Abstract
Like charges stabilize emulsions, whereas opposite charges break emulsions. This is the fundamental principle for many industrial and practical processes. Using micrometer-sized pH-sensitive polymeric hydrogel particles as emulsion stabilizers, we prepare emulsions that consist of oppositely charged droplets, which do not coalesce. We observe noncoalescence of oppositely charged droplets in bulk emulsification as well as in microfluidic devices, where oppositely charged droplets are forced to collide within channel junctions. The results demonstrate that electrostatic interactions between droplets do not determine their stability and reveal the unique pH-dependent properties of emulsions stabilized by soft microgel particles. The noncoalescence can be switched to coalescence by neutralizing the microgels, and the emulsion can be broken on demand. This unusual feature of the microgel-stabilized emulsions offers fascinating opportunities for future applications of these systems.
Keywords: stimuli-sensitive emulsions, limited coalescence, poly (N-isopropylacrylamide) microgels, Pickering emulsions, lab-on-a-chip
Emulsions are multiphase systems, which consist of two immiscible liquids, one dispersed in another in the form of small droplets with submicron to micron sizes. Emulsions are used in food, cosmetics, medicine, coatings, paints, and a large number of other technical applications (1). Due to their large interfacial area, emulsions are thermodynamically unstable and have a tendency to undergo coalescence, a process during which two droplets merge into a bigger one, minimizing the surface energy (2). Certain molecules or materials, such as surfactants or solid particles, can be added to prevent coalescence, making emulsions kinetically stable. These emulsion stabilizers usually provide electrostatic repulsion, steric repulsion, and/or strength to the interfacial layer of the droplets, according to widely accepted theories. For example, when emulsion droplets are covered with an ionic surfactant, they carry like charges; these charges repel each other due to electrostatic interaction, which prevents droplet aggregation or coalescence. By contrast, when oppositely charged droplets are generated in an emulsion, they attract each other and coalescence is hastened; this phenomenon is the key event in processes like electrocoalescence (3, 4).
In contrast to this classical behavior, unexpected noncoalescence of two oppositely charged droplets has been reported very recently (5, 6); these investigations show that under the influence of an electric field of sufficient strength, two oppositely charged water droplets recoil after contact rather than coalesce, depending on the curvature and local capillary pressure at the liquid bridge between two adjacent droplets. However, these studies were conducted with drops that were formed temporarily under the influence of an electric field in a continuous phase of air rather than with drops that are dispersed in a liquid. By contrast, stable emulsions, which consist of oppositely charged droplets dispersed in a fluid have not been reported to date.
In this paper, we show that it is possible to prepare stable emulsions that contain oppositely charged droplets, using pH-sensitive polymer microgels as interfacial stabilizers. We directly image the noncoalescence of these oppositely charged droplets in a microfluidic device (7–9). In our study, the charges of the droplets originate from the chemical composition of the stabilizing microgel particles that sit on the droplet surface (10). The stabilization of emulsions through the use of these microgels is at first glance very similar to Pickering emulsions (11–14), which are solid-stabilized emulsions discovered a century ago (15, 16). However, in contrast to solid particles (17–22), microgels are highly deformable, and the unexpected noncoalescence of the oppositely charged droplets in such systems might be due to the soft and porous structure of microgels at the interface. More interestingly, by changing the pH, the noncoalescence can be switched to coalescence. The resultant pH-triggered coalescence holds promises for the invention of new “smart” materials and process enhancement in many emulsion-related applications (23–25).
Results
Bulk Emulsion Mixing.
We obtain stable emulsions with oppositely charged droplets in two steps: First, we prepare an emulsion with negatively charged droplets and a second emulsion with positively charged droplets. Then we combine these two emulsions. The charges of the droplets are introduced by pH-sensitive microgels that are used as emulsion stabilizers. Microgels are nano- to micrometer-sized porous polymer particles, which consist of a cross-linked polymer network that is swollen with solvent. pH and temperature-sensitive microgels are able to change their size by swelling or shrinking in response to changes in their environment, and they provide a new way to stabilize emulsions, which has been studied only in recent years (26–39).
We prepare the first emulsion, MAA-emulsion, with Sudan-Yellow-dyed heptane as the organic dispersed phase and microgel dispersion M1, which consists of poly(N-isopropylacrylamide-co-methacrylic acid) (in the following termed MAA microgels) in water at pH 7, as the aqueous continuous phase. Similarly, we prepare the second emulsion, AEM-emulsion, with Sudan-Blue-dyed heptane and microgel dispersion M2, which consists of poly(N-isopropylacrylamide-co-2-aminoethyl methacrylate) (termed AEM microgels) in water at the same pH. The AEM microgels are covalently labeled with a fluorescent rhodamine dye to distinguish them from the MAA microgels, which are not labeled.
We use two different emulsification procedures: First, we explore the method of limited coalescence (39, 40). We use 0.05 wt.% of MAA microgel and 0.04 wt.% of AEM microgel to prepare two bulk emulsions using an Ultra-Turrax high-speed homogenizer. The homogenizer creates oil drops that are not stable but coalesce; this produces larger, stable drops fully covered by the microgels. The important advantage of this limited coalescence procedure is that there are no free excess microgels in the aqueous phase. However, the process is time-consuming and leads to large drops, and thus this technique is not of high practical relevance. Therefore, we also prepare emulsions with the high-speed homogenizer at higher microgel concentration, which directly leads to stable droplets but with excess microgels in the aqueous phase.
