Abstract
Protein-trafficking pathways are targeted here in human melanoma cells using methods independent of oncogene mutational status, and the ability to up-regulate and down-regulate tumor treatment sensitivity is demonstrated. Sensitivity of melanoma cells to cis-diaminedichloroplatinum II (cDDP, cis-platin), carboplatin, dacarbazine, or temozolomide together with velaparib, an inhibitor of poly (ADP ribose) polymerase 1, is increased by up to 10-fold by targeting genes that regulate both protein trafficking and the formation of melanosomes, intracellular organelles unique to melanocytes and melanoma cells. Melanoma cells depleted of either of the protein-trafficking regulators vacuolar protein sorting 33A protein (VPS33A) or cappuccino protein (CNO) have increased nuclear localization of cDDP, increased nuclear DNA damage by platination, and increased apoptosis, resulting in increased treatment sensitivity. Depleted cells also exhibit a decreased proportion of intracellular, mature melanosomes compared with undepleted cells. Modulation of protein trafficking via cell-surface signaling by binding the melanocortin 1 receptor with the antagonist agouti-signaling protein decreased the proportion of mature melanosomes formed and increased cDDP sensitivity, whereas receptor binding with the agonist melanocyte-stimulating hormone resulted in an increased proportion of mature melanosomes formed and in decreased sensitivity (i.e., increased resistance) to cDDP. Mutation of the protein-trafficking gene Hps6, known to impair the formation of mature melanosomes, also increased cDDP sensitivity. Together, these results indicate that targeting protein-trafficking molecules markedly increases melanoma treatment sensitivity and influences the degree of melanosomes available for sequestration of therapeutic agents.
Keywords: targeted therapy, treatment resistance, chemotherapy, agouti-signaling peptide
Molecules regulating trafficking pathways present possible therapeutic targets for melanoma treatment, but the function of these proteins in melanoma biology and the potential for treatment response remains largely unexamined to date. Evidence has been accumulating regarding the importance of regulators of protein and vesicular trafficking in melanomas. Trafficking molecules compose a newly recognized class of oncoproteins that serve as molecular drivers of tumor proliferation (1, 2), and reports of physical associations between trafficking and signaling molecules (3) suggest crosstalk and regulatory interplay between protein-trafficking and signaling pathways. However, many protein-trafficking molecules have a generalized cellular distribution, and targeting these may have unintended and severe side effects. Here we focus on the initial assessment of the utility of targeting trafficking molecules that regulate selected pathways functionally important in only a few specific cell types, predominantly melanocytes, to enhance melanoma therapy.
A critical barrier impeding effective therapy of metastatic melanoma is the high rate of tumor resistance to a wide variety of therapies, including inhibitors of signaling and immunomodulatory pathways (4, 5). A strategy of targeting trafficking pathways for melanoma therapy has several advantages that are useful to exploit. These pathways are essential for many cellular processes and are responsible for directing integral membrane proteins (e.g., receptor tyrosine kinases), membrane-associated proteins (e.g., RAS), and soluble cargo proteins to their final cellular destination (6, 7). Targeting these trafficking pathways can potentially influence the function of more than one downstream tumorigenic driver molecule because these pathways traffic many diverse molecules that mediate various cellular processes. Selected pathway machinery molecules are cell lineage specific and function predominantly in melanocytes (8), potentially serving as cell type-specific therapeutic targets in melanomas, with associated adverse side effects anticipated to be minimal. Finally, a therapeutic approach targeting trafficking molecules is not dependent on the presence of mutations in oncogenes.
The VPS33A molecule has been implicated in mediating protein trafficking (9). Homozygous mutation of Vps33A in the buff mouse strain causes a lightened coat color and aberrantly formed eumelanosomes, the intracellular organelles in which brown/black melanin pigment is synthesized (10), suggesting a role for VPS33A in trafficking proteins targeted to eumelanosomes. The cappuccino (CNO) protein is a subunit of the heteromeric biogenesis of lysosome-related organelle complex-1 (BLOC-1), which mediates trafficking at the endosome (11, 12). Mutations in VPS33A and CNO cause cell type-specific symptoms in patients and mice largely limited to melanocytes and platelets resulting in pigment dilution and nonlife-threatening prolonged bleeding times (8). Mutations in the Vps33a or Cno gene both resulted in increased sensitivity to cDDP, etoposide, and vinblastine (13) and blocked the normal process of eumelanosome maturation through four morphological stages (stages I–IV) (10, 14), indicating a fundamental role for VPS33A and CNO in contributing to cellular resistance to multiple cytotoxic agents.
We report here that the sensitivity of human melanoma cells to multiple agents can be significantly increased or decreased by manipulating the regulation of protein trafficking. We used three approaches to perturb protein trafficking, all of which resulted in increased or decreased melanoma treatment sensitivity: (i) RNA silencing to deplete cells of molecules that regulate protein trafficking, (ii) signaling via the cell-surface receptor melanocortin 1 receptor (MC1R), and (iii) gene mutation.
Results
Silencing the Protein-Trafficking Genes VPS33A and CNO Resulted in Aberrant Protein Trafficking and Impaired Melanosome Formation.
