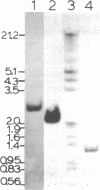
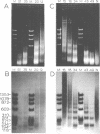
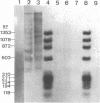
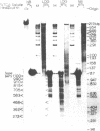
Abstract
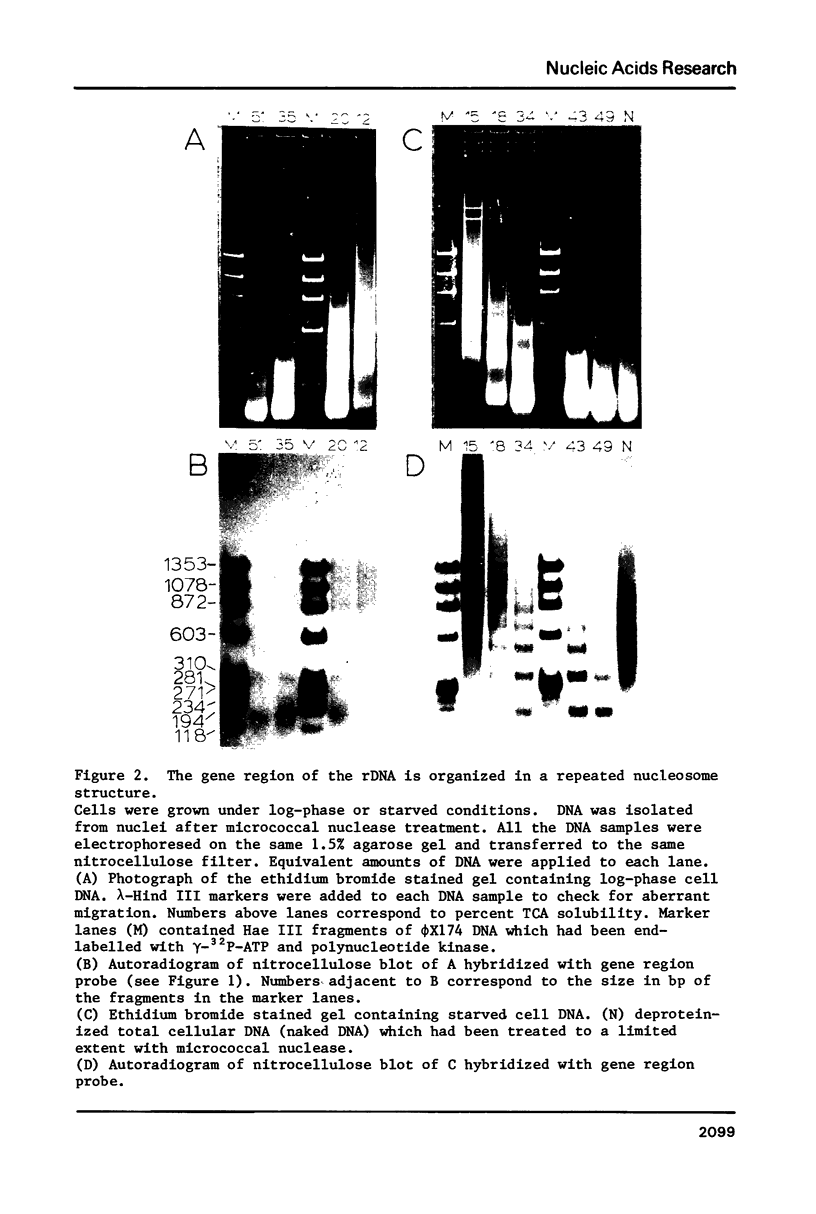
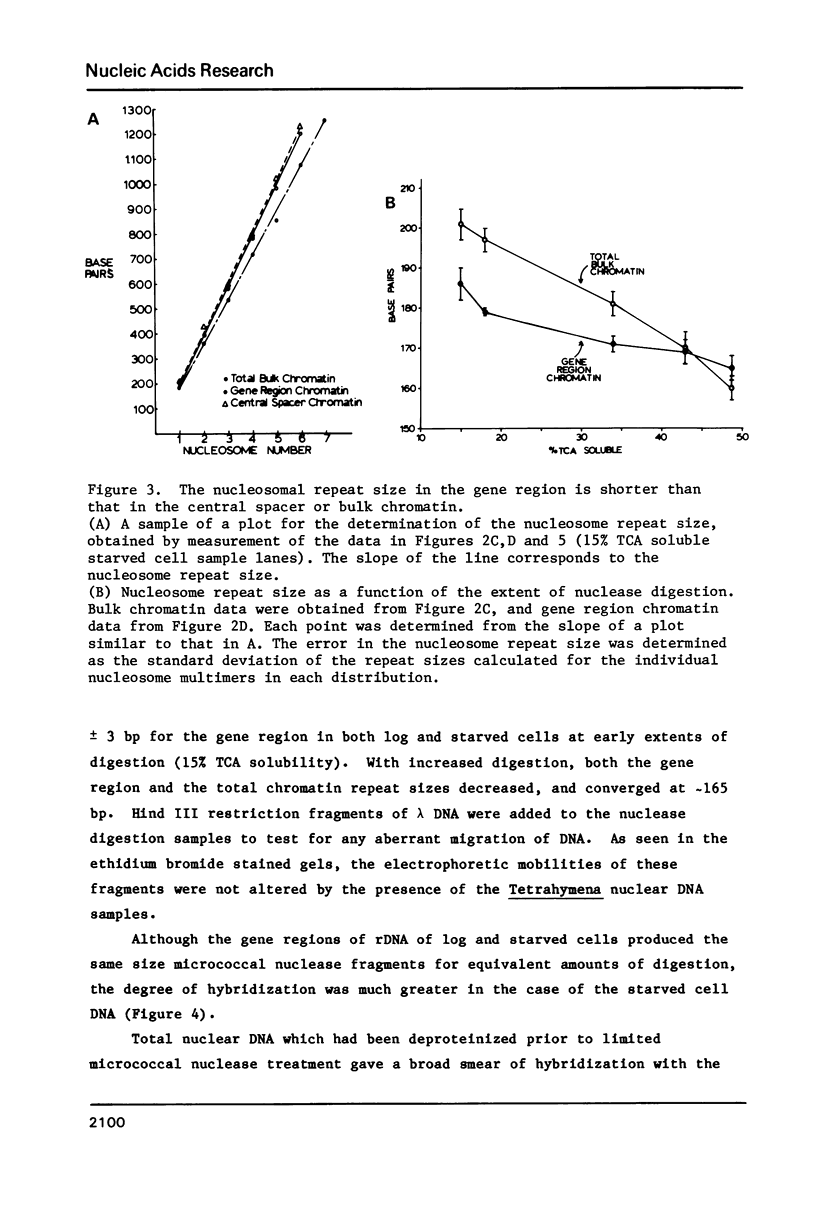
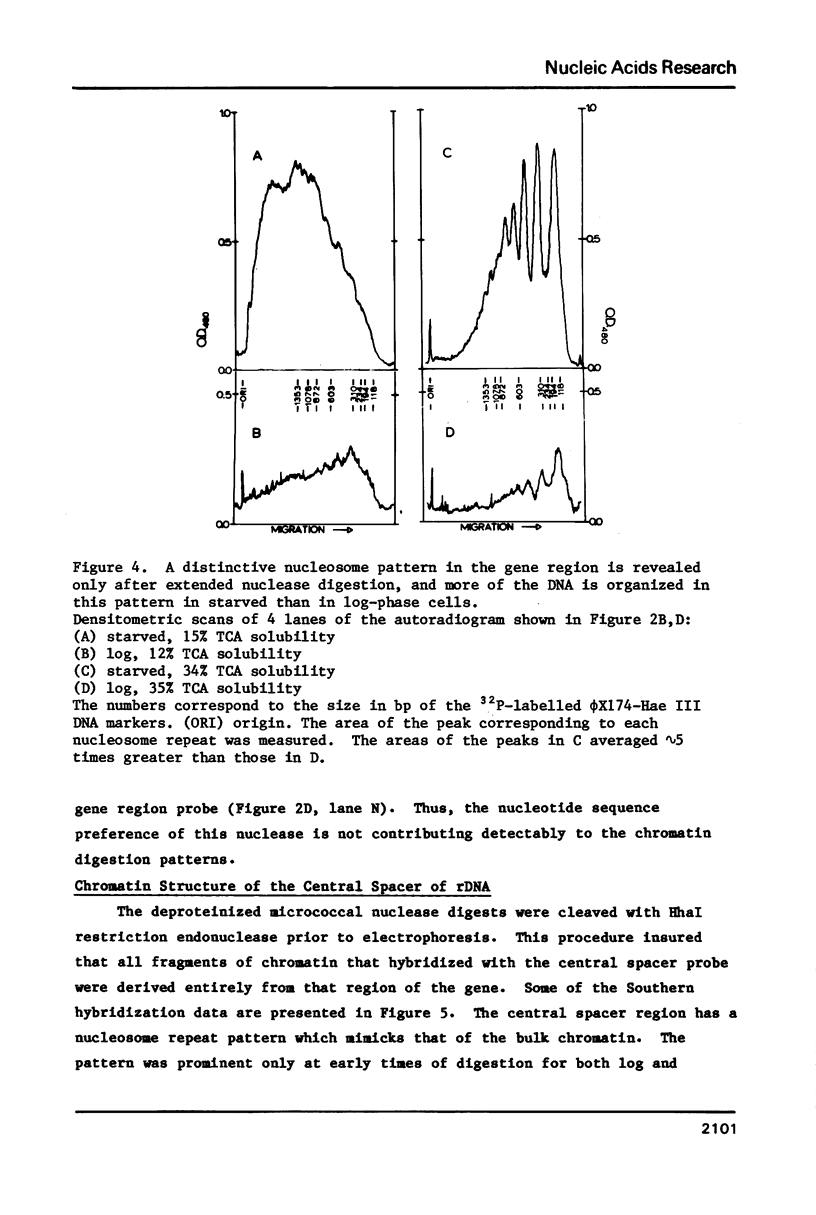
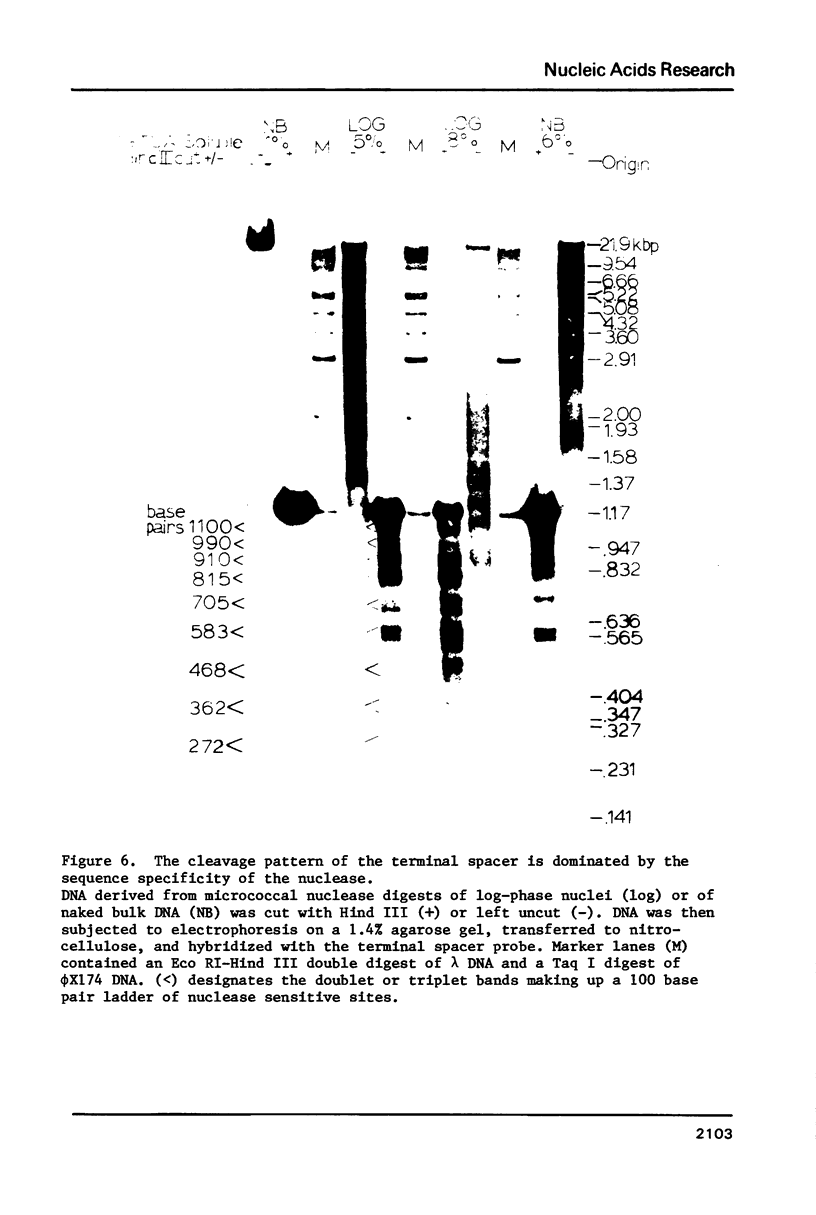
The chromatin structure of the palindromic macronuclear ribosomal RNA genes of Tetrahymena thermophila was probed with micrococcal nuclease. Independent of the state of transcriptional activity, the transcribed region had a shorter nucleosome repeat (184 +/- 3 base pairs) than the non-transcribed central spacer or bulk chromatin (both 200 base pairs). The transcribed region displayed an increased sensitivity to micrococcal nuclease in rapidly growing cells, which suggested an altered chromatin structure during transcription. At early stages of nuclease digestion, the central spacer appeared to be in a highly structured nucleosomal array. Based on the differences in nucleosome repeat distance and sensitivity to nuclease, we conclude that quite different chromatin structures are maintained in two adjacent regions of the Tetrahymena ribosomal RNA gene. The DNA of the non-transcribed terminal spacer was found to contain sequences which are highly susceptible to micrococcal nuclease, precluding any conclusions about nucleosome structure in this region.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allis C. D., Glover C. V., Bowen J. K., Gorovsky M. A. Histone variants specific to the transcriptionally active, amitotically dividing macronucleus of the unicellular eucaryote, Tetrahymena thermophila. Cell. 1980 Jul;20(3):609–617. doi: 10.1016/0092-8674(80)90307-4. [DOI] [PubMed] [Google Scholar]
