders in Microsoft Excel format.
Field Collection of Naturally Spawned Herring Eggs.
Locations of naturally deposited herring spawn were determined by a combination of vessel-based vegetation rake sampling, direct observation by free diving, or direct observation of intertidal spawn from shore at low tide. The turbidity of San Francisco Bay hinders direct visual observation of deposited subtidal spawn by divers. Although major deposits of subtidal spawn were not detected in the vicinity of the cage moorings at any of the study sites in 2008, intertidal spawning was estimated to have occurred on or close to February 19, 2008, at the SRB reference site, spreading westward through the range of the study area through February 22. Natural spawn samples were collected at the reference site SRB (February 22), and oiled sites SA (February 27), KC (February 28), and PP (February 29). Although natural spawning was observed at the PSQ reference site, the spawning density was very light, occurred concurrently with a sewage spill from the adjacent San Quentin prison, and was concurrent with much more dense spawn deposition at SRB. As a result of personnel exposure considerations and logistics of processing samples, it was decided to not collect samples at PSQ. Intertidal spawning was observed in only one location in 2009: PC, a reference site not sampled in 2008. Spawning occurred beginning the week of January 19, 2009, at PC, and spawn was sampled on January 28. In 2010, intertidal spawning occurred at some study sites starting January 26. Spawn samples were collected from oiled sites PP (February 4), SA (February 4), KC (February 5), and reference site PC (February 6).
At all sites, samples were collected 7 d after the natural spawning event had originally been detected by rake or shore-based surveys, or based on field examination of the embryo developmental stage at each site by visual inspection after fixation in Stockard solution. At each location, attempts were made to collect marine vegetation with spawned herring eggs attached from the middle to lower intertidal zone. These efforts were successful in most situations. At some sites, marine vegetation was largely absent in the lower intertidal zone, and the herring had primarily spawned in the upper intertidal zone. In these situations, samples were collected as low in the intertidal zone as possible, and in no cases were samples collected above the waterline.
The protocol for collection of natural spawn was performed uniformly at all sampling sites. At each site (Fig. S1), samples were collected along a 100-m transect at positions shoreward of the cage deployment positions (i.e., C1–C5) and parallel to the shore within the intertidal zone. Each 100-m transect was divided into 10 distinct 10-m subtransects from which vegetation samples with attached spawn were pooled into eight distinct samples (i.e., N1–N8); two subtransects within the 100-m transect were randomly skipped at each site. GPS coordinates were recorded at the midpoint of each subtransect. Samples were collected from the shoreline by personnel wearing chest waders and/or by free diving. Algal holdfasts were cut with a knife, and the entire sample placed in heavy-duty zip-lock bags containing ambient seawater. When a sufficient sample size for processing the required laboratory subsamples had been collected at each subtransect, the zip-lock bag was filled with ambient water at the same subtransect, sealed, and placed in a large cooler lined with freshly frozen blue ice. Individual samples from the same site were separated from one another by frozen blue ice blocks, to maintain an ambient, or lower than ambient, water temperature during transport back to the laboratory. In all cases, processing of the natural spawn samples in the laboratory was begun within 6 h after the last natural spawn subtransect site was collected in the field.
Laboratory Processing of Embryos for Analytical Chemistry, Imaging, and Hatching Assay.
Upon arrival, general condition of samples were assessed. Nylon sheets with caged embryos were removed, cut into thirds, and placed in 150-mm plastic Petri dishes filled with 16 psu seawater on ice. From one subsample, 2 to 3 g of embryos for PAC analysis were scraped with an inverted 100-mm Petri dish into a precleaned 240-mL glass jar (I-Chem) and frozen at −20 °C. The other subsamples were used for dechorionation and assessment of live embryos and incubation to hatch. Embryos were dechorionated with no. 55 Dumont forceps and anesthetized with a dose of MS-222 titrated just to achieve immobilization. At least 30 undamaged embryos were collected from each cage (150 total per site), and imaged in 16 psu seawater in a 100-mm Petri dish containing 1% agarose molded with slots to accommodate the eye and yolk sack for lateral views. Embryos were examined on Nikon SMZ-1000 stereoscopes maintained at 12 °C with temperature-controlled stages (Brook Industries). Digital imaging was performed with Fire-i400 digital video cameras, with still images acquired on Apple PowerBook G4 laptops using BTV Carbon Pro-5.4.1, and 20-s video clips of the heart were collected with iMovie HD (Apple). Processing of natural spawn samples was similar, with vegetation divided into the three subsamples (collection of 100 embryos for freezing intact in liquid nitrogen, 2–3-g composite samples for analytical chemistry, and imaging and hatching). Embryos were removed from vegetation with forceps individually or in clumps. For each of the eight transects in 2008 and 2009 samples, at least 20 embryos were imaged, for a total of 160 per site. In 2010, natural spawn samples were assessed more broadly while still attached to vegetation. Three subsamples of algae with attached eggs were taken from each of the eight transects, and scanned microscopically for the presence of necrotic or grossly abnormal embryos. Images of eight random fields were collected for each subsample, with each image containing at least 10 embryos (total of ≥240 embryos per site). Because essentially no gross defects or abnormal mortality were observed in 2010 samples, embryos were not dechorionated for detailed morphological analysis, but morphology was assessed in hatched larvae.