We previously demonstrated that primary mouse melanocytes with mutations in Vps33a or Cno had aberrant melanosome formation and increased sensitivity to cis-platin (cDDP) compared with melanocytes from the congenic parental mouse strains (13). To assess whether depletion of VPS33A or CNO proteins in human melanoma cells affects drug sensitivity, we established stable isogenic cell lines by selecting clones of the pigmented human melanoma cell line MNT-1 after transduction with shRNA targeted to either VPS33A or CNO. Decreased VPS33A expression was detected by RT-PCR in selected clones, and decreased expression of CNO was also achieved (Fig. S1).
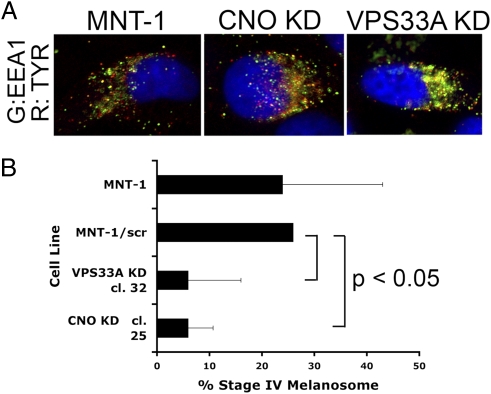
Depletion of either VPS33A or CNO affected protein trafficking (Fig. 1A). The tyrosinase protein (TYR) normally transits through endosomes to melanosomes (11), and in control cells, a small amount of colocalization of tyrosinase was observed with the endosomal marker EEA1, likely due to the brief residence time of TYR in endosomes. After depletion of either VPS33A or CNO, markedly increased colocalization of TYR with EEA1 was evident in a perinuclear vesicular distribution, suggesting that the egress of TYR from endosomes was blocked. TYR egress appeared more dependent on VPS33A function than on CNO function because significantly more colocalization of TYR with EEA1 in the perinuclear vesicular distribution was seen with VPS33A depletion compared with CNO depletion, consistent with a previous report that tyrosinase trafficking is only partially dependent on the function of the BLOC-1 complex, of which CNO is a subunit (11). In the images, the level of TYR in VPS33A- or CNO-depleted cells appeared increased over that seen in control cells; immunoblotting confirmed an increased TYR abundance (Fig. S2), suggesting that blocking the egress of TYR from endosomes had inhibited its degradation in lysosomes.
Fig. 1.
Depletion of VPS33A or CNO in human melanoma cells results in aberrant protein trafficking and loss of stage IV eumelanosome formation. (A) Distribution of TYR protein is altered with depletion of VPS33A or CNO. Immunofluorescence microscopy of MNT-1 cells transduced with scrambled shRNA or depleted of VPS33A (clone 32) or CNO (clone 25) is shown. EEA1 (endosomal marker), green; TYR, red. Original magnification, 60×. (B) Summary of quantitative electron microscopy of mature stage IV eumelanosomes in control cells vs. MNT-1 cells depleted of CNO or VPS33A.
Quantitative electron microscopy revealed that the steady-state percentage of stage IV, fully melanized, mature eumelanosomes was decreased in cells depleted of either VPS33A or CNO (Fig. 1B and Fig. S3), and both VPS33A- and CNO-depleted cells had less melanin compared with control cells (Fig. S4). These results confirm a regulatory role for VPS33A and CNO proteins in governing protein trafficking and melanosome formation and function in human melanoma cells.
Depletion of VPS33A or CNO Resulted in Increased Melanoma Cell Chemosensitivity.
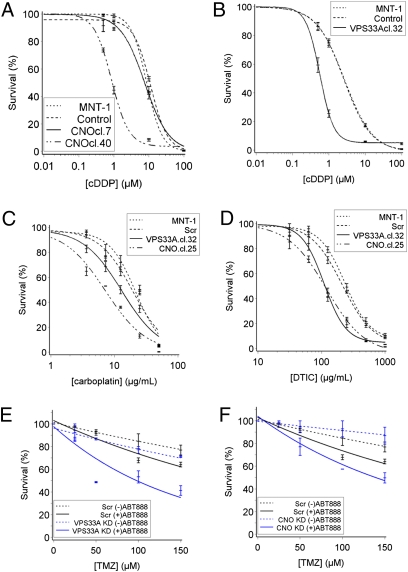
In CNO-depleted melanoma cells, we found that the sensitivity to cDDP was inversely correlated with the level of CNO expression and the percentage of mature stage IV eumelanosomes present in cells. As shown in Fig. 2A, MNT-1 cells transduced with shRNA encoding a randomly scrambled nucleotide sequence were unchanged in cDDP sensitivity compared with untransduced MNT-1 cells. Clone 7, which was weakly depleted of CNO (Fig. S1), also had no significant change in cDDP sensitivity, whereas the efficiently depleted clone 40 had decreased expression levels of CNO by RT-PCR (Fig. S1), a decreased proportion of stage IV mature eumelanosomes, and a 10-fold increase in sensitivity to cDDP (P < 0.0001) (Fig. 2A and Table 1). MNT-1 cells depleted of VPS33A, which also had a decreased proportion of stage IV mature eumelanosomes (Fig. 1C), had a fivefold increase in cDDP sensitivity (P < 0.0001) (Fig. 2B and Table 1). Increased sensitivity to carboplatin and dacarbazine (DTIC) was also observed in depleted cells compared with undepleted cells (Fig. 2 C and D and Table 1). Sensitivity to temozolomide (TMZ) alone did not differ in depleted compared with undepleted cells, but when temozolomide treatment was combined with the poly (ADP ribose) polymerase 1 (PARP-1) inhibitor velaparib, significantly increased sensitivity was observed in depleted cells compared with undepleted cells (Fig. 2 E and F and Table 1). These data demonstrate that, in human melanoma cells, depletion of either trafficking regulator VPS33A or CNO resulted in a significantly increased sensitivity to cDDP, carboplatin, DTIC, and the combination of TMZ/velaparib, and this increased sensitivity correlated with a decreased proportion of mature eumelanosomes being formed.
