Abstract
Release of substance P (SP) from nociceptive nerve fibers and activation of its receptor neurokinin 1 (NK1) are important effectors in the transmission of pain signals. Nonetheless, the role of SP in muscle pain remains unknown. Here we show that a single i.m. acid injection in mice lacking SP signaling by deletion of the tachykinin precursor 1 (Tac1) gene or coadministration of NK1 receptor antagonists produces long-lasting hyperalgesia rather than the transient hyperalgesia seen in control animals. The inhibitory effect of SP was found exclusively in neurons expressing acid-sensing ion channel 3, where SP enhances M-channel–like potassium currents through the NK1 receptor in a G protein-independent but tyrosine kinase-dependent manner. Furthermore, the SP signaling could alter action potential thresholds and modulate the expression of TTX-resistant sodium currents in medium-sized muscle nociceptors. Thus, i.m. SP mediates an unconventional NK1 receptor signal pathway to inhibit acid activation in muscle nociceptors, resulting in an unexpected antinociceptive effect against chronic mechanical hyperalgesia, here induced by repeated i.m. acid injection.
Keywords: dorsal root ganglion, fibromyalgia, genistein, G-protein-coupled receptor, Src
Substance P (SP) is an undecapeptide belonging to the tachykinin small-peptide family (1). SP is generated in primary nociceptive sensory neurons (nociceptors) and is released with noxious stimulation (2). The release from cutaneous peripheral terminals induces neurogenic inflammation and release from central terminals enhances the glutamate-dependent excitatory postsynaptic potential, thus leading to central sensitization (3–5). Although sensory neurons in muscle also contain SP, i.m. SP injection evokes a very low level of neurogenic inflammation and no pain (6, 7).
Accumulating evidence has suggested that muscle pain might be closely related to acidosis and activation of proton-sensing ion channels (8–10). Lactate and ATP sensitize this acid activation (11, 12). Human and animal studies have revealed that acidosis is an effective trigger of muscle pain. Thus, chronic muscle pain can be induced in rodents by repeated i.m. injection of acid (13, 14), by i.m. injection of complete Freund's adjuvant (CFA), carrageenan, capsaicin, or proinflammatory cytokines (15–18), by arterial occlusion (19), and by eccentric muscle contraction (20). These stimuli might correspond to muscle pain related to acidosis, inflammatory and ischemic myalgia, or delayed-onset muscle soreness. Although these models might not reflect fully the complicated human pain conditions, and although repetitive acidosis is not known to produce chronic pain or central sensitization in humans, these rodent models are useful for probing the underlying mechanisms and analgesic modulation of chronic muscle pain. For instance, acid-sensing ion channel 3 (ASIC3) is essential for triggering acid-induced mechanical hyperalgesia in models of i.m. injection of acid, CFA, or carrageenan (13, 17, 21–23). Coinjection of neurotrophin-3 reverses the acid-induced chronic hyperalgesia (24). Also, some muscle-derived pain can be attenuated by treatment with pregabalin or with the voltage-dependent potassium M-channel openers retigabine and flupirtine (25, 26). Flupirtine already is in use as an analgesic for chronic muscle pain. In addition to acid, ATP, capsaicin, and nerve growth factor (NGF) also can activate muscle nociceptors (27, 28). However, the role of SP in muscle pain is unknown.
We adopted the mouse model of muscle pain induced by repeated i.m. acid injection to test the role of SP in acid-evoked hyperalgesia and then used whole-cell patch-clamp recording to probe the SP-mediated signal pathway in muscle-afferent neurons.
Results
Loss of SP Signal Facilitates Referred Hyperalgesia Induced by I.M. Acid Injection.
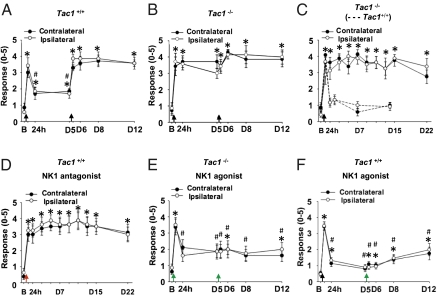
As described by Sluka et al. (13), repeated injections of acid solution (pH 4.0) to one side of the gastrocnemius muscle (GM) caused bilateral, long-lasting (>2 wk) mechanical hyperalgesia in mouse hind paws (Fig. 1A). The first acid injection induced rapid, transient referred hyperalgesia (an increased response to a noxious stimulus outside the injured site), which declined after 24 h in wild-type mice. A second acid injection administered 5 d after the first induced long-lasting referred hyperalgesia. Mice lacking the tachykinin 1 gene (Tac1−/−; no SP and no neurokinin A production) showed persistent referred hyperalgesia that lasted for at least 22 d (Fig. 1 B and C). Therefore, the reversal of the acid-induced hyperalgesia seemed to depend on SP or neurokinin A. To evaluate the role of the SP receptor neurokinin 1 (NK1) in this process, we coinjected acid and 2-[1-imino-2-(2-methoxyphenyl)ethyl]-7,7-diphenyl-4-perhydroisoindolone (3aR, 7aR) (RP-67580), a selective NK1 antagonist, into the muscle of wild-type mice. This coinjection also induced long-lasting mechanical hyperalgesia and produced an exact phenocopy of the Tac1 gene deletion (Fig. 1D). Therefore, SP–NK1 signaling was activated by i.m. injection of acid and counteracted the acid-induced hyperexcitation of muscle nociceptors.
Fig. 1.