Very stable oil-in-water (O/W) emulsions can be generated in both cases, and the oil phase inside the droplets shows yellow and blue color, respectively. The droplets in MAA-emulsion are negatively charged, whereas the droplets of AEM-emulsion are positively charged. This is because at pH 7, the MAA microgels are negatively charged due to partial dissociation of H+ from the methacrylic acid (MAA) units, whereas the AEM microgels are positively charged due to partial association of H+ to the amine-containing (AEM) units. The sign of the charges of the emulsion droplets is confirmed by electrophoretic mobility measurements (Tables S1 and S2).
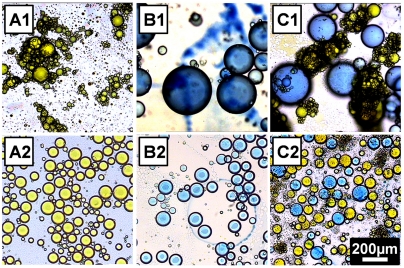
Fig. 1 shows the stable emulsions obtained by both procedures. The pictures in the top panel (Fig. 1 A1 and B1) show emulsions obtained via limited coalescence. The AEM droplets appear to be larger than the MAA droplets, possibly because of the different microgel size.
Fig. 1.
Optical micrographs of microgel-stabilized heptane-in-water (O/W) emulsions. (Top) Emulsions prepared via limited coalescence: (A1) MAA-emulsion made from Sudan-Yellow-dyed-heptane and a 0.05 wt.% microgel dispersion. (B1) AEM-emulsion made from Sudan-Blue-dyed-heptane and a 0.04 wt.% microgel dispersion. (C1) Mixed emulsion made from mixing equal volumes of the MAA- and the AEM- emulsions. (Bottom) Emulsions prepared with excess microgels: (A2) MAA-emulsion made from Sudan-Yellow-dyed-heptane and a 1 wt.% microgel dispersion. (B2) AEM-emulsion made from Sudan-Blue-dyed-heptane and a 1 wt.% microgel dispersion. (C2) Mixed emulsion made from mixing equal volumes of the MAA- and the AEM-emulsions. The scale bar applies to all six images.
Mixed emulsions with oppositely charged droplets are obtained by combining equal volumes of MAA-emulsion and AEM-emulsion. The oppositely charged droplets in these mixed emulsions do not coalesce, as shown in Fig. 1 C1 and C2. The mixed emulsions are stable at least for several weeks.
It is obvious that the drops prepared via the limited coalescence carry opposite charges, and the fact that they do not coalescence clearly demonstrates the counterintuitive behavior of these microgel-stabilized emulsions.
The emulsions prepared with the homogenizer at higher microgel concentration contain excess microgels in the continuous water phase, which could matter when the emulsions prepared with oppositely charged microgels are mixed. Immediate heterocoagulation occurs when the plain microgel dispersions M1 and M2 are mixed, as it is expected for oppositely charged colloidal particles (41–43). Similarly, excess free microgels in the continuous phase of the emulsions also flocculate when these two emulsions are mixed; however, the stability of the mixed emulsion droplets is not reduced. Moreover, the micrograph C2 in Fig. 1 indicates that there is no tendency for the oppositely charged droplets to form aggregates.
To check if microgels dispersed in the aqueous phase adsorb to emulsions droplets stabilized by oppositely charged microgels, we investigate the electrophoresis of mixtures of the MAA-emulsion with M2 and of the AEM-emulsion with M1: At pH 7, charge reversal is observed for both emulsions after adding the other type of microgels, changing from negative to positive electrophoretic mobility for the MAA-stabilized emulsion and from positive to negative for the AEM-stabilized emulsion (Tables S1 and S2). Obviously, the electrostatic attraction is sufficiently strong to induce flocculation of oppositely charged microgels as well as adsorption of charged microgels onto oppositely charged emulsion droplets. The aspect of heteroaggregation has been studied in more detail, and these experiments are described in SI Text.
To this end we can conclude that the different bulk mixing experiments reveal that microgels allow preparing emulsions with droplets that carry opposite charges, and that these droplets do not coalesce upon mixing.
Microfluidic Mixing.
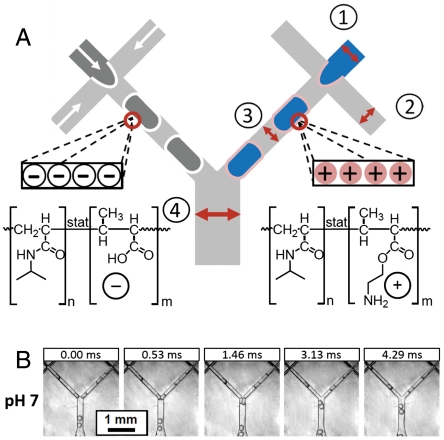
The coexistence of oppositely charged droplets in an emulsion implies that these droplets interact in a way that is different from classical expectations. However, it is rather difficult to observe individual droplet interactions in bulk mixing experiments by optical means. To allow for such detailed investigations, we apply a method that allows us to form, handle, and observe individual droplets and individual mixing events with high precision: droplet-based microfluidics (7–9). We use a microfluidic device to prepare O/W droplets stabilized with either the MAA or the AEM microgels and mix the two types of droplets immediately after their formation. For this purpose, we produce these two different kinds of droplets in two separate, drop-forming cross-junction channels. A few hundred micrometers downstream, these two channels merge at a mixing junction, squeezing two independent, freshly formed droplets against each other, as shown in Fig. 2A. A high-speed camera enables the observation of this process. Despite an earlier report on the microfluidic generation of alternating pairs of oppositely charged polyelectrolyte droplets (44), direct interaction of pairs of oppositely charged emulsion droplets has not been observed before.