Fig. 2.
Depletion of VPS33A or CNO increases sensitivity to cDDP, carboplatin, DTIC, and TMZ/velaparib. (A) cDDP sensitivity of CNO-depleted MNT-1. MNT-1, untreated; control, MNT-1 transduced with scrambled shRNA; CNOcl.7 and CNOcl.40, CNO knockdown clones 7 and 40. Clone 7 was inefficiently silenced whereas clone 40 was efficiently silenced (Fig. S1). Only clone 40 shows significantly increased cDDP sensitivity (P < 0.0001). (B) cDDP sensitivity of VPS33A-depleted MNT-1. VPS33Acl.32, VPS33A knockdown clone 32. Note that the curves for the MNT-1 and the control cell line overlie almost exactly; depletion of VPS33A results in significantly increased cDDP sensitivity (P < 0.0001). (C) Increased carboplatin sensitivity in VPS33A-depleted (P < 0.005) or CNO-depleted (P < 0.0001) cells. (D) Increased DTIC sensitivity in VPS33A- or CNO-depleted cells (P < 0.0001). (E and F) Increased sensitivity of VPS33A-depleted (P < 0.05) (E) and CNO-depleted cells (P < 0.005) (F) to combined treatment with TMZ and 50 μM of velaparib (ABT888, Abbott Laboratories).
Table 1.
IC50 for VPS- or CNO-depleted vs. control cells
| Treatment | Cell line | Relative IC50 | P value |
| As below | MNT-1 + scr shRNA | 1 | NA |
| cDDP | VPS33A KD | 0.2 | <0.0001 |
| CNO KD | 0.1 | <0.0001 | |
| Carboplatin | VPS33A KD | 0.7 | <0.005 |
| CNO KD | 0.4 | <0.0001 | |
| DTIC | VPS33A KD | 0.5 | <0.0001 |
| CNO KD | 0.6 | <0.0001 | |
| TMZ | VPS33A KD | 0.7 | 0.2 |
| CNO KD | 2 | 0.2 | |
| TMZ + velaparib | VPS33A KD | 0.2 | <0.05 |
| TMZ + velaparib | CNO KD | 0.3 | <0.005 |
cDDP, cis-platin; DTIC, dacarbazine; TMZ, temozolomide; velaparib, a PARP-1 inhibitor; KD, knockdown. NA, not applicable.
VPS33A- or CNO-Depleted Cells Have an Increased Nuclear Localization of cDDP, Increased Nuclear Platinum-DNA Conjugates, and Increased Apoptosis.
cDDP cytotoxicity is thought to be mediated in part by the conjugation of the cDDP platinum moiety to nuclear DNA, leading to intrastrand DNA cross-linking followed by cellular apoptosis (15). One possible mechanism of resistance could be that undepleted MNT-1 melanoma cells have a more rapid rate of DNA repair compared with depleted cells. However, when global DNA repair was measured for UV-treated VPS33A- or CNO-depleted cells, no difference in the repair of cyclobutane pyrimidine dimers (Fig. S5A) or 6,4-photoproducts was detected in depleted compared with undepleted cells. In fact, no difference in sensitivity to UV irradiation was detected in depleted vs. undepleted cells (Fig. S5B), consistent with the finding of no difference in DNA repair and also suggesting no difference in the resultant apoptosis. Measurement of baseline apoptosis was comparable in untreated depleted and undepleted cells (Fig. 4C), indicating that depletion of VPS33A or CNO did not alter inherent rates of apoptosis.
Fig. 4.