Effect of SP signaling on i.m. acid-induced mechanical hyperalgesia. The withdrawal responses of mouse hind paws to a 0.2-mN bending force is shown in Tac1+/+ and Tac1−/− mice before and after i.m. acid injection. A response was defined as foot lifting when a 0.2-mN von Frey filament was applied. (A) Tac1+/+ and (B) Tac1−/− mice were injected with pH 4.0 saline on days 0 and 5 (n = 7). (C) A single i.m. acid injection evoked bilateral chronic hyperalgesia in Tac1−/− mice (n = 8) but only transient hyperalgesia in Tac1+/+ mice (n = 6, dotted lines). (D) Coinjection of acid with 100 μM NK1 antagonist (RP-67580) in Tac1+/+ mice induced long-lasting mechanical hyperalgesia similar to that in Tac1−/− mice receiving a single acid injection on day 0 (n = 8). (E) Coinjection of acid and the 40 μM NK1 receptor agonist SM-SP prevented i.m. acid-induced chronic referred hyperalgesia in Tac1−/− mice; only transient hyperalgesia developed 4 h after the first coinjection (n = 8). (F) Coinjection of acid and 40 μM SM-SP prevented the chronic hyperalgesia induced by the second acid injection in Tac1+/+ mice (n = 21). Black arrows indicate the time of i.m. acid injection. Red and green arrows indicate the time at which mice received the coinjection of acid with the NK1 antagonist and agonist, respectively. B, baseline on day 0; D, day. *P < 0.05 compared with baseline; #P < 0.05 compared with the response at 4 h.
SP Prevents the Development of Referred Hyperalgesia Induced by I.M. Acid Injection.
We next determined whether the Tac1−/− phenotype could be reversed by coinjection of the acid solution and an NK1-selective agonist, [Sar9, Met(O2)11]-SP (SM-SP) (Fig. 1E). Indeed, we observed transient hyperalgesia in Tac1−/− mice 4 h after the coinjection, which returned to the same low level observed in wild-type mice. Thus, a single activation of the NK1 receptor completely reversed the Tac1−/− phenotype. Strikingly, when acid and SM-SP were coinjected again in these mice, they did not show the long-lasting hyperalgesia that we previously observed. Thus, the activation of NK1 receptor signaling protects against acid-induced chronic hyperalgesia. We also could prevent the chronic hyperalgesia induced by the second acid injection by coinjection of acid and SM-SP (40 μM) in wild-type mice (Fig. 1F). The antinociceptive effect of SM-SP at the second acid injection was dose dependent. SM-SP was as effective at 10 μM as at 40 μM, but a lower concentration, 4 μM, induced a transient hyperalgesia that lasted at least 24 h after the second acid injection (Fig. S1 A–C). These data suggest that muscle nociceptors release SP in response to i.m. acid stimulation. This SP release was sufficient to reduce the hypersensitivity after the first injection but not after repeat acid administration.
Because referred mechanical hyperalgesia induced by repeated i.m. acid treatment requires ASIC3 as a transducer (13), we next examined the effect of SP deficiency in the absence of ASIC3. As expected, mice lacking Asic3 showed no response to i.m. acid injections (Fig. S1D). Similarly, i.m. acid injection did not induce hyperalgesia in Asic3 and Tac1 double-knockout mice (Fig. S1E), so the antinociceptive effect of SP in muscle is observed only with ASIC3-mediated acid-induced hyperalgesia.
SP Attenuates ASIC3-Mediated Current by a G Protein-Independent Pathway in Muscle-Afferent Dorsal Root Ganglion Neurons.
To determine the i.m. sites of SP action, we first investigated SP and ASIC3 immunoreactivity in muscle nerve fibers. As shown in Fig. S2A, most SP-positive nerve fibers also expressed ASIC3 channels. Acids may trigger SP release from these fibers by activating ASIC3. However, compared with saline injection, neither acid (pH 4.0) nor SM-SP injection evoked significant plasma extravasation in muscle (Fig. S2B). In contrast, injection of mustard oil into muscle significantly increased the level of plasma extravasation (Fig. S2B), indicating that muscle nociceptors can induce neurogenic inflammation that might involve a non-SP pathway.
We next used whole-cell patch-clamp recording to examine whether SP could modulate ASIC3-mediated depolarization in muscle-afferent neurons. With voltage-clamp mode, we determined ASIC3-expressing dorsal root ganglion (DRG) neurons, while the acid-induced current was inhibited by salicylic acid (SA) (29, 30). We thus named these neurons SA-sensitive (SAS) neurons (Fig. 2A). In contrast, other acid-sensitive DRG neurons expressed an SA-resistant sustained current in response to acid stimulation. Interestingly, SP specifically attenuated the acid-induced inward current in GM SAS neurons but had mixed effects on non-GM SAS neurons (Fig. 2A). Of the eight non-GM SAS neurons we recorded, two had acid-induced current positively modulated by SP (1.25 ± 0.08; normalized to baseline), and two were negatively modulated (0.60 ± 0.13; normalized to baseline); the other four neurons were SP insensitive. The cell-type–specific SP effect required NK1 receptors, because SP had no effect on ASIC3-mediated current when RP-67580 was applied (Fig. 2B). Because the NK1 receptor is a G protein-coupled receptor (GPCR), to examine the effect of G proteins, we replaced GTP with a nonhydrolysable analog, GDP-β-S in the internal pipette solution. Surprisingly, in GM SAS neurons, the effect of SP on ASIC3-mediated current was resistant to GDP-β-S dialysis, indicating the involvement of an NK1 receptor-dependent, G protein-independent pathway (Fig. 2C). In contrast, GDP-β-S dialysis significantly inhibited a baclofen-induced GABA-B current (Fig. S3). We then examined the effect of phosphotyrosine kinase (PTK) on the SP-mediated inhibition; PTK is involved in a G protein-independent pathway (31). Bath application of a PTK inhibitor, genistein, effectively abolished the SP-mediated inhibition of the ASIC3-selective current (Fig. 2D), suggesting an unconventional G protein-independent SP–NK1 signaling pathway in GM SAS DRG neurons.
Fig. 2.