Fig. 2.
Microfluidic device used to study the interaction of microgel-stabilized emulsion droplets. (A) Two drop-forming cross-junctions and one mixing junction are used to produce and mix different types of emulsion droplets. The left cross-junction is used to form colorless heptane droplets stabilized by nonlabeled, negatively charged MAA microgels. The right cross-junction is used to form Sudan-Blue-dyed heptane droplets stabilized by rhodamine-labeled, positively charged AEM microgels. The dimensions of the channels are: (1) 50 μm; (2) 50 μm; (3) 100 μm; (4) 200 μm. All channels have a uniform height of 100 μm. Negative charges are generated by the deprotonation of the carboxylic groups of the MAA microgels at intermediate and high pH; positive charges are generated by the protonation of the amine groups of the AEM microgels at intermediate and low pH. (B) Interaction of oppositely charged droplet at pH 7. The scale bar denotes 1 mm and applies to all micrographs.
Again, we distinguish the two different droplet species that are formed in the microfluidic experiment by the presence or absence of fluorescent labels. In the left cross-junction of the microfluidic device, we inject colorless heptane as the inner phase and a 0.5 wt.% MAA microgel dispersion in water as the outer phase to form negatively charged droplets. In the right cross-junction, we inject Sudan-Blue-dyed heptane as the inner phase and 0.5 wt.% AEM microgel dispersion as outer phase to form positively charged droplets. The pH of the outer phase is 7 in both cases, the same as in the bulk mixing experiment, rendering both microgels charged. A series of micrographs taken from a high-speed video that documents the mixing process (Movie S1) exemplifies that the two types of droplets do not coalesce at the mixing junction, even though they are strongly pushed against each other, causing strong deformation of both droplets, as shown in Fig. 2B. Upon close observation, there might even be a repulsive tendency between the two types of droplets, judging by the observation that the droplets bounce off each other and separate again as they flow downstream in the channel. As a result of the noncoalescence, the emulsion droplets formed in this experiment can be collected and remain stable at least for several days.
pH-Dependent Droplet Coalescence.
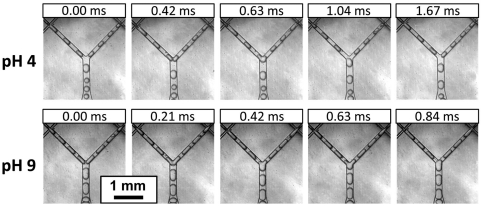
Now that we can prepare stable, heterogeneous emulsions with pH-sensitive microgels, we investigate how these emulsions behave in response to a pH change. Therefore we study the mixing of MAA microgel-stabilized and AEM microgel-stabilized droplets using the same microfluidic procedure but at a lower pH (pH = 4) and at a higher pH (pH = 9), respectively (see Fig. 3 and Movies S2 and S3). When two droplets come together at these pH conditions, the interfaces show little resilience to deformation, and coalescence takes place immediately upon contact of the two droplets. At pH 4, all droplet pairs merge; at pH 9, similarly, nearly all droplets merge. This pH sensitivity is opposite to an emulsion stabilized by rigid pH-sensitive particles, which is stable when the stabilizers are uncharged and destabilizes when they are charged (17–22).
Fig. 3.
pH-dependent interaction of oppositely charged, microgel-stabilized emulsion droplets. (Top) Coalescence of noncharged (left) and positively charged (right) droplets at pH 4. (Bottom) Coalescence of negatively charged (left) and noncharged (right) droplets at pH 9. Scale bar, 1 mm.
To understand the pH influence on the mixed emulsions, the intrinsic stability of each of the droplet species has to be considered: At pH 4 or pH 9, one of the microgel species will be uncharged and the emulsion formed with these uncharged microgels becomes intrinsically unstable. At pH 4, the MAA microgels become neutral, but the AEM microgels remain positively charged; at pH 9, the AEM microgels turn neutral, and the MAA microgels remain negatively charged. Neutralized microgels also adsorb to the oil–water interface, but in contrast to charged microgels, they form densely packed, brittle interfacial layers, which lead to a less stable emulsion as it has been shown previously (36–38). For this reason, emulsions prepared with the MAA microgels are highly stable at high pH but are unstable at pH < 7. Similarly, emulsions prepared with the AEM microgels are stable at low pH and unstable at pH > 7. With bulk emulsification, no stable emulsion can be formed with neutralized microgels, because droplets generated by the homogenizer quickly aggregate irreversibly and coalesce, and phase separation follows. By contrast, droplets with neutralized microgels can be formed in the microfluidic channels because these droplets are produced in a sequential manner and are spaced out by the continuous phase as they flow downstream in the channel. These particular experimental conditions prevent direct droplet contact, such that the droplets do not coalesce; however, their interface is vulnerable. Therefore, when subjected to sufficient mechanical forces, their weak interfaces coalesce, while interfaces stabilized by charged microgels remain intact under the same conditions. Moreover, immediate droplet coalescence takes place if two droplets meet and if at least one of these two droplets has a vulnerable interface. This finding indicates that the noncoalescence in the emulsion prepared at pH 7 can be converted into coalescence by the change of pH, a feature that is valuable for processes in which the control of the stabilizing or breaking of the emulsion is important.