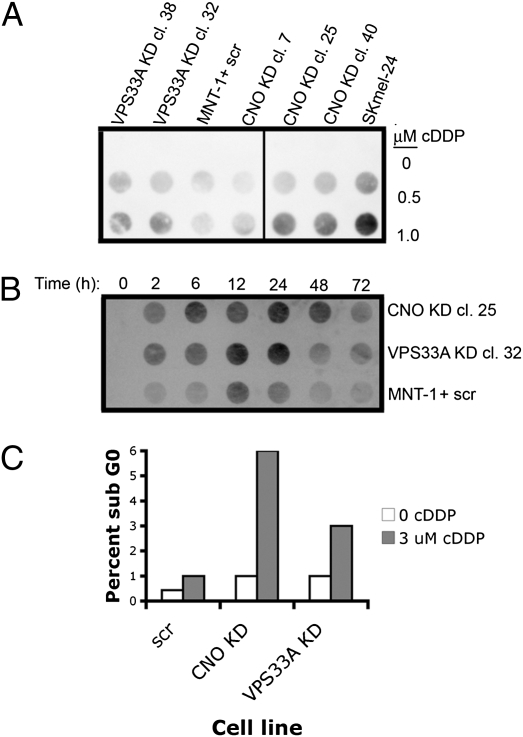
Increased nuclear Pt-DNA conjugates in VPS33A- or CNO-depleted MNT-1 cells. (A) Dose-dependent increase in Pt-DNA and increased detection in depleted compared with undepleted or inefficiently depleted [CNO KD cl. 7] MNT-1 cells. SK-MEL-24, unpigmented human melanoma cell containing immature stage I melanosomes (13), is included as a positive control for Pt-DNA detection. (B) Increased levels of Pt-DNA in VPS33A or CNO-depleted cells incubated with 1 μM cDDP. (C) Increased apoptosis in depleted vs. undepleted MNT-1 cells incubated with cDDP for 2 h, stained with propidium iodide, and then analyzed by flow cytometry.
Another possibility is that, because depletion of VPS33A or CNO impairs eumelanosome maturation, mature eumelanosomes may protect melanoma cells from treatment agents by sequestering the agents away from target molecules such as nuclear DNA, followed possibly by exocytosis and secretion of the sequestered agent. For example, in the case of treatment with cDDP, this model of a melanosomal protective function predicts that, in MNT-1 cells depleted of VPS33A or CNO compared with undepleted cells, there will be (i) an increased nuclear localization of cDDP, (ii) an increase in platinum-DNA (Pt-DNA), and (iii) an increase in the proportion of apoptotic cells.
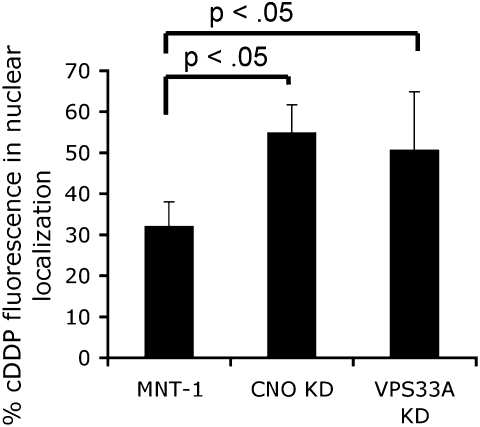
To test this, fluorescence confocal microscopy was used to examine the steady-state distribution of fluorescently tagged cDDP, comparing its localization in VPS33A- or CNO-depleted MNT-1 cells with that in control cells. In control melanoma cells, a minority of labeled cDDP was found in a nuclear localization (vs. in cytoplasmic vesicular structures) whereas in cells depleted of either CNO or VPS33A, an increased proportion of cDDP signal was found localized within the nucleus (Fig. 3 and Fig. S6). Next, the degree to which nuclear DNA was modified by cDDP was compared in depleted vs. undepleted cells using an antibody that specifically recognizes Pt-DNA conjugates. After incubation with cDDP, there was an increased detection of Pt-DNA observed in depleted cells compared with undepleted and weakly depleted cells (Fig. 4A). An unrelated melanoma cell line, SK-MEL-24, known to have only immature melanosomes (13), was observed to also have a marked increase in detection of Pt-DNA compared with control MNT-1 cells (Fig. 4A), which have a predominance of mature eumelanosomes (13), consistent with mature eumelanosomes having a protective role against cDDP. Pt-DNA conjugates accumulated at earlier time points in depleted cells and were detected at higher levels compared with undepleted cells (Fig. 4B). Increased apoptosis was observed in cDDP-treated depleted MNT-1 cells compared with undepleted cells (Fig. 4C). Taken together, these results are consistent with eumelanosomes having a role in protecting melanoma cells from the cytotoxic effects of cDDP by sequestering cDDP, preventing DNA modification and damage and subsequent apoptosis.
Fig. 3.
Increased nuclear localization of cDDP in depleted MNT-1 cells. Quantitation of percentage of total fluorescence localized to the nucleus.
Chemosensitivity Is Up-Regulated or Down-Regulated by Receptor-Mediated Modulation of Protein Trafficking and Eumelanosome Formation.
To further test the role of protein trafficking and eumelanosome formation in influencing melanoma drug sensitivity, we used an alternative method to moderate both processes. The binding of the melanocyte-stimulating hormone (MSH) to the MC1R on the cell surface of melanocytes has been shown to increase mature eumelanosome number, whereas binding of the antagonist agouti-signaling peptide (ASIP) to the receptor causes a decrease in mature eumelanosome formation (16–18). MSH binding to the MC1R has also been shown to influence the trafficking of the catalase protein (19). To assess whether using this alternative method to modulate eumelanosome formation could alter the trafficking of other proteins and also influence melanoma drug sensitivity, we treated MNT-1 cells with either MSH or ASIP-YY, a synthetic homolog of ASIP, and then incubated the cells with cDDP.
