Abstract
Varicella-zoster virus (VZV) is a human α-herpesvirus that causes varicella (chickenpox) during primary infection and zoster (shingles) upon reactivation. Like other viruses, VZV must subvert the intrinsic antiviral defenses of differentiated human cells to produce progeny virions. Accordingly, VZV inhibits the activation of the cellular transcription factors IFN regulatory factor 3 (IRF3) and signal transducers and activators of transcription 1 (STAT1), thereby downregulating antiviral factors, including IFNs. Conversely, in this study, we found that VZV triggers STAT3 phosphorylation in cells infected in vitro and in human skin xenografts in SCID mice in vivo and that STAT3 activation induces the anti-apoptotic protein survivin. Small-molecule inhibitors of STAT3 phosphorylation and survivin restrict VZV replication in vitro, and VZV infection of skin xenografts in vivo is markedly impaired by the administration of the phospho-STAT3 inhibitor S3I-201. STAT3 and survivin are required for malignant transformation caused by γ-herpesviruses, such as Kaposi's sarcoma virus. We show that STAT3 activation is also critical for VZV, a nononcogenic herpesvirus, via a survivin-dependent mechanism. Furthermore, STAT3 activation is critical for the life cycle of the virus because VZV skin infection is necessary for viral transmission and persistence in the human population. Therefore, we conclude that takeover of this major cell-signaling pathway is necessary, independent of cell transformation, for herpesvirus pathogenesis and that STAT3 activation and up-regulation of survivin is a common mechanism important for the pathogenesis of lytic as well as tumorigenic herpesviruses.
The life cycle of varicella-zoster virus (VZV) in the human host depends on its tropism for T cells, skin, and neurons within sensory ganglia (1). As shown in the SCID mouse model of VZV pathogenesis, infected human T cells transport the virus to epidermal cells in human skin xenografts and to neural cells in dorsal root ganglia xenografts (2, 3). VZV establishes latency in sensory ganglia; upon reactivation, the virus migrates to the skin via axonal transport to cause zoster.
VZV modulates several signaling pathways to replicate efficiently in vitro, and these regulatory effects are especially important in differentiated skin cells infected in vivo. VZV interferes with IFN induction and signaling via inhibition of IFN regulatory factor 3 (IRF3), NFκB, and STAT1 in vitro and in skin (4, 5). However, the pathogenesis of VZV skin infection requires a mechanism to overcome the constitutive IFNα expression by epidermal cells that accounts for the 10- to 21-d interval between VZV transfer into skin and the appearance of lesions at skin surfaces (6). How VZV overcomes this cutaneous IFN barrier and produces skin vesicles is not known.
The STATs are ubiquitous transcription factors with many cellular functions and are at the junction of several cytokine-signaling pathways (7, 8). Of the seven STAT family proteins, STAT3 exerts widespread effects through transcriptional up-regulation of genes encoding proteins involved in cell survival, cell cycle progression, and homeostasis (9, 10). STAT3 is activated by phosphorylation via several receptor and nonreceptor cellular kinases, including the Jak and Src family kinases and growth factor receptor tyrosine kinases (11). STAT3 activation leads to overexpression of genes involved in tumorigenesis and is constitutive in most primary human tumors (11–13). STAT3 plays a significant role in the pathogenesis of the γ-herpesviruses, Kaposi's sarcoma-associated herpesvirus (KSHV), Epstein–Barr virus (EBV), and herpesvirus saimiri (14–16), all of which exploit the oncogenic effects of phosphorylated STAT3 (pSTAT3).
Transcription mediated by pSTAT3 controls several apoptotic pathway genes, including the Bcl family and the inhibitors of apoptosis (IAP) family genes. Survivin, an inducible member of the IAP family, is abundant in cancers and tissues that contain proliferating cells (17, 18). STAT3 binds to the survivin promoter, and inhibition of STAT3 signaling reduces survivin expression (19, 20). In these experiments, we found that survivin mediates a necessary virus-enhancing effect of STAT3 activation on VZV, a lytic herpesvirus.
Results
VZV Directs STAT3 Phosphorylation in Infected Cells Independently of Secreted Factors.
pSTAT3 expression measured by mean fluorescence intensity (MFI) was reproducibly approximately twofold higher in human embryonic lung fibroblasts (HELF) infected with VZV-GFP virus compared with uninfected HELF from the same monolayer or control uninfected (UI) HELF (Fig. 1A and Fig. S1 A and B). pSTAT3 was comparable in VZV+ HELF and in IFNα-treated, uninfected HELF, and expression in VZV− HELF was similar to uninfected HELF. When equal numbers of sorted VZV+ and VZV− populations were analyzed for pSTAT3 by immunoblot, pSTAT3 expression was enhanced in the VZV+ HELF, and total STAT3 levels were similar in both populations (Fig. 1B). VZV glycoprotein E (gE) expression confirmed the efficiency of cell separation. When pSTAT3 expression was analyzed in human tonsil T cells, VZV+ CD3+ T cells exhibited ∼1.5-fold higher pSTAT3 compared with control T cells cocultured with uninfected HELF (Fig. S1C). In contrast, pSTAT3 in VZV− CD3+ T cells was similar to mock-infected T cells. Thus, STAT3 activation was induced in both HELF and T cells and only in the VZV-infected cell populations in vitro.