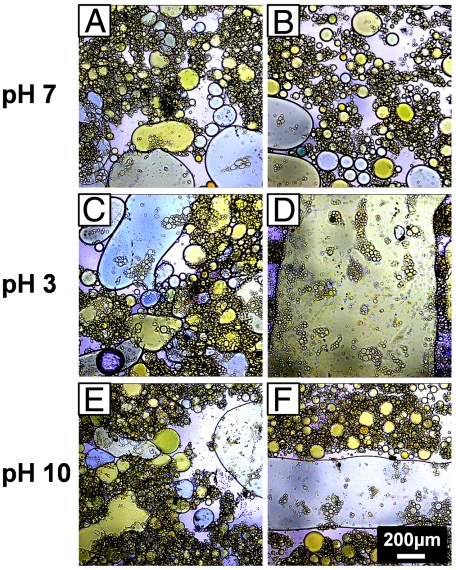
We also investigate the stability of mixed emulsions prepared via the limited coalescence procedure at different pH in a bulk experiment. Shear flow experiments are performed with the mixed emulsion at pH 3, pH 7, and pH 10, and the emulsions are observed with an optical microscope. For each sample, at each pH, a series of large amplitude oscillatory shear deformation is applied at different strains and frequencies (see SI Text for details). The stability of the emulsion is indicated by the strain and frequency at which the sample undergoes phase separation. At pH 7, the mixed emulsion remains stable even at a strain of 1,200 and a frequency of 9 Hz (Fig. 4 A and B). Conversely, at pH 3, phase separation occurs already at strain 400 and frequency 1 Hz (Fig. 4 C and D). The absence of both yellow and blue droplets after shear shows that both the MAA- and AEM-emulsion droplets coalesce. At pH 10, phase separation is observed at strain = 1,200 and frequency = 9 Hz (Fig. 4E and F). Evidently, the emulsion is less stable at pH 10 as compared to pH 7. Coalescence between MAA droplets and AEM droplets is observed similar to pH 3, (see also Fig. S1E). However, macroscopic phase separation appears to involve mainly the AEM droplets (blue), as several MAA-emulsion droplets (yellow) remain stable after shear (Fig. 4F). The difference between the stability of the MAA droplets and the AEM droplets when they are not charged might be due to the different microgel sizes and thus the different droplet sizes obtained during the limited coalescence process.
Fig. 4.
Optical micrographs of the mixed MAA- and AEM-emulsions at pH 7, pH 3, and pH 10 before (Left) and after (Right) shear forces are applied (A) at pH 7 before shear; (B) at pH 7 after shear (strain 1200; frequency 9 Hz; 1 min); (C) at pH 3 before shear; (D) at pH 3 after shear (strain 400; frequency 1 Hz 1 min); (E) at pH 10 before shear; (F) at pH 10 after shear (strain 1,200; frequency 9 Hz 1 min). The scale bar applies to all images. The MAA- and AEM-emulsions were prepared by limited coalescence. The Sudan-Yellow-dyed MAA-droplets are in bright yellow; the Sudan-Blue-dyed AEM-droplets are in bright blue.
Discussion
In contrast to accepted concepts, stable emulsions that consist of oppositely charged droplets can be obtained by using pH-sensitive poly(N-isopropylacrylamide-co-methacrylic acid) (MAA microgels) and poly(N-isopropylacrylamide-co-2-aminoethyl methacrylate) (AEM microgels) as stabilizers. Direct visualization of individual mixing events of oppositely charged droplets is possible in microfluidic devices, and noncoalescence of these droplets is observed. Bulk and microfluidic mixing experiments provide consistent information about the droplet stability, which is important to notice as different time scales are involved in microfluidic and bulk experiments, respectively.
The findings show that Coulomb attraction of two oppositely charged droplets cannot make them coalesce when the interface is packed with charged, swollen, elastic microgels. Even the presence of excess microgels in the continuous phase does not influence the stability and noncoalescence of the oppositely charged droplets. Thus, simple bulk mixing processes such as those employed in standard industrial routines can be used to prepare stable emulsions with oppositely charged droplets.
The fact that oppositely charged droplets do not coalesce is counterintuitive. The attractive Coulomb interaction between the oppositely charged droplets could be ineffective, given that the Debye length (ca. 5 nm) is much shorter than the size of the microgels (300–800 nm) and the size of the droplets (> 10 μm). In an additional bulk experiment, we lower the ionic strength in the aqueous phases by treating them with ion-exchange resins prior to the emulsification and the mixing; however, the noncoalescence effect remains. Apparently, electrostatic interactions between droplets do not determine the droplet stability.
On the other hand, oppositely charged microgels dissolved in the aqueous phase do flocculate. The aggregation of microgels in bulk is driven by the release of counterions, when the chains of the microgels partially interpenetrate (similar to the formation of polyelectrolyte complexes). Apparently this does not occur between microgels adsorbed at the oil–water interface of emulsion droplets. Microgels adsorbed to the oil–water interface are hardly mobile. We assess this by perfoming a fluorescence photobleaching experiment with the mixed emulsion: After preparing a mixed emulsion with the microfluidic device at pH 7, we bleach an area on the surface of one droplet by high intensity laser irradiation. Subsequently, the sample is observed for 10 min. The confocal images (Movie S4 and Fig. S1B) reveal no fluorescence recovery. This observation implies that on this time scale the microgels do not diffuse within the layer and that there is no exchange with microgels in the bulk phase; thus, microgels do not desorb. In addition, it has been shown previously by means of cryogenic field emission scanning electron microscopy (Cryo-FESEM) that microgels are strongly deformed at the interface (34, 37).