Confocal immunofluorescence microscopy confirmed that the steady-state distribution of TYRP1, a protein normally trafficked from the trans-Golgi network (TGN) to melanosomes (20), was markedly altered in both MSH- and ASIP-YY–treated cells, with the distribution pattern differing depending on the treatment agent. With no treatment, a moderate amount of colocalization of TYRP1 and a marker for the TGN was observed (Fig. 5A). After ASIP-YY treatment, there was a dramatic increase in colocalization, suggesting that ASIP-YY treatment caused TYRP-1 to accumulate in the TGN and prevented trafficking to the melanosome. In contrast, after treatment with MSH, very little colocalization of TYRP1 with the TGN marker was observed; however, vesicular structures staining for TYRP1 were seen in a more peripheral localization compared with control MNT-1 cells, suggesting that TYRP-1 was accumulating in peripherally localized structures such as melanosomes. Thus, TYRP-1 trafficking was differentially altered by treatment with ASIP-YY compared with treatment with MSH.
Fig. 5.
Receptor-mediated signaling regulates protein trafficking, melanosome formation, and drug sensitivity. (A) Five days of incubation with 20 nM ASIP-YY increased colocalization of TYRP1 with trans-Golgi marker TGN38. Incubation with 40 nM α-MSH decreased colocalization, and TYRP1-labeled vesicles are more peripherally localized. (B) Incubation of MNT-1 cells with 40 nM of α- MSH for 5 d increased the percentage of mature eumelanosomes; treatment with 20 nM ASIP-YY decreased the percentage. (C) MNT-1 cells treated with 20 or 40 nM of ASIP-YY increased cDDP sensitivity (P < 0.0001). The curves for 20 or 40 nM treated cells overlie almost exactly. (D) MNT-1 cells treated with 40 nM ASIP-YY have increased sensitivity to DTIC (P < 0.0001). (E) MNT-1 cells treated with 20 or 40 nM of α-MSH exhibited an increase in resistance to cDDP (P = 0.0005).
Pretreatment of MNT-1 cells with ASIP-YY decreased the percentage of mature eumelanosomes formed and increased chemosensitivity to cDDP compared with that in cells without ASIP-YY pretreatment (Fig. 5 B and C); IC50 (concentration that inhibits response by 50%) decreased by more than threefold in ASIP-YY–treated cells (P < 0.0001) (Table 2). Sensitivity to DTIC was also increased by ASIP-YY pretreatment (P < 0.0001) (Fig. 5D and Table 2). In contrast, pretreatment with MSH increased the percentage of mature eumelanosomes and, strikingly, increased resistance to cDDP; the IC50 increased by twofold (P = 0.0005) (Fig. 5 B and E and Table 2). These data indicate that exogenous signaling via MC1R can significantly alter the sensitivity of melanoma cells to cDDP or DTIC and that modulating the formation and function of an intracellular organelle by influencing protein trafficking can markedly alter cancer cell treatment response.
Table 2.
IC50 with and without MC1R signaling
| Treatment | Cell line | Relative IC50 | P value |
| As below | MNT-1 | 1 | NA |
| cDDP + ASIP | MNT-1 | 0.3 | <0.0001 |
| cDDP + MSH | MNT-1 | 2.1 | 0.0005 |
| DTIC + ASIP | MNT-1 | 0.5 | <0.0001 |
ASIP, agouti signaling peptide; MSH, melanocyte stimulating hormone.
Mutation of the Protein-Trafficking Regulator Gene Hps6 Increased cDDP Sensitivity.
ASP, the murine homolog to ASIP, has been shown to regulate the transcription of melanosome-trafficking genes, including Hps3 (21), suggesting a mechanism by which binding of ASIP-YY alters protein trafficking to the melanosome. The Hps3 protein is part of a multimeric complex, BLOC-2, that regulates trafficking from the endosome; BLOC-2 also includes the subunit Hps5 and Hps6 proteins (22). We previously demonstrated that mutation of Hps3, Hps5, or Hps6 genes resulted in aberrant melanosome formation (14). To test if alteration of the function of BLOC-2 could influence chemosensitivity, we examined the effects of mutation of Hps6 on treatment sensitivity to cDDP. We observed that immortal ruby-eye (Hps6ru/Hps6ru) melanocytes, derived from mice carrying a mutation in Hps6 (23), have an increased cDDP sensitivity compared with congenic C57BL/6 immortal melanocytes with wild-type Hps6; IC50 was decreased by threefold (P < 0.0001) (Fig. 6 and Table 3), demonstrating that disruption of a third regulator of protein trafficking causes increased chemosensitivity. We conclude that targeting protein-trafficking pathways is an effective strategy for influencing melanoma treatment sensitivity.
Fig. 6.
Mutation of the protein traffic regulator Hps6 gene increases cDDP sensitivity. Immortal melanocytes derived from ruby-eye mice, which have a mutation in the Hps6 gene (Ruby), have increased sensitivity to cDDP compared with congenic melanocytes derived from C57BL/6 mice (Ink4a) (P < 0.0001).
Table 3.
IC50 for immortal murine melanocytes
| Treatment | Cell line | Relative IC50 | P value |
| cDDP | Ink4a | 1 | NA |
| cDDP | Ruby | 0.3 | <0.0001 |
Discussion
We target protein-trafficking pathways found in melanoma cells and report that this approach, which has the advantage of being mutation independent and relatively cell lineage specific, significantly increases the sensitivity to treatment with a number of cytotoxic agents and that treatment sensitivity can be up-regulated or down-regulated via cell-surface signaling. Our data indicate that the efficacy of chemotherapeutics used for melanoma therapy can be greatly enhanced and that, combined with therapies targeting signaling or immunomodulatory pathways, this enhanced chemotherapy has a potentially useful role in melanoma treatment, particularly in light of recently observed high rates of resistance to treatment with small molecule inhibitors alone.