Fig. 1.
VZV infection directs STAT3 phosphorylation in human fibroblasts independently of secreted factors. (A) Infected and uninfected HELF were analyzed at 24 hpi by phosphoflow for pSTAT3 expression. The MFI of pSTAT3 expression from different experiments was plotted and represented as a bar chart; uninfected HELF (UI) and HELF treated with IFNα were the negative and positive controls, respectively. (B) Total and pSTAT3 expression in FACS-sorted GFP+ and GFP− populations. (C) FACS analysis for detection of pSTAT3 expression in VZV-GFP–infected cells treated or untreated with IFNα. (D) Conditioned media from uninfected and VZV-infected HELF were tested for the presence of secreted cytokines by Luminex assay at 24 hpi. (E) Culture supernatants from uninfected and VZV-infected HELF (24 hpi) were added to fresh monolayers of HELF. Lysates from untreated control HELF (“C”), HELF treated with OSM, HELF treated with two increasing volumes of supernatant from uninfected HELF (UI), and VZV-infected HELF (VZV) were probed for pSTAT3 expression by immunoblotting.
To determine whether VZV-infected cells retained responsiveness to exogenous STAT3 activation, VZV-infected HELF were treated with IFNα for 30 min before phosphoflow analysis. Both VZV+ and VZV− cells responded to IFNα with a shift in MFI (Fig. 1C), but pSTAT3 expression was higher in VZV+ cells exposed to IFNα than in VZV− cells. Thus, although VZV robustly inhibits IFN induction (4), the ability of exogenous IFNα to trigger STAT3 phosphorylation in infected cells is unimpaired.
To further substantiate that STAT3 activation was not a secondary effect, supernatants collected 24 h after VZV or mock infection of HELF were tested using a 51-plex human cytokine array (Fig. 1D), which included interleukin 6 (IL6) and leukemia inhibitory factor (LIF), which are known to trigger pSTAT3. Secreted IL6 was reduced whereas LIF showed a slight 1.4-fold increase in supernatant from VZV-infected HELF; IFNα and IFNβ controls were not detected. Conditioned media from uninfected and VZV-infected HELF were also evaluated to determine whether pSTAT3 might result from secreted factors below detection or not included in the multiplex assay and if the minor increase in LIF secretion was biologically relevant. Supernatants recovered at 24 h were added to HELF for 24 h; oncostatin M (OSM) was a positive control. When lysates were tested for pSTAT3 by immunoblot, media containing OSM induced pSTAT3, but conditioned media from VZV-infected and uninfected HELF did not (Fig. 1E). Thus, secreted factors released from VZV-infected cells, including LIF, were unlikely to constitute a major mechanism for pSTAT3 up-regulation during VZV replication. The data also confirmed that STAT3 was phosphorylated in the infected but not in the uninfected cell population.
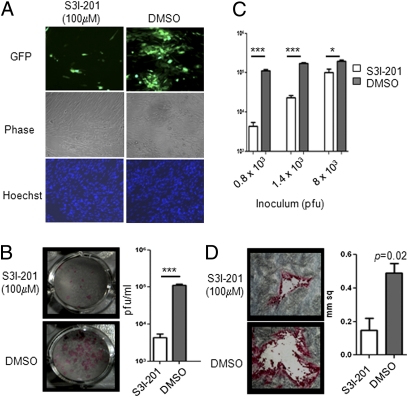
Inhibition of STAT3 Phosphorylation Restricts VZV Replication and Cell–Cell Spread in Vitro.
S3I-201, an amidosalicylic compound, inhibits STAT3 phosphorylation and dimerization, thereby blocking its transactivating function (21). VZV spread was restricted significantly in the presence of S3I-201 compared with DMSO (Fig. 2A) whereas no effect was observed in untreated and DMSO-treated HELF (Fig. S2). Inhibitor treated and untreated cells did not differ in cell morphology, viability, or number. The mean VZV titer was ∼1.5-fold less in HELF infected in the presence of S3I-201 compared with DMSO at 48 hours post infection (hpi) (Fig. 2B). Inhibition of pSTAT3 had more pronounced effects when the inoculum titer was lower (Fig. 2C). With an inoculum of 0.8 × 103 pfu, the difference in viral titers between treated and control HELF was ∼1.5-fold lower compared with <0.5 with an inoculum of 8.0 × 103 pfu. S3I-201 treatment also reduced VZV plaque size significantly in the titration plates (Fig. 2D). Furthermore, pSTAT3 inhibition reduced VZV infection of tonsil T cells; 50% fewer T cells were infected in the presence of S3I-201 compared with DMSO (Fig. S3).