For hatching rates, a section of mesh from cages containing as many as 200 embryos (assessed macroscopically) was placed into 250-mL glass culture dishes containing 200 mL 16 psu seawater and incubated in a 12 °C incubator. Most of the sites showed evidence of hatching (i.e., empty chorions observed microscopically) before retrieval, so initial actual numbers of embryos were lower. Daily water changes were performed for the duration of incubation and counts were performed daily for 2 to 6 d, based on the variability in days to hatching observed between sites. For natural spawn samples, several strands of vegetation with attached embryos were placed into 11 × 21-mm rectangular glass dishes containing 600 to 700 mL 16 psu seawater and incubated in a 12 °C incubator. As many as 100 embryos were carefully removed from the vegetation into six-well culture plates (20–30 embryos per well) and incubated at 12 °C with daily water changes. Forty-eight hours after retrieval, embryos, larvae, and empty chorions (i.e., egg shells) were enumerated as follows: eyed nonhatched embryos, dead or unfertilized embryos, number of empty chorions, and normal and abnormal larvae. Subsequent daily counts enumerated only eyed nonhatched embryos, partially hatched embryos, and abnormal larvae. As a result of adherence of sediments or other suspended particles to caged embryos, it was difficult to determine incidence of nonfertilized vs. embryos with arrested development; thus, these embryos were counted as dead/unfertilized. Partially hatched larvae (i.e., embryos/larvae that had partially exited the chorion but were nonviable) were counted as nonhatched embryos. Larvae were defined as normal if they had straight body axes, lack of pericardial or yolk sac edema, regularly beating hearts, and ability to swim and respond to stimuli (i.e., touch). Normal hatching for both cages and natural spawn was defined as the number of morphologically normal larvae per total number of hatched and unhatched embryos combined.
Analyses of PACs and POPs in Herring Tissues, and PEMDs.
Herring tissues (embryos, adult bodies, and ovaries) were extracted and analyzed for PACs and POPs by using a GC/MS method described elsewhere (52, 53). Briefly, this method involves (i) extraction of tissues using an accelerated solvent extraction procedure, (ii) clean-up of the entire methylene chloride extract on a single stacked silica gel/alumina column, (iii) separation of PACs and POPs from the bulk lipid and other biogenic material by high-performance size-exclusion liquid chromatography, and (iv) analysis on a low-resolution quadrupole GC/MS system equipped with a 60-m DB-5 GC capillary column. The instrument was calibrated by using sets of as many as six multilevel calibration standards of known concentrations. PEMDs were extracted after applying an internal standard solution to each sample, with a spiking solution of analytes added to a subset. The samples were extracted in 20%/80% methylene chloride/pentane by sonication, and extracts eluted through silica columns by using 20%/80% methylene chloride/pentane to remove any interfering biogenic compounds. The cleaned-up extracts were concentrated to 100 μL and analyzed as described for eggs and sediments.
Statistics.
Statistical analyses were carried out by using JMP 6.0.2 (SAS Institute) for Macintosh (Apple). Heart rate data were analyzed by a mixed-model nested ANOVA, with cages nested under site as a random effect, site nested under oiled state, and oiled state as an independent variable. PAC levels were compared by one-way ANOVA. In cases in which ANOVA results were significant, differences among sites were compared post hoc by using the Tukey–Kramer HSD test, which compares all means to each other. The probabilities of a nonrandom distribution for the incidence of edema and levels of alkyl-dibenzothiophenes and fluoranthene were determined by using the nonparametric Wilcoxon rank-sum test with the site mean values. For analyses of PAC concentration data, values that were below the limits of quantification were treated as 0 in calculations of means.