We demonstrate that depleting components of the BLOC-1 (CNO) or BLOC-2 (Hps6) trafficking complexes or the VPS33A protein can increase human melanoma sensitivity to cDDP, carboplatin, DTIC, or the combination of TMZ/velaparib. Melanosomal sequestration of cDDP prevents DNA damage and subsequent apoptosis. Interestingly, although response to TMZ treatment did not differ in VPS33A- or CNO-depleted melanoma cells compared with undepleted control cells, incubation with the PARP-1 inhibitor velaparib increased sensitivity to TMZ in depleted cells, suggesting that velaparib, but not TMZ, is sequestered in melanosomes. It is anticipated that various treatment agents will have different propensities for sequestration, depending on specific molecular properties. This model is further supported by the lack of difference in UV response observed in control and depleted cells because UV light cannot be sequestered in melanosomes. We also demonstrate that cell-surface signaling via the MC1R can either up-regulate or down-regulate response to cDDP or DTIC. This last finding may have clinical implications because it suggests that, in the setting of higher endogenous levels of MSH, treatment of melanoma with certain cytotoxic agents may be less effective.
Targeting selected trafficking proteins in melanoma cells is not anticipated to cause much toxicity in itself because individuals with spontaneously occurring mutations in these proteins have specific cell-type effects limited to melanocytes and platelets, such as pigment dilution and increased bleeding times (8, 24). Although intact melanosome formation early in development is required for normal neurologic function of the eyes and ears, targeting melanosome formation in adulthood is not anticipated to affect hearing or vision because melanosomes in the inner ear and retinal pigment epithelium are stably retained intracellularly, rather than secreted as in cutaneous pigment cells (25, 26), and lack the requirement for replenishment, so minimal ocular or otic toxicity is anticipated.
It was previously noted that melanomas are resistant to apoptosis. The data here indicate that melanomas can be protected from apoptosis by melanosomal sequestration of treatment agents, consistent with our and other groups’ previous findings that melanosome function plays a role in melanoma treatment resistance (13, 27–31). Gottesman and colleagues (29) have shown that chemosensitivity is independent of melanoma pigment content, consistent with a melanosome function alternative to that of melanin synthesis (e.g., sequestration) influencing chemosensitivity. Sequestration may also contribute to melanoma resistance to newer treatment agents, e.g., inhibitors of signaling pathways, by preventing interaction with target molecules and thus limiting the resultant proapoptotic signaling. This may also be a protective mechanism in nonmelanoma tumor cells that have regulated secretory vesicles related to or similar to melanosomes. Targeting protein-trafficking pathways to alter organelle function and to enhance treatment efficacy may be useful in other malignancies that are difficult to treat—specifically, malignancies derived from secretory tissues that have intracellular organelles related to the melanosome, i.e., lysosome-related organelles (32).
In summary, targeting protein-trafficking regulators in melanoma cells caused a redistribution of intracellular cDDP, increased cDDP-mediated DNA damage, and caused a 2- to 10-fold increased sensitivity to cDDP, carboplatin, DTIC, or the combination of TMZ/velaparib. Regulators of protein trafficking can play an influential role in melanoma chemotherapy response, likely by facilitating drug sequestration, and are candidates for targeted therapy to significantly increase cancer treatment efficacy. Further studies are needed to assess if melanosomal sequestration plays a role in melanoma resistance to other classes of treatment agents, such as small molecule inhibitors of signaling pathways.
Materials and Methods
Cell Culture.
Human melanoma cells MNT-1 and SK-MEL-24 were cultured as previously published (13). The mutant mouse melanocyte line melan-ru1-3 was obtained by crossing ruby-eye (Hps6ru/Hps6ru) mice to C57BL/6 Cdkn2a (Ink4a-Arf) null mice, yielding cells that are immortal (33). The ruby-eye mice were congenic on the C57BL/6 background. The melan-Ink4a-Arf1 was used as the control immortal C57BL/6 line (33). All experiments complied with local and European legislation concerning the use of animals for research purposes. Mouse melanocyte lines were established and cultured as previously published (33, 34).
RNA-Mediated Gene Silencing.
MNT-1 melanoma cells were transduced with scrambled sequence shRNA or shRNA targeting VPS33A or CNO in lentiviral vectors (Santa Cruz Biotechnology) following the manufacturer's instructions (see SI Materials and Methods for details).
Drug Sensitivity Studies.
Drug sensitivity studies were performed as previously published (13). To test UV sensitivity, cells were drained of medium and exposed to ultraviolet C radiation (254 nm, 1.4 J⋅m−2/s). Calibration with UV endonuclease on a plasmid gave a pyrimidine dimer yield approximately sevenfold higher than ultraviolet B radiation for the same fluence.
Electron Microscopy.