Effect of SP on ASIC3-mediated currents. (A) Representative current traces showing the effect of SP on acid-induced currents in SAS neurons that innervate into GM and in non-GM SAS neurons. The pH 6.8 acid-induced currents on fluorogold-labeled GM and nonlabeled DRG neurons were screened for the presence of ASIC3 with 500 μM SA in the bath; then the perfusion medium was switched to a bath containing 3 μM SP to record any change in acid-induced current. At the end of the recording, the same neurons were treated with 3 μM SP administered by puff to determine the effect of SP treatment alone. (B) GM SAS neurons were treated with the NK1 receptor antagonist RP-67580 to test whether SP-mediated modulation of acid-induced current depended on the NK1 receptor. The neurons were treated with (n = 6) or without (n = 5) a GDP-β-S–containing pipette solution to validate the participation of G proteins in SP-mediated modulation. The acid-induced currents were recorded in ACSF for 2 min as baseline, in a bath containing RP-67580 for 2 min, in a bath containing SP and RP-67580 for another 2 min, and then in a bath containing RP-67580 for 1 min. During the final minutes, neurons were treated with ACSF for washout. (C) Effect of G proteins on SP-mediated inhibition of acid-induced current. SAS neurons were treated with (n = 9 for GM neurons) or without (n = 8 for GM neurons, n = 6 for non-GM neurons) a pipette solution containing GDP-β-S. Acid-induced currents were recorded before (−2 min), during (3 min), and after (3 min) SP treatment. *P < 0.05 and **P < 0.01, GM SAS (GTP) vs. non-GM SAS (GTP) groups; ##P < 0.01, GM SAS (GDP) vs. non-GM SAS (GTP) groups. (D) The involvement of PTKs in SP-mediated modulation of acid-induced current. GM SAS neurons were recorded for 2 min as baseline and then were treated with SP alone (n = 8) as a positive control, with genistein alone (an inhibitor of PTK; n = 6) as a negative control, with SP + genistein (n = 6), or with SP + daidzein (an inactive structural analog of genistein; n = 9) for 3 min. Then recorded neurons were washed out with ACSF for another 3 min. *P < 0.05; **P < 0.01 vs. SP alone.
SP Induces an Outward Current in Most Muscle-Afferent DRG Neurons.
To probe further the mechanism of SP-mediated antinociception, we investigated the SP-mediated electrophysiological response in muscle nociceptors. SP (3 μM) induced a transient inward current in 6.7% of 210 fluorogold-labeled GM-afferent neurons or a slow inactivating outward current (18.4 ± 0.9 s before returning to the baseline) in 50.5% of the neurons; the other 42.9% of the neurons did not respond to SP (Fig. 3A). In contrast, SP induced a transient inward current in 30.6% of 124 fluorogold-negative neurons and a slow inactivating outward current in 16.1%; the other 53.2% of the neurons did not respond to SP. Therefore, SP mediated an outward current (ISP-O) in most muscle-afferent DRG neurons. The GM DRG neurons expressing ISP-O were in a wide range of cell sizes; most were 30–40 μm in diameter (Fig. S4), and most (9 of 14) were ASIC3-expressing neurons (Fig. S5).
Fig. 3.
SP-mediated currents in muscle DRG neurons. (A) SP-induced electrophysiological responses resulting from superfusion of 3 μM SP for 4 s in GM-afferent DRG neurons (fluorogold positive) and non–GM-afferent DRG neurons (fluorogold negative). The number of neurons and percentage for each type of response are shown. (B) Peak whole-cell current amplitude as a function of SP (ISP-O). Dose-dependent (0.1, 1, 3, and 10 μM) response of ISP-O with an EC50 of 2.6 μM is shown (n = 27). (C) Representative traces of sustained outward current induced by repeated SP administration (ISP-O, with >10 pA cutoff). (D) Representative traces of transient inward current induced by repeat SP administration (ISP-I, with >10 pA cutoff). (E) Comparison of the participation of each tachykinin receptor subtype in the formation of ISP-O by their respective receptor antagonists (10 μM RP-67580 for NK1, 3 μM GR-159897 for NK2, and 3 μM SB-218795 for NK3). **P < 0.01 vs. NK1 antagonist. (F–H) Representative traces showing the effect of the NK1 (F), NK2 (G), and NK3 (H) receptor antagonist on ISP-O. All neurons were held at −70 mV for recording.
The ISP-O was elicited in a concentration-dependent manner (0.1–10 μM), with an EC50 of 2.6 μM (n = 27), and did not show a substantial decrease after repeated SP application (Fig. 3 B and C). In contrast, the SP-induced transient inward current was desensitized after SP administration (Fig. 3D). While 3 μM SP was superfused, the ISP-O was reversibly blocked by the NK1-selective antagonist RP-67580 (10 μM), with a mean inhibition of 86.0 ± 7.6% (Fig. 3 E and F). In contrast, selective antagonists for the NK2 receptor [5-fluoro-3-(2-(4-methoxy-4-([(R)-phenylsulphinyl]methyl)-1-piperidinyl)ethyl)-1H-indole (GR-159897);3 μM] and NK3 receptor [N-(α-(methoxycarbonyl)benzyl)-2-phenylquinoline-4-carboxamide (SB-218795); 3 μM] only partly inhibited the ISP-O, with mean inhibitions of 34.4 ± 4.1% and 34.4 ± 4.2%, respectively (Fig. 3 E, G, and H).
Involvement of PTK Signal Pathway in ISP-O.
Similar to the effect of SP on ASIC3-mediated current, the ISP-O was not altered after neurons were dialyzed with GDP-β-S for 10 min (Fig. 4A). To elucidate the downstream effectors of the NK1 receptor that mediated the ISP-O, we examined the participation of PTK by treating neurons with genistein. Genistein significantly reduced the ISP-O (Fig. 4B). Although genistein is a potent PTK inhibitor and is the most frequently used PTK inhibitor, its specificity has raised concerns, because both PTK-dependent and -independent effects have been found (32, 33). We verified the PTK dependency of ISP-O on GM DRG neurons by using an inactive structural analog of genistein, daidzein, which had no effect on ISP-O (Fig. 4C). The PTK-dependent effect involved Src tyrosine kinase, because 1-(1,1-Dimethylethyl)-1-(4-methylphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (PP1), an Src tyrosine kinase inhibitor, reduced the amplitude of ISP-O (Fig. 4 D and E). Moreover, if ISP-O were generated in a PTK-dependent manner, application of sodium orthovanadate, a protein phosphatase inhibitor, should enhance the magnitude of ISP-O. Indeed, the amplitude of ISP-O increased significantly when vanadate was added to the bath solution, compared with the normal response in a bath of artificial cerebrospinal fluid (ACSF) (Fig. 5A). Vanadate also partially reversed the inhibitory effect of genistein (Fig. 5B). These results demonstrate the participation of PTK in generating ISP-O (Fig. 5C).