Fig. 2.
Inhibition of STAT3 phosphorylation restricts VZV replication and spread in vitro. (A) VZV-GFP–infected HELF were treated with DMSO or S3I-201; virus spread was determined at 48 hpi by fluorescence microscopy, and nuclei were stained with Hoechst. (B) Infectious virus yields from HELF infected with VZV-GFP in the presence of S3I-201 or DMSO were determined at 48 hpi by titration on melanoma cells; plaques were detected by staining with human polyclonal anti-VZV IgG. Plaques from representative wells are shown; graph shows mean titers ± SEM in the presence and absence of the pSTAT3 inhibitor. (C) DMSO- and S3I-201–treated HELF were inoculated with increasing titers of VZV, and replication was assessed at 48 hpi. (D) Plaque sizes in melanoma cells infected with VZV propagated in DMSO- and S3I-201–treated HELF for 48 h were determined using ImageJ; graph shows the mean size ± SEM of 10 plaques from each condition.
Role of STAT3 Phosphorylation in the Pathogenesis of VZV Skin Infection.
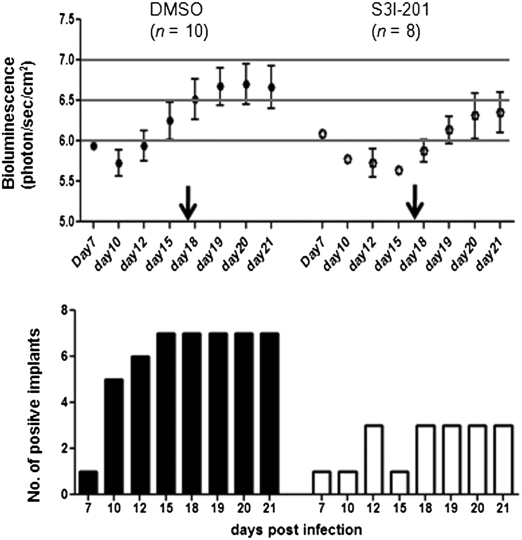
To establish biological relevance, we investigated the consequences of interfering with STAT3 phosphorylation on VZV infection of human skin xenografts in vivo. Skin xenografts in two groups of five mice (two xenografts/mouse) were inoculated with a VZV recombinant expressing firefly luciferase (22). Group I received DMSO and group II was treated with S3I-201 every other day from day 0–16 after VZV inoculation, and all were imaged daily for 21 d (Fig. 3). Xenografts were not infected in one S3I-201 animal (#8); the inhibitor did not affect skin cell morphology or total STAT3 (Fig. S4).
Fig. 3.
Assessment of the effects of inhibiting STAT3 phosphorylation on VZV replication in skin xenografts in vivo. Human skin xenografts in SCID mice were infected with VZV expressing firefly luciferase. Mice, with two xenografts each, were treated with DMSO (group I: mouse #1–5) or S3I-201 (group II: mouse #6–10) beginning on the day of inoculation through day 16. Xenografts in mouse #8 were left uninfected to assess the effects of S3I-201 treatment on human skin. VZV replication in the xenografts was monitored by bioluminescence imaging for 21 d; images shown were taken on the days post infection as indicated.
By day 10, 5/10 (50%) xenografts in the DMSO group showed bioluminescence compared with only 1/8 (12.5%) in the S3I-201 group (P = 0.028 at the 5% level; two-sample Z test to compare proportion of positives) (Fig. 3). On day 12, the number of positive xenografts increased to 6/10 (60%) in the DMSO group vs. 3/8 (38%) in the S3I-201 group. By day 15, the S3I-201 group continued to have fewer positive xenografts compared with the DMSO group (1/8 vs. 7/10; P = 0.001), and signal intensity was lower in those that were positive.
S3I-201 treatment reduced VZV replication on the basis of the bioluminescence signal and the number of positive xenografts compared with DMSO-treated mice throughout the 21-d period (Fig. 4). The kinetics of VZV replication in DMSO-treated animals peaked at day 19–21, as expected from prior studies (23). The biological activity of the inhibitor was confirmed because withdrawing the drug resulted in increased VZV replication in 3/8 xenografts in the S3I-201 group (Fig. 4). At 21 d, VZV was recovered from 6/7 bioluminescence-positive xenografts in the DMSO group (titer: 0.3 × 102 – 2 × 104 pfu) compared with only 1 (titer: 1.4 × 103) in the S3I-201 group. VZV DNA was detected by PCR in all bioluminescence-positive xenografts and in none of those that were negative. Thus, VZV infection was typically blocked completely in the S31-201 group. These experiments indicated that STAT3 phosphorylation was required for efficient VZV replication and spread in human skin in vivo. Notably, blocking STAT3 activation had a much more substantial effect on VZV infection in vivo than could be detected in cultured cells in vitro.
Fig. 4.