Analyses of PAC Patterns in PEMDs.
The relative abundance of 34 PAC compounds in PEMDs was compared between sites by using multidimensional scaling analysis (MDS) (54) in the software package Primer version 6 (55). In brief, the MDS algorithm attempts to satisfy conditions prescribed by a PAC-similarity matrix to place samples with similar PAC patterns together, and dissimilar samples apart in low-dimensional (i.e., 2D) space with the least amount of stress. PAC data were pretreated by standardizing (i.e., computing the proportional contribution of each PAC compound concentration to the total PAC concentration in each sample), and then transforming by taking square root, to reduce the contribution of dominant compounds. The total number of PACs input to the MDS procedure (34) was reduced to five compounds that most efficiently described the PAC patterns, by using the BEST procedure (Primer version 6) to eliminate compounds that did not contribute to explaining the observed PAC patterns. Subsequent Bray–Curtis similarity data for the five compounds selected by the BEST procedure were plotted in 2D, on unitless axes. Pairwise site comparisons of PEMD patterns were conducted with ANOSIM, using the R statistic to identify the main between-site differences. Values of the ANOSIM R statistic range from 0 (i.e., no separation, or complete similarity) to 1.0 (i.e., complete separation, or no similarity) of sites.
Supplementary Material
Acknowledgments
The authors thank Nick Adams, Jennie Bolton, Daryle Boyd, Doug Burrows, Cathy Laetz, Dan Lomax, Ron Pearce, Carla Stehr, Maryjean Willis, Gladys Yanagida, Jason Herum, Dawn Meeks, Joe Newman, and Devon Stephens for excellent technical assistance; Ryan Watanabe and Ryan Bartling for providing ripe adult herring and advice on the location of prespawn herring schools; Mike Anderson and Greg Baker for their inputs on study design and advice throughout the project; and Sandie O'Neill for critical review of the manuscript. Analysis of persistent organic pollutants in 2008 natural spawn samples was carried out by Alpha Analytical Woods Hole Division, Mansfield, MA. This work was funded as a study contributing to the Cosco Busan Oil Spill Cooperative Natural Resource Damage Assessment.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.A.K. is a guest editor invited by the Editorial Board.
See Author Summary on page 357.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108884109/-/DCSupplemental.
References
- 1.Watters DL, Brown HM, Griffin FJ, Larson EJ, Cherr GN. Pacific herring spawning grounds in San Francisco Bay: 1973–2000. In: Feyrer F, Brown LR, Brown RL, Orsi JJ, editors. Early Life History of Fishes in the San Francisco Estuary and Watershed. Bethesda, MD: American Fisheries Society; 2004. pp. 3–36. [Google Scholar]
- 2.US Coast Guard Incident Specific Preparedness Review (ISPR) M/V Cosco Busan Oil Spill in San Francisco Bay: Report on Initial Response Phase, 11 January 2008. 2008. Available at www.uscg.mil/foia/CoscoBuscan/CoscoBusanISPRFinalx.pdf.