Fig. 4.
Effect of PTK on ISP-O. (A) Effect of GDP-β-S dialysis on ISP-O. Representative traces of ISP-O at baseline (0–1 min) and after 10 min of GDP-β-S (1 mM in internal solution) dialysis in the same GM DRG neuron. The dialysis did not alter the peak amplitude of ISP-O significantly (n = 17). (B) Inhibition of ISP-O by PTK inhibitor (n = 13). Traces represent the ISP-O from the same neuron before and during superfusion with 30 μM genistein. (C) Effect of an inactive structural analog of genistein (30 μM daidzein) on ISP-O (n = 14). (D) Effect of the Src-selective antagonist on ISP-O (n = 12). Traces represent the ISP-O from the same neuron before and during superfusion with 20 μM PP1. (E) Normalized ISP-O currents under different treatments. Data represent mean ± SEM, **P < 0.01, during vs. before treatment. N.S., not significant.
Fig. 5.
Effect of protein phosphatase inhibitor on ISP-O. (A) Effect of 1 μM sodium orthovanadate on ISP-O (n = 12). (B) The inhibitory effect of genistein (Gen) on ISP-O was reversed in the presence of sodium orthovanadate (n = 12). Van, vanadate. (C) Normalized ISP-O currents. Peak amplitude of ISP-O obtained during the treatment normalized to the peak amplitude before treatment in the same neurons. Data are mean ± SEM; **P < 0.01 during vs. before treatment; ##P < 0.01 second vs. first treatment.
Involvement of M-Channel–Like Activity in SP-Mediated Antinociception.
We next probed the ion channel mediating the ISP-O. In the current-clamp mode, SP (3 μM) induced hyperpolarization in most GM DRG neurons (38 of 49), suggesting that potassium ions may be the major charge carriers (Fig. 6A). To test this hypothesis further, we measured the SP response in ACSF containing two different potassium concentrations in voltage-clamp mode. In neurons expressing ISP-O, the SP response was switched to a sustained inward current when the potassium concentration was increased from 5 to 10 mM (Fig. 6B and Fig. S6A). We thus tested whether the ISP-O in GM DRG neurons was mediated by the opening of potassium channels. The ISP-O was abolished with tetraethylammonium chloride (TEA-Cl) treatment and reappeared again when the bath solution was switched back to normal ACSF (Fig. 6C and Fig. S6B). We then tested the possibility of SP opening M channels in muscle DRG neurons. In 21 of 24 GM DRG neurons, a potent M-channel blocker, 10,10-bis(4-Pyridinylmethyl)-9(10H)-anthracenone dihydrochloride (XE991) significantly inhibited the ISP-O, suggesting that M channels might be the downstream effectors activated by NK1 receptor and PTK signaling (Fig. 6D and Fig. S6C). Another M-channel blocker, linopiridine, significantly inhibited ISP-O, further supporting the involvement of the M channel (Fig. 6E, and Fig. S6D). We next examined whether SP could modulate M-channel activity directly. In all GM DRG neurons expressing ISP-O (n = 15), SP showed increased M-channel activity (Fig. 6 F–H and Fig. S6E). We then tested the effect of blocking M-channel activity on acid-induced mechanical hyperalgesia in mice. Coinjection of acid with XE991 (200 μM) into the mouse GM produced bilateral hyperalgesia at 4 h, as seen on von Frey assay, that lasted for more than 1 wk (Fig. 6 I and J). Therefore, blocking M-channel activity in muscle-afferent DRG neurons produced a hyperalgesic effect on the acid-induced chronic muscle pain similar to the effect of NK1-selective antagonism.
Fig. 6.
Involvement of potassium-channel activity in ISP-O and pain behaviors. (A) SP (3 μM) induced hyperpolarization in most GM DRG neurons in current-clamp mode (n = 38 of 49). (B–E) Additional experiments to verify the participation of K+ channel in the formation of ISP-O; neurons were held at −70 mV. (B) Effect of altering extracellular potassium concentration on SP-mediated current (n = 15). (C) Effect of TEA on ISP-O. TEA-Cl (130 mM) replaced NaCl in the bath solution (n = 7). (D) Effect of the M-channel blocker XE991 (10 μM) on ISP-O (n = 21). (E) Effect of M-channel blocker linopiridine dihydrochloride (30 μM) on ISP-O (n = 10). (F) Voltage protocol used for recording M currents with and without SP. Neurons were held at −20 mV and were given a 1-s hyperpolarizing pulse to −50 mV. (G and H) Neurons were held at −70 mV to test the presence of ISP-O before recording M currents. (G) SP had no effect on M currents in GM DRG neurons without ISP-O (n = 7). (H) GM DRG neurons with ISP-O showed enhancement of M currents with 3 μM SP (n = 15). The enhanced effect of SP on M currents could be inhibited by 10 μM XE991 (n = 15). (I) Effect of coinjection of acid and XE991 on acid-induced mechanical hyperalgesia in the ipsilateral mouse hind paw (n = 6 in both groups). (J) Effect of the coinjection of acid and XE991 on acid-induced mechanical hyperalgesia in the contralateral hind paw (n = 6 in both groups). B, baseline; D, day. Data are mean ± SEM; **P < 0.01 vs. baseline. Fig. S6 shows the statistical analyses of B–E, G, and H.
Modulation of Neuronal Excitability in Acid-Induced Muscle Pain.