Inhibition of STAT3 phosphorylation restricts VZV replication in skin in vivo. The average bioluminescence from the skin xenografts that had a positive signal (y axis) was plotted against the days post infection (x axis) in the DMSO- and S3I-201–treated mice (Upper). Arrows indicate discontinuation of DMSO and S3I-201 treatment on day 16. (Lower) The number of skin xenografts with a positive bioluminescence signal on each day.
VZV Infection of Skin Is Associated with STAT3 Phosphorylation in Vivo.
When skin sections were analyzed for pSTAT3 and IE63, an abundant VZV protein, pSTAT3 expression was unique to the infected skin tissues and was undetectable in sham inoculated xenografts (Fig. 5A). In skin, the populations of cells that expressed pSTAT3 and those that showed IE63 expression did not overlap completely; pSTAT3 was also detected in cells surrounding the foci of VZV-infected cells.
Fig. 5.
STAT3 phosphorylation in VZV-infected skin in vivo. (A) VZV-infected and uninfected skin sections obtained 21 days post inoculation were analyzed by immunohistochemistry. Sections were probed with antibodies to VZV-IE63 (to identify foci of infected cells) and pSTAT3; treatment with calf intestinal phosphatase confirmed the specificity of pSTAT3 detection. (B) For immunofluorescence, VZV-infected skin sections were stained with antibodies to the viral protein ORF61 (Alexa Fluor 488; green) and pSTAT3 (Alexa Fluor 594; red) and examined by confocal microscopy. Nuclei were stained with Hoechst.
To further verify pSTAT3 induction, skin sections were stained for ORF61 protein and pSTAT3 (Fig. 5B). ORF61 is expressed in infected cell nuclei very early after infection (24). ORF61-positive cells (green) that also expressed pSTAT3 (red) were observed. However, cells immediately adjacent to VZV lesions that had no detectable ORF61 expressed pSTAT3, consistent with results using IE63 to identify VZV-infected skin cells. These observations suggest that STAT3 activation may be induced before expression of viral proteins at detectable levels.
VZV Infection Leads to Up-Regulation of Survivin Expression in Vitro and in Vivo.
We next determined if enhanced STAT3 phosphorylation had functional effects in VZV-infected cells by assessing expression of target genes regulated by pSTAT3. Although STAT3 is itself such a target gene, STAT3 transcript levels were unchanged in VZV-infected HELF (Fig. 6A), consistent with no change in total STAT3 protein (Fig. 1B and Fig. S5A). To identify other downstream targets of pSTAT3 that are potentially regulated during VZV infection, the transcription of LIF, cyclin D1, survivin, and Bcl-xl genes was evaluated (Table S1). At 24 h after VZV infection, survivin transcripts increased in VZV-infected compared with uninfected HELF by RT-PCR (∼2.8-fold) whereas transcription of Bcl-xl and cyclin D1 genes remained largely unchanged (Fig. 6A). LIF gene transcripts increased slightly (1.3-fold), consistent with the low level of LIF protein secretion (Fig. 1E). When confirmed by quantitative PCR, survivin gene transcription increased by 16-fold in VZV-infected HELF compared with uninfected cells, which was comparable to the 15-fold increase observed in IL6-treated HELF (Fig. S5B).
Fig 6.
VZV infection leads to up-regulation of survivin transcription and translation in vitro and in vivo. (A) Assessment of indicated pSTAT3-regulated cellular transcripts by RT-PCR in uninfected and VZV-infected HELF at 24 hpi. (B) VZV-GFP–infected HELF were flow-sorted to recover GFP+ and GFP− populations. RNA from each sorted cell population was processed for detection of indicated transcripts. (C) Detection of survivin transcripts in HELF infected with VZV in the presence of DMSO and S3I-201. (D) Immunoblot detection of survivin, pSTAT3, and IκBα expression from untreated, uninfected HELF (lane 1), IL6-treated HELF (lane 2), VZV-infected HELF + DMSO (lane 3), and VZV-infected HELF + S3I-201 (lane 4). (E) VZV-infected and uninfected skin sections were stained with antibodies to the viral glycoprotein gE (Alexa Fluor 594; red) and survivin (Alexa Fluor 488; green) and examined by confocal microscopy. Nuclei were stained with Hoechst.
Survivin gene up-regulation was further evaluated in HELF that were inoculated with VZV-GFP, sorted into VZV+ and VZV− cell populations after 24 h, and analyzed by RT-PCR. The up-regulation of survivin gene transcription occurred predominantly within VZV-infected cells whereas cyclin D1 gene transcripts were only marginally increased compared with uninfected cells (Fig. 6B). Survivin gene transcription was reduced when VZV-infected HELF were treated with the pSTAT3 inhibitor S3I-201 compared with DMSO (Fig. 6C). Furthermore, survivin gene transcription was associated with induction of survivin protein. Survivin expression was enhanced in VZV-infected cell lysates compared with the uninfected lysate (lane 3 vs. lane 1 in Fig. 6D) and induction of survivin protein was blocked by the pSTAT3 inhibitor (lane 4 in Fig. 6D). No degradation of IκBα was observed compared with the uninfected control, indicating that survivin increase is unlikely to be NFκB-dependent and that VZV is known to inhibit NFκB (5).