The ability of positively or negatively charged rigid polymer particles to stabilize emulsions or foams has been demonstrated by a number of groups (17–22). Their results show that rigid particles behave very differently from the soft microgels studied in this paper: pH-sensitive rigid particles are only stable in the aqueous bulk phase at a pH when they are charged. However, the fully charged state prevents strong adsorption to the oil–water interface and thus fully charged particles cannot serve as emulsion stabilizers. By contrast, soft microgels are lyophilic colloids and are colloidally stable in the charged and uncharged states, and thus they are stable in the aqueous bulk phase in the entire pH range. In addition, the microgels exhibit a special property that they adsorb to the oil–water interface independent of pH (36).
There is no study in the literature on the mixing of oppositely charged droplets that are stabilized by common Pickering stabilizers. To compare the behavior of deformable microgels to that of rigid particles as emulsions stabilizers, we explore a bulk mixing experiment with carboxyl and amine latex particles at pH 7. While the amine latex can stabilize the heptane-water emulsion, the carboxyl particle cannot. Hence, we were not able to prepare emulsions with common Pickering stabilizers that have the same pH-dependent features as the microgel-stabilized emulsions.
Systems with oppositely charged species at the interface have been reported before; we can now compare these interfaces to those in MAA and AEM microgel-stabilized emulsions. For example, it is known in detergent systems that mixing anionic and cationic surfactants has a synergistic effect, because these oppositely charged surfactants can complex and pack more efficiently at the air–water interface (45). It has also been reported recently that preformed heteroaggregates from oppositely charged silica particles can stabilize O/W emulsions, thanks to the reduced hydrophilicity by heteroaggregation (46). Furthermore, adsorption of oppositely charged polyelectrolytes on oil–water interface has been used to prepared micro- and nanocontainers (47). However, in all these studies, the interfacial structure of each foam bubble or each droplet is identical, whereas in the mixed microgel-stabilized emulsions, two droplet species with opposite charges coexist.
Microgel-stabilized emulsions are more complex as compared to Pickering emulsions stabilized rigid particle due to the deformability of the soft and porous microgels. Accurate theoretical descriptions of microgel-stabilized emulsions, especially considerations of the driving forces that lead to deformation of the microgels, the viscoelasticity and the particular packing structures at the interface, are challenging. The special behaviors of microgel-stabilized emulsions make them very interesting materials for fundamental and applied studies. One can, for example, encapsulate different substances for a reaction or ingredients of a formulation in separated compartments within one stable emulsion and store them together for extended period of time (at least several months) without the risk of getting in contact with each other prematurely. However, because the noncoalescence can be switched to coalescence, leading to breakage of the emulsion by neutralizing these microgels, these components can be mixed on demand. In addition, model systems with tailored spatial distribution of charged moieties can be designed that enable further fundamental studies on the behavior of soft, charged particles at oil–water interfaces. We hope that the observations reported in this paper will stimulate both further research to the fundamental understanding and the exploration of interesting applications of such emulsions.
Materials and Methods
Materials.
N-isopropylacrylamide (NiPAM, > 99.0%, Acros Chemicals), methacrylic acid (MAA, > 99.0%, ABCR-GmbH), 2-aminoethyl methacrylate hydrochloride (AEM, Polysciences), N,N′- methylenebisacrylamide (BIS, > 99.5%), potassium peroxodisulfate (KPS, 99.0%, Merck), n-heptane (> 99%, Merck), Sudan Blue (powder, TCI America), Sudan Yellow (Sudan I, 1-phenylazo-2-naphthol, TCI) and the fluorescent label methacryloxyethylthiocarbamoylrhodamine B (MRB, Polysciences) were used as received. Bidistilled Milli-Q water was used for both the synthesis and the characterization of the microgels and the bulk preparations of the emulsions. Milli-Q water was used for emulsion preparations in microfluidic channels. The MAA and the AEM microgels were synthesized by surfactant-free precipitation polymerization, using a protocol reported before (30, 36, 48). The dye MRB was added during the synthesis of the positively charged AEM microgels.
Characterization of the MAA and the AEM microgels.
The hydrodynamic radius (Rh), diffusion coefficient (D), the electrophoretic mobility (μe), and the amount of charge of the MAA and the AEM microgels are listed in Table S3. The diffusion coefficient (D) and hydrodynamic radius (Rh) of the microgels were determined by dynamic light scattering (DLS) conducted with an ALV-5000 Instrument with light of wavelength of 632.8 nm. Electrophoretic mobility measurements were performed with a NANO ZS zetasizer (Malvern Instruments). The amount of charge was determined by acid-base titration.
Preparation and observation of emulsions made by UltraTurrax.
Oil and aqueous microgel dispersions at pH 7 were added together at the desired ratio in a sample glass. The total volume of the mixture was 10 mL. A fixed water/oil ratio of 7∶3 was used. The oil was used as received or dyed with Sudan Blue or Sudan Yellow. Additional emulsion samples were prepared with a mixture of heptane and dichloromethane as organic phase that leads to density-matched droplets. The mixing was done with an IKA UltraTurrax T-25 with a 10 mm head at a speed of around 8,000 rpm for 2 min. The optical and fluorescence micrographs of the emulsions were taken with a Leica microscope equipped with a camera. For the shear experiments on the emulsions, a Linkam Optical Shearing system (CSS 450) was employed in addition to the optical microscope. The oscillation mode was used.
Preparation of PDMS microfluidic devices.