Because SP mediated the activation of M-channel activity, we further examined whether SP could alter the neural excitability in current-clamp mode in GM DRG neurons. In about half the GM DRG neurons (15/29), SP increased the action potential (AP) threshold by 6.0 ± 0.7 mV (Fig. 7A). In a small proportion of GM DRG neurons (6/29), SP also lowered AP threshold slightly, by 4.3 ± 0.4 mV. We next examined whether modulation of M-channel activity could alter neuronal excitability, similar to the effect of SP in the acid-induced muscle pain model. We compared the voltage-gated sodium currents (INav) in GM DRG neurons 2 d after i.m. injection of acid (pH 4.0 saline) or pH 7.4 saline with or without RP-67580 (100 μM) or XE991 (200 μM) (Fig. 7 B and C). All recordings involved fluorogold-positive small (20- to 30-μm diameter) and medium (30- to 40-μm diameter) GM DRG neurons. Medium-sized GM DRG neurons showed a significant alteration in voltage-gated sodium channel (Nav) excitability under different treatments. Compared with pH 7.4 saline injection, medium-sized GM DRG neurons showed significantly larger total INav and TTX-resistant INav after single (n = 23) or double (n = 29) injection of pH 4.0 saline (Fig. 7D). Compared with acid injection alone, medium-sized GM DRG neurons showed further enhanced total INav and TTX-resistant INav after coinjection of acid and RP-67580 (Fig. 7E). In contrast, acid injection had little effect on Nav excitability in small-sized GM DRG neurons. Compared with pH 7.4 saline injection, small neurons showed significantly enhanced TTX-resistant INav with pH 4.0 saline injection (Fig. S7A). The effect was not enhanced with coinjection of RP-67580 (Fig. S7B). Interestingly, coinjection of XE991 and acid produced a similar potentiation in terms of amplitude of INav and specificity of the neuronal cell population, indicating that inhibition of M-channel activity and the NK1 receptor in muscle afferents produced a similar modulation of neuronal excitability specific to medium-sized GM DRG neurons (Fig. 7E and Fig. S7B). To elucidate further the effect of M-channel modulation on neural excitability, we investigated the AP threshold of medium-sized GM DRG neurons in current-clamp mode. Compared with pH 7.4 saline preinjection, medium-sized GM DRG neurons showed significantly decreased AP thresholds with pH 4.0 saline preinjection, and preinjection of acid and RP-67580 or XE991 further decreased the AP threshold (Fig. 7F). Thus, SP may modulate the opening of M channels and consequently the neuronal excitability, especially of TTX-resistant sodium currents, in medium-sized GM DRG neurons.
Fig. 7.
Effect of SP signaling on INav and APs in the acid-induced muscle pain model. (A) Effect of SP (3 μM) on AP threshold in GM DRG neurons (n = 29). GM DRG neurons were injected with depolarizing currents of various magnitudes from resting membrane potential until an AP was formed. The AP threshold was determined by measuring the point of sharp upward rise of the AP. The delta AP threshold was calculated by subtracting the value obtained from each neuron with SP from the value without SP and was assigned to a group based on the relative change in AP threshold (higher, the same, or lower). (B) Representative current trace shows TTX-resistant INav peak amplitude. (C) Representative TTX-resistant INav traces in different experimental groups. (D) Effect of i.m. acid injections on INav in medium-sized GM DRG neurons. Two days before DRG isolation, mice were injected with 20 μL pH 7.4 saline (Saline), or with pH 4.0 saline (Acid), or with two injections of pH 4.0 saline 2 d apart (Acid×2). Neurons were recorded in ACSF to obtain total INav and then were recorded in ACSF containing TTX to obtain the TTXr INav. The value for TTX-sensitive (TTXs) INav was calculated by subtracting TTS-resistant (TTXr) INav from total INav. (E) INav in medium-sized GM DRG neurons isolated from mice that received coinjection of pH 4.0 saline and RP-67580 (100 μM) or XE991 (200 μM) 2 d before. (F) The AP threshold of GM DRG neurons from mice preinjected with pH 7.4 saline (n = 29), with pH 4.0 saline (n = 26), with pH 4.0 saline and RP-67580 (n = 26), or with pH 4.0 saline and XE991 (n = 26). Data are mean ± SEM; *P < 0.05 and **P < 0.01 vs. the saline group (D and F); #P < 0.05 and ##P < 0.01 vs. the acid group (E and F). RP, RP-67580; XE, XE991.
Discussion
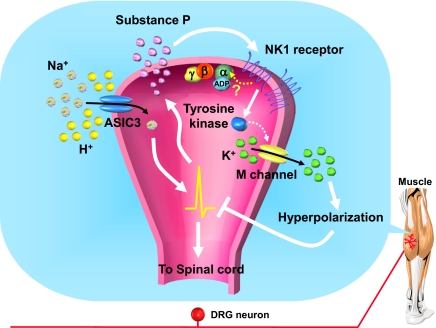
The role of SP in the transmission of cutaneous nociceptive stimuli has been well studied (34–36). Substances that inhibit SP signaling pathways generally show antinociceptive effects in animal models (37–40), although NK1 antagonists failed to show an effect in most clinical trials (41). Here we uncovered an unexpected antinociceptive role for SP involving an unconventional NK1 signal pathway in muscle-afferent DRG neurons. Intramuscular release of SP seems to have an important physiological role in nociceptive plasticity by limiting the acid-induced activation of muscle nociceptors leading to referred and mirror-image hyperalgesia to a transient effect (most probably no direct effect of local SP on central sensitization). This physiological mechanism may be therapeutically useful, because application of a selective NK1 agonist prevents long-lasting hyperalgesia after repeated acid-injection induction. The antinociceptive effect of SP seems to be mediated by ASIC3-expressing muscle-afferent neurons by attenuating the acid-induced depolarization. Our results suggest a model in which the activation of acid-sensitive muscle nociceptors triggers the local release of SP, which may act by an autocrine mechanism or specifically on ASIC3-expressing neurons, to attenuate the acid-induced depolarization by triggering M-channel–like activity. This effect involves an NK1 receptor- and a G protein-independent but PTK-dependent pathway (Fig. 8). This mechanism is not sufficient to prevent the hypersensitivity of muscle nociceptors after repeated acid stimulation.
Fig. 8.
A schematic model of SP-mediated inhibition of ASIC3 signaling. When tissue acidosis occurs in muscle, protons depolarize the muscle afferents by activating ASIC3 channels and fire APs, which can trigger the release of SP in the nerve terminals. SP acts on the NK1 receptor in the local nerve terminals. The NK1 receptors on muscle afferents are coupled with an unconventional signal pathway by activating tyrosine kinase and M channels to inhibit the ASIC3-mediated neural firing.