We next determined if survivin expression was also up-regulated during VZV infection of skin in vivo. As expected, VZV glycoprotein E (red) was expressed prominently on infected cell membranes (Fig. 6E). Survivin expression (green) was detected in the nuclei and cytoplasm of gE-expressing cells (Fig. 6E and Fig. S5C). Some nearby cells that did not express gE also contained survivin (Fig. S5D). This pattern of survivin expression was as observed for pSTAT3, which was found in VZV-infected cells and in cells in close proximity to infected skin cells.
Inhibition of Survivin Restricts VZV Replication and Spread in Vitro.
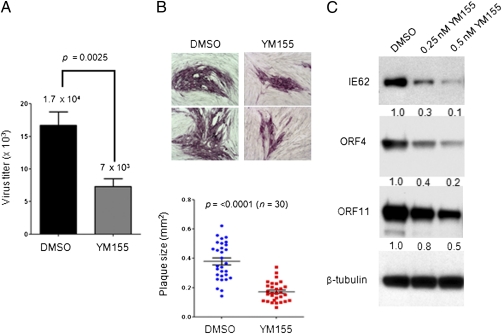
Given these findings, YM155, a small-molecule inhibitor of survivin transcription, was used to assess whether increased survivin expression was required for VZV replication and spread (25). VZV titers decreased in the presence of YM155- compared with DMSO-treated HELF at 48 hpi (Fig. 7A). VZV spread in YM155-treated HELF was also much more restricted compared with DMSO-treated HELF (Fig. 7B); mean plaque sizes were ∼0.2 mm2 with YM155 compared with ∼0.4 mm2 with DMSO. Impaired VZV replication in the absence of survivin was further confirmed by decreased expression of VZV proteins IE62, ORF4, and ORF11 at 24 hpi in the presence of increasing concentrations of the survivin inhibitor (Fig. 7C).
Fig. 7.
Inhibition of survivin expression restricts VZV replication and spread in vitro. (A) HELF were infected with VZV-GFP in the presence of YM155 or DMSO, and infectious virus yields were determined at 48 hpi by titration on melanoma cells. (B) VZV-GFP–infected HELF were treated with DMSO or YM155; plaques were detected by staining with anti-VZV IgG at 48 hpi. Plaque sizes were determined using ImageJ; the graph shows the mean size ± SEM of 30 plaques. (C) Expression of VZV proteins was assessed in VZV-infected HELF lysates (24 hpi) treated with YM155 or DMSO.
Discussion
Our study demonstrates that VZV activation of STAT3 is a critical determinant of the pathogenesis of skin infection in vivo. Furthermore, VZV-induced activation of STAT3 resulted in the expression of survivin in virus-infected cells, and survivin was necessary for optimal VZV replication. Interference either with STAT3 phosphorylation or directly with survivin using small-molecule inhibitors impaired the cell–cell spread of VZV and diminished or blocked the production of infectious virus progeny. VZV also up-regulated pSTAT3 in primary human tonsil T cells, and inhibiting STAT3 activation was associated with markedly diminished T-cell infection. The pathogenic potential of VZV depends on infection of T cells, which transfer the virus to skin. Thus, VZV regulation of STAT3 is important in virus tropism for T cells as well as skin cells, both of which are essential targets for VZV's life cycle in the human host.
Constitutive expression of pSTAT3 and survivin is a well-known characteristic of tumor cells and cells transformed by oncogenic viruses, including KSHV and EBV (14, 26–28). Our study of VZV shows that STAT3 activation and survivin expression also support lytic herpesvirus replication not only in vitro but also in differentiated human skin cells within their tissue microenvironment in vivo. Most oncogenic viruses induce a persistent activation of STAT3. In contrast, the process of α-herpesvirus takeover of the host cell is directed toward assembly and release of large quantities of infectious virus particles and requires only short-term preservation of the infected cell. VZV has no mechanism to abort its lytic program in skin or T cells. Thus, these experiments indicate that STAT3 activation need not be prolonged or support oncogenesis to have important proviral effects for herpesviruses.
Investigating STAT3 regulation by VZV has the advantage that consequences for pathogenesis can be established in the SCID mouse model in vivo. VZV interactions with differentiated human cells within tissues can be examined directly without modulation by adaptive immunity and signaling pathways involved in host cell defense, and changes in cell signaling elicited by VZV replication can be assessed functionally by treating animals with monoclonal antibodies or small-molecule inhibitors in vivo (6). In these experiments, inhibition of STAT3 by i.p. administration of S3I-201 dramatically reduced the capacity of VZV to establish or maintain infection in human skin xenografts. As reported in studies of S3I-201 in tumor models (29), mice given the drug had no adverse effects, and skin xenografts showed no drug-related changes. Therefore, the refractoriness to VZV infection can be attributed to interference with STAT3 phosphorylation.