- 3.Brown ED, et al. Injury to the early life history stages of Pacific herring in Prince William Sound after the Exxon Valdez oil spill. In: Rice SD, Spies RB, Wolfe DA, Wright BA, editors. Proceedings of the Exxon Valdez Oil Spill Symposium. Vol. 18. Bethesda, MD: American Fisheries Society; 1996. pp. 448–462. [Google Scholar]
- 4.Hose JE, et al. Sublethal effects of the Exxon Valdez oil spill on herring embryos and larvae: Morphological, cytogenetic, and histopathological assessments, 1989–1991. Can J Fish Aquat Sci. 1996;53:2355–2365. [Google Scholar]
- 5.McGurk MD, Brown ED. Egg-larval mortality of Pacific herring in Prince William Sound, Alaska, after the Exxon Valdez oil spill. Can J Fish Aquat Sci. 1996;53:2343–2354. [Google Scholar]
- 6.Norcross BL, Hose JE, Frandsen M, Brown ED. Distribution, abundance, morphological condition, and cytogenetic abnormalities of larval herring in Prince William Sound, Alaska, following the Exxon Valdez oil spill. Can J Fish Aquat Sci. 1996;53:2376–2387. [Google Scholar]
- 7.Peterson CH, et al. Long-term ecosystem response to the Exxon Valdez oil spill. Science. 2003;302:2082–2086. doi: 10.1126/science.1084282. [DOI] [PubMed] [Google Scholar]
- 8.Kocan RM, Hose JE, Brown ED, Baker TT. Pacific herring (Clupea pallasi) embryo sensitivity to Prudhoe Bay petroleum hydrocarbons: Laboratory evaluation and in situ exposure at oiled and unoiled sites in Prince William Sound. Can J Fish Aquat Sci. 1996;53:2366–2387. [Google Scholar]
- 9.Marty GD, Hose JE, McGurk MD, Brown ED, Hinton DE. Histopathology and cytogenetic evaluation of Pacific herring larvae exposed to petroleum hydrocarbons in the laboratory or in Prince William Sound, Alaska, after the Exxon Valdez oil spill. Can J Fish Aquat Sci. 1997;54:1846–1857. [Google Scholar]
- 10.Marty GD, et al. Ascites, premature emergence, increased gonadal cell apoptosis, and cytochrome P4501A induction in pink salmon larvae continuously exposed to oil-contaminated gravel during development. Can J Zool Rev Can Zool. 1997;75:989–1007. [Google Scholar]
- 11.Carls MG, Rice SD, Hose JE. Sensitivity of fish embryos to weathered crude oil: Part I. Low-level exposure during incubation causes malformations, genetic damage, and mortality in larval Pacific herring (Clupea pallasi) Environ Toxicol Chem. 1999;18:481–493. [Google Scholar]
- 12.Heintz RA, Short JW, Rice SD. Sensitivity of fish embryos to weathered crude oil: Part II. Increased mortality of pink salmon (Oncorhynchus gorbuscha) embryos incubating downstream from weathered Exxon Valdez crude oil. Environ Toxicol Chem. 1999;18:494–503. [Google Scholar]
- 13.Couillard CM. A microscale test to measure petroleum oil toxicity to mummichog embryos. Environ Toxicol. 2002;17:195–202. doi: 10.1002/tox.10049. [DOI] [PubMed] [Google Scholar]
- 14.Pollino CA, Holdway DA. Toxicity testing of crude oil and related compounds using early life stages of the crimson-spotted rainbowfish (Melanotaenia fluviatilis) Ecotoxicol Environ Saf. 2002;52:180–189. doi: 10.1006/eesa.2002.2190. [DOI] [PubMed] [Google Scholar]
- 15.Colavecchia MV, Backus SM, Hodson PV, Parrott JL. Toxicity of oil sands to early life stages of fathead minnows (Pimephales promelas) Environ Toxicol Chem. 2004;23:1709–1718. doi: 10.1897/03-412. [DOI] [PubMed] [Google Scholar]
- 16.Colavecchia MV, Hodson PV, Parrott JL. CYP1A induction and blue sac disease in early life stages of white suckers (Catostomus commersoni) exposed to oil sands. J Toxicol Environ Health A. 2006;69:967–994. doi: 10.1080/15287390500362154. [DOI] [PubMed] [Google Scholar]
- 17.Incardona JP, et al. Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development. Environ Health Perspect. 2005;113:1755–1762. doi: 10.1289/ehp.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carls MG, et al. Fish embryos are damaged by dissolved PAHs, not oil particles. Aquat Toxicol. 2008;88:121–127. doi: 10.1016/j.aquatox.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Incardona JP, et al. Cardiac arrhythmia is the primary response of embryonic Pacific herring (Clupea pallasi) exposed to crude oil during weathering. Environ Sci Technol. 2009;43:201–207. doi: 10.1021/es802270t. [DOI] [PubMed] [Google Scholar]