A photomask with a schematic of the microfluidic channels was drawn in AutoCAD and printed on transparency plastic in UV-absorbent ink. The microchannel structure was then developed onto a 3″ silicon wafer using photolithography and replicated using soft lithography in PDMS (Sylgard 184 Silicone Elastomer Kit, Dow Corning, base : crosslinker = 10∶1) (49). The PDMS replica was bonded to a glass slide after oxygen plasma treatment in a PlasmaPrep2 (GALA Instrument). To enable the use of organic solvents, the PDMS device was coated with a durable glass-like layer using sol-gel chemistry. The sol-gel is intrinsically hydrophobic but can be made hydrophilic. To accomplish this, we modified the inner channel walls by grafting poly(acrylic acid); this was achieved by incorporating methacrylate-silanes into the initial sol-gel coating which enabled hydrophilic poly(acrylic acid) to be grafted onto the sol-gel (50, 51). An alternative means to achieve solvent-resistance of the channels is to coat them with parylene-c (52, 53). This coating is very hydrophobic by default, but can be rendered hydrophilic by extensive plasma oxidation.
Emulsification in the microfluidic device and microscopy observation.
The channels on the PDMS device were connected to PE tubing (PE/2 micro medical tubing, I.D. = 0.015″, O.D. = 0.043″, Scientific Commodities Inc.) through holes that were punched into the PDMS elastomer. The tubing was then connected to syringes with the liquid of interest. The flow rate for different phases was controlled individually by syringe pumps (Harvard Apparatus PHD 2000 series). The device was placed on a microscope stage. The emulsification was performed on a Leica DM IRB microscope equipped with a Phantom V9 fast camera (Vision Research).
Before the differently prepared droplets come together in the microfluidic device, they are generated at the drop-forming cross-junctions and flow downstream for 1.7 × 103 μm. The MAA droplets flow at a speed of 3.5 × 104 μm/s (volume flow rate 2,500 μL/h) and the AEM droplets flow at 9 × 104 μm/s (volume flow rate 6,500 μL/h). In further experiments, we use a microfluidic device that allows the excess microgels to be washed away before the droplets are mixed. This is achieved by adding a fivefold excess of plain aqueous phase immediately after forming the droplets, thereby reducing the concentration of microgels in the continuous phase (Movie S5).
Fluorescence confocal microscopy.
Confocal imaging was performed using a Leica TCS SP5 confocal laser scanning microscope. Under a 10× DRY objective of NA = 0.3, the fluorophore was excited in the scanning mode with the 543 nm line of a HeNe laser at 50% of its maximum power (0.31 mW at the object level, as measured by the manufacturer). Samples were prepared by placing a droplet of the emulsions to be studied between two thin glass coverslips (VWR) separated by a rubbery gasket (Molecular Probes, press-to-seal™ silicon isolator). The confocal plane was set to be approximately in the middle of the sample. The experiments were conducted at room temperature.
Supplementary Material
Acknowledgments.
We thank Charlotte Knittel for the preparation of the microgels. This project was funded by the Deutsche Forschungsgemeinschaft, the National Science Foundation (DMR-0602684), and the Harvard Materials Research Science and Engineering Center (DMR-0820484), which is gratefully acknowledged. S.S. was a research fellow of the German Academy of Sciences Leopoldina (BMBF-LPD 9901/8-186) and is now a Liebig fellow of the Fund of the German Chemical Industry (FCI). J.T. received funding from FCI.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019196109/-/DCSupplemental.
References
- 1.Lissant KJ. Emulsions and emulsion technology. vol 6. New York: Marcel Dekker; 1974. [Google Scholar]
- 2.deGennes PG, Brochard-Wyart F, Quéré D. Capillarity and Wetting Phenomena. New York: Springer; 2004. [Google Scholar]
- 3.Eow JS, Ghadiri M, Sharif AO, Williams TJ. Electrostatic enhancement of coalescence of water droplets in oil: A review of the current understanding. Chem Eng J. 2001;84:173–192. [Google Scholar]
- 4.Chabert M, Dorfman KD, Viovy JL. Droplet fusion by alternating current (AC) field electrocoalescence in microchannels. Electrophoresis. 2005;26:3706–3715. doi: 10.1002/elps.200500109. [DOI] [PubMed] [Google Scholar]
- 5.Ristenpart WD, Bird JC, Belmonte A, Dollar F, Stone HA. Non-coalescence of oppositely charged drops. Nature. 2009;461:377–380. doi: 10.1038/nature08294. [DOI] [PubMed] [Google Scholar]
- 6.Bird JC, Ristenpart WD, Belmonte A, Stone HA. Critical angle for electrically driven coalescence of two conical droplets. Phys Rev Lett. 2009;103:164502–164505. doi: 10.1103/PhysRevLett.103.164502. [DOI] [PubMed] [Google Scholar]
- 7.Quake SR, Scherer A. From micro- to nanofabrication with soft materials. Science. 2000;290:1536–1540. doi: 10.1126/science.290.5496.1536. [DOI] [PubMed] [Google Scholar]