The importance of epidermal cell production of type I IFNs in innate control of VZV was demonstrated by extensive lesion formation when anti-IFNα/β antibody was given to SCID mice with infected skin xenografts (6). Although STAT1 and STAT3 activation can be synergistic, some studies show that these proteins may be antagonistic (30). pSTAT1 is not detected in VZV-infected epidermal cells whereas nuclear pSTAT1 is prominent in the uninfected cells (6). Conversely, STAT3 activation in VZV-infected epidermal cells and impaired skin infection when STAT3 phosphorylation is inhibited now identify a mechanism by which VZV can overcome this substantial IFN barrier in skin. Interestingly, pSTAT3 nuclear expression was detected in some epidermal cells that did not express VZV proteins but were in close proximity to pSTAT3-positive infected cells, suggesting that these cells may be in an early stage of infection. Because IFNα is present in VZV-infected skin tissues, it is also of interest that exogenous stimulation of VZV-infected HELF with IFNα had an additive effect on pSTAT3 expression. Once STAT3 activation has occurred in VZV-infected skin cells, local IFNα might contribute to the change in the pSTAT expression pattern. Thus, VZV-mediated STAT3 phosphorylation in epidermal cells may suppress antiviral responses mediated by pSTAT1 through a negative feedback signaling loop, which permits cell–cell spread and eventually produces vesicles containing infectious virions at the skin surface.
Although STAT3 activation has varied effects, survivin expression is a well-known consequence (31). Survivin was the only one of five STAT3-responsive genes, including Bcl-xl, another anti-apoptotic factor, induced in VZV-infected cells. Inhibiting survivin with YM155 had effects similar to blocking STAT3 activation. It is also possible that nuclear survivin observed in some VZV-infected cells modulates the cell cycle to benefit viral replication. Nuclear survivin has been associated with virus-mediated induction of the S phase of the cell cycle that supports adenovirus replication (32), and an increase in progression to the S/G2 phase has been observed in VZV-infected cells (33). Survivin also modulates cell proliferation; human cytomegalovirus was recently shown to mediate endothelial cell proliferation and angiogenesis associated with survivin expression (34, 35). However, we observed no difference in expression of the Ki67 proliferation marker in VZV-infected and uninfected skin xenografts (36). From these observations, we propose that the anti-apoptotic activity of survivin is of primary importance for VZV skin pathogenesis by maintaining the VZV-infected cell long enough to allow sufficient production and transfer of infectious virus particles to neighboring epidermal cells. Interestingly, survivin is expressed in human keratinocytes that compose the outer root sheath of hair follicles (37). Because these are the first cells that express VZV proteins after T-cell transfer of the virus, VZV may take advantage of the proviral function of this endogenous survivin to support the initial phase of skin infection.
Whereas the oncogenic γ-herpesviruses have mechanisms that induce survivin directly, our experiments indicate that survivin expression in VZV-infected cells is secondary to STAT3 activation although a direct mechanism is not excluded. Survivin is stimulated by KSHV through the binding of latency-associated nuclear antigen (LANA) to the survivin promoter and by EBV latent membrane protein 1 (LMP1) via the NFκB and AP1 pathways (27, 28). Both LANA and LMP1 also induce pSTAT3 but have not been linked to survivin induction by either of these two γ-herpesvirus proteins. Survivin up-regulation in VZV-infected cells did not correlate with NFκB activation, and VZV blocks NFκB activation in vitro and in vivo, indicating that this pathway is not an alternative to survivin induction through pSTAT3 (5). pSTAT3 in virus-infected cells has been associated with interactions between various host cell kinases and viral factors. A role for cellular kinases in STAT3 activation in VZV-infected cells is suggested by the observation that resveratrol, which inhibits pSTAT3 mediated by cellular kinases, has antiviral activity against VZV (38, 39). The role of cellular and viral kinases in pSTAT3 and survivin up-regulation during VZV infection warrants investigation.
In summary, these observations document the activation of STAT3 by VZV and its importance for herpesvirus replication not only in vitro but also in vivo. We suggest that pSTAT3 antagonism of pSTAT1 facilitates spread of the virus in skin despite the presence of IFNα and, in addition, that VZV-induced STAT3 activation enhances infection via survivin expression and inhibition of apoptosis (Fig. S6). Identifying host and viral proteins involved in the control of this complex STAT3-signaling pathway is of interest because its biological relevance for pathogenesis is established. From this work, we conclude that STAT3 activation and the induction of survivin is a common mechanism necessary for the pathogenesis of lytic as well as tumorigenic herpesviruses.
Materials and Methods
Viruses.
ORF10-GFP (VZV-GFP; gift from T. C. Heineman, GlaxoSmithKline Biologicals) and rOkaF62/63RL-expressing luciferase (22) were used for in vitro and in vivo studies, respectively.
Reagents.