- Allis C. D., Gorovsky M. A. Histone phosphorylation in macro- and micronuclei of Tetrahymena thermophila. Biochemistry. 1981 Jun 23;20(13):3828–3833. doi: 10.1021/bi00516a025. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H., Chiou S. S. Non-nucleosomal packaging of a tandemly repeated DNA sequence at termini of extrachromosomal DNA coding for rRNA in Tetrahymena. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2263–2267. doi: 10.1073/pnas.78.4.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H., Gall J. G. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978 Mar 25;120(1):33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- Borchsenius S., Bonven B., Leer J. C., Westergaard O. Nuclease-sensitive regions on the extrachromosomal r-chromatin from Tetrahymena pyriformis. Eur J Biochem. 1981 Jul;117(2):245–250. doi: 10.1111/j.1432-1033.1981.tb06329.x. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Zasloff M. A. Nucleosomal packaging of the thymidine kinase gene of herpes simplex virus transferred into mouse cells: an actively expressed single-copy gene. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5079–5083. doi: 10.1073/pnas.77.9.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Brehm S. L. Replication of the extrachromosomal ribosomal RNA genes of Tetrahymena thermophilia. Nucleic Acids Res. 1981 Jul 24;9(14):3531–3543. doi: 10.1093/nar/9.14.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Karrer K. M. Chromatin structure of the ribosomal RNA genes of Tetrahymena thermophila as analyzed by trimethylpsoralen crosslinking in vivo. J Mol Biol. 1980 Feb 5;136(4):395–416. doi: 10.1016/0022-2836(80)90397-6. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Rio D. C. Localization of transcribed regions on extrachromosomal ribosomal RNA genes of Tetrahymena thermophila by R-loop mapping. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5051–5055. doi: 10.1073/pnas.76.10.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Lomonossoff G. P., Laskey R. A. High sequence specificity of micrococcal nuclease. Nucleic Acids Res. 1981 Jun 25;9(12):2659–2673. doi: 10.1093/nar/9.12.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Eckert W. A., Kaffenberger W. Regulation of rRNA metabolism in Tetrahymena pyriformis. I. Nutritional shift-down. Eur J Cell Biol. 1980 Apr;21(1):53–62. [PubMed] [Google Scholar]
- Engberg J., Andersson P., Leick V., Collins J. Free ribosomal DNA molecules from Tetrahymena pyriformis GL are giant palindromes. J Mol Biol. 1976 Jun 25;104(2):455–470. doi: 10.1016/0022-2836(76)90281-3. [DOI] [PubMed] [Google Scholar]
- Engberg J., Nasir-ud-Din, Eckert W. A., Kaffenberger W., Pearlman R. E. Detailed transcription map of the extrachromosomal ribosomal RNA genes in Tetrahymena thermophila. J Mol Biol. 1980 Sep 25;142(3):289–313. doi: 10.1016/0022-2836(80)90274-0. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Foe V. E. Modulation of ribosomal RNA synthesis in Oncopeltus fasciatus: an electron microscopic study of the relationship between changes in chromatin structure and transcriptional activity. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):723–740. doi: 10.1101/sqb.1978.042.01.074. [DOI] [PubMed] [Google Scholar]
- Higashinakagawa T., Tashiro F., Mita T. DNA-dependent RNA polymerase from a protozoan, Tetrahymena pyriformis. Extraction and partial characterization. J Biochem. 1975 Apr;77(4):783–793. doi: 10.1093/oxfordjournals.jbchem.a130783. [DOI] [PubMed] [Google Scholar]
- Hörz W., Altenburger W. Sequence specific cleavage of DNA by micrococcal nuclease. Nucleic Acids Res. 1981 Jun 25;9(12):2643–2658. doi: 10.1093/nar/9.12.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Campbell G. R., Allfrey V. G. Different nucleosome structures on transcribing and nontranscribing ribosomal gene sequences. Science. 1979 Dec 7;206(4423):1192–1194. doi: 10.1126/science.505006. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Matthews H. R., Littau V. C., Lothstein L., Bradbury E. M., Allfrey V. G. The structure of chromatin containing DNA complementary to 19 S and 26 S ribosomal RNA in active and inactive stages of Physarum polycephalum. Arch Biochem Biophys. 1978 Dec;191(2):537–560. doi: 10.1016/0003-9861(78)90392-2. [DOI] [PubMed] [Google Scholar]
- Karrer K. M., Gall J. G. The macronuclear ribosomal DNA of Tetrahymena pyriformis is a palindrome. J Mol Biol. 1976 Jun 25;104(2):421–453. doi: 10.1016/0022-2836(76)90280-1. [DOI] [PubMed] [Google Scholar]