- 20.Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Hicken CE, et al. Sublethal exposure to crude oil during embryonic development alters cardiac morphology and reduces aerobic capacity in adult fish. Proc Natl Acad Sci USA. 2011;108:7086–7090. doi: 10.1073/pnas.1019031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West JE, O'Neill SM, Ylitalo GM. Spatial extent, magnitude, and patterns of persistent organochlorine pollutants in Pacific herring (Clupea pallasi) populations in the Puget Sound (USA) and Strait of Georgia (Canada) Sci Total Environ. 2008;394:369–378. doi: 10.1016/j.scitotenv.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Bence AE, Kvenvolden KA, Kennicutt MC. Organic geochemistry applied to environmental assessments of Prince William Sound, Alaska, after the Exxon Valdez oil spill - A review. Org Geochem. 1996;24:7–42. [Google Scholar]
- 24.Douglas GS, Bence AE, Prince RC, McMillen SJ, Butler EL. Environmental stability of selected petroleum hydrocarbon source and weathering ratios. Environ Sci Technol. 1996;30:2332–2339. [Google Scholar]
- 25.Page DS, et al. The natural petroleum hydrocarbon background in subtidal sediments of Prince William Sound, Alaska, USA. Environ Toxicol Chem. 1996;15:1266–1281. [Google Scholar]
- 26.Page DS, et al. Pyrogenic polycyclic aromatic hydrocarbons in sediments record past human activity: A case study in Prince William Sound, Alaska. Mar Pollut Bull. 1999;38:247–260. [Google Scholar]
- 27.Lima ALC, Farrington JW, Reddy CM. Combustion-derived polycyclic aromatic hydrocarbons in the environment - A review. Environ Forensics. 2005;6:109–131. [Google Scholar]
- 28.Hay D. A stock hypothesis based on spawn and winter distribution. Proceedings of the Fifth Pacific Coast Herring Workshop. Can MS Rep Fish Aquat Sci. 1985;1871:145–148. [Google Scholar]
- 29.Galkina LA. Survival of spawn of the Pacific herring (Clupea harengus pallasii Val.) related to the abundance of the spawning stock. Rapp P-v Reun Cons Int Explor Mer. 1971;160:30–33. [Google Scholar]
- 30.Taylor FHC. Variation in hatching success in Pacific herring (Clupea pallasii) eggs with water depth, temperature, salinity and egg mass thickness. Rapp P-v Reun Cons Int Explor Mer. 1971;160:34–41. [Google Scholar]
- 31.Hourston AS, Rosenthal H, von Westernhagen H. Viable hatch from eggs of Pacific herring (Clupea harengus pallasi) deposited at different intensities on a variety of substrates. Can Tech Rep Fish Aquat Sci. 1984;1274:19. [Google Scholar]
- 32.Alderdice DF, Hourston AS. Factors influencing development and survival of pacific herring (Clupea harengus pallasi) eggs and larvae to beginning of exogenous feeding. Can J Fish Aquat Sci. 1985;42:56–68. [Google Scholar]
- 33.Stratoudakis Y, Gallego A, Morrison JA. Spatial distribution of developmental egg ages within a herring Clupea harengus spawning ground. Mar Ecol Prog Ser. 1998;174:27–32. [Google Scholar]
- 34.Alderdice DF, Velsen FPJ. Some effects of salinity and temperature on early development of Pacific herring (Clupea pallasi) J Fish Res Board Can. 1971;28:1545–1562. [Google Scholar]
- 35.Griffin FJ, et al. Effects of salinity on sperm motility, fertilization, and development in the Pacific herring, Clupea pallasi. Biol Bull. 1998;194:25–35. doi: 10.2307/1542510. [DOI] [PubMed] [Google Scholar]
- 36.Dinnel P, Hoover R, Lechuga L, Tobiason K, Elphick J. Development of larval Pacific Herring, Clupea Pallasi, Bioassay Protocols: Refinement, Validation, Effluent and Cherry Point Ambient Water Testing During 2007. Olympia, WA: Washington Department of Ecology; 2008. [Google Scholar]
- 37.Kiorboe T, Frantsen E, Jensen C, Sorensen G. Effects of suspended sediment on development and hatching of herring (Clupea harengus) eggs. Estuar Coast Shelf Sci. 1981;13:107–111. [Google Scholar]