- 8.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 9.Teh S-Y, Lin R, Hung L-H, Lee AP. Droplet microfluidics. Lab Chip. 2008;8:198–220. doi: 10.1039/b715524g. [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Nieves A, Fernández-Barbero A, Vincent B, de las Nieves FJ. Charge controlled swelling of microgel particles. Macromolecules. 2000;33:2114–2118. [Google Scholar]
- 11.Binks BP. Particles as surfactants-similarities and differences. Curr Opin Colloid Interface Sci. 2002;7:21–41. [Google Scholar]
- 12.Aveyard R, Binks BP, Clint JH. Emulsions stabilised solely by colloidal particles. Adv Colloid Interface Sci. 2003;100-102:503–546. [Google Scholar]
- 13.Boroudjerdi H, et al. Statics and dynamics of strongly charged soft matter. Phys Rep. 2005;416:129–199. [Google Scholar]
- 14.Binks BP, Murakami R. Phase inversion of particle-stabilized materials from foams to dry water. Nat Mater. 2006;5:865–869. doi: 10.1038/nmat1757. [DOI] [PubMed] [Google Scholar]
- 15.Ramsden W. Separation of solids in the surface layers of solutions and suspensions-preliminary account. Proc R Soc London. 1903;72:156–164. [Google Scholar]
- 16.Pickering SU. Emulsions. J Chem Soc Trans. 1907;91:2001–2021. [Google Scholar]
- 17.Read ES, Fujii S, Amalvy JI, Randall DP, Armes SP. Effect of varying the oil phase on the behavior of pH-responsive latex-based emulsifiers: Demulsification versus transitional phase inversion. Langmuir. 2004;20:7422–7429. doi: 10.1021/la049431b. [DOI] [PubMed] [Google Scholar]
- 18.Fujii S, Randall DP, Armes SP. Synthesis of polystyrene/poly[2-(dimethylamino)ethyl methacrylate-stat-ethylene glycol dimethacrylate] core-shell latex particles by seeded emulsion polymerization and their application as stimulus-responsive particulate emulsifiers for oil-in-water emulsions. Langmuir. 2004;20:11329–11335. doi: 10.1021/la048473x. [DOI] [PubMed] [Google Scholar]
- 19.Amalvy JI, Unali G-F, Li Y, Granger-Bevan S, Armes SP. Synthesis of sterically stabilized polystyrene latex particles using cationic block copolymers and macromonomers and their application as stimulus-responsive particulate emulsifiers for oil-in-water emulsions. Langmuir. 2004;20:4345–4354. doi: 10.1021/la035921c. [DOI] [PubMed] [Google Scholar]
- 20.Amalvy JI, Armes SP, Binks BP, Rodrigues JA, Unali G-F. Use of sterically-stabilized polystyrene latex particles as a pH-responsive particulate emulsifier to prepare surfactant-free oil-in-water emulsions. Chem Commun. 2003:1826–1827. doi: 10.1039/b304967a. [DOI] [PubMed] [Google Scholar]
- 21.Binks BP, Murakami R, Armes SP, Fujii S, Schmid A. pH-responsive aqueous foams stabilized by ionizable latex particles. Langmuir. 2007;23:8691–8694. doi: 10.1021/la700444a. [DOI] [PubMed] [Google Scholar]
- 22.He X-D, Ge X-W, Liu H-R, Deng M-G, Zhang Z-C. Self-assembly of pH-responsive acrylate latex particles at emulsion droplets interface. J Appl Polym Sci. 2007;105:1018–1024. [Google Scholar]
- 23.Brugger B, Richtering W. Magnetic, thermosensitive microgels as stimuli-responsive emulsifiers allowing for remote control of separability and stability of oil in water-emulsions. Adv Mater. 2007;19:2973–2978. [Google Scholar]
- 24.Dinsmore AD, et al. Colloidosomes: Selectively permeable capsules composed of colloidal particles. Science. 2002;298:1006–1009. doi: 10.1126/science.1074868. [DOI] [PubMed] [Google Scholar]
- 25.Shah RK, Kim J-W, Weitz DA. Monodisperse stimuli-responsive colloidosomes by self-assembly of microgels in droplets. Langmuir. 2010;26:1561–1565. doi: 10.1021/la9041327. [DOI] [PubMed] [Google Scholar]
- 26.Fujii S, Read ES, Binks BP, Armes SP. Stimulus-responsive emulsifiers based on nanocomposite microgel particles. Adv Mater. 2005;17:1014–1018. [Google Scholar]
- 27.Binks BP, Murakami R, Armes SP, Fujii S. Effects of pH and salt concentration on oil-in-water emulsions stabilized solely by nanocomposite microgel particles. Langmuir. 2006;22:2050–2057. doi: 10.1021/la053017+. [DOI] [PubMed] [Google Scholar]
- 28.Dupin D, Armes SP, Connan C, Reeve P, Baxter SM. How does the nature of the steric stabilizer affect the Pickering emulsifier performance of lightly cross-linked, acid-swellablepoly(2-vinylpyridine) latexes? Langmuir. 2007;23:6903–6910. doi: 10.1021/la063170j. [DOI] [PubMed] [Google Scholar]
- 29.Ngai T, Behrens SH, Auweter H. Novel emulsions stabilized by pH and temperature sensitive microgels. Chem Commun. 2005 doi: 10.1039/b412330a. [DOI] [PubMed] [Google Scholar]
- 30.Brugger B, Rosen BA, Richtering W. Microgels as stimuli responsive stabilizers for emulsionsmicrogels as stimuli responsive stabilizers for emulsions. Langmuir. 2008;24:12202–12208. doi: 10.1021/la8015854. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Ming T, Wang J, Ngai T. High internal phase emulsions stabilized solely by microgel particles. Angew Chem. 2009;121:8642–8645. doi: 10.1002/anie.200902103. Angew Chem Int Ed Engl (2009) 48:8490–8493. [DOI] [PubMed] [Google Scholar]