S3I-201 (Calbiochem) was used at 100 μM for 48 h in vitro and 5 mg/kg for in vivo i.p. administration into the xenotransplanted mice (29). YM155 (Selleck Chemical) was used at 0.25 or 0.5 nM for 9 h. OSM (0.5 µg/mL; Biovision), IFNα (104 U/mL; Sigma), and IL6 (100 ng/mL; Cell Signaling Technology) were used to induce pSTAT3.
Flow Cytometry.
Single-cell suspensions of VZV-GFP–infected HELF were made in FACs buffer, fixed in paraformaldehyde (1.5%), permeabilized in methanol, stained using CD3-PE and Alexa Fluor 647-pSTAT3-Y705 and analyzed using BD Biosciences LSR II.
In Vivo Imaging.
Xenografts prepared from human fetal tissues (obtained from Advanced Bioscience Resources with informed consent according to federal and state regulations) were engrafted in male homozygous C.B-17 scid mice and injected with VZV-infected HELF at 6 wks; mice were injected i.p. with S3I-201 or DMSO every other day from day 0–16 (29).
Bioluminescence was measured from day 0–21 in mice given 3 mg d-luciferin (Caliper Life Sciences) and imaged after 5 min (Xenogen IVIS 200). Luminescence values were calculated with Living Image software-2.50.1.
RNA Isolation and RT-PCR Assay.
Total RNA isolated from infected and uninfected HELF (TRIzol Reagent;Invitrogen) was reverse-transcribed with a first-strand cDNA synthesis kit (Invitrogen); 5% of the first-strand reaction was used for RT-PCR to detect survivin, Bcl-xl, STAT3, LIF, cyclin D1, and GAPDH using primers listed in Table S1 (40). HELF infected with VZV-GFP for 24 h were sorted into GFP+ and GFP− populations using Vantoo sorter. An equal number of cells from each population were used for cDNA synthesis (SuperscriptIII Cell Direct cDNA Synthesis System; Invitrogen) and RT-PCR.
Luminex Assay.
Secreted cytokines were detected using the Luminex 200 IS System.
Supplementary Material
Acknowledgments
We thank Dr. Adrish Sen for helpful discussions and Tim Knaak and Yael Rosenberg for technical assistance. This work was supported by NIH Grants AI20459 and AI053846.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114232109/-/DCSupplemental.
References
- 1.Cohen JI, Straus SE, Arvin AM. In: Varicella-zoster virus replication, pathogenesis, and management. Fields Virology. 5th Ed. Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Strause SE, editors. Vol 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 2773–2818. [Google Scholar]
- 2.Arvin AM, et al. Varicella-zoster virus T cell tropism and the pathogenesis of skin infection. Curr Top Microbiol Immunol. 2010;342:189–209. doi: 10.1007/82_2010_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zerboni L, Ku CC, Jones CD, Zehnder JL, Arvin AM. Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc Natl Acad Sci USA. 2005;102:6490–6495. doi: 10.1073/pnas.0501045102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen N, et al. Varicella-zoster virus immediate-early protein 62 blocks interferon regulatory factor 3 (IRF3) phosphorylation at key serine residues: A novel mechanism of IRF3 inhibition among herpesviruses. J Virol. 2010;84:9240–9253. doi: 10.1128/JVI.01147-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones JO, Arvin AM. Inhibition of the NF-kappaB pathway by varicella-zoster virus in vitro and in human epidermal cells in vivo. J Virol. 2006;80:5113–5124. doi: 10.1128/JVI.01956-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ku CC, et al. Varicella-zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-alpha. J Exp Med. 2004;200:917–925. doi: 10.1084/jem.20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 8.Levy DE, Darnell JE., Jr Stats: Transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 9.Jarnicki A, Putoczki T, Ernst M. Stat3: Linking inflammation to epithelial cancer—More than a “gut” feeling? Cell Div. 2010;5:14. doi: 10.1186/1747-1028-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akira S. Functional roles of STAT family proteins: Lessons from knockout mice. Stem Cells. 1999;17(13):138–146. doi: 10.1002/stem.170138. [DOI] [PubMed] [Google Scholar]
- 11.Al Zaid Siddiquee K, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008;18:254–267. doi: 10.1038/cr.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell JE., Jr Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18:2553–2558. doi: 10.1128/mcb.18.5.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Punjabi AS, Carroll PA, Chen L, Lagunoff M. Persistent activation of STAT3 by latent Kaposi's sarcoma-associated herpesvirus infection of endothelial cells. J Virol. 2007;81:2449–2458. doi: 10.1128/JVI.01769-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, et al. STAT3 activation induced by Epstein-Barr virus latent membrane protein1 causes vascular endothelial growth factor expression and cellular invasiveness via JAK3 and ERK signaling. Eur J Cancer. 2010;46:2996–3006. doi: 10.1016/j.ejca.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Chung YH, et al. Activation of Stat3 transcription factor by Herpesvirus saimiri STP-A oncoprotein. J Virol. 