- 38.Boehlert GW. Abrasive effects of mount saint helens ash upon epidermis of yolk-sac larvae of pacific herring Clupea harengus pallasi. Mar Environ Res. 1984;12:113–126. [Google Scholar]
- 39.Messieh SN, Wildish DJ, Peterson RH. Possible impact of sediment from dredging and spoil disposal on the Miramichi Bay herring fishery. Can Tech Rep Fish Aquat Sci. 1981;1008:33. [Google Scholar]
- 40.Morgan JD, Levings CD. Effects of suspended sediment on eggs and larvae of lingcod Ophiodon elongatus, Pacific herring Clupea harengus pallasi, and surf smelt Hypomesus pretiosus. Can Tech Rep Fish Aquat Sci. 1989;1729:1–31. [Google Scholar]
- 41.Griffin FJ, Smith EH, Vines CA, Cherr GN. Impacts of suspended sediments on fertilization, embryonic development, and early larval life stages of the pacific herring, Clupea pallasi. Biol Bull. 2009;216:175–187. doi: 10.1086/BBLv216n2p175. [DOI] [PubMed] [Google Scholar]
- 42.Costello MJ, Gamble JC. Effects of sewage sludge on marine fish embryos and larvae. Mar Environ Res. 1992;33:49–74. [Google Scholar]
- 43.Hansen PD, von Westernhagen H, Rosenthal H. Chlorinated hydrocarbons and hatching success in Baltic herring spring spawners. Mar Environ Res. 1985;15:59–76. [Google Scholar]
- 44.Barron MG, Carls MG, Heintz R, Rice SD. Evaluation of fish early life-stage toxicity models of chronic embryonic exposures to complex polycyclic aromatic hydrocarbon mixtures. Toxicol Sci. 2004;78:60–67. doi: 10.1093/toxsci/kfh051. [DOI] [PubMed] [Google Scholar]
- 45.Barron MG, Carls MG, Short JW, Rice SD. Photoenhanced toxicity of aqueous phase and chemically dispersed weathered Alaska North Slope crude oil to Pacific herring eggs and larvae. Environ Toxicol Chem. 2003;22:650–660. [PubMed] [Google Scholar]
- 46.Arfsten DP, Schaeffer DJ, Mulveny DC. The effects of near ultraviolet radiation on the toxic effects of polycyclic aromatic hydrocarbons in animals and plants: A review. Ecotoxicol Environ Saf. 1996;33:1–24. doi: 10.1006/eesa.1996.0001. [DOI] [PubMed] [Google Scholar]
- 47.Little EE, Cleveland L, Calfee RD, Barron MG. Assessment of the photoenhanced toxicity of a weathered oil to the tidewater silverside. Environ Toxicol Chem. 2000;19:926–932. [Google Scholar]
- 48.Pelletier MC, et al. Phototoxicity of individual polycyclic aromatic hydrocarbons and petroleum to marine invertebrate larvae and juveniles. Environ Toxicol Chem. 1997;16:2190–2199. [Google Scholar]
- 49.Hatlen K, et al. Natural sunlight and residual fuel oils are an acutely lethal combination for fish embryos. Aquat Toxicol. 2010;99:56–64. doi: 10.1016/j.aquatox.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Incardona J, et al. Potent phototoxicity of marine bunker oil to translucent herring embryos after prolonged weathering. PLoS One. 2011 doi: 10.1371/journal.pone.0030116. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uhler AD, Stout SA, Douglas GS. Chemical heterogeneity in modern marine residual fuel oils. In: Wang Z, Stout SA, editors. Oil Spill Environmental Forensics. London: Academic; 2007. pp. 327–348. [Google Scholar]
- 52.Sloan CA, et al. Determining aromatic hydrocarbons and chlorinated hydro-carbons in sediments and tissues using accelerated solvent extraction and gas chromatography/mass spectrometry. In: Ostrander GK, editor. Techniques in Aquatic Toxicology. Vol. 2. Boca Raton, FL: CRC Press; 2005. pp. 631–651. [Google Scholar]
- 53.Sloan CA, et al. Extraction, Cleanup and Gas Chromatography/Mass Spectrometry Analysis of Sediments and Tissues for Organic Contaminants. NOAA Technical Memorandum NMFS-NWFSC-59. Washington, DC: US Department of Commerce; 2004. [Google Scholar]
- 54.Clarke KR, Warwick RM. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation. 2nd Ed. Plymouth, UK: PRIMER-E; 2001. [Google Scholar]
- 55.Clarke KR, Gorley RN. Primer v. 5: User Manual/Tutorial. Plymouth, UK: PRIMER-E; 2001. [Google Scholar]