- 32.Sun G, Li Z, Ngai T. Inversion of particle-stabilized emulsions to form high-internal-phase emulsions. Angew Chem. 2010;122:2209–2212. doi: 10.1002/anie.200907175. Angew Chem Int Ed Engl (2010) 49:2163–2166. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Ngai T. Macroporous polymer from core—shell particle-stabilized pickering emulsions. Langmuir. 2010;26:5088–5092. doi: 10.1021/la903546g. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt S, et al. Influence of microgel architecture and oil polarity on stabilization of emulsions by stimuli-sensitive core-shell poly(N-isopropylacrylamide-co-methacrylic acid) microgels: Mickering versus Pickering behavior? Langmuir. 2011;27:9801–9806. doi: 10.1021/la201823b. [DOI] [PubMed] [Google Scholar]
- 35.Monteux C, et al. Poly(N-isopropylacrylamide) microgels at the oil-water interface: Interfacial properties as a function of temperature. Langmuir. 2010;26:13839–13846. doi: 10.1021/la1019982. [DOI] [PubMed] [Google Scholar]
- 36.Brugger B, Richtering W. Emulsions stabilized by stimuli-sensitive poly(N-isopropylacrylamide)-co-methacrylic acid polymers: Microgels versus low molecular weight polymers. Langmuir. 2008;24:7769–7777. doi: 10.1021/la800522h. [DOI] [PubMed] [Google Scholar]
- 37.Brugger B, Rütten S, Phan K-H, Möller M, Richtering W. The colloidal suprastructure of smart microgels at oil-water interfaces. Angew Chem. 2009;121:4038–4041. doi: 10.1002/anie.200900239. Angew Chem Int Ed Engl (2009) 48:3978–3981. [DOI] [PubMed] [Google Scholar]
- 38.Brugger B, Vermant J, Richtering W. Interfacial layers of stimuli-responsive poly-(N-isopropylacrylamide-co-methacrylicacid) (PNIPAM-co-MAA) microgels characterized by interfacial rheology and compression isotherms. Phys Chem Chem Phys. 2010;12:14573–14578. doi: 10.1039/c0cp01022g. [DOI] [PubMed] [Google Scholar]
- 39.Destribats M, et al. Soft microgels as Pickering emulsion stabilizers: Role of particle deformability. Soft Matter. 2011;7:7689–7698. [Google Scholar]
- 40.Arditty S, Whitby CP, Binks BP, Schmitt V, Leal-Calderon F. Some general features of limited coalescence in solid-stabilized emulsions. Eur Phys J E Soft Matter. 2003;11:273–281. doi: 10.1140/epje/i2003-10018-6. [DOI] [PubMed] [Google Scholar]
- 41.Miyake M, Ogawa K, Kokufuta E. Light-scattering study of polyelectrolyte complex formation between anionic and cationic nanogels in an aqueous salt-free system. Langmuir. 2006;22:7335–7341. doi: 10.1021/la060701v. [DOI] [PubMed] [Google Scholar]
- 42.Hou Y, Ye J, Wei X, Zhang G. Effects of cations on the sorting of oppositely charged microgels. J Phys Chem B. 2009;113:7457–7461. doi: 10.1021/jp902556m. [DOI] [PubMed] [Google Scholar]
- 43.Fernández-Barbero A, Vincent B. Charge heteroaggregation between hard and soft particles. Phys Rev E. 2000;63:011509–011515. doi: 10.1103/PhysRevE.63.011509. [DOI] [PubMed] [Google Scholar]
- 44.Lee M, et al. Multilayer deposition on patterned posts using alternating polyelectrolyte droplets in a microfluidic device. Lab Chip. 2010;10:1160–1166. doi: 10.1039/b919753b. [DOI] [PubMed] [Google Scholar]
- 45.Patish A, Devi S, Shah DO. Importance of 1∶3 molecular ratio on the interfacial properties of mixed surfactant systems. Langmuir. 1999;15:7403–7405. [Google Scholar]
- 46.Binks BP, Liu W, Rodrigues JA. Novel stabilization of emulsions via the heteroaggregation of nanoparticles. Langmuir. 2008;24:4443–4446. doi: 10.1021/la800084d. [DOI] [PubMed] [Google Scholar]
- 47.Tong W, Gao C, Möhwald H. Stable weak polyelectrolyte microcapsules with pH-responsive permeability. Macromolecules. 2006;39:335–340. [Google Scholar]
- 48.Pelton R. Temperature-sensitive aqueous microgels. Adv Colloid Interface Sci. 2000;85:1–33. doi: 10.1016/s0001-8686(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 49.McDonald JC, et al. Fabrication of microfluidic systems in poly(dimethylsiloxane) Electrophoresis. 2000;21:27–40. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 50.Abate AR, Lee D, Do T, Holtze C, Weitz DA. Glass coating for PDMS microfluidic channels by sol-gel methods. Lab Chip. 2008;8:516–518. doi: 10.1039/b800001h. [DOI] [PubMed] [Google Scholar]
- 51.Abate AR, Thiele J, Weinhart M, Weitz DA. Patterning microfluidic device wettability using flow confinement. Lab Chip. 2010;10:1774–1776. doi: 10.1039/c004124f. [DOI] [PubMed] [Google Scholar]
- 52.Chen PJ, Shih CY, Tai YC. Design, fabrication and characterization of monolithic embedded parylenemicrochannels in silicon substrate. Lab Chip. 2006;6:803–810. doi: 10.1039/b600224b. [DOI] [PubMed] [Google Scholar]
- 53.Hua ZS, et al. A versatile microreactor platform featuring a chemical-resistant microvalve array for addressable multiplex syntheses and assays. J Micromech Microeng. 2006;16:1433–1443. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.