- Kiss G. B., Amin A. A., Pearlman R. E. Two separate regions of the extrachromosomal ribosomal deoxyribonucleic acid of Tetrahymena thermophila enable autonomous replication of plasmids in Saccharomyces cerevisiae. Mol Cell Biol. 1981 Jun;1(6):535–543. doi: 10.1128/mcb.1.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFond R. E., Goguen J., Einck L., Woodcock C. L. Micrococcal nuclease cleavage of chromatin displays nonrandom properties. Biochemistry. 1981 Apr 14;20(8):2127–2132. doi: 10.1021/bi00511a009. [DOI] [PubMed] [Google Scholar]
- Leick V., Andersen S. B. Polols and turnover rates of nuclear ribosomal RNA in Tetrahymena pyriformis. Eur J Biochem. 1970 Jul;14(3):460–464. doi: 10.1111/j.1432-1033.1970.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D. J., Gorovsky M. A. Subunit structure of rDNA-containing chromatin. Biochemistry. 1976 Feb 24;15(4):750–755. doi: 10.1021/bi00649a005. [DOI] [PubMed] [Google Scholar]
- Piper P. W., Celis J., Kaltoft K., Leer J. C., Nielsen O. F., Westergaard O. Tetrahymena ribosomal RNA gene chromatin is digested by micrococcal nuclease at sites which have the same regular spacing on the DNA as corresponding sites in the bulk nuclear chromatin. Nucleic Acids Res. 1976 Feb;3(2):493–505. doi: 10.1093/nar/3.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt S. C., Grainger R. M. A mosaicism in the higher order structure of Xenopus oocyte nucleolar chromatin prior to and during ribosomal gene transcription. Cell. 1981 Mar;23(3):711–720. doi: 10.1016/0092-8674(81)90434-7. [DOI] [PubMed] [Google Scholar]