2004;78:6489–6497. doi: 10.1128/JVI.78.12.6489-6497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Connor DS, et al. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci USA. 2000;97:13103–13107. doi: 10.1073/pnas.240390697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 19.Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 20.Gu L, Chiang KY, Zhu N, Findley HW, Zhou M. Contribution of STAT3 to the activation of survivin by GM-CSF in CD34+ cell lines. Exp Hematol. 2007;35:957–966. doi: 10.1016/j.exphem.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Siddiquee K, et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci USA. 2007;104:7391–7396. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones JO, Sommer M, Stamatis S, Arvin AM. Mutational analysis of the varicella-zoster virus ORF62/63 intergenic region. J Virol. 2006;80:3116–3121. doi: 10.1128/JVI.80.6.3116-3121.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moffat JF, Stein MD, Kaneshima H, Arvin AM. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J Virol. 1995;69:5236–5242. doi: 10.1128/jvi.69.9.5236-5242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichelt M, Brady J, Arvin AM. The replication cycle of varicella-zoster virus: Analysis of the kinetics of viral protein expression, genome synthesis, and virion assembly at the single-cell level. J Virol. 2009;83:3904–3918. doi: 10.1128/JVI.02137-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakahara T, et al. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007;67:8014–8021. doi: 10.1158/0008-5472.CAN-07-1343. [DOI] [PubMed] [Google Scholar]
- 26.Kung CP, Meckes DG, Jr, Raab-Traub N. Epstein-Barr virus LMP1 activates EGFR, STAT3, and ERK through effects on PKCdelta. J Virol. 2011;85:4399–4408. doi: 10.1128/JVI.01703-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faqing T, et al. Epstein-Barr virus LMP1 initiates cell proliferation and apoptosis inhibition via regulating expression of Survivin in nasopharyngeal carcinoma. Exp Oncol. 2005;27(2):96–101. [PubMed] [Google Scholar]
- 28.Lu J, et al. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (KSHV) upregulates survivin expression in KSHV-associated B-lymphoma cells and contributes to their proliferation. J Virol. 2009;83:7129–7141. doi: 10.1128/JVI.00397-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin L, et al. The STAT3 inhibitor NSC 74859 is effective in hepatocellular cancers with disrupted TGF-beta signaling. Oncogene. 2009;28:961–972. doi: 10.1038/onc.2008.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Regis G, Pensa S, Boselli D, Novelli F, Poli V. Ups and downs: The STAT1:STAT3 seesaw of Interferon and gp130 receptor signalling. Semin Cell Dev Biol. 2008;19:351–359. doi: 10.1016/j.semcdb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Gritsko T, et al. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12(1):11–19. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 32.Connell CM, Wheatley SP, McNeish IA. Nuclear survivin abrogates multiple cell cycle checkpoints and enhances viral oncolysis. Cancer Res. 2008;68:7923–7931. doi: 10.1158/0008-5472.CAN-08-0817. [DOI] [PubMed] [Google Scholar]
- 33.Moffat JF, Greenblatt RJ. Effects of varicella-zoster virus on cell cycle regulatory pathways. Curr Top Microbiol Immunol. 2010;342:67–77. doi: 10.1007/82_2010_28. [DOI] [PubMed] [Google Scholar]
- 34.Botto S, et al. IL-6 in human cytomegalovirus secretome promotes angiogenesis and survival of endothelial cells through the stimulation of survivin. Blood. 2011;117:352–361. doi: 10.1182/blood-2010-06-291245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slinger E, et al. HCMV-encoded chemokine receptor US28 mediates proliferative signaling through the IL-6-STAT3 axis. Sci Signal. 2010;3:ra58. doi: 10.1126/scisignal.2001180. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, et al. Disruption of PML nuclear bodies is mediated by ORF61 SUMO-interacting motifs and required for varicella-zoster virus pathogenesis in skin. PLoS Pathog. 2011;7:e1002157. doi: 10.1371/journal.ppat.1002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Botchkareva NV, Kahn M, Ahluwalia G, Shander D. Survivin in the human hair follicle. J Invest Dermatol. 2007;127:479–482. doi: 10.1038/sj.jid.5700537. [DOI] [PubMed] [Google Scholar]
- 38.Docherty JJ, Sweet TJ, Bailey E, Faith SA, Booth T. Resveratrol inhibition of varicella-zoster virus replication in vitro. Antiviral Res. 2006;72(3):171–177. doi: 10.1016/j.antiviral.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Yu LJ, et al. Inhibition of STAT3 expression and signaling in resveratrol-differentiated medulloblastoma cells. Neoplasia. 2008;10:736–744. doi: 10.1593/neo.08304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, Zhang J, Wang L, Wei H, Tian Z. Therapeutic effects of STAT3 decoy oligodeoxynucleotide on human lung cancer in xenograft mice. BMC Cancer. 2007;7:149. doi: 10.1186/1471-2407-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.