Samples were prepared (with minor modifications as noted in the SI Materials and Methods), and the percentage of melanosomes was determined as previously published (14). At least 10 individual melanoma cells were evaluated for each cell line or condition.
Measurement of Global DNA Repair.
Measurement was done as previously described (35, 36). See SI Materials and Methods for details.
Detection of Pt-DNA Conjugates.
Pt-DNA conjugates were detected by DNA dot blotting followed by immunostaining with an antibody specific for Pt-(GG) intrastrand DNA adducts (Oncolyze), as previously published (37) with some modifications (SI Materials and Methods).
Fluorescence Microscopy.
Antibodies and reagents are listed in SI Materials and Methods.
ASIP-YY Protein Synthesis.
The agouti-signaling peptide sequence, acetyl-KKVVRPRTPLSAPCVATRNSCKPPAPACCDPCASCYCRFFRSACYCRVLSLNC–amidation, was produced on an Applied Biosystems 433A peptide synthesizer using standard Fmoc chemistry (38). This specific sequence corresponds to the double-mutant ASIP(80–132, Q115Y, S124Y) in which the two X → Y mutations are included to enhance folding and stability. Referred to as ASIP-YY, this protein exhibits MC1R affinity and selectivity equivalent to that of wild-type ASIP (16).
Statistics.
Different nonlinear models were compared with fit dose–response curves to calculate IC50 for the survival curves. Four-parameter or three-parameter logistic equations were used for data that exhibited a sigmoidal relationship between concentration and survival and exponential decay equations for data that did not follow a sigmoidal pattern but appeared to gradually plateau. All calculations were performed using Proc NLIN from the SAS system, version 9.1 (SAS Institute).
Supplementary Material
Acknowledgments
Thanks to Debbie Crumrine, Ivy Hsieh, Lucy Huang, and Jerelyn Magnusson for excellent technical assistance; to Lynn Lamoreux (Texas A&M University) for crossing mouse mutants and providing tissue; and to Lynn Plowright for preparation of the new melanocyte cultures. Work was supported by the American Cancer Society; the Melanoma Research Alliance (M.L.W.); Veterans Administration Merit Award (D.H.O.); University of California Cancer Research Campaign (J.E.C.); National Institutes of Health Grant DK064265 (to G.L.M.); and Wellcome Trust Program Grant 078327 (to D.C.B., E.V.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118366109/-/DCSupplemental.
References
- 1.Scott KL, Chin L. Signaling from the Golgi: Mechanisms and models for Golgi phosphoprotein 3-mediated oncogenesis. Clin Cancer Res. 2010;16:2229–2234. doi: 10.1158/1078-0432.CCR-09-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akavia UD, et al. An integrated approach to uncover drivers of cancer. Cell. 2010;143:1005–1017. doi: 10.1016/j.cell.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kedlaya R, et al. Interactions between GIPC-APPL and GIPC-TRP1 regulate melanosomal protein trafficking and melanogenesis in human melanocytes. Arch Biochem Biophys. 2011;508:227–233. doi: 10.1016/j.abb.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 5.Chapman PB, et al. BRIM-3 Study Group Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamberlain MD, et al. Deregulation of Rab5 and Rab4 proteins in p85R274A-expressing cells alters PDGFR trafficking. Cell Signal. 2010;22:1562–1575. doi: 10.1016/j.cellsig.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Misaki R, et al. Palmitoylated Ras proteins traffic through recycling endosomes to the plasma membrane during exocytosis. J Cell Biol. 2010;191(1):23–29. doi: 10.1083/jcb.200911143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei ML. Hermansky-Pudlak syndrome: A disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19(1):19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 9.Guo X, et al. Involvement of vps33a in the fusion of uroplakin-degrading multivesicular bodies with lysosomes. Traffic. 2009;10:1350–1361. doi: 10.1111/j.1600-0854.2009.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen T, Wei ML. Characterization of melanosomes in murine Hermansky-Pudlak syndrome: Mechanisms of hypopigmentation. J Invest Dermatol. 2004;122:452–460. doi: 10.1046/j.0022-202X.2004.22117.x. [DOI] [PubMed] [Google Scholar]
- 11.Setty SR, et al. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol Biol Cell. 2007;18:768–780. doi: 10.1091/mbc.E06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Pietro SM, et al. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol Biol Cell. 2006;17:4027–4038. doi: 10.1091/mbc.E06-05-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie T, Nguyen T, Hupe M, Wei ML. Multidrug resistance decreases with mutations of melanosomal regulatory genes. Cancer Res. 2009;69:992–999. doi: 10.1158/0008-5472.CAN-08-0506. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen T, et al. Melanosome morphologies in murine models of Hermansky-Pudlak syndrome reflect blocks in organelle development. J Invest Dermatol. 2002;119:1156–1164. doi: 10.1046/j.1523-1747.2002.19535.x. [DOI] [PubMed] [Google Scholar]