- Reeder R. H., Wahn H. L., Botchan P., Hipskind R., Sollner-Webb B. Ribosomal genes and their proteins from Xenopus. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1167–1177. doi: 10.1101/sqb.1978.042.01.117. [DOI] [PubMed] [Google Scholar]
- Reeves R. Structure of Xenopus ribosomal gene chromatin during changes in genomic transcription rates. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):709–722. doi: 10.1101/sqb.1978.042.01.073. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stalder J., Seebeck T., Braun R. Accessibility of the ribosomal genes to micrococcal nuclease in Physarum polycephalum. Biochim Biophys Acta. 1979 Feb 27;561(2):452–463. doi: 10.1016/0005-2787(79)90153-9. [DOI] [PubMed] [Google Scholar]
- Sutton C. A., Sylvan P., Hallberg R. L. Ribosome biosynthesis in Tetrahymena thermophila. IV. Regulation of ribosomal RNA synthesis in growing and growth arrested cells. J Cell Physiol. 1979 Dec;101(3):503–513. doi: 10.1002/jcp.1041010316. [DOI] [PubMed] [Google Scholar]
- Vavra K. J., Allis C. D., Gorovsky M. A. Regulation of histone acetylation in Tetrahymena macro- and micronuclei. J Biol Chem. 1982 Mar 10;257(5):2591–2598. [PubMed] [Google Scholar]
- Wu C., Bingham P. M., Livak K. J., Holmgren R., Elgin S. C. The chromatin structure of specific genes: I. Evidence for higher order domains of defined DNA sequence. Cell. 1979 Apr;16(4):797–806. doi: 10.1016/0092-8674(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Zaug A. J., Cech T. R. In vitro splicing of the ribosomal RNA precursor in nuclei of Tetrahymena. Cell. 1980 Feb;19(2):331–338. doi: 10.1016/0092-8674(80)90507-3. [DOI] [PubMed] [Google Scholar]