SP signaling in muscle-afferent neurons is unique in its inhibitory transduction via a G protein-independent pathway, in contrast to the usually excitatory effects of SP signaling in almost all neuronal cells, including neurons in the spinal dorsal horn, brainstem A7 cell group, hippocampus, ventral tegmental area, and DRG (5, 31, 42, 43). One exception is SP mediating an outward current in the vagal sensory neurons in ferrets (44). However, no other study has focused on this phenomenon. Therefore, whether the vagal sensory neurons have a biological effect similar to that of muscle-afferent neurons in terms of SP signaling is an unknown but an intriguing possibility. SP signaling through the NK1 receptor is mediated by various G protein–dependent second-messenger systems, including phospholipase C, phospholipase A2, and adenylate cyclase (45). Recently SP was found to open a cation channel complex, sodium leak channel nonselective (NALCN), which is responsible for background Na+ leak conductance, via the activation of Src family kinases without the aid of G proteins (31). The ISP generated by NALCN shares similarities with the ISP-O we identified. None of these SP-induced currents required the direct participation of G proteins, but both depended on PTK. That SP antinociception in muscle acts via an Src kinase is intriguing because Src kinases can be activated downstream of the neurotrophin receptor (46, 47). Given that i.m. administration of neurotrophin 3 reverses the acid-induced hyperalgesia, SP may work through or in parallel with neurotrophin receptors (24). However, neurotrophin (e.g., NGF) receptors also are implicated in mediating hyperalgesia in delayed-onset muscle soreness (48). Further studies should probe whether the antinociceptive effects of SP are blocked by neurotrophin receptor antagonists.
Another surprise of the study was that activation of an M-channel–like current contributed to ISP-O in muscle-afferent DRG neurons. Many GPCRs with G proteins Gq/G11 (including the NK1 receptor) are known to close M channels (Kv7) in neurons of both the peripheral and central nervous systems, although the underlying mechanisms still are not clear (49). The SP-mediated M-channel–like activation is intriguing, because inhibition of the M current in nociceptors may be one of the general mechanisms underlying pain produced by inflammatory mediators (50, 51).
Although an antinociceptive role for SP is not a new idea, this concept has been almost ignored for decades. In early studies, SP at low doses could release endorphins in the brain and thus mediate analgesia (52, 53). However, the SP analgesia occurred only when tested animals were hypersensitive to noxious stimuli (54). Therefore, the SP analgesia was interpreted as an action of normalizing responsiveness to pain. In contrast, we revealed SP antinociception for acid pain in peripheral sites of muscle nociceptors. Intriguingly, the effect of endogenous SP release failed during the second acid challenge. One possible explanation is that muscle nociceptors become more resistant to SP after i.m. acid challenge, perhaps because of the SP-mediated Src activation, which could interact with β-arrestins and affect GPCR trafficking and down-regulation, although the involvement of other proton-sensing GPCR signaling is possible also (55, 56). Further studies should probe how the first acid challenge alters SP–NK1 signaling in a delayed phase.
Increased SP levels often are associated with chronic muscle pain. Most patients with fibromyalgia show SP levels in cerebrospinal fluid above the highest normal control value (57). In patients with continuous idiopathic cervical pain, muscle tissues associated with myofascial trigger points show higher SP levels and lower pH values than do muscle tissues with latent myofascial trigger points in patients with no neck pain or normal controls (58). Likewise, increased SP immunopositive muscle-afferent neurons are found in rats with muscle inflammation (18, 59). However, the biological relevance of the increased SP levels has never been examined in detail. Here, we suggest that chronic muscle pain might elicit increased SP as a feedback control system counteracting the sensitization of muscle nociceptors and the acid-induced pain. Given the specific antinociceptive effect of SP on muscle nociceptors, NK1 receptor antagonists might worsen the muscle pain condition and thus compromise their clinical efficacy in treating pain (41, 60). Our finding offers insights for ongoing clinical trials testing NK1 antagonists in fibromyalgia patients (61). In particular, blocking SP–NK1 signaling might increase the risk of muscle-originated chronic hyperalgesia. In contrast, using SP as a peripheral analgesic might be conceivable, although SP (in the micromolar range) is equally potent as a spinal-sensitizing mediator in vitro (5, 62). Local application of SP could inhibit the excitability of muscle nociceptors effectively, and the local SP dosage would be largely diluted and have little effect on nociceptive transmission in the spinal cord.
Materials and Methods
Animals.
We used adult (8- to 12-wk-old) C57/BL6 mice. All procedures followed the Guide for the Use of Laboratory Animals (National Academies Press, Washington, DC) and were approved by the Institutional Animal Care and Use Committee of Academia Sinica. We aimed to minimize the number of animals used and their suffering without compromising the quality of the experiments. Tac1−/− mice (40) and Asic3−/− mice (63) were generated and genotyped as described. Both null-mutant mice were backcrossed to C57/BL6 mice for 10 generations to establish a congenic strain. Congenic Tac1−/−Asic3−/− mice were offspring of Tac1+/− and Asic3+/− intercrosses.
Behavioral Assays.
The mouse model of chronic mechanical hyperalgesia induced by repeated i.m. acid injection was modified from the Sluka et al. model (SI Material and Methods) (13).
Plasma Extravasation.
Extravasation levels were evaluated by quantifying Evans blue staining in tissues as described (SI Material and Methods) (64).
DRG Primary Culture.
DRG neurons of all lumbar segments were cultured on poly-l-lysine–coated cover slides as previously described (SI Material and Methods) (30, 65).
Whole-Cell Patch-Clamp Recording.
Details of whole-cell patch-clamp recording are given in SI Material and Methods.
Data Analysis.
Results are presented as mean ± SEM and were analyzed by use of Origin 8.0 (OriginLab). One-way ANOVA and then a Fisher least significant difference post hoc test were used to calculate differences between groups (Fig. 2). Other electrophysiological data were analyzed by paired or unpaired Student's t test as appropriate. The Mann–Whitney u test was used to compare withdrawal responses to von Frey filament in mice before acid injection (baseline) and at each time point after i.m. acid injection. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Drs. Ming-Yuan Min and Cheng-Chang Lien for constructive discussions and critical comments on the manuscript. This work was supported by the Institute of Biomedical Sciences, Academia Sinica, Taiwan, and by Grant NSC98-2320-B-001-019-MY3 from the National Science Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 363.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108903108/-/DCSupplemental.
References
- 1.Hökfelt T, Pernow B, Wahren J. Substance P: A pioneer amongst neuropeptides. J Intern Med. 2001;249:27–40. doi: 10.1046/j.0954-6820.2000.00773.x. [DOI] [PubMed] [Google Scholar]
- 2.Hökfelt T, Kellerth JO, Nilsson G, Pernow B. Substance P: Localization in the central nervous system and in some primary sensory neurons. Science. 1975;190:889–890. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- 3.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: Do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda H, Heinke B, Ruscheweyh R, Sandkühler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. 2003;299:1237–1240. doi: 10.1126/science.1080659. [DOI] [PubMed] [Google Scholar]
- 6.McMahon SB, Sykova E, Wall PD, Woolf CJ, Gibson SJ. Neurogenic extravasation and substance P levels are low in muscle as compared to skin the rat hindlimb. Neurosci Lett. 1984;52:235–240. doi: 10.1016/0304-3940(84)90167-8. [DOI] [PubMed] [Google Scholar]
- 7.Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain. 1993;54:241–289. doi: 10.1016/0304-3959(93)90027-M. [DOI] [PubMed] [Google Scholar]
- 8.Issberner U, Reeh PW, Steen KH. Pain due to tissue acidosis: A mechanism for inflammatory and ischemic myalgia? Neurosci Lett. 1996;208:191–194. doi: 10.1016/0304-3940(96)12576-3. [DOI] [PubMed] [Google Scholar]
- 9.Molliver DC, et al. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mense S. Muscle pain: Mechanisms and clinical significance. Dtsch Arztebl Int. 2008;105:214–219. doi: 10.3238/artzebl.2008.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 12.Birdsong WT, et al. Sensing muscle ischemia: Coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68:739–749. doi: 10.1016/j.neuron.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sluka KA, et al. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 14.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Sluka KA. Stimulation of deep somatic tissue with capsaicin produces long-lasting mechanical allodynia and heat hypoalgesia that depends on early activation of the cAMP pathway. J Neurosci. 2002;22:5687–5693. doi: 10.1523/JNEUROSCI.22-13-05687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schäfers M, Sorkin LS, Sommer C. Intramuscular injection of tumor necrosis factor-alpha induces muscle hyperalgesia in rats. Pain. 2003;104:579–588. doi: 10.1016/S0304-3959(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 17.Yen YT, et al. Role of acid-sensing ion channel 3 in sub-acute-phase inflammation. Mol Pain. 2009;5:1. doi: 10.1186/1744-8069-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambalavanar R, et al. Deep tissue inflammation upregulates neuropeptides and evokes nociceptive behaviors which are modulated by a neuropeptide antagonist. Pain. 2006;120:53–68. doi: 10.1016/j.pain.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. J Appl Physiol. 1998;84:1827–1833. doi: 10.1152/jappl.1998.84.6.1827. [DOI] [PubMed] [Google Scholar]
- 20.Taguchi T, Matsuda T, Tamura R, Sato J, Mizumura K. Muscular mechanical hyperalgesia revealed by behavioural pain test and c-Fos expression in the spinal dorsal horn after eccentric contraction in rats. J Physiol. 2005;564:259–268. doi: 10.1113/jphysiol.2004.079483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sluka KA, et al. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deval E, et al. Acid-sensing ion channels (ASICs): Pharmacology and implication in pain. Pharmacol Ther. 2010;128:549–558. doi: 10.1016/j.pharmthera.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Walder RY, et al. ASIC1 and ASIC3 play different roles in the development of Hyperalgesia after inflammatory muscle injury. J Pain. 2010;11:210–218. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandhi R, Ryals JM, Wright DE. Neurotrophin-3 reverses chronic mechanical hyperalgesia induced by intramuscular acid injection. J Neurosci. 2004;24:9405–9413. doi: 10.1523/JNEUROSCI.0899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen AN, Mathiesen C, Blackburn-Munro G. Pharmacological characterisation of acid-induced muscle allodynia in rats. Eur J Pharmacol. 2004;487:93–103. doi: 10.1016/j.ejphar.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama T, Maeda Y, Audette KM, Sluka KA. Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J Pain. 2007;8:422–429. doi: 10.1016/j.jpain.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Hoheisel U, Unger T, Mense S. Excitatory and modulatory effects of inflammatory cytokines and neurotrophins on mechanosensitive group IV muscle afferents in the rat. Pain. 2005;114:168–176. doi: 10.1016/j.pain.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Mense S. Algesic agents exciting muscle nociceptors. Exp Brain Res. 2009;196:89–100. doi: 10.1007/s00221-008-1674-4. [DOI] [PubMed] [Google Scholar]
- 29.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin YW, et al. Identification and characterization of a subset of mouse sensory neurons that express acid-sensing ion channel 3. Neuroscience. 2008;151:544–557. doi: 10.1016/j.neuroscience.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Lu B, et al. Peptide neurotransmitters activate a cation channel complex of NALCN and UNC-80. Nature. 2009;457:741–744. doi: 10.1038/nature07579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia Z, et al. Genistein inhibits voltage-gated sodium currents in SCG neurons through protein tyrosine kinase-dependent and kinase-independent mechanisms. Pflugers Arch. 2008;456:857–866. doi: 10.1007/s00424-008-0444-2. [DOI] [PubMed] [Google Scholar]
- 33.Belevych AE, Warrier S, Harvey RD. Genistein inhibits cardiac L-type Ca(2+) channel activity by a tyrosine kinase-independent mechanism. Mol Pharmacol. 2002;62:554–565. doi: 10.1124/mol.62.3.554. [DOI] [PubMed] [Google Scholar]
- 34.Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360–364. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- 35.Park TJ, et al. Selective inflammatory pain insensitivity in the African naked mole-rat (Heterocephalus glaber) PLoS Biol. 2008;6:e13. doi: 10.1371/journal.pbio.0060013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brand A, Smith ES, Lewin GR, Park TJ. Functional neurokinin and NMDA receptor activity in an animal naturally lacking substance P: The naked mole-rat. PLoS ONE. 2010;5:e15162. doi: 10.1371/journal.pone.0015162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantyh PW, et al. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 38.Cao YQ, et al. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- 39.De Felipe C, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- 40.Zimmer A, et al. Hypoalgesia in mice with a targeted deletion of the tachykinin 1 gene. Proc Natl Acad Sci USA. 1998;95:2630–2635. doi: 10.1073/pnas.95.5.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill R. NK1 (substance P) receptor antagonists—why are they not analgesic in humans? Trends Pharmacol Sci. 2000;21:244–246. doi: 10.1016/s0165-6147(00)01502-9. [DOI] [PubMed] [Google Scholar]
- 42.Min MY, et al. Physiological and morphological properties of, and effect of substance P on, neurons in the A7 catecholamine cell group in rats. Neuroscience. 2008;153:1020–1033. doi: 10.1016/j.neuroscience.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Sculptoreanu A, de Groat WC. Neurokinins enhance excitability in capsaicin-responsive DRG neurons. Exp Neurol. 2007;205:92–100. doi: 10.1016/j.expneurol.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jafri MS, Weinreich D. Substance P hyperpolarizes vagal sensory neurones of the ferret. J Physiol. 1996;493:157–166. doi: 10.1113/jphysiol.1996.sp021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quartara L, Maggi CA. The tachykinin NK1 receptor. Part I: Ligands and mechanisms of cellular activation. Neuropeptides. 1997;31:537–563. doi: 10.1016/s0143-4179(97)90001-9. [DOI] [PubMed] [Google Scholar]
- 46.Ahn M, Beacham D, Westenbroek RE, Scheuer T, Catterall WA. Regulation of Na(v)1.2 channels by brain-derived neurotrophic factor, TrkB, and associated Fyn kinase. J Neurosci. 2007;27:11533–11542. doi: 10.1523/JNEUROSCI.5005-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwasaki Y, Gay B, Wada K, Koizumi S. Association of the Src family tyrosine kinase Fyn with TrkB. J Neurochem. 1998;71:106–111. doi: 10.1046/j.1471-4159.1998.71010106.x. [DOI] [PubMed] [Google Scholar]
- 48.Murase S, et al. Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise (delayed-onset muscle soreness) J Neurosci. 2010;30:3752–3761. doi: 10.1523/JNEUROSCI.3803-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linley JE, et al. Inhibition of M current in sensory neurons by exogenous proteases: A signaling pathway mediating inflammatory nociception. J Neurosci. 2008;28:11240–11249. doi: 10.1523/JNEUROSCI.2297-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu B, et al. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl- channels. J Clin Invest. 2010;120:1240–1252. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart JM, et al. Substance P and analgesia. Nature. 1976;262:784–785. doi: 10.1038/262784a0. [DOI] [PubMed] [Google Scholar]
- 53.Frederickson RC, Burgis V, Harrell CE, Edwards JD. Dual actions of substance P on nociception: Possible role of endogenous opioids. Science. 1978;199:1359–1362. doi: 10.1126/science.204012. [DOI] [PubMed] [Google Scholar]
- 54.Oehme P, Hilse H, Morgenstern E, Göres E. Substance P: Does it produce analgesia or hyperalgesia? Science. 1980;208:305–307. doi: 10.1126/science.6154313. [DOI] [PubMed] [Google Scholar]
- 55.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 56.Chen YJ, Huang CW, Lin CS, Chang WH, Sun WH. Expression and function of proton-sensing G-protein-coupled receptors in inflammatory pain. Mol Pain. 2009;5:39. doi: 10.1186/1744-8069-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russell IJ. Neurochemical pathogenesis of fibromyalgia. Z Rheumatol. 1998;57(Suppl 2):63–66. doi: 10.1007/s003930050238. [DOI] [PubMed] [Google Scholar]
- 58.Shah JP, Phillips TM, Danoff JV, Gerber LH. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol. 2005;99:1977–1984. doi: 10.1152/japplphysiol.00419.2005. [DOI] [PubMed] [Google Scholar]
- 59.Reinert A, Kaske A, Mense S. Inflammation-induced increase in the density of neuropeptide-immunoreactive nerve endings in rat skeletal muscle. Exp Brain Res. 1998;121:174–180. doi: 10.1007/s002210050449. [DOI] [PubMed] [Google Scholar]
- 60.Urban LA, Fox AJ. NK1 receptor antagonists—are they really without effect in the pain clinic? Trends Pharmacol Sci. 2000;21:462–464, author reply 465. doi: 10.1016/s0165-6147(00)01578-9. [DOI] [PubMed] [Google Scholar]
- 61.Rao SG. Current progress in the pharmacological therapy of fibromyalgia. Expert Opin Investig Drugs. 2009;18:1479–1493. doi: 10.1517/13543780903203771. [DOI] [PubMed] [Google Scholar]
- 62.Murase K, Randić M. Actions of substance P on rat spinal dorsal horn neurones. J Physiol. 1984;346:203–217. doi: 10.1113/jphysiol.1984.sp015017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen CC, et al. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci USA. 2002;99:8992–8997. doi: 10.1073/pnas.122245999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamer PW, McGeachie JM, Davies MJ, Grounds MD. Evans Blue Dye as an in vivo marker of myofibre damage: Optimising parameters for detecting initial myofibre membrane permeability. J Anat. 2002;200:69–79. doi: 10.1046/j.0021-8782.2001.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng CM, et al. Probing localized neural mechanotransduction through surface-modified elastomeric matrices and electrophysiology. Nat Protoc. 2010;5:714–724. doi: 10.1038/nprot.2010.15. [DOI] [PubMed] [Google Scholar]