- 15.Todd RC, Lippard SJ. Inhibition of transcription by platinum antitumor compounds. Metallomics. 2009;1:280–291. doi: 10.1039/b907567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNulty JC, et al. Structures of the agouti signaling protein. J Mol Biol. 2005;346:1059–1070. doi: 10.1016/j.jmb.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki I, Cone RD, Im S, Nordlund J, Abdel-Malek ZA. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology. 1996;137:1627–1633. doi: 10.1210/endo.137.5.8612494. [DOI] [PubMed] [Google Scholar]
- 18.Sakai C, et al. Modulation of murine melanocyte function in vitro by agouti signal protein. EMBO J. 1997;16:3544–3552. doi: 10.1093/emboj/16.12.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maresca V, et al. MC1R stimulation by alpha-MSH induces catalase and promotes its re-distribution to the cell periphery and dendrites Pigment Cell. Melanoma Res. 2010;23:263–275. doi: 10.1111/j.1755-148X.2010.00673.x. [DOI] [PubMed] [Google Scholar]
- 20.Truschel ST, et al. ESCRT-I function is required for Tyrp1 transport from early endosomes to the melanosome limiting membrane. Traffic. 2009;10:1318–1336. doi: 10.1111/j.1600-0854.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Pape E, et al. Microarray analysis sheds light on the dedifferentiating role of agouti signal protein in murine melanocytes via the Mc1r. Proc Natl Acad Sci USA. 2009;106:1802–1807. doi: 10.1073/pnas.0806753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, et al. The BLOC interactomes form a network in endosomal transport. J Genet Genomics. 2007;34:669–682. doi: 10.1016/S1673-8527(07)60076-9. [DOI] [PubMed] [Google Scholar]
- 23.Gautam R, et al. The Hermansky-Pudlak syndrome 3 (cocoa) protein is a component of the biogenesis of lysosome-related organelles complex-2 (BLOC-2) J Biol Chem. 2004;279:12935–12942. doi: 10.1074/jbc.M311311200. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki T, et al. The mouse organellar biogenesis mutant buff results from a mutation in Vps33a, a homologue of yeast vps33 and Drosophila carnation. Proc Natl Acad Sci USA. 2003;100:1146–1150. doi: 10.1073/pnas.0237292100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopes VS, Wasmeier C, Seabra MC, Futter CE. Melanosome maturation defect in Rab38-deficient retinal pigment epithelium results in instability of immature melanosomes during transient melanogenesis. Mol Biol Cell. 2007;18:3914–3927. doi: 10.1091/mbc.E07-03-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters TA, Kuijpers W, Tonnaer EL, van Muijen GN, Jap PH. Distribution and features of melanocytes during inner ear development in pigmented and albino rats. Hear Res. 1995;85(1-2):169–180. doi: 10.1016/0378-5955(95)00043-4. [DOI] [PubMed] [Google Scholar]
- 27.Chen KG, et al. Melanosomal sequestration of cytotoxic drugs contributes to the intractability of malignant melanomas. Proc Natl Acad Sci USA. 2006;103:9903–9907. doi: 10.1073/pnas.0600213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen KG, et al. Involvement of ABC transporters in melanogenesis and the development of multidrug resistance of melanoma Pigment Cell. Melanoma Res. 2009;22:740–749. doi: 10.1111/j.1755-148X.2009.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen KG, et al. Influence of melanosome dynamics on melanoma drug sensitivity. J Natl Cancer Inst. 2009;101:1259–1271. doi: 10.1093/jnci/djp259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brozyna AA, VanMiddlesworth L, Slominski AT. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int J Cancer. 2008;123:1448–1456. doi: 10.1002/ijc.23664. [DOI] [PubMed] [Google Scholar]
- 31.Slominski A, Zbytek B, Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int J Cancer. 2009;124:1470–1477. doi: 10.1002/ijc.24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, Gahl WA. Disorders of lysosome-related organelle biogenesis: Clinical and molecular genetics. Annu Rev Genomics Hum Genet. 2008;9:359–386. doi: 10.1146/annurev.genom.9.081307.164303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sviderskaya EV, et al. p16(Ink4a) in melanocyte senescence and differentiation. J Natl Cancer Inst. 2002;94:446–454. doi: 10.1093/jnci/94.6.446. [DOI] [PubMed] [Google Scholar]
- 34.Sviderskaya EV, et al. Functional neurons and melanocytes induced from immortal lines of postnatal neural crest-like stem cells. FASEB J. 2009;23:3179–3192. doi: 10.1096/fj.08-123596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh DH, Yeh K. Differentiating human keratinocytes are deficient in p53 but retain global nucleotide excision repair following ultraviolet radiation. DNA Repair (Amst) 2005;4:1149–1159. doi: 10.1016/j.dnarep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson BE, Oh DH. Proficient global nucleotide excision repair in human keratinocytes but not in fibroblasts deficient in p53. Cancer Res. 2005;65:8723–8729. doi: 10.1158/0008-5472.CAN-05-1457. [DOI] [PubMed] [Google Scholar]
- 37.Rebillard A, et al. Cisplatin-induced apoptosis involves membrane fluidification via inhibition of NHE1 in human colon cancer cells. Cancer Res. 2007;67:7865–7874. doi: 10.1158/0008-5472.CAN-07-0353. [DOI] [PubMed] [Google Scholar]
- 38.Patel MP, et al. Loop-swapped chimeras of the agouti-related protein and the agouti signaling protein identify contacts required for melanocortin 1 receptor selectivity and antagonism. J Mol Biol. 2010;404(1):45–55. doi: 10.1016/j.jmb